Abstract
Optogenetic manipulations are widely used for investigating the contribution of genetically identified cell types to behavior. Simultaneous electrophysiological recordings are less common, although they are critical for characterizing the specific impact of optogenetic manipulations on neural circuits in vivo. This is at least in part because combining photostimulation with large-scale electrophysiological recordings remains technically challenging, which also poses a limitation for performing extracellular identification experiments. Currently available interfaces that guide light of the appropriate wavelength into the brain combined with an electrophysiological modality suffer from various drawbacks such as a bulky size, low spatial resolution, heat dissipation, or photovoltaic artifacts. To address these challenges, we have designed and fabricated an integrated ultrathin neural interface with 12 optical outputs and 24 electrodes. We used the device to measure the effect of localized stimulation in the anterior olfactory cortex, a paleocortical structure involved in olfactory processing. Our experiments in adult mice demonstrate that because of its small dimensions, our novel tool causes far less tissue damage than commercially available devices. Moreover, optical stimulation and recording can be performed simultaneously, with no measurable electrical artifact during optical stimulation. Importantly, optical stimulation can be confined to small volumes with approximately single-cortical layer thickness. Finally, we find that even highly localized optical stimulation causes inhibition at more distant sites.
NEW & NOTEWORTHY In this study, we establish a novel tool for simultaneous extracellular recording and optogenetic photostimulation. Because the device is built using established microchip technology, it can be fabricated with high reproducibility and reliability. We further show that even very localized stimulation affects neural firing far beyond the stimulation site. This demonstrates the difficulty in predicting circuit-level effects of optogenetic manipulations and highlights the importance of closely monitoring neural activity in optogenetic experiments.
Keywords: grating coupler, optogenetics, optoprobe, silicon photonics, waveguide
INTRODUCTION
A key experimental approach to investigate how the dynamic activity in neural circuits gives rise to perception and behavior involves the manipulation of specific neural cell types with optogenetics. To critically evaluate the impact of optogenetic manipulations on neural circuits in vivo or for identifying the response properties of specific neural subtypes (Cohen et al. 2012), it is necessary to illuminate brain tissue while simultaneously monitoring the activity of many neurons. However, combining multisite photostimulation with extracellular electrophysiology for interrogating neural circuits at high resolution remains technically challenging, and the yield of optogenetically targeted recordings is typically low, despite great progress in the development of neural interfaces for optogenetic applications (Fan and Li 2015).
Devices that use optical fibers suffer from several limitations such as mechanical stiffness, impractical connection to an external light source, and limited spatial addressability (Anikeeva et al. 2011; Canales et al. 2015; Gagnon-Turcotte et al. 2015; Tamura et al. 2012). Optical fibers also can be mounted to conventional silicone probes, to achieve a larger number of recording channels (Stark et al. 2012), but such devices have a large cross-sectional area and thus cause undesirable levels of tissue damage.
Advanced nano- and microtechnology have enabled the fabrication of waveguides, which convey light inside confined structures. Waveguides fabricated in standard laboratories are typically relatively large such that only one to two waveguides can be integrated per electrode shank, which limits the density of stimulation sites (Im et al. 2011; Schwaerzle et al. 2013; Son et al. 2015; Wu et al. 2013).
The integration of micro light-emitting diodes (µLEDs) on the shank itself obviates the need for waveguides and in-coupling of light (Kim et al. 2013; Wu et al. 2015), but this approach also has limitations due to heat dissemination in the brain, in particular when larger light intensities are required. Also, µLEDs small enough to be monolithically integrated are today only available for blue light. A promising approach involves the integration of thin-film LEDs into optoelectronic devices (Goßler et al. 2014), although not easily compatible with the complementary metal-oxide semiconductor (CMOS)-related process required to create high-density electrodes (Jun et al. 2017). Finally, the occurrence of electrical artifacts due to LED control currents is a problem for single-unit recordings in optogenetic studies (Wu et al. 2015).
In conclusion, despite great progress in the development of optogenetic interfaces, combining multichannel recordings with multisite optical illumination at high spatial resolution remains a challenge today. Therefore, relatively little is known about the effects of localized illumination and the spatial extent of photoactivation on spiking activity in a circuit.
We have designed and fabricated an ultrathin (30 μm) optical neural probe, co-integrating 12 silicon nitride (SixNy) waveguides and 24 biocompatible titanium nitride (TiN) electrodes on a 100-µm-wide shank (Hoffman et al. 2016). In this study, we used this new device to investigate the effect of spatially confined optogenetic stimulation with blue light (450 nm) in vivo. Our measurements in the anterior olfactory cortex (or nucleus, AON) of anesthetized and awake behaving mice reveal that because of its small dimensions, our novel tool causes far less tissue damage than a commercially available device (NeuroNexus A1×8-4mm-200-1250-OA16-1; Royer et al. 2010). We further demonstrate that optical stimulation and recording can be performed simultaneously, with no measurable electrical artifact during optical stimulation. Optical stimulation can be confined to small volumes with approximately single-cortical layer thickness. Finally, we find that highly localized optical stimulation causes inhibition at more distant sites, suggesting recruitment of inhibitory networks (Kay and Brunjes 2014).
Our work establishes a novel tool for optogenetics that can be fabricated with high reproducibility and reliability because it is based on a CMOS-compatible technology (Jun et al. 2017). Moreover, we show that even very localized photostimulation has wide-ranging effects beyond the stimulation site. This demonstrates the difficulty in predicting circuit-level effects of optogenetic manipulations and highlights the need for closely monitoring neural activity in optogenetic experiments.
MATERIALS AND METHODS
Probe fabrication and characterization.
The optoelectrical chips have been fabricated at Imec on 200-mm silicon wafers using a back-end CMOS-compatible process as follows. 1) First, a 400-nm layer of SixOy is deposited using plasma-enhanced chemical vapor deposition (PECVD). 2) Next, 800 nm of aluminum and 60 nm of TiN are deposited and patterned by means of dry etching to form the connection lines. 3a) The bottom cladding layer (SixOy) is then deposited by PECVD and 3b) planarized using chemical mechanical polishing (CMP) down to 400 nm. The CMP step reduces the losses by decreasing the roughness of the horizontal walls. 4) The waveguide core layer (220 nm of SixNy) is subsequently deposited by PECVD. 5) The grating couplers (GCs) are defined on top of the core layer using 193-nm optical lithography and a partial dry etching (132 nm) of the SixNy. 6) The waveguide cores are defined with 193-nm optical lithography and a dry etching of SixNy down to the SixOy (bottom cladding). 7a) The remaining SixNy is covered by a PECVD layer of SixOy (top cladding), which is 7b) planarized using CMP down to 150 nm. Thereafter, an extra SixOy layer of 300 nm is deposited for passivation. 8) The electrodes are formed on vias of 1-µm width that are etched in the SixOy. 9) Subsequently, they are filled with TiN, which is deposited with reactive physical vapor deposition and patterned with reactive ion etching (RIE). 10) Next, the bond pads are defined by etching wide openings in the SixOy down to the metallization layer. 11) The optoprobe outline is defined by RIE and a Bosch-type silicon etching 80 µm deep. 12) Finally, the wafers that are destined for in vivo systems are back-side ground on tape to a thickness of 30 or 50 µm, which also separates each of the probes from the main chip.
The waveguide propagation loss in the devices was measured using the following setup. Each input coupler was illuminated with a transistor outline (TO)-can packaged laser diode (PL450B; Osram). The emitting window of the TO package was placed as close as possible on top of the input grating with a motorized micromanipulator (SM5; Luigs-Neumann). Because the laser diode was still inside of the package, the emitting point is located at least 900 µm above the input grating. The light emitted by the output couplers was collected by a 100-µm-core optical fiber (FG105ACA; Thorlabs) and measured by a photo sensor (S151C; Thorlabs) connected to an optical power meter (PM200; Thorlabs). The optical fiber was placed on top of the output coupler by means of a manual micromanipulator (MS3/M; Thorlabs). The measurements taken from two different chips reveal a mean waveguide loss of 1.1 ± 0.4 dB/cm.
To quantify the reliability of coupling the laser diode onto the device, we repeatedly disconnected and reconnected the laser diode (n = 20), measuring the power intensity after each step. With the device measured, we observed a standard error of 0.2 µW at a mean power of 3.2 µW, suggesting overall reliable and repeatable coupling.
Integrated optoelectronic system.
The system is composed of two parts: one that electrically connects the probe to external amplifiers (probe carrier) and another that carries the laser diode. The laser diode carrier and the probe carrier are latched together through a custom-made two-part magnetic fastener. During assembly time, one side of the fastener is glued to the probe carrier. Subsequently, the laser diode carrier is slid back and forth on top of the free side of the fastener with a micromanipulator until the light beam is aligned to a desired input grating. During this time, the corresponding output is monitored with a power meter and displaced around the input grating until the output power is maximized.
After the laser diode is aligned, a layer of ultraviolet curable glue is applied between the laser diode carrier and the free side of the fastener, and UV light is applied to glue the parts together. After this process, the laser diode and the probe carrier can be detached and reattached by means of the magnetic link. The laser diode carrier can be interchanged with other laser diode carriers that have been previously aligned for different input gratings. In this way the system can achieve a manual activation of different light outputs. Importantly, input grating couplers on the base of the probe are sufficiently separated to ensure the illumination field generated by the laser diode does not reach two input gratings at the same time.
Viral vector construction and production.
A rAAV2/7 CamKII (0.4)–ChR2(L132C/T159C)–mCherry vector was produced by the Leuven Viral Vector Core as described earlier (Van der Perren et al. 2011). In short, rAAV2-based vectors packaged in AAV7-capsid were produced on the basis of the triple transfection method with a transfer plasmid, the AAV serotype 7 plasmid (pAAV7), and an AAV helper plasmid. The ChR2(L132C/T159C)–mCherry construct was a kind gift of Dr. Peter Hegemann (Humboldt University of Berlin) (Prigge et al. 2012) and cloned in the in-house transfer plasmid. A viral titer of 7.32 × 1012 GCs/ml was obtained as determined by real-time quantitative PCR.
Stereotactic injection.
All animal experiments were performed in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and approved by the Bioethical Committee of the KU Leuven (permit no. p067/2016). Adult female C57Bl/6J mice were housed under a 12:12-h light-dark cycle with food and water ad libitum.
Anesthesia was induced by intraperitoneal injection of a mixture of ketamine (70 mg/kg, Nimatek; Eurovet) and medetomidine (1 mg/kg, Domitor; Pfizer). The mice were fixed in a stereotactic head frame (Stoelting), a midline incision of the skin was made, and a small craniotomy was drilled at the location of the AON (2.9 mm anterior-posterior, 1.0 mm lateral-medial relative to bregma). A total volume of 30 nl of viral vector was injected through a borosilicate glass capillary with a Nanoject II injector (Drummond) at a depth of 2.8 mm relative to the dura. After the injection, the needle was left in place for an additional 5 min to allow diffusion before being slowly withdrawn from the brain. The skin was sutured, and anesthesia was reversed with an intraperitoneal injection of atipamezol (0.5 mg/kg, Antisedan; Pfizer). As a postoperative analgesic, buprenorphine (0.09 g/kg, Vetergesic; Patheon UK) was administered intraperitoneally.
Extracellular recording and optical stimulation.
Anesthesia was induced by intraperitoneal injection of a mixture of ketamine (70 mg/kg, Nimatek; Eurovet) and medetomidine (1 mg/kg, Domitor; Pfizer). The mouse was fixed in a stereotactic frame (Stoelting), and a craniotomy was made above the AON. Two bone screws were placed posterior to the craniotomy for grounding and reference purposes. The optoelectrical neural probe was fixed onto a stereotactic holder and slowly lowered into the brain. The laser diode (PL450B; Osram) was powered by a Mightex laser driver (Sirius universal LED controller; Mightex Systems), which was modulated by a stimulator (STG2000; MultiChannel Systems).
To perform electrophysiological recordings in awake animals, a head plate was fixed onto the skull using dental cement (Espe RelyX luting, 3M; acrylic dental repair powder, Lang) at least 2 wk before the recording session. During at least 1 wk, the animals were habituated for head restraining every day.
Recordings of neural signals and timestamps of light delivery were obtained with the Digital Lynx recording system (Neuralynx) at 32 kHz and bandpass filtered between 600 and 6,000 Hz. Spikes were sorted online and offline using the KlustaKwik built-in tool of Spikesort3D (Neuralynx). A recording session included 15-min baseline recording before any stimulation.
Odor delivery.
Odorants were delivered with a custom-made, constant-air flow olfactometer, described previously (Esquivelzeta Rabell et al. 2017). An odor flow of clean air (0.1 l/min) passed through small vials (4 ml) containing an odorant chemical dissolved in mineral oil and was delivered to the animal in a small angle, avoiding airflow to the animal’s eyes. The olfactometer was controlled with custom LabVIEW software (National instruments). Odorants were presented for 2 s and in a random order and number of repeats. The experiment was performed inside a sound- and light-isolated box (75 cm3) with constant exhaust to ensure rapid clearance of odorant stimuli and prevent distraction of the animal.
Data analysis.
Data were analyzed using custom MATLAB (The MathWorks) scripts. We calculated the likelihood (L) ratio and isolation distance for all sorted spikes. Only units with isolation distance above 10 and an L ratio below 0.3 were included in the data set (Anikeeva et al. 2011; Schmitzer-Torbert et al. 2005). A normalized firing rate per unit was calculated for each stimulation paradigm as the median firing rate during light on subtracted by the basal firing rate obtained in the baseline recording (15-min prestimulation recording). For every unit, significant stimulation or inhibition was tested on the firing rate during light on vs. baseline firing using a two-tailed Kruskal-Wallis test with a 95% confidence interval. Post hoc comparison was performed using Dunn’s test, and the results were corrected for multiple testing using Benjamini-Hochberg correction. The spike latency and fidelity were determined using the 20-Hz stimulation paradigm. For calculation of the spike latency, the mean time of onset of the first spike after light on was calculated across all light pulses during the paradigm. Spike fidelity was determined as the percentage of light pulses that were followed by a spike in the 15 ms after light onset.
To compare the waveform of spontaneous and light-evoked spikes, the raw signal of the light-evoked spikes was extracted from the light-on periods of the 20-Hz stimulation paradigm. Spontaneously occurring spike waveforms were extracted from the baseline recording.
Odorant responses were normalized to baseline firing rate before odor onset. Significant responses were identified using a one-way ANOVA and post hoc Bonferroni testing. The results were corrected for multiple testing using Benjamini-Hochberg correction.
Perfusion and immunohistochemistry.
After the recording session, mice were perfused for histological analysis. Mice were deeply anesthetized by intraperitoneal injection of pentobarbital sodium (60 mg/kg, Nembutal; Ceva Sante Animale) and perfused transcardially with saline followed by ice-cold 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Animals prepared for CamKII analysis were perfused with a mix of 4% PFA and 0.1% glutaraldehyde. After overnight fixation in 4% PFA, 50-µm-thick coronal brain sections were cut with a vibratome (HM 650 V; Microm). To identify the location of the probe tract, immunohistochemical staining against mCherry and counterstaining with hematoxylin were performed on every second section throughout the AON.
For the mCherry staining, the free-floating sections were pretreated with 3% hydrogen peroxide (Chem-Laboratory) in PBS-Triton X-100 (PBS/T) 0.1% for 10 min and incubated overnight with rabbit anti-red fluorescent protein (anti-RFP; 1:5,000; Rockland Immunochemicals) in PBS/T 0.1% with 10% normal swine serum (Dako). Next, a biotinylated swine anti-rabbit Ig (1:300; Dako) was used, followed by incubation with a streptavidin-horseradish peroxidase complex (1:1,000; Dako). Immunoreactivity was visualized using diaminobenzidine (0.4 mg/ml; Sigma-Aldrich) as a chromogen. The sections were mounted on coverslips and counterstained with hematoxylin (Leica Biosystems). After a dehydration series, stained sections were mounted with DPX (Sigma-Aldrich) and visualized with a light microscope (Leica Microsystems).
For immunofluorescent staining, sections were washed in PBS, preblocked with 10% normal horse serum in PBS/T 0.2%, and incubated overnight with rabbit anti-RFP (1:1,000; Rockland Immunochemicals) and mouse anti-CamKII (1:200; Abcam) in PBS/T 0.2% with 10% horse serum. After being washed with PBS, sections were incubated for 2 h with donkey anti-mouse Alexa Fluor 488 (Molecular Probes, Invitrogen) and donkey anti-rabbit Alexa Fluor 555 (Molecular Probes, Invitrogen) in PBS/T 0.2%. Next, the sections were washed in PBS and mounted with Mowiol (Sigma) and DAPI (1:10 000 Invitrogen). The sections were analyzed using a laser scanning confocal microscope (Zeiss LSM 710) with a laser of wavelengths 455, 488, and 543 nm and the Zen 2010 software.
RESULTS
Probe design and physical properties.
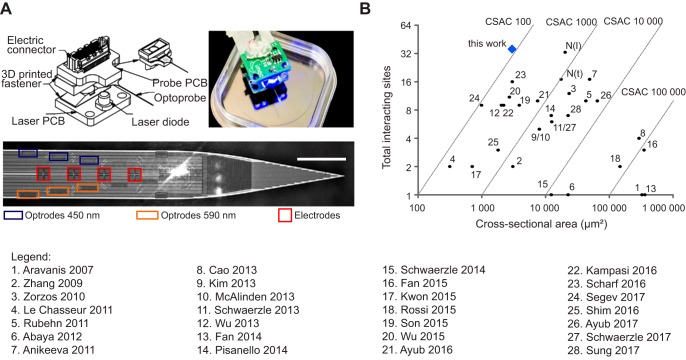
We designed a new optoelectrical neural interface, which incorporates 12 optical outputs and 24 biocompatible titanium nitride electrodes on a shank of 100-µm width, 30-µm thickness and 10-mm length (Hoffman et al. 2016). Briefly, a neural shank was connected to an electrical printed circuit board (PCB) that provided the interface to an external recording amplifier (Fig. 1A). Another PCB containing a miniature laser diode was connected via a detachable thermal insulator that prevents heat dissipation into the brain. The light was coupled in and out of fully integrated SixNy waveguides using grating couplers, designed and optimized for use in optogenetics applications. Grating couplers are common photonic devices that are used to efficiently couple light in and out of planar waveguides at defined angles. Light outlets were placed symmetrically on the probe shank with half of them optimized for blue light (450 nm) and half for amber light (590 nm). Light exits the grating couplers in an angle of 15–20°, avoiding direct light illumination on the electrodes. The size of the electrodes and optical outlets (respectively 10 × 10 and 6 × 20 µm2) is comparable to a neuronal cell body, enabling spatially defined stimulation on the cellular level while reaching the necessary optical power for ChR2 activation (1 mW/mm2; Boyden et al. 2005). The electrodes were placed in a tetrode (TT) configuration with a vertical pitch of 62 µm. Optical outputs were placed symmetrically between two TTs, resulting in a distance of 31 µm between the TT and the next optical output in either direction. During assembly of the optoelectronic system, we aligned one single blue light laser diode over selected grating couplers to enable stimulation through a single optical outlet. After alignment, the laser diode was fixed to the probe with a three-dimensional (3-D) printed magnetic reversible latch mechanism. In this way, the diode will self-align when attached to the probe during stimulation experiments. The overall weight of the neural probe was kept below 2 g to enable chronic implantation.
Fig. 1.
Optoelectrode with high density of interacting sites. A: schematic overview of probe. Top left, the optoprobe is glued on top of an electrical printed circuit board (PCB; probe PCB). A laser diode (LD) is soldered onto another PCB (laser PCB), which is joined to the probe PCB by a detachable thermal insulator. Top right, image of a fully assembled system. Bottom, close-up of the probe shank with the different optical outputs (470 nm, blue rectangles; 590 nm, orange rectangles) and electrodes (red squares). Each electrode is 10 × 10 µm; interelectrode distance is 1 µm. Scale bar, 100 µm. B: comparison of the density of interactive sites to existing neural interfaces, with numbers indicating their source (1, Aravanis et al. 2007; 2, Zhang et al. 2009; 3, Zorzos et al. 2010; 4, LeChasseur et al. 2011; 5, Rubehn et al. 2011; 6, Abaya et al. 2012; 7, Anikeeva et al. 2011; 8, Cao et al. 2013; 9,Kim et al. 2013; 10, McAlinden et al. 2013; 11, Schwaerzle et al. 2013; 12, Wu et al. 2013; 13, Fan et al. 2014; 14, Pisanello et al. 2014; 15, Schwaerzle et al. 2014; 16, Fan et al. 2015; 17, Kwon et al. 2015; 18, Rossi et al. 2015; 19, Son et al. 2015; 20, Wu et al. 2015; 21, Ayub et al. 2016; 22, Kampasi et al. 2016; 23, Scharf et al. 2016; 24, Segev et al. 2017; 25, Shim et al. 2016; 26, Ayub et al. 2017; 27, Schwaerzle et al. 2017; 28, Sung et al. 2017). N(l) and N(t) refer to a standard NeuroNexus probe with a linear or tetrode configuration, respectively. The total number of interacting sites was obtained by summation of all optical outlets and electrodes. The cross-sectional area is measured as the coronal section through the probe shank. The cross-sectional area coefficient (CSAC) is defined as the ratio of the cross-sectional area to the combined number of optical outlets and electrode sites.
To allow for a quantitative comparison of the relative density of electrodes and optical sites on our novel interface with existing neural probes, we determined the cross-sectional area coefficient (CSAC), defined as the ratio of the cross-sectional area to the combined number of optical outlets and electrode sites (Lopez et al. 2014). This coefficient reflects the advantage of a high number of interactive sites to study a large subset of neurons in a complex circuit while reducing the insertional damage. Compared with the existing neural interfaces, including the commonly used probes from NeuroNexus (Royer et al. 2010), this newly developed optoelectrical neural probe has the best CSAC (Fig. 1B).
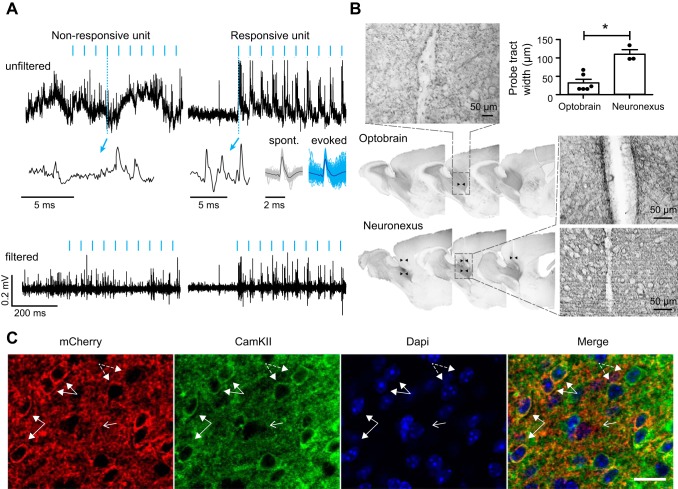
The subsequent in vivo characterization of the optoelectrode was performed in the dorsal AON, a paleocortical structure involved in olfactory processing. The AON is characterized by only two tangential cell layers that encircle the anterior portion of the anterior commissure, an outer plexiform layer (layer I) dense with fibers, and an inner cell zone (layer II; see Fig. 3C). Each layer is roughly 400 µm thick. The AON layers gradually merge with three-layered piriform cortex (Brunjes et al. 2005; Brunjes and Kenerson 2010). Thus, compared with neocortex, the AON has a more simplified anatomical organization, which facilitated our analysis. We used animals injected with recombinant AAV expressing a double mutant channelrhodopsin, ChR2(L132C/T159C), for improved sensitivity and ion conductance (Prigge et al. 2012), fused to mCherry under the control of the CamKII promoter. Immunohistochemical analysis for mCherry and CamKII confirmed robust transduction of ChR2 in CamKII+ cells (Fig. 2C). The CamKII promoter has been demonstrated to drive ChR2 expression in pyramidal cells in visual cortex (Scheyltjens et al. 2015). Because pyramidal cells in the AON pars principalis have similar morphological, electrophysiological, and molecular properties to pyramidal cells found in neocortex and hippocampus (Kay and Brunjes 2014), it seems plausible ChR2 expression was largely confined to pyramidal cells in the AON.
Fig. 3.
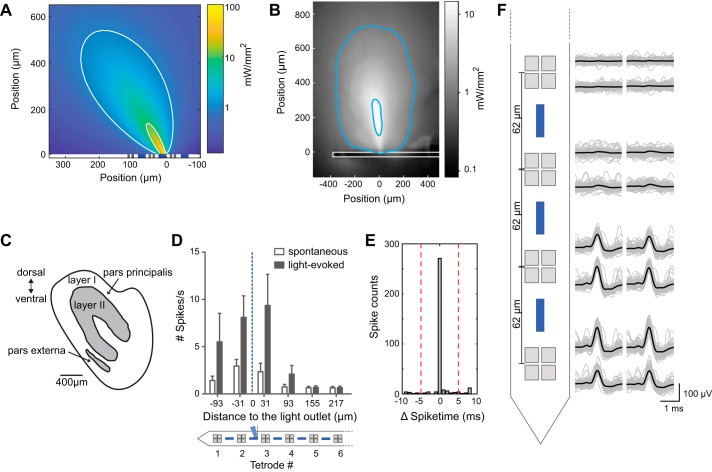
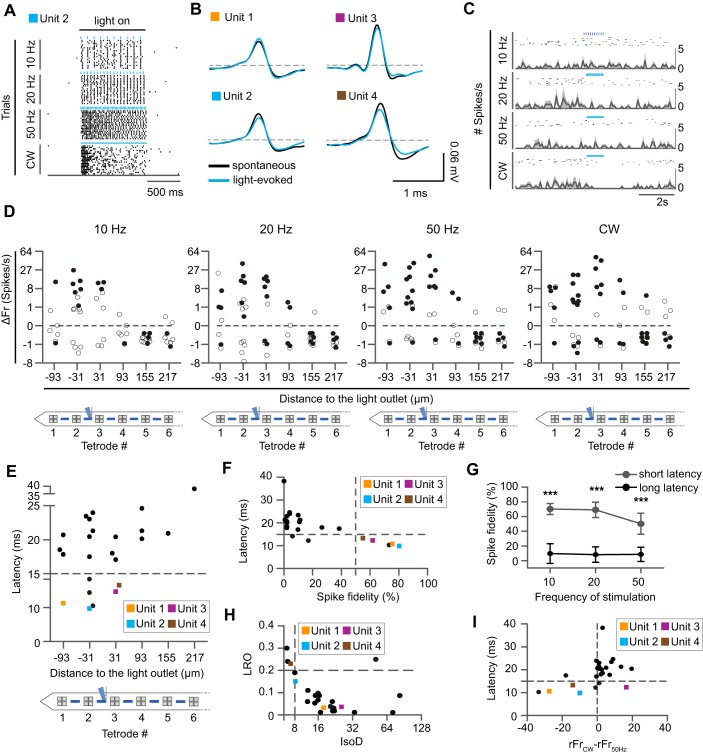
Spatially confined optogenetic stimulation in vivo. A: simulation of light distribution in the brain. White contour lines correspond to power densities of 10 and 1 mW/mm2. Gray and blue squares indicate the size and position of electrodes and optical outlets, respectively. B: illumination profile in brain tissue. The probe (outlined in white) was inserted into brain tissue and placed under a confocal microscope. On the basis of known light power at the outlet, we calculated the power density in the tissue to delineate experimentally determined contour lines (blue) corresponding to power densities of 10 and 1 mW/mm2. Aspect ratio is different from that in A. C: schematic overview of the anterior olfactory nucleus, in which recordings were performed. D: summary of mean firing rate (spikes/s, ±SE) of all single units (n = 60) recorded in all recording sessions in anesthetized mice (n = 6) in relation to the distance of the tetrode (TT) to the light outlet. TTs are numbered, beginning with TT1 from the shank tip. E: in one recording session, the same unit was recorded in 2 different TTs. The correlogram of spontaneous spikes recorded with these 2 TTs shows simultaneous occurrence of spikes within a narrow time window (light-induced spikes were excluded from analysis). F: spike amplitude distribution across the probe for the recording in E. Raw (gray) and mean (black) voltage are shown from 4 TTs. The 2 TTs at bottom show simultaneously recorded spikes (see also correlogram in E). Probe geometry is not in scale.
Fig. 2.
Absence of a light artifact and limited tissue damage. A: no photo-induced light artifact was observed in vivo. The anterior olfactory nucleus was transduced with rAAV2/7-CamKII-ChR2(L132C/T159C)-mCherry, and electrophysiological recordings were performed in awake head-restrained mice. Top and bottom graphs represent raw and filtered (600–6,000 Hz) signals, respectively, of a nonresponsive (left) and a responsive unit (right) recorded on the same tetrode during 20-Hz stimulation with 5-ms light pulses of blue light (470 nm). Blue arrows indicate a magnified view of a raw signal fragment during optical stimulation. Spontaneous (black) and light-evoked (blue) waveforms are represented for the responsive unit (cross-correlation ~98%). B: reduced insertional damage compared with that for a commercially available probe (NeuroNexus) with 125-µm optical fiber. Sagittal sections of diaminobenzidine staining for mCherry are shown surrounding the probe tract of both probes (indicated with black arrowheads). Insets show an enlargement of the probe tract of both devices. At top right, the mean width (±SD) of the probe tract is represented for the optoelectrode (n = 6) and NeuroNexus probe (n = 3). *P < 0.01 (one-way ANOVA, P < 0.05, df = 2). C: selective expression of ChR2-mCherry in CamKII+ cells. Confocal images show mCherry and CamKII expression at ×40 magnification. Example cells positive for both mCherry and CamKII are highlighted by closed arrows. Example cells positive for CamKII but not mCherry are highlighted by dashed arrows. Arrow with open head points to a cell that is negative for mCherry and CamKII. Scale bar, 30 µm.
To demonstrate the optoelectrode can elicit action potentials when the brain is illuminated with blue light through the waveguide, we performed simultaneous extracellular recording and optogenetic stimulation experiments. During stimulation, clear stimulation-locked spiking activity could be observed. The waveforms of spontaneous and photostimulated spikes were highly correlated (≥98%; Fig. 2A), suggesting the same neuron was recorded during and outside stimulation. Importantly, we did not find any evidence for electrical artifacts in the recordings (Fig. 2A). Our histological analysis further revealed that our optoelectrode causes far less insertion damage compared with the commonly used, commercially available NeuroNexus probe, which features a 125-µm optical fiber glued on top of an electrode shank (50 µm). Insertional damage by the NeuroNexus probes was detected across 300 µm of brain tissue, compared with 50 µm for our probe (P = 0.024, Mann-Whitney U-test; Fig. 2B).
Spatially confined optogenetic stimulation.
Next, we evaluated the spatial characteristics of the illumination pattern obtained through a single light outlet. Light exits the waveguide through a grating coupler and enters the brain in an angle that maximizes out-coupling efficiency. We simulated the tissue illumination pattern from one light output with a Monte Carlo simulation (Fang and Boas 2009) (Fig. 3A). We set the total light intensity in the simulation to 4 µW, based on measurements obtained with the optoelectrode (3.7 ± 0.19, mean ± SE). At a distance of several hundred micrometers, the light intensity drops to 1 mW/mm2, which, on the basis of in vitro studies (Aravanis et al. 2007; Zhang et al. 2006), would result in only 50% chance of eliciting spikes. For effective and reliable light-induced spiking, a higher light intensity of 10 mW/mm2 is typically required (Boyden et al. 2005; Han and Boyden 2007; Zhang et al. 2007). According to the simulation, robust optogenetic activation would thus be confined to a maximum distance of 133 µm and a maximum width of 30 µm.
To validate the results from the simulation, we also measured the beam profile from a single outlet in brain tissue (Fig. 3B). On the basis of known light power at the outlet, we further calculated the power density in the tissue to delineate experimentally determined contour lines corresponding to power densities of 10 and 1 mW/mm2, respectively. We found that the illumination pattern is largely consistent with the simulation, although it seems that the light propagates slightly further into the tissue than predicted by the simulation. The high-power density zone with 10 mW/mm2, which is expected to enable robust optogenetic activation, was confined to roughly 50 µm around the light outlet in the horizontal direction and up to 300 µm in the vertical direction, respectively.
To measure the impact of this illumination pattern on spiking patterns in vivo, we performed extracellular recordings in anesthetized C57BL/6J mice and applied different light stimulation paradigms through the second light outlet from the shank tip. We stimulated with 5-ms pulses for 1 s at 10, 20, or 50 Hz or with a continuous wave (CW), repeating each paradigm 20 times while recording a total of 60 signal units in 6 animals. Pooling all units from all experiments and across all stimulation paradigms demonstrates that optical stimulation causes a net increase in spiking activity (mean spike/s of all single units across) at the TTs surrounding the light outlet, but not at more distant TTs (Fig. 3D).
To estimate the detection range of our electrodes in the AON, we identified a neuron that was clearly recorded simultaneously on two neighboring tetrodes, indicated by the simultaneous occurrence of spikes in two separate TTs (Fig. 3E). On the basis of the probe geometry, this suggests a minimum detection range of >80 µm (inter-TT distance 62 µm + 10 µm for each electrode; Fig. 3F).
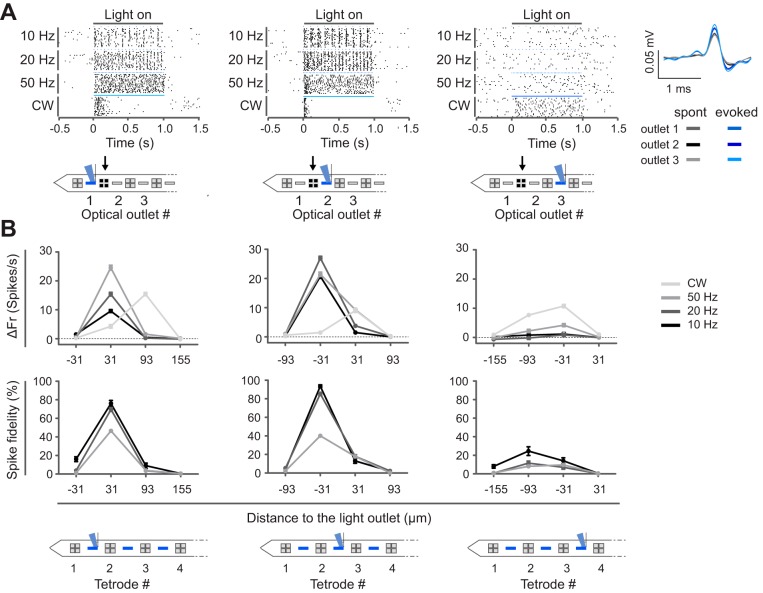
Next, we explored the spatial illumination profile in more detail by performing a recording session in an anesthetized animal (n = 1) in which the stimulation light outlet was sequentially moved through all possible positions (Fig. 4). During the experiment, we sequentially placed the laser diode onto different input grating coupler positions while continuously recording neural signals from all TTs. In this way, we could follow up the response of the same units while varying the position of stimulation across the shank. When we stimulated through the second light outlet from the shank tip, a highly responsive unit was identified in TT2 (Fig. 4A). This unit responded to optical stimulation with a robust increase in firing rate (optogenetic response, firing rate during light stimulation subtracted with the basal firing rate) that occurred with high spike fidelity. Of note, both light-evoked and spontaneously occurring spike waveforms were identical (cross-correlation >99%) throughout the recording sessions (~45 min each), indicating the absence of probe drift during the experiment (Fig. 4A). Strikingly, when we stimulated through the adjacent outlet (light outlet 3), 93 µm further away from the recording TT2, the optogenetic response and spike fidelity dropped significantly for all stimulation paradigms, except for CW stimulation (P < 0.0001, Kruskal Wallis and Dunn’s post test, Bonferroni correction for multiple testing). The fact that direct photoactivation was abolished when the light source was moved more than 93 µm away implies that the probe enables highly localized stimulation. CW illumination caused lower optogenetic responses with less fidelity when the light was emitted proximal to the TT compared with illumination through a more distal light output (P < 0.0001, Kruskal Wallis and Dunn’s post test, Bonferroni correction for multiple testing). This peculiar pattern might suggest that excess stimulation drives the unit on TT2 into a depolarization block (Herman et al. 2014), also illustrated in the raster plot (Fig. 4A). Interestingly, when light outlet 3 was used no additional, clearly responsive units appeared in the vicinity of the light outlet (Fig. 4B). Given the dense labeling of CamKII+ cells in the AON, this provides further evidence for a highly localized stimulation profile of the optoelectrode.
Fig. 4.
Highly localized neuronal activations. A: continuous single-unit recording during sequential activation of one light outlet after another. Spike raster plots are for an example unit recorded at TT2 (indicated with black arrow, activated light outlet indicated in blue). Every stimulation paradigm was repeated 20 times. The waveforms of the light-induced spikes (blue) were identical to the spontaneously occurring spikes (gray) across the different recording sessions (cross-correlation ~99%, quantified during 20-Hz stimulation). B: baseline normalized firing rate (ΔFr; median ± interquartile range of 20 repetitions) and spike fidelity of one recorded unit per TT in TT1–TT4 across all stimulation paradigms (different shades of gray) for a given position of the light outlet (indicated in blue). Reliable spiking in TT2 is only evoked when light is emitted in close proximity, from either light outlet 1 or 2, but not from light outlet 3.
Local direct stimulation and bidirectional modulation.
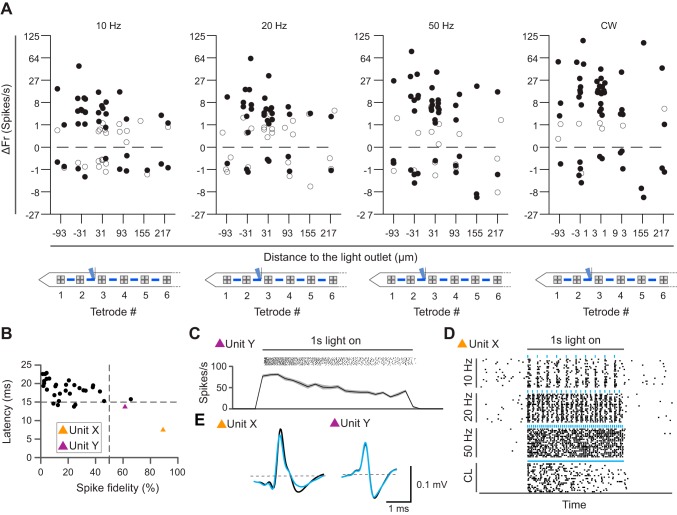
To evaluate the broader effect of spatially confined, local stimulation on the microcircuit in vivo, we analyzed all single units from all TTs in our data set from anesthetized mice, obtained during stimulation through a single light outlet (n = 6). In line with our earlier findings (Fig. 4B), we measured significant optogenetic activation at the TT closest to the light outlet (Fig. 5, A, B, and D). With CW, significantly activated units could be found up to three TTs further away from the activated light outlet, suggesting that the increased power-density of CW recruits more distal neurons. At the same time, we also found an increasing number of units proximal to the light outlet, which were significantly depressed by CW, in line with a higher probability for a depolarization block.
Fig. 5.
Proximal and distal modulation of neural activity in anesthetized animals. A: spike raster plot of a significantly activated unit (unit 2; blue square) recorded in the second TT from the probe tip at 10, 20, or 50 Hz or with a continuous wave (CW). B: mean spontaneous (black) and light-induced (blue) spiking of examples of 4 highly responsive units (cross-correlation ~99%), labeled units 1–4, respectively, and marked with orange, blue, purple, and brown squares, respectively, throughout the figure. C: spike raster plot and peristimulus time histograms of a significantly inhibited example unit. D: median baseline-normalized firing rate (ΔFr) of all units (n = 60) recorded in anesthetized animals (n = 6) during different light stimulation paradigms. Significantly activated or inhibited units are marked by closed circles (Kruskal Wallis and Dunn’s post hoc test, P < 0.05, Benjamini-Hochberg correction for multiple testing). All other units are marked by open circles. CW results in greater spread of significantly activated neurons but also increases the number of significantly inhibited neurons. E: mean latency of all significantly activated units relative to their respective distance from the light outlet. Shortest latencies are observed close to the light outlet. F: mean latency of all significantly activated units vs. spike fidelity. Units 1–4 are characterized by a spike fidelity >50% and a latency <15 ms. These units are presumed directly activated neurons. G: presumed directly activated units spike reliably in response to optical stimulation across stimulation frequencies. Values are mean spike fidelity(±SD) of all presumed directly activated units (gray) and all other units (black; 2-way ANOVA and Bonferroni post hoc, dflatency = 1, dffrequency = 2, P < 0.0). ***P < 0.0001. H: quality metrics of all significantly activated units as measured by likelihood ratio (LRO) and isolation distance (IsoD). I: correlation between mean latency of all significantly activated units and the inhibiting effect of CW (calculated as the difference of the normalized firing rate during CW and 50-Hz stimulation, rFrCW−rFr50Hz).
Significant inhibition during optical stimulation was not restricted to units proximal to the light outlet under CW illumination. Rather, many units positioned distal to the light outlet were significantly inhibited, including during pulsed stimulation (Fig. 5D). The fact, that inhibition was observed along the entire probe shank suggests that spatially confined local neuronal stimulation caused inhibition at the network level in the AON.
To address the question as to which units were excited directly vs. indirectly through synaptic connections, we investigated spike latencies (time to first spike, Fig. 5E) and spike fidelity (reliability of evoking spikes) in all activated units (Fig. 5F). Units with short spike latency and high spike fidelity are typically considered to be directly activated and thus to originate from the genetically targeted cell type expressing ChR2 (Zhang et al. 2013). In our data set, we found 5 neurons with a latency below 15 ms (during 20-Hz stimulation), which we consider CamKII+ cells (Fig. 5F, mean latency 10.25 ± 0.4 ms). These relatively long spike latencies in presumed directly activated neurons may reflect gradual opening of opsin channels under low light intensity (Wu et al. 2015). When the stimulation frequency was increased to 50 Hz, the spike fidelity of these units decreased slightly, but not significantly (Fig. 5G, Kruskal-Wallis and Dunn’s post hoc test, P = 0.2319). Their light-evoked waveforms had a correlation of >99% to the spontaneously occurring spikes (Fig. 5B). Presumed directly activated units were well isolated (Fig. 5H), and they exhibited markedly reduced firing rates during CW application, supporting the view they entered depolarization block. Consistent with this, we found a significant correlation between the spike latency during 20-Hz stimulation and the observed decreased firing rate during CW (Spearman’s r = 0.526, P < 0.01) throughout the data set (Fig. 5I).
Optogenetic modulation profile is maintained in the awake state.
Given that both spontaneous and optogenetically evoked firing rates are affected by anesthesia (Cardin et al. 2010), we also studied circuit-level effects of local optogenetic stimulation in awake, head-restrained animals (n = 5). As expected, the mean baseline firing rate across all units was higher in awake (3.4 ± 0.6 spikes/s) compared with anesthetized animals (1.7 ± 0.3 spikes/s). The profile of optogenetic responses across the different TTs was broadly similar (Fig. 6A). The effect of optogenetic stimulation on firing rates was more pronounced, as indicated by larger inhibition and excitation across stimulation frequencies, compared with that in anesthetized mice. Among all recorded units, we considered two units as directly activated (latency time 9.6 ± 2.2 ms, spike fidelity 82.7 ± 10.8%; Fig. 6B). In line with the recordings in anaesthetized mice, these two units also decreased their firing rate during CW stimulation (Fig. 6, C and D).
Fig. 6.
Pattern of optogenetic circuit modulation is maintained in awake animals. A: median normalized firing rate (ΔFr) of all single units (n = 58) recorded in awake (n = 5) animals during various light stimulation paradigms. Significantly activated and inhibited units are indicated by closed circles; all other units are open circles. B: mean latency of all significantly stimulated units (determined with Kruskal-Wallis and Dunn’s post hoc test, P < 0.05; Benjamini-Hochberg correction for multiple testing) vs. the spike fidelity. Presumed directly activated units (units X and Y) are indicated with orange and purple triangles, respectively. C: spike raster and peristimulus time histogram of a presumed directly stimulated unit (unit Y, purple in B) during CW application. D: spike raster and peristimulus time histogram of the presumed directly stimulated unit (unit X, orange in B) during different stimulation paradigms. Both presumed directly activated units seem to undergo depolarization block. E: overlay of mean spontaneous (black) and light-induced (blue) spike waveforms of presumed directly stimulated units (correlation ~98%).
Response of an identified and nonidentified neurons during behavior.
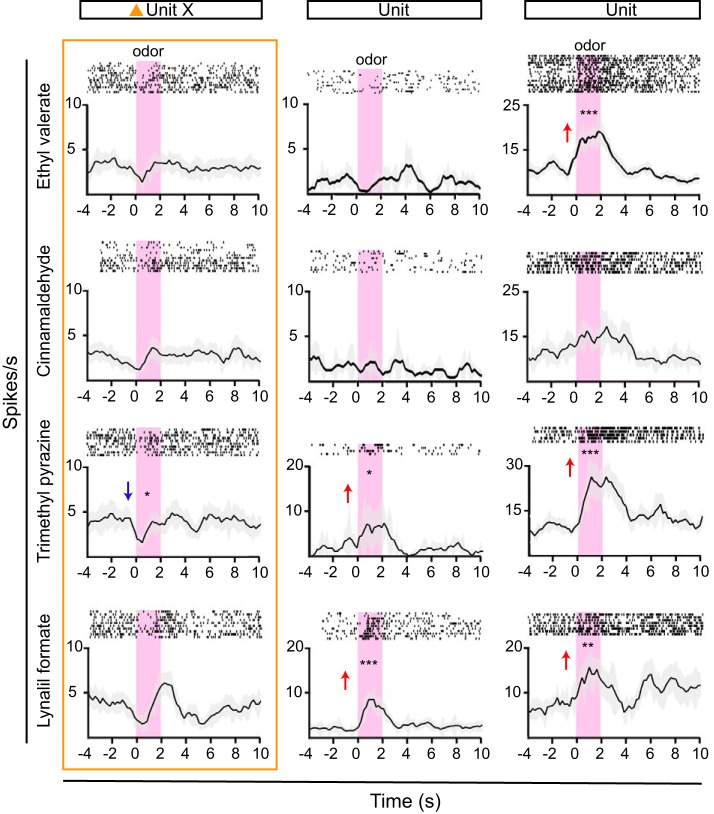
We finally used our optoelectrode to record neural response profiles in behaving mice. To a subset of the animals recorded in awake head-restrained state (n = 5), we presented a panel of four odorants. Not surprisingly, we observed robust odorant responses in the AON pars principalis after odorant stimulation. This is in line with recordings performed previously in anesthetized rats (Kikuta et al. 2008). An example recording from one animal is shown in Fig. 7. One unit (unit X, also shown in Fig. 6) was presumably directly activated, whereas two other simultaneously recorded neurons were not. Interestingly, the identified CamKII+ neuron appeared to have a specific inhibitory response to odorant presentation (Fig. 7, unit X), whereas the other two units were activated by specific odorants (Fig. 7). In conclusion, this proof-of-concept experiment shows the novel optoelectrode is well suited for investigating neuronal response properties in the brain of awake, behaving mice.
Fig. 7.
Diverse odorant responses in anterior olfactory nucleus neurons of awake, behaving mice. Peristimulus time histograms and spike raster plots of 3 example units and their responses to a panel of 4 odorants are shown. Odorants are presented for 2 s (pink shading). Units responded to specific odorants or multiple odorants with increasing (red arrow) or decreasing (blue arrow) firing rates, compared with baseline (significant responses: *P < 0.05; **P < 0.005; ***P < 0.005. Unit X (orange triangle, same unit as in Fig. 6, B–E) is presumed directly activated and thus appears to reflect an optogenetically identified CamKII+ neuron. This unit appears to be inhibited by odorant stimulation (one-way ANOVA and Bonferroni post hoc test, df = 11, P < 0.05).
DISCUSSION
We have designed and fabricated an ultrathin, high-density optical-electrical interface for combined optogenetic stimulation and extracellular recording. We used this new device to investigate the effect of spatially confined optogenetic stimulation in vivo. Our measurements in the anterior olfactory cortex of anesthetized and awake, behaving mice reveal that because of its small dimensions, the probe causes far less tissue damage than commercially available devices. Moreover, we show that optical stimulation and recording can be performed simultaneously, with no measurable electrical artifact during optical stimulation. Optical stimulation can be confined to small volumes with approximately single-cortical layer thickness. Finally, we find that such highly localized optical stimulation causes inhibition at more distant sites.
Advantages and limitations of the optoelectrode.
A key technical achievement of our optoelectrode is the realization of a small pitch between the electrodes and optical outlets. This allows for good axial coverage and enables high spatial resolution while keeping the cross-sectional area small. These features are particularly useful for studying dense layered structures such as the AON, hippocampus, or neocortex. By limiting tissue damage, the optoelectrode also allows for a more precise histological reconstruction of recording sites, which improves the resolution for assigning neural response properties to particular anatomical subdivisions. Notably, the advantage of small dimension is common to all approaches, which circumvents the need for inserting optical fibers into the brain, including those using LEDs incorporated in the probe shank (Kampasi et al. 2016). The fact that light is emitted from the light outlet through a grating coupler at a defined angle, nearly perpendicular to the probe, prevents the direct illumination of the electrodes, which in turn abolishes electrical artifacts caused by the photovoltaic effect (Welkenhuysen et al. 2016). Being able to record neural discharges without electrical artifacts is a unique feature of the optoelectrical interface presented.
Another advantage of the optoelectrode technology is that it is based on established CMOS-related processes, which allow for highly reproducible, reliable fabrication. This is in contrast to most interfaces described previously, which exist as (published) prototypes but cannot readily be fabricated at large volumes (e.g., Kim et al. 2013).
A major limitation of the current system is that only one single light source at a time can be mounted to the probe base because of the large dimensions of the laser diode. Accordingly, switching between different light outlets requires handling the laser diode on the animal. Our current development addresses this limitation to expand the number of independently addressable outlets. Possible solutions include integrating multiple bare laser diodes on the probe base or using a multifiber connector, connected to an optoelectronic switch board and an external light source.
Spatially confined illumination.
The characterization of the spatial profile, using sequential illumination through different light outlets, reveals that the probe can stimulate CamKII+ AON neurons with a narrowly confined spatial resolution. In both anesthetized and awake animals, presumed directly activated units were only observed in the TT flanking the activated light outlet, at a horizontal distance of merely 31 µm. Only 1 unit of 5 directly modulated units was found at a horizontal distance of 93 µm. This is remarkably consistent with our simulation, which suggested spikes can be reliably evoked at a maximal horizontal distance of 30 µm from the light outlet. It also fits the experimental observation of brain tissue illumination, suggesting a similar stimulation range. Taken together, these findings highlight the advantage of grating couplers for precisely out-coupling light in a narrow band at a defined angle.
In the vertical dimension, the simulation estimated reliable optogenetic activation at a maximum distance of 133 µm, whereas the experimental data indicated maximal illumination distance of ~300 µm in tissue. It has previously been demonstrated, with the use of similar low-impedance electrodes in the hippocampus, that spike activity could be detected at least over a 200-µm vertical span (Henze et al. 2000). On the basis of capture of a neuron simultaneously recorded on two neighboring tetrodes, we estimate that the recording range was likely at least 100 µm. Given the empirical illumination range of up to 300 µm in the vertical direction, it is therefore possible, in principle, that we stimulated neurons that were not recorded. One way to address this issue experimentally would be to reduce the light intensity. However, our recordings also show that even with the chosen power settings, only a few neurons were activated with high fidelity, suggesting we were already operating in a suboptimal stimulation regime. In our view, the most plausible explanation for this apparent discrepancy is that threshold power densities, which are mainly derived from in vitro work, underestimate the light intensities required to robustly elicit action potentials in vivo.
In addition to evoking spikes close to the cell bodies from which we also recorded extracellular discharges, we likely also illuminated passing axons or dendrites, possibly evoking action potentials in far more distal neurons, whose discharge were clearly out of the recording range.
We further demonstrate increasing recruitment of activated neurons along the probe with increasing stimulation frequencies (and hence increasing power densities). CW illumination, on the other hand, can induce a hyperpolarization block in neurons close to the light outlet. These findings highlight the inherent complexity associated with illuminating specific cells within a neural circuit. They further illustrate the delicate balance of tuning light intensities and stimulation frequencies in optogenetic experiments, even when illumination is spatially fairly well controlled.
Local stimulation and global modulation.
In both anesthetized and awake mice, we observed widespread inhibition across the probe axis (Figs. 5D and 6A), induced by local stimulation from a single outlet. What mechanisms might account for this inhibitory effect? ChR2 expression was confined to CamKII+ neurons, most of which are expected to be pyramidal cells in layer II of the AON. Stimulation of these excitatory neurons, which are similar to neocortical pyramidal cells (Brunjes et al. 2005), likely recruited inhibitory networks through postsynaptic inhibition. Although the precise connectivity rules within the AON remain to be determined, at least five inhibitory cell types have been identified (Kay and Brunjes 2014), which might contribute to the observed widespread inhibition. Of particular interest is a subclass of so-called horizontal cells found in layer 2, whose dendrites are in fact not oriented horizontally to the pial surface, but vertically instead. These “vertical” horizontal cells project both to the deep portions of layer 2 and the superficial portions of layer 1. Cells with a similar morphology have also been identified in the piriform cortex, but not among the many inhibitory cell classes in the hippocampus or neocortex. It will be interesting to further characterize these horizontal cells, which do not express any of the known genetic markers for inhibitory neuron classes (Kay and Brunjes 2014).
Our awake recordings with mice in the acute preparation confirm the optoelectrode can be used for performing optogenetically targeted recordings. Moreover, we provide in this report, to our knowledge, the first single-unit recordings in the AON of awake animals. Odor responses recorded in the AON pars principalis in our proof-of-principle experiment were heterogeneous, consistent with the view of a distributed representation of odors similar to what has been observed in the piriform cortex (Kay et al. 2011). Further work is currently ongoing to comprehensively characterize response properties of AON neurons to olfactory stimulation, to better understand the functional properties of this understudied olfactory structure.
In conclusion, this work establishes a novel tool combining spatially confined stimulation and extracellular recording. Our findings further demonstrate the new optoelectrode is useful for characterizing microcircuits with spatially defined illumination in vivo. Finally, the fact that even very localized photostimulation has wide-ranging effects beyond the stimulation site, demonstrates the difficulty in predicting circuit-level effects of optogenetic manipulations, and highlights the need for closely monitoring neural activity in optogenetic experiments. Further technological improvements will facilitate such experiments and enable dissection of neural circuits at high-resolution in vivo.
GRANTS
This work was supported by Institute for the Promotion of Innovation by Science and Technology in Flanders Project SBO110068 Optobrain, European Commission Project FP7-ICT-2011-C ENLIGHTENMENT, and a personal fellowship from the Fund for Scientific Research Flanders (to S. Libbrecht) S. Haesler was supported by Career Development Award CDA00029/2013 from the Human Frontier Science Program and a Marie-Curie Career Integration Grant from the European Union (DopaPredict).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L., L.H., M.W., V.B., D.B., and S.H. conceived and designed research; S.L., L.H., M.W., and C.V.d.H. performed experiments; S.L., L.H., and S.H. analyzed data; S.L., L.H., C.V.d.H., and S.H. interpreted results of experiments; S.L., L.H., and S.H. prepared figures; S.L. and S.H. drafted manuscript; S.L., L.H., M.W., D.B., and S.H. edited and revised manuscript; S.L., L.H., M.W., C.V.d.H., V.B., D.B., and S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Leuven Viral Vector Core for the construction and production of the rAAV vectors.
REFERENCES
- Abaya TVF, Blair S, Tathireddy P, Rieth L, Solzbacher F. A 3D glass optrode array for optical neural stimulation. Biomed Opt Express 3: 3087–3104, 2012. doi: 10.1364/BOE.3.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci 15: 163–170, 2011. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Ayub S, Gentet LJ, Fiáth R, Schwaerzle M, Borel M, David F, Barthó P, Ulbert I, Paul O, Ruther P. Hybrid intracerebral probe with integrated bare LED chips for optogenetic studies. Biomed Microdevices 19: 49, 2017. doi: 10.1007/s10544-017-0190-3. [DOI] [PubMed] [Google Scholar]
- Ayub S, Gossler C, Schwaerzle M, Klein E, Paul O, Schwarz UT, Ruther P. . High-density probe with integrated thin-film micro light emitting diodes (μLEDs) for optogenetic applications. IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS). Shanghai, China, January 24–28, 2016, p. 379–382. doi: 10.1109/MEMSYS.2016.7421640. [DOI] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex). Brain Res Brain Res Rev 50: 305–335, 2005. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Kenerson MC. The anterior olfactory nucleus: quantitative study of dendritic morphology. J Comp Neurol 518: 1603–1616, 2010. doi: 10.1002/cne.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol 33: 277–284, 2015. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- Cao H, Gu L, Mohanty SK, Chiao JC. An integrated μLED optrode for optogenetic stimulation and electrical recording. IEEE Trans Biomed Eng 60: 225–229, 2013. doi: 10.1109/TBME.2012.2217395. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of channelrhodopsin-2. Nat Protoc 5: 247–254, 2010. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482: 85–88, 2012. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivelzeta Rabell J, Mutlu K, Noutel J, Martin Del Olmo P, Haesler S. Spontaneous rapid odor source localization behavior requires interhemispheric communication. Curr Biol 27: 1542–1548.e4, 2017. doi: 10.1016/j.cub.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Fan B, Kwon K, Rechenberg R, Khomenko A, Haq M, Becker MF, Weber AJ, Li W. A polycrystalline diamond-based, hybrid neural interfacing probe for optogenetics. 28th IEEE International Conference on Micro Electro Mechanical Systems. Estoril, Portugal, January 18–22, 2015, p. 616–619. doi: 10.1109/MEMSYS.2015.7051031. [DOI] [Google Scholar]
- Fan B, Kwon KY, Weber AJ, Li W. An implantable, miniaturized SU-8 optical probe for optogenetics-based deep brain stimulation. Conf Proc IEEE Eng Med Biol Soc 2014: 450–453, 2014. doi: 10.1109/EMBC.2014.6943625. [DOI] [PubMed] [Google Scholar]
- Fan B, Li W. Miniaturized optogenetic neural implants: a review. Lab Chip 15: 3838–3855, 2015. doi: 10.1039/C5LC00588D. [DOI] [PubMed] [Google Scholar]
- Fang Q, Boas DA. Monte Carlo simulation of photon migration in 3D turbid media accelerated by graphics processing units. Opt Express 17: 20178–20190, 2009. doi: 10.1364/OE.17.020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon-Turcotte G, Kisomi AA, Ameli R, Camaro CO, LeChasseur Y, Néron JL, Bareil PB, Fortier P, Bories C, de Koninck Y, Gosselin B. A wireless optogenetic headstage with multichannel electrophysiological recording capability. Sensors (Basel) 15: 22776–22797, 2015. doi: 10.3390/s150922776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goßler C, Bierbrauer C, Moser R, Kunzer M, Holc K, Pletschen W, Köhler K, Wagner J, Schwaerzle M, Ruther P, Paul O, Neef J, Keppeler D, Hoch G, Moser T, Schwarz UT. GaN-based micro-LED arrays on flexible substrates for optical cochlear implants. J Phys D Appl Phys 47: 205401, 2014. doi: 10.1088/0022-3727/47/20/205401. [DOI] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One 2: e299, 2007. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing channelrhodopsin-2. eLife 3: e01481, 2014. doi: 10.7554/eLife.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Welkenhuysen M, Andrei A, Musa S, Luo Z, Libbrecht S, Severi S, Soussan P, Baekelandt V, Haesler S, Gielen G, Puers R, Braeken D.. High-density optrode-electrode neural probe using SixNy photonics for in vivo optogenetics. IEEE International Electron Devices Meeting (IEDM). Washington, DC, December 7–9, 2016, p. 29.5.1–29.5.4. doi: 10.1109/IEDM.2015.7409795. [DOI] [Google Scholar]
- Im M, Cho IJ, Wu F, Wise KD, Yoon E. Neural probes integrated with optical mixer/splitter waveguides and multiple stimulation sites. IEEE 24th International Conference on Micro Electro Mechanical Systems (MEMS). Cancun, Mexico, Jan. 23–27, 2011, p. 1051–1054. doi: 10.1109/MEMSYS.2011.5734609. [DOI] [Google Scholar]
- Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydın Ç, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Häusser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun WL, Svoboda K, Carandini M, Harris KD, Koch C, O’Keefe J, Harris TD. Fully integrated silicon probes for high-density recording of neural activity. Nature 551: 232–236, 2017. doi: 10.1038/nature24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampasi K, Stark E, Seymour J, Na K, Winful HG, Buzsáki G, Wise KD, Yoon E. Fiberless multicolor neural optoelectrode for in vivo circuit analysis. Sci Rep 6: 30961, 2016. doi: 10.1038/srep30961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RB, Brunjes PC. Diversity among principal and GABAergic neurons of the anterior olfactory nucleus. Front Cell Neurosci 8: 111, 2014. doi: 10.3389/fncel.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial distribution of neural activity in the anterior olfactory nucleus evoked by odor and electrical stimulation. J Comp Neurol 519: 277–289, 2011. doi: 10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Kashiwadani H, Mori K. Compensatory rapid switching of binasal inputs in the olfactory cortex. J Neurosci 28: 11989–11997, 2008. doi: 10.1523/JNEUROSCI.3106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim RH, Lu C, Lee SD, Song IS, Shin G, Al-Hasani R, Kim S, Tan MP, Huang Y, Omenetto FG, Rogers JA, Bruchas MR. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340: 211–216, 2013. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KY, Lee HM, Ghovanloo M, Weber A, Li W. Design, fabrication, and packaging of an integrated, wirelessly-powered optrode array for optogenetics application. Front Syst Neurosci 9: 69, 2015. doi: 10.3389/fnsys.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChasseur Y, Dufour S, Lavertu G, Bories C, Deschênes M, Vallée R, De Koninck Y. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat Methods 8: 319–325, 2011. doi: 10.1038/nmeth.1572. [DOI] [PubMed] [Google Scholar]
- Lopez CM, Andrei A, Mitra S, Welkenhuysen M, Eberle W, Bartic C, Puers R, Yazicioglu RF, Gielen GGE. An implantable 455-active-electrode 52-channel CMOS neural probe. IEEE J Solid-State Circuits 49: 248–261, 2014. doi: 10.1109/JSSC.2013.2284347. [DOI] [Google Scholar]
- McAlinden N, Massoubre D, Richardson E, Gu E, Sakata S, Dawson MD, Mathieson K. Thermal and optical characterization of micro-LED probes for in vivo optogenetic neural stimulation. Opt Lett 38: 992–994, 2013. doi: 10.1364/OL.38.000992. [DOI] [PubMed] [Google Scholar]
- Pisanello F, Sileo L, Oldenburg IA, Pisanello M, Martiradonna L, Assad JA, Sabatini BL, De Vittorio M. Multipoint-emitting optical fibers for spatially addressable in vivo optogenetics. Neuron 82: 1245–1254, 2014. doi: 10.1016/j.neuron.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M, Schneider F, Tsunoda SP, Shilyansky C, Wietek J, Deisseroth K, Hegemann P. Color-tuned channelrhodopsins for multiwavelength optogenetics. J Biol Chem 287: 31804–31812, 2012. doi: 10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Go V, Murphy T, Fu Q, Morizio J, Yin HH. A wirelessly controlled implantable LED system for deep brain optogenetic stimulation. Front Integr Neurosci 9: 8, 2015. doi: 10.3389/fnint.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsáki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci 31: 2279–2291, 2010. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubehn B, Wolff SB, Tovote P, Schuettler M, Lüthi A, Stieglitz T. Polymer-based shaft microelectrodes with optical and fluidic capabilities as a tool for optogenetics. Conf Proc IEEE Eng Med Biol Soc 2011: 2969–2972, 2011. [DOI] [PubMed] [Google Scholar]
- Scharf R, Tsunematsu T, McAlinden N, Dawson MD, Sakata S, Mathieson K. Depth-specific optogenetic control in vivo with a scalable, high-density μLED neural probe. Sci Rep 6: 28381, 2016. doi: 10.1038/srep28381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyltjens I, Laramée ME, Van den Haute C, Gijsbers R, Debyser Z, Baekelandt V, Vreysen S, Arckens L. Evaluation of the expression pattern of rAAV2/1, 2/5, 2/7, 2/8, and 2/9 serotypes with different promoters in the mouse visual cortex. J Comp Neurol 523: 2019–2042, 2015. doi: 10.1002/cne.23819. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- Schwaerzle M, Elmlinger P, Paul O, Ruther P. Miniaturized tool for optogenetics based on an LED and an optical fiber interfaced by a silicon housing. Conf Proc IEEE Eng Med Biol Soc 2014: 5252–5255, 2014. doi: 10.1109/EMBC.2014.6944810. [DOI] [PubMed] [Google Scholar]
- Schwaerzle M, Paul O, Ruther P. Compact silicon-based optrode with integrated laser diode chips, SU-8 waveguides and platinum electrodes for optogenetic applications. J Micromech Microeng 27: 065004, 2017. doi: 10.1088/1361-6439/aa6ad4. [DOI] [Google Scholar]
- Schwaerzle M, Seidl K, Schwarz UT, Paul O, Ruther P. Ultracompact optrode with integrated laser diode chips and SU-8 waveguides for optogenetic applications. IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS). Taipei, Taiwan, Jan. 20–24, 2013, p. 1029–1032. doi: 10.1109/MEMSYS.2013.6474424 [DOI] [Google Scholar]
- Segev E, Reimer J, Moreaux LC, Fowler TM, Chi D, Sacher WD, Lo M, Deisseroth K, Tolias AS, Faraon A, Roukes ML. Patterned photostimulation via visible-wavelength photonic probes for deep brain optogenetics. Neurophotonics 4: 011002, 2017. doi: 10.1117/1.NPh.4.1.011002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim E, Chen Y, Masmanidis S, Li M. Multisite silicon neural probes with integrated silicon nitride waveguides and gratings for optogenetic applications. Sci Rep 6: 22693, 2016. doi: 10.1038/srep22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Lee HJ, Kim J, Shin H, Choi N, Lee CJ, Yoon ES, Yoon E, Wise KD, Kim TG, Cho IJ. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays. Sci Rep 5: 15466, 2015. doi: 10.1038/srep15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Koos T, Buzsáki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J Neurophysiol 108: 349–363, 2012. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HK, Lee HK, Wang C, Kim NY. Design and fabrication of implantable neural probes with monolithically integrated light-emitting diodes for optogenetic applications. J Nanosci Nanotechnol 17: 2582–2584, 2017. doi: 10.1166/jnn.2017.13071. [DOI] [PubMed] [Google Scholar]
- Tamura K, Ohashi Y, Tsubota T, Takeuchi D, Hirabayashi T, Yaguchi M, Matsuyama M, Sekine T, Miyashita Y. A glass-coated tungsten microelectrode enclosing optical fibers for optogenetic exploration in primate deep brain structures. J Neurosci Methods 211: 49–57, 2012. doi: 10.1016/j.jneumeth.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Van der Perren A, Toelen J, Carlon M, Van den Haute C, Coun F, Heeman B, Reumers V, Vandenberghe LH, Wilson JM, Debyser Z, Baekelandt V. Efficient and stable transduction of dopaminergic neurons in rat substantia nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther 18: 517–527, 2011. doi: 10.1038/gt.2010.179. [DOI] [PubMed] [Google Scholar]
- Welkenhuysen M, Hoffman L, Luo Z, De Proft A, Van den Haute C, Baekelandt V, Debyser Z, Gielen G, Puers R, Braeken D. An integrated multi-electrode-optrode array for in vitro optogenetics. Sci Rep 6: 20353, 2016. doi: 10.1038/srep20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Stark E, Im M, Cho IJ, Yoon ES, Buzsáki G, Wise KD, Yoon E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. J Neural Eng 10: 056012, 2013. doi: 10.1088/1741-2560/10/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Stark E, Ku PC, Wise KD, Buzsáki G, Yoon E. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88: 1136–1148, 2015. doi: 10.1016/j.neuron.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3: 785–792, 2006. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature 446: 633–639, 2007. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang J, Laiwalla F, Kim JA, Urabe H, Van Wagenen R, Song YK, Connors BW, Zhang F, Deisseroth K, Nurmikko AV. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue. J Neural Eng 6: 055007, 2009. doi: 10.1088/1741-2560/6/5/055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser MB, Moser EI. Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science 340: 1232627, 2013. doi: 10.1126/science.1232627. [DOI] [PubMed] [Google Scholar]
- Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt Lett 35: 4133–4135, 2010. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]