Abstract
The dorsal lateral geniculate nucleus (dLGN) of the thalamus is the exclusive relay of retinal information en route to the visual cortex. Although much of our understanding about dLGN comes from studies done in higher mammals, such as the cat and primate, the mouse as a model organism has moved to the forefront as a tractable experimental platform to examine cell type-specific relations. This review highlights our current knowledge about the development, structure, and function of the mouse dLGN.
Keywords: dorsal lateral geniculate nucleus, mouse, retina, retinogeniculate, thalamus, vision
INTRODUCTION
The dorsal lateral geniculate nucleus (dLGN) is the thalamic relay linking the retina to the visual cortex. Much of our understanding about this nucleus comes from studies done in carnivores and primates, such as the cat and macaque. However, within the past decade, the mouse has emerged as a model system to understand many aspects about the development, structural composition, and functional operations of the dLGN. Aided primarily by the advance of molecular tools and the widespread availability of transgenic lines, studies in the mouse allow unprecedented access to interrogate specific cell types, circuits, and projections associated with dLGN. Although the mouse has laterally placed eyes and poor acuity, the neuronal architecture of its dLGN is highly conserved, making this species a premier experimental platform to elucidate thalamic circuit structure and function. Indeed, studies in the mouse provide further support that dLGN is more than a simple relay of visual information. The visual response properties of dLGN neurons are far more diverse and sophisticated than previously recognized, and much like its higher mammalian counterparts, the mouse dLGN receives most of its input from nonretinal sources that generally operate to modulate the gain of signal transmission. The purpose of this review is to provide an in-depth examination of the development, structure, and function of the mouse dLGN, citing work done primarily on pigmented, wild-type, and transgenic strains. Topics to be covered include the organization of retinal and nonretinal projections to dLGN, the structural and functional composition of dLGN neurons, and their associated patterns of connectivity and visual response properties.
ORGANIZATION OF RETINAL PROJECTIONS TO dLGN
In the mouse, the vast majority of retinal ganglion cells (RGCs) project to two subcortical structures, the superior colliculus (SC) and dLGN (Ellis et al. 2016). Estimates in rodents suggest ~40% of all RGCs project to dLGN (Martin 1986; Reese 1988). Retinal axon terminal fields within the dLGN are organized along three dimensions: retinal topography, eye of origin, and RGC type (Fig. 1).
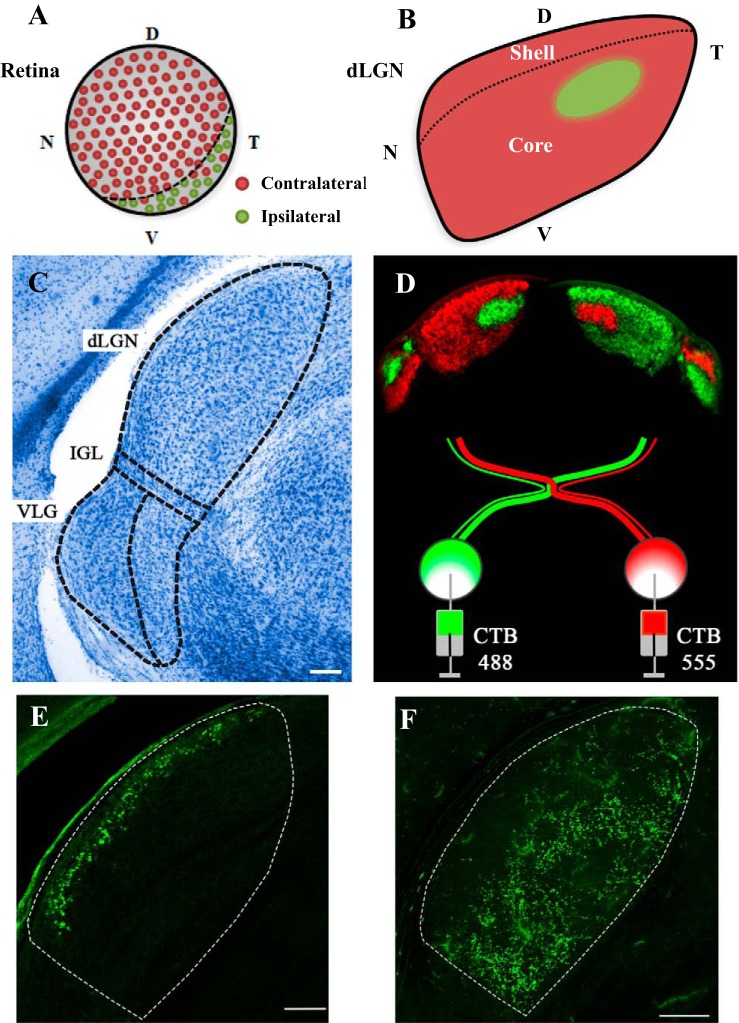
Fig. 1.
Organization of the mouse retinogeniculate pathway. A: schematic illustrating the location of retinal ganglion cells that project to contralateral (red) and ipsilateral (green) dorsal lateral geniculate nucleus (dLGN). Dashed line depicts the ventro-temporal crescent, where the ipsilateral-projecting retinal ganglion cells (RGCs) reside. Letters refer to regions of retina: N, nasal; T, temporal; D, dorsal; V, ventral. B: drawing portraying the “hidden laminae” organization of dLGN. Dashed lines mark the shell and core subdivision. Retinal projections from the contralateral eye are in red, and those from the ipsilateral eye in green. C: Nissl-stained section of dLGN as a homogenous, bean-shaped structure. Dashed lines delineate the boundaries of dLGN from the intrageniculate leaflet (IGL) and ventral geniculate nuclei (vLGN). Scale bar: 100 μm. D: anterograde labeling of retinal projections with cholera toxin beta-subunit (CTB) reveals eye-specific patterning in dLGN and vLGN. Injecting two different fluorescent conjugates of CTB (Alexa Fluor 488 green, 555 red) into one or the other eye reveals within-section visualization of crossed and uncrossed retinal terminal fields in dLGN. Scale bar: 100 μm. E: section of dLGN from a thyrotropin-releasing hormone receptor (TRHR) mouse showing the intrinsic green fluorescent protein (GFP) (green) labeling of retinal projections to the shell, arising from a group of ON/OFF direction-selective RGCs that respond to posterior motion. F: section of dLGN from a Calretinin-E GFP mouse (CB2-GFP), illustrating the GFP labeling of retinal projections to the core, arising from a complete mosaic of an RGC type that responds transiently to light offset (tOFF-αRGC). All sections are in the coronal plane.
Retinotopy and eye-specific projections.
The axons of neighboring RGCs project to the dLGN in an orderly fashion to provide a visual representation of the contralateral hemifield (Grubb and Thompson 2003; Pfeiffenberger et al. 2006; Piscopo et al. 2013). The nasal-temporal visual axis is positioned in a medial-to-lateral plane of dLGN, with the upper to lower visual fields mapping in a dorsal to ventral manner (Fig. 1B). However, there is evidence to suggest that lower visual fields may be overrepresented (Piscopo et al. 2013). Embedded in this visual map are the retinal projections from the two eyes (Fig. 1, B and D). In carnivores and primates, the inputs from the two eyes are separated into distinct laminae, and dLGN neurons within each lamina receive monocular input from the contralateral or ipsilateral eye. However, like other nocturnal rodents, the mouse dLGN lacks a discernible lamination pattern (Reese 1988). Instead, retinal projections are organized into nonoverlapping eye-specific domains that can be visualized by anterograde labeling of RGCs (Fig. 1D; Godement et al. 1984; Jaubert-Miazza et al. 2005; Muir-Robinson et al. 2002; Pfeiffenberger et al. 2005).
In pigmented strains the vast majority (95%) of retinal axons cross at the optic chiasm (Dräger and Olson 1980; Petros et al. 2008). Those from nasal and most of the temporal retina cross to project to the contralateral hemisphere, and have terminal fields that occupy as much as 90% of the total area in dLGN (Fig. 1, A and B). Additionally, a small group of retinal axons from the ventro-temporal crescent of retina do not cross at the optic chiasm and project to the ipsilateral dLGN. These projections terminate in the dLGN to form an irregularly shaped cylinder through the anteromedial region, occupying as little as 10% of the total area, and showing little if any overlap with contralateral eye projections (Fig. 1, B and D).
Such eye-specific patterning is not evident during the early stages of retinal axon innervation, but begins to take shape near the end of the first postnatal (P) week (Fig. 2; Godement et al. 1984; Jaubert-Miazza et al. 2005). RGC axons innervate dLGN at perinatal ages, with those from the contralateral eye arriving (E16-P0) before those from the ipsilateral eye (P0–P2). At these ages, terminal fields from the contralateral eye occupy almost the entire LGN, and as ipsilateral eye projections arrive, they begin to form a large overlapping domain within the dorsal medial sector of dLGN. During the first postnatal week, these overlapping eye domains retract, so that by end of the first week, they show clear signs of segregation. By natural eye opening (~P12), they are well separated and resemble an adult-like pattern of innervation. At the level of a single retinal axon, the refinement of these eye-specific, retinotopic maps is accomplished by the remodeling of axon arbors, where those projecting to inappropriate target areas are eliminated and those projecting to proper locations grow and elaborate (Dhande et al. 2011). As axon arbors stabilize, presynaptic boutons (the presumed site of retinogeniculate synapses) continue to grow and cluster into spatially restricted regions on axon arbors (Hong et al. 2014).
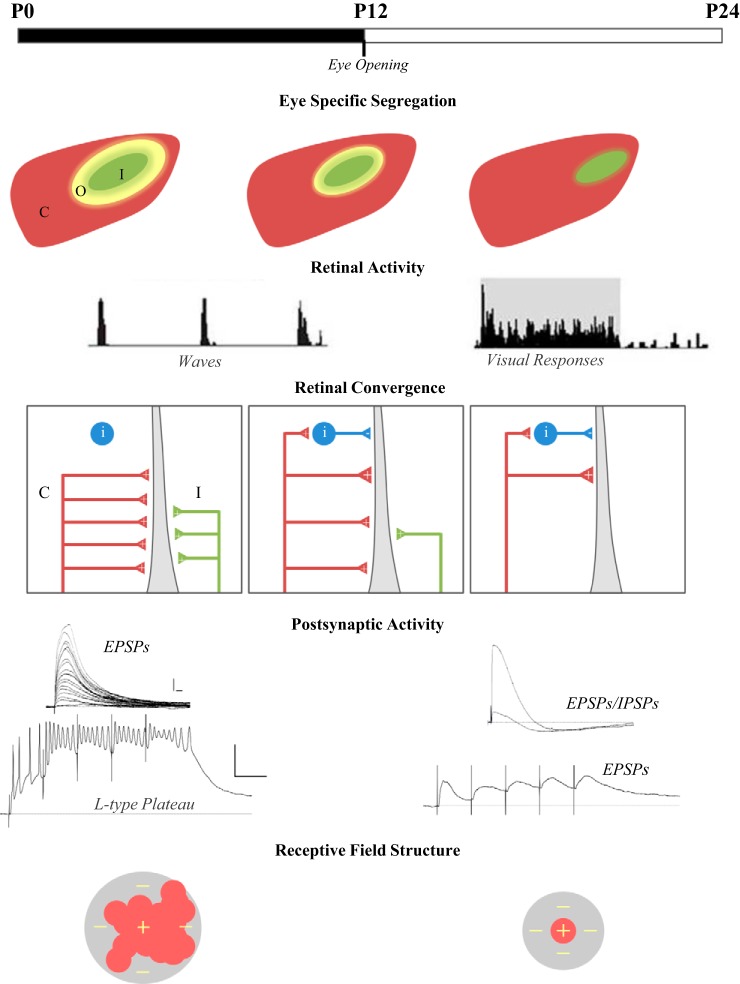
Fig. 2.
Developmental events occurring in the retinogeniculate pathway. Top to bottom: timeline showing retinogeniculate refinement in relation to postnatal (P) age and natural eye opening. Eye-specific segregation of retinal projections in the dorsal lateral geniculate nucleus (dLGN): illustrations of dLGN portray the refinement of retinogeniculate projections. Colors depict the spatial extent of contralateral (C; red), ipsilateral (I; green), and overlapping (O; yellow) retinal projections. Retinal activity: spike frequency histograms showing the nature of retinal activity during the period of spontaneous retinal waves, and when after eye opening, waves are replaced by visually evoked activity (gray). Retinal convergence: wiring diagram illustrating the pattern of retinal convergence onto the dendrite of a relay neuron. Developing relay neurons receive weak binocular excitatory input from multiple retinal ganglion cells (RGCs) that originate from the contralateral (C; red) and ipsilateral (I; green) eye. Mature neurons receive strong monocular input from just a few RGCs, as well as inhibitory input from interneurons (i; blue). Postsynaptic activity: in developing dLGN relay neurons, increasing the stimulus intensity of optic tract stimulation evokes a progressive increase in the amplitude of excitatory postsynaptic potentials (EPSPs), whereas repetitive stimulation (below) evokes summating EPSPS that activate L-type plateau potentials. In mature neurons, optic tract stimulation activates far fewer, but larger, EPSPs followed by inhibitory postsynaptic potentials (IPSPs). Repetitive stimulation triggers a train of EPSPs, but not plateaus. Scale bars: top: 10 mV, 20 ms; bottom: 20 mV, 50 ms. Receptive field structure: schematic portrays the refinement in receptive field size and the center (red +) surround (gray −) organization of dLGN receptive fields.
The mechanisms underlying visual map formation and eye-specific axon segregation have been well studied, and much of our present understanding can be attributed to the utilization of genetically modified mouse models (Cang and Feldheim 2013; Huberman et al. 2008a). These studies reveal that the refinement of the retinogeniculate pathway is brought about by an interaction between molecular guidance cues and neural activity. Molecular signals in the form of the Eph family of receptor tyrosine kinases and their cell surface-bound ligands (ephrin A’s) determine the initial position of retinal axons and help to establish a coarse topographic map and the approximate location of eye-specific domains (Cang et al. 2008; Pfeiffenberger et al. 2005, 2006). However, the refinement of these maps requires neural activity. Before the onset of visually evoked activity, which happens around the time of natural eye opening (P10–P12), neighboring RGCs fire spontaneously in rhythmic bursts that sweep across the retina in a wave-like manner (Fig. 2; Ackman et al. 2012; Demas et al. 2003). When the spatio-temporal patterns of these waves are perturbed, retinal axon arbors from the two eyes remain diffusely organized and/or fail to segregate properly in dLGN (Huberman et al. 2008a; Muir-Robinson et al. 2002; Torborg and Feller 2005). Functionally, disrupting retinal waves also affect the refinement of visual maps in the dLGN. While coarse topography is preserved, there is a breakdown in fine-scale visual topography across the naso-temporal axis and aberrant clustering of ON/OFF responses in dLGN (Grubb et al. 2003).
Accompanying the anatomical rearrangements is a remodeling of synaptic connections (Fig. 2; Chen and Regehr 2000; Guido 2008; Hong and Chen 2011). Initially, a single dLGN neuron receives input from many RGCs, and over the course of the first three postnatal weeks, synaptic pruning occurs, leaving only a few functional inputs. Interestingly, the period of synaptic pruning continues well after eye-specific segregation, suggesting that the remodeling of the retinogeniculate pathway is a protracted process comprising distinct phases (Fig. 2). There is an initial coarse-scale phase that involves axonal mapping and eye-specific segregation that is followed by a fine-scale period of refinement, in which weak synaptic inputs are eliminated and the remaining ones strengthened. Studies employing a late (P20–P32) dark rearing procedure, introduced well after the period of natural eye opening and the cessation of spontaneous waves, also indicate the existence of a third, maintenance stage, where the continued strengthening and stabilization of adult-like patterns of connectivity require visually evoked activity (Hooks and Chen 2006, 2008). Visual deprivation introduced during this phase destabilizes retinogeniculate connectivity, whereby dLGN neurons revert to an immature state in which they receive many, relatively weak RGC inputs. Interestingly, the disruption of corticothalamic activity originating from layer V1 of the visual cortex also perturbs this final stage of refinement, suggesting that both feedforward and feedback visual activity plays an instrumental role in the final sculpting of retinogeniculate connections (Thompson et al. 2016). Finally, the remodeling of retinogeniculate connections is likely to contribute to the postnatal maturation of receptive field properties. In species such as cat and ferret, developing dLGN neurons are often binocularly driven, having highly unstructured receptive fields that are large, irregularly shaped, and lack orderly ON- and OFF- subregions. However, after retinogeniculate refinement, dLGN receptive fields are monocularly driven and much smaller, with well-defined concentric center-surround organization (Shatz and Kirkwood 1984; Tavazoie and Reid 2000). A recent study in the mouse shows that the developmental sharpening of receptive field structure (decrease in size and increase in surround suppression) accompany a pairing of decreased excitatory and increased feed-forward inhibitory convergence in dLGN connectivity (Tschetter et al. 2018).
RGC type-specific projections.
The plethora of transgenic mouse lines reveals great diversity among RGCs, and to date, close to 35 different types have been identified (Baden et al. 2016; Dhande et al. 2015; Sanes and Masland 2015). Tracing studies indicate many of these different types project to the dLGN (Ellis et al. 2016; Martersteck et al. 2017), giving rise to a remarkable assortment of visual response properties among dLGN neurons (see visual response properties of dlgn neurons). Many also terminate in dLGN in a manner that conforms to the shell and core divisions (Figs. 2, E and F and 3; Dhande et al. 2015; Kerschensteiner and Guido 2017; Seabrook et al. 2017). For example, several identified types of direction selective ganglion cells (DSGC; e.g., ON-OFF, OFF, J-, F-miniON, F-miniOFF) innervate the shell, a domain that occupies a small dorsolateral strip of dLGN, just below the optic tract (Cruz-Martín et al. 2014; Huberman et al. 2009; Kim et al. 2010; Rivlin-Etzion et al. 2011; Rousso et al. 2016). Not all DSGCs have arbors that are strictly confined to the shell. One type of ON-OFF DSGC (BD-RGC) that responds to ventral motion has terminal fields that straddle the border of shell and core division (Kay et al. 2011; Kim et al. 2010). By contrast, RGCs that have a conventional center-surround organization, responding in a sustained or transient manner to light increments or decrements (e.g., ONs, OFFs, OFFT, SbC), terminate in the core, a much larger region of dLGN that is situated beneath the shell (Dhande et al. 2015). Among these, perhaps the most notable is a transgenic line (CB2) that labels a complete retinal mosaic of OFF transient α-cells and has a large terminal field domain that delineates the core (Huberman et al. 2008b).
Fig. 3.
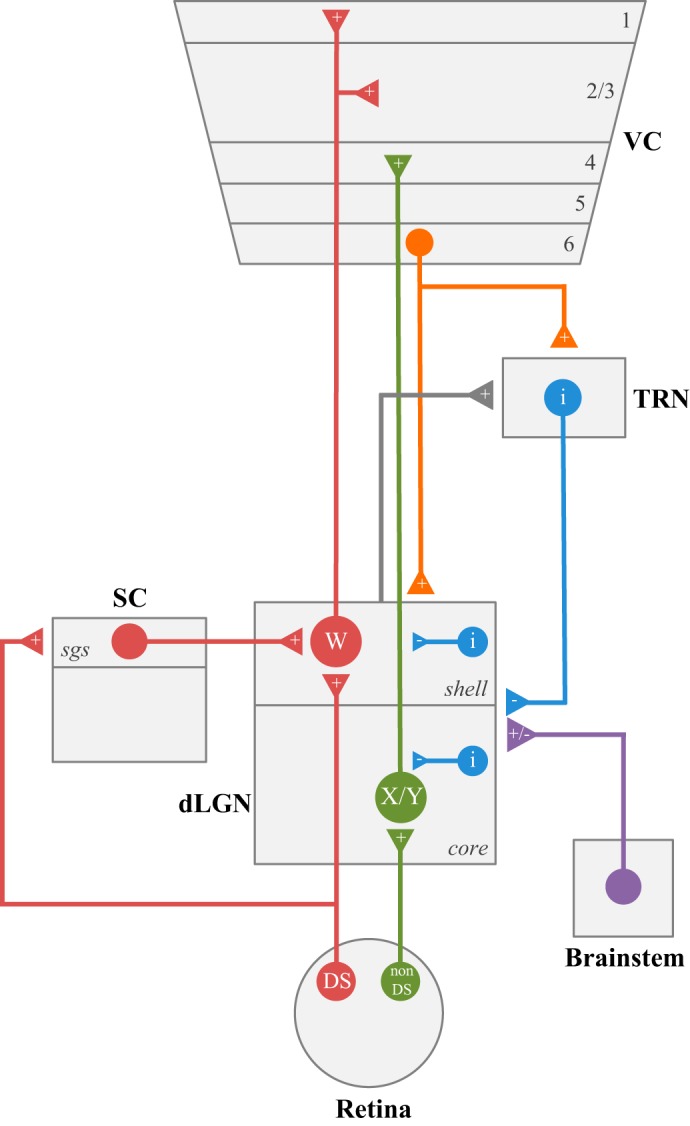
Neural circuits associated with dorsal lateral geniculate nucleus (dLGN). Wiring diagram showing the pattern of connectivity between neurons of dLGN, retina, brain stem, visual cortex (VC), thalamic reticular nucleus (TRN), and superior colliculus (SC). DS, direction selective retinal ganglion cells; i, inhibitory neurons; X, Y and W, different classes of relay neurons; sgs, stratum griseum superficialis; +/−, excitatory or inhibitory input.
These orderly arrangements suggest that the retinogeniculate pathway comprises, in part, separate parallel visual channels (Dhande et al. 2015; Kerschensteiner and Guido 2017; Seabrook et al. 2017). Whether these channels are preserved through dLGN remains unresolved, but the existence of morphologically distinct classes of relay neurons provide further support for this possibility (see Relay neurons).
ORGANIZATION OF NONRETINAL PROJECTIONS TO dLGN
In the mouse, like other mammals, retinal inputs provide the primary excitatory drive for dLGN neurons, but comprise only ~10% of the total number of synapses in this nucleus (Bickford et al. 2010). The vast majority of input arises from structures that do not receive direct retinal projections. These include three principal sources: layer VI neurons of visual cortex, GABAergic neurons of the thalamic reticular nucleus (TRN), and a collection of brain stem nuclei comprised primarily of cholinergic neurons (Figs. 3 and 4). These nonretinal inputs project diffusely throughout all of dLGN, suggesting they have little impact in shaping the receptive field structure of dLGN neurons. Instead, they act more as modulators of retinogeniculate signaling, altering the gain of signal transfer in diverse ways, including improving receptive field structure, regulating burst and tonic response modes, and controlling network oscillatory activity during different behavioral states (Sherman and Guillery 2002, 2011). However, not all nonretinal projections to dLGN operate in this manner. One other input from a midbrain retinorecipient structure, the SC resembles and operates more like a retinal driver, rather than a nonretinal modulator (Bickford et al. 2015).
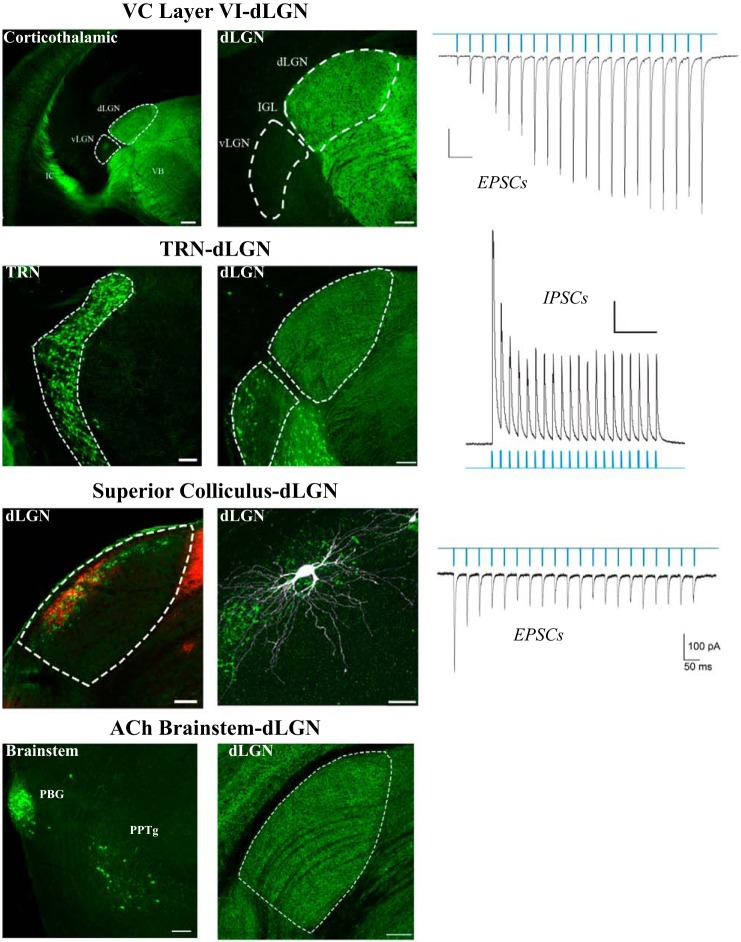
Fig. 4.
Sources of nonretinal input to dorsal lateral geniculate nucleus (dLGN). Top to bottom, visual cortex-VC-dLGN: projections from layer VI of neocortex to dLGN taken from a Golli-tau mouse, in which the expression of a green fluorescent protein (GFP) is restricted to layer VI cortical neurons. Optogenetic stimulation (blue light pulses) of corticogeniculate terminals evokes a train of excitatory postsynaptic currents (EPSCs) in a dLGN relay neuron. Scale bars: 200 μm, 100 μm, 50 pA, and 200 ms. TRN-dLGN: projections from thalamic reticular nucleus (TRN) to dLGN taken from a GAD-65 mouse in which the expression of GFP is confined to GABAergic neurons of the TRN. Optogenetic stimulation of TRN terminals in dLGN evokes a train of postsynaptic inhibitory responses (IPSCs) in a relay neuron. Scale bars: 100 μm, 100 μm, 200 pA, and 500 ms. Superior colliculus-dLGN: section of dLGN from a thyrotropin-releasing hormone receptor (TRHR) mouse in which GFP is expressed in the retinal terminal fields of ON/OFF in direction-selective ganglion cells (DSGCs). An anterograde tracer was placed into SC labels tectogeniculate terminals (red) in overlapping regions of the shell. Example of a biocytin-filled W-cell located in the shell of TRHR mouse. Optogenetic stimulation of tectogeniculate terminals evokes a train of EPSCs in a dLGN W cell. Scale bars: 100 μm, 100 μm, 100 pA, and 50 ms. ACh brain stem-dLGN: projections from pedunculopontine (PPTg) and parabigeminal (PBG) brain stem nuclei to dLGN taken from a ChAT-Cre mouse crossed to an Ai9 reporter line (pseudo-colored green). Scale bars: 100 μm and 100 μm. All sections are in the coronal plane. All recordings are done in voltage-clamp mode (EPSCs at −70 mV; IPSCs at 0 mV).
Corticogeniculate projections.
In the mouse, perhaps the most studied nonretinal projection is from layer VI of visual cortex (Figs. 3 and 4). Transgenic lines (e.g., Golli-tau GFP, and NTSR1-Cre) that label layer VI neurons, reveal that corticothalamic axons begin to innervate the dorsal thalamus at early postnatal ages (Grant et al. 2016; Jacobs et al. 2007), but corticogeniculate innervation and connectivity take place mainly after the first postnatal week, well after the early phases of retinogeniculate refinement (Seabrook et al. 2013a). Loss-of-function studies in a mutant mouse that lacks a transcription factor responsible for the differentiation of retinal ganglion cells (Math 5−/−) show that the absence of retinal input to dLGN accelerates corticogeniculate innervation (Seabrook et al., 2013a). Moreover, retinal axon innervation controls the timing of corticogeniculate input by regulating the expression of the chondroitin sulfate proteoglycan aggrecan, a repulsive extracellular matrix molecule that inhibits cortical projections from entering dLGN prematurely (Brooks et al. 2013). In fact, such sequencing and reliance on retinal innervation may be part of a conserved developmental plan that orchestrates the late postnatal arrival of other nonretinal inputs to dLGN, including those from brain stem and TRN (W. Guido, unpublished observations). Despite the late arrival of corticogeniculate inputs, these projections somehow regulate the initial targeting of some RGCs to dLGN (Diao et al. 2017; Shanks et al. 2016). In genetically modified mice lacking a visual cortex, some RGCs fail to project to dLGN. Many of these seem to be RGCs that mediate spatial vision (i.e., image-forming RGCs vs. intrinsically photosensitive RGCs). These axons normally bifurcate and project to both dLGN and SC, but in the absence of the visual cortex, they only target SC (Shanks et al. 2016). How such guidance is accomplished remains unclear, since corticothalamic axons, although present in medial thalamus, have yet to arrive in dLGN at perinatal ages when RGCs target and innervate dLGN (Seabrook et al. 2013a).
Cortical layer VI neurons are one of the largest sources of nonretinal input to dLGN, forming small excitatory synapses on the distal dendritic segments of dLGN relay neurons (Bickford et al. 2010). Using an in vitro slice preparation and optogenetics to photoactivate cortical terminals in dLGN reveals this input evokes glutamatergic excitatory postsynaptic currents (EPSCs) in relay neurons that increase in amplitude with each successive pulse of repetitive stimulation (Fig. 4; Jurgens et al. 2012). Such facilitation is a hallmark feature of modulator input, whereby sustained periods of stimulation give rise to steady and increasing levels of depolarization (Petrof and Sherman 2013). Nonetheless, understanding how corticogeniculate inputs influence retinogeniculate signaling during visual stimulation has been challenging. For example, in vivo optogenetic silencing of mouse layer VI neurons leads to both increases and decreases of visually evoked dLGN activity (Denman and Contreras 2015). Studies done in a number of mammalian species report subtle and variable influences and suggest that corticogeniculate activation can regulate the response mode, sharpen receptive field structure, and alter spike timing (Hasse and Briggs 2017). The difficulty in deciphering a more definitive role is, in part, attributed to the complex circuitry and diverse cell types belonging to this pathway. In addition to providing direct excitatory input onto dLGN relay neurons, cortical projections can send collaterals to GABAergic neurons of TRN and project directly onto intrinsic interneurons of dLGN. Indeed, optogenetic stimulation of these elements gives rise to powerful excitation (Jurgens et al. 2012), which, in turn, inhibit the activity of relay neurons. Thus, future application of optogenetic and/or chemogenetic manipulations is needed to isolate specific cell types and circuits influenced by corticothalamic stimulation.
Brain stem cholinergic (ACh) projections.
Tracing studies in rodents, and more recently in transgenic mice (ChAT-Cre), indicate that ACh projections to dLGN arise from the pedunculopontine and laterodorsal tegmentum, as well as the parabigeminal nucleus (Figs. 3 and 4; Hallanger et al. 1987; Harting et al. 1991b; Sokhadze et al. 2014). During development, ACh projections slowly innervate dLGN throughout the first postnatal month (Ballesteros et al. 2005; Sokhadze et al. 2014). Studies done in different mammalian species reveal that these projections have a substantial influence on retinogeniculate transmission, converting the response mode of relay neurons from burst to tonic during visual stimulation (Lu et al. 1993), establishing network states during sleep, wakefulness, and arousal (Gut and Winn 2016; Mena-Segovia and Bolam 2017), and modulating visuomotor responses (Cui and Malpeli 2003). The cellular bases of these effects are complicated, in part, because of the different ACh receptor subtypes, and how they are distributed among relay neurons and interneurons. Pharmacological studies done in rodents show that for relay neurons, ACh operates through nicotinic, as well as M1 and M3 muscarinic receptor, which together mediate a graded and sustained depolarization (Zhu and Uhlrich 1997, 1998). However, interneurons possess an M2 muscarinic receptor subtype, and their activation leads to membrane hyperpolarization and a subsequent reduction in feedforward inhibition onto relay neurons (Antal et al. 2010; Zhu and Heggelund 2001).
TRN projections.
The TRN is a shell-like structure containing GABAergic neurons that surround the dorsolateral aspect of the thalamus. While providing inhibitory input to thalamocortical relay neurons of primary sensory thalamic nuclei, the TRN receives ascending excitatory input from axon collaterals of relay neurons, as well as descending input from layer VI of neocortex (Fig. 3; Guillery et al. 1998; Pinault 2004; Sherman 2001). Thus, TRN serves as an inhibitory interface between ascending thalamic and descending cortical signaling. The direct impact of TRN inhibition on mouse retinogeniculate signal transmission remains largely unexplored, although optogenetic stimulation of TRN terminals in dLGN evokes robust and sustained inhibition of relay neurons (Fig. 4; Campbell and Guido 2016). However, the inhibitory influences of TRN on thalamocortical function have been well studied. Recent optogenetic manipulations in mice have led to a number of important discoveries implicating TRN circuits in the control of tonic and burst firing mode, the attentional modulation of thalamic signal transmission, the generation and propagation of rhythms during sleep and wakefulness, and the prevention of hyper-synchronous oscillations associated with certain disease states (Fogerson and Huguenard 2016; Halassa and Acsády 2016).
Tectogeniculate projections.
In addition to the modulatory projections described above, dLGN also receives input from the superficial layers of SC (Fig. 3). However, unlike the modulatory nonretinal projections, which project diffusely throughout dLGN, tectogeniculate inputs are restricted to the dorsolateral shell region of dLGN, and they overlap extensively with the projections of DSGCs (Fig. 4; Bickford et al. 2015). Indeed, tectogeniculate and retinogeniculate inputs have a similar synaptic ultrastructure and tend to cluster, along with retinal terminals, on proximal regions of dLGN relay cell dendrites. In vitro recordings and optogenetic stimulation of tectogeniculate terminals evoke large, fast-rising EPSCs that decrease in amplitude during repetitive stimulation, and thus have a driver-like retinal profile (Fig. 4). Transsynaptic labeling of DSGCs and reconstructions of tectorecipient dLGN neurons reveal they comprise a specific class of relay neurons (W-cell, see Relay neurons) that reside in the shell and project to the superficial layers of visual cortex (Bickford et al. 2015; Cruz-Martín et al. 2014). In fact, the shell of mouse dLGN bears some resemblance to the C-laminae of cats and the koniocellular division of some primates (Cheong et al. 2013; Demeulemeester et al. 1991; Harting et al. 1991a), suggesting that it is part of a conserved channel that conveys information about stimulus motion and eye position.
ORGANIZATION OF dLGN
In the coronal plane of Nissl-stained material, the mouse dLGN appears as a bean-shaped homogeneous structure with boundaries that separate it from the ventrobasal complex, medial geniculate nucleus, the intrageniculate leaflet, and the ventral geniculate nuclei (Fig. 1C). The dLGN takes shape at late embryonic (E) ages, and by E17, the nucleus comprises a narrow strip of neurons along the dorsolateral edge of the thalamus (Angevine 1970; Godement et al. 1984; Vue et al. 2007). During postnatal weeks 1–3, dLGN undergoes a threefold increase in size to take on an adult-like appearance located in the dorsolateral aspect of the thalamus (El-Danaf et al. 2015). As discussed, “hidden laminae” exist in the form of eye-specific patterns of retinal innervation and as a dorsolateral shell and core region (Reese 1988). The shell receives input from DSGCs and the SC and occupies a small strip of dLGN parallel to, and just below, the optic tract. The much larger core division lies beneath the shell and contains the retinal projections from the ipsilateral eye (i.e., ipsi patch), as well as an assortment of projections from non-DSGCs.
Neuronal composition and intrinsic circuitry.
Mouse dLGN contains two principal cell types, thalamocortical relay neurons and intrinsic interneurons (Rafols and Valverde 1973). Estimates reveal that 90% are relay neurons and the remainder interneurons (Jaubert-Miazza et al. 2005). While both cell types receive excitatory retinal input, only relay neurons have axons that exit the dLGN and project to the visual cortex. By contrast, intrinsic interneurons have poorly defined axonal and dendritic fields that are restricted to dLGN and make feedforward inhibitory connections with relay neurons (Fig. 5). As a result, optic tract stimulation often evokes a monosynaptic excitatory postsynaptic potentials (EPSP) that is followed by inhibitory postsynaptic potentials (IPSP) activity (Bickford et al. 2010; Blitz and Regehr 2005; Ziburkus et al. 2003). Underlying IPSP activity in dLGN are the unique synaptic arrangements between interneurons and relay neurons. For example, in addition to a conventional axodendritic synapse (F1), interneurons have presynaptic terminals (F2) that arise from their dendrites. In many instances, these specialized terminals are part of a glomerulus involving three synapses: one between a retinal and F2 terminal, one between the same retinal terminal and the dendrite of a relay cell, and another between the F2 terminal and same dendritic appendage of a relay cell (Bickford 2018; Guillery 1969; Hamos et al. 1985; Sherman 2004). Such an arrangement bypasses the normal route of soma to axon communication and transforms each dendritic branch of an interneuron into an independent processor, thus providing a substrate for both widespread and focal inhibition among relay neurons (Cox and Beatty 2017). In vivo studies of these inhibitory interactions reveal a role in contrast gain control, the sharpening of receptive field structure, and preserving the temporal fidelity of retinally mediated spike trains (Hirsch et al. 2015).
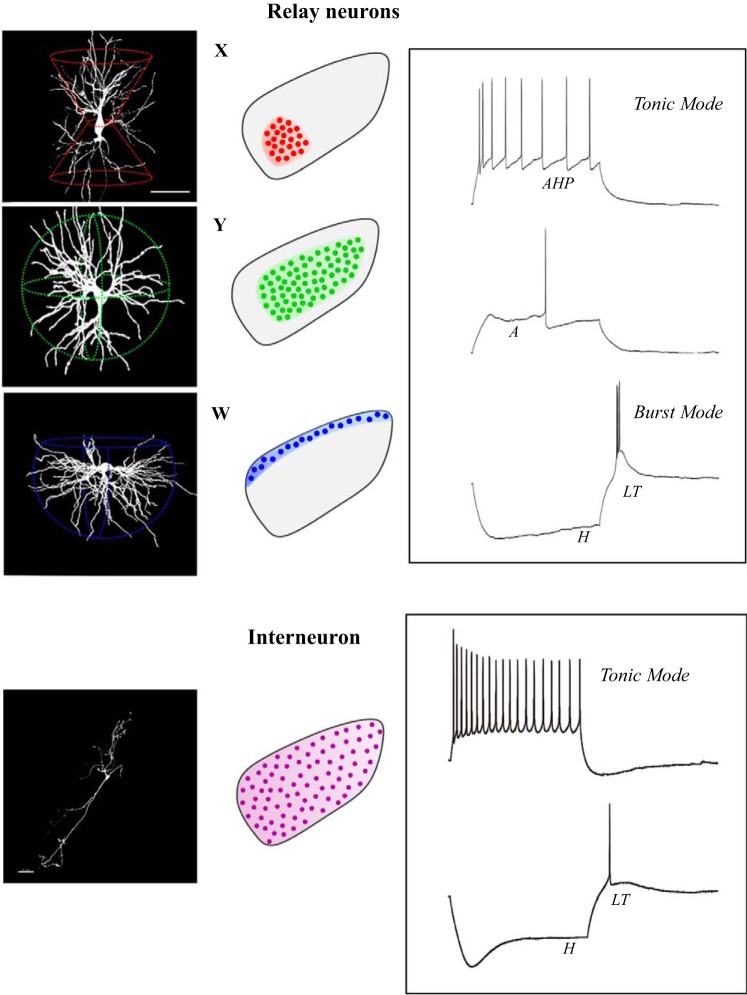
Fig. 5.
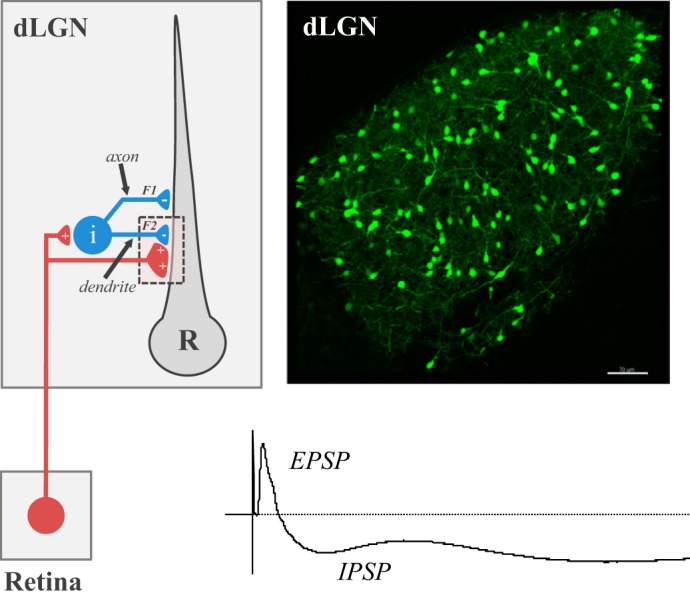
Feed-forward inhibition in dorsal lateral geniculate nucleus (dLGN). Left: circuit diagram showing the pattern of synaptic connectivity between a retinal axon, intrinsic interneuron (i), and relay (R) neuron in dLGN. Retinal (red) synapses make glutamatergic excitatory connections onto an interneuron and relay neuron. Interneurons form GABAergic inhibitory connections with relay neurons through axodendritic (F1) and dendrodendritic (F2) synapses. Right: coronal section through dLGN of a GAD-67 green fluorescent protein (GFP) mouse where GFP is expressed in intrinsic interneurons. Scale bar: 100 μm. Bottom: voltage response from a relay neuron showing the excitatory (EPSP) and inhibitory (IPSP) postsynaptic activity evoked by electrical stimulation of the optic tract.
Relay neurons.
Biocytin fills conducted during in vitro recording experiments confirm earlier Golgi impregnated studies showing that relay neurons have type I or Class A morphology, which consists of a thick unbranched axon, large round soma, and complex multipolar dendritic arbors (El-Danaf et al. 2015; Jaubert-Miazza et al. 2005; Krahe et al. 2011). Three-dimensional reconstructions and quantitative assessment of their dendritic architecture reveal relay neurons possess highly stereotypic shapes that cluster into three distinct morphological groups that bear a striking resemblance to X-, Y-, and W- cells of the cat (Fig. 6; Friedlander et al. 1981; Krahe et al. 2011; Stanford et al. 1981, 1983). Moreover, each class has a regional preference within dLGN (Krahe et al. 2011). X-cells have a biconical dendritic tree and are confined to the ventral region of dLGN. Y-cells have a radially symmetric dendritic tree and reside largely in the binocularly innervated central core region of dLGN, with some having dendritic fields that extend into both the contralateral and ipsilateral retinal terminal domains. W-cells possess a hemispheric shape, occupy the outer perimeter of dLGN and appear to be the exclusive inhabitants of the shell (Bickford et al. 2015). Thus, these regional preferences align well with the projection patterns of certain types of RGCs (e.g., W-cells and DSGCs; Y-cells and OFF alpha transient), and suggest that rodents possess a system-wide parallel organization that extends beyond the retina (Dhande et al. 2015; Martin 1986; Reese 1988; Seabrook et al. 2017). However, an important caveat that remains largely unexplored is whether these morphologically distinct classes of relay neurons have separate and nonoverlapping visual response properties. In a strict sense this possibility seems unlikely given the highly diverse population of RGCs that project to dLGN that drives their visual response properties (see visual response properties of dlgn neurons).
Fig. 6.
Dendritic morphology and membrane properties of dorsal lateral geniculate nucleus (dLGN) neurons. Left to right: reconstructions of relay neurons (X-,Y-,W cells) and an interneuron, along with their corresponding regional preferences, membrane properties, and firing characteristics. Traces reflect voltage responses to current injection. Letters represent the activation of different voltage-gated conductances. AHP, after-hyperpolarization; A, A-type K+; LT, low-threshold Ca2+; H, mixed cation.
Thalamocortical relay neurons destined for dLGN originate from a specific progenitor domain in the caudal thalamic ventricular zone (Vue et al. 2007). The majority of thalamocortical neurons arrive in dLGN by E17, at a time when retinal axons begin to innervate the nucleus (Altman and Bayer 1989; Godement et al. 1984; Vue et al. 2007). Relay neurons of dLGN undergo at least two growth spurts (El-Danaf et al. 2015; Parnavelas et al. 1977). The first one occurs during the first postnatal week, as dendritic branches elaborate and expand in length and complexity to form highly stereotypic architecture that leads to their class-specific identity. During the second spurt at postnatal weeks 2 and 3, relay neurons experience an overall increase in dendritic field, but branch number and complexity remain roughly the same. Interestingly, the timing of these growth spurts corresponds to the expansion in dLGN area, so by end of postnatal week 3, both the maturation of relay neurons and dLGN size stabilize to assume an adult profile. The absence of retinal input disrupts the dendritic growth of relay neurons (El-Danaf et al. 2015). In vitro recordings and biocytin fills in mutant (Math 5−/−) mice that lack retinofugal projections show that visually naive dLGN relay neurons undergo a period of exuberant dendritic growth and branching, followed by branch elimination, and an overall reduction in dendritic field size. These results suggest that retinal signaling provides some form of trophic support for relay neurons, perhaps by regulating the release of certain neurotrophic factors implicated in cell survival, growth, and repair (Caleo et al. 2003; Cohen-Cory and Lom 2004; Menna et al. 2003). Despite the deleterious effects brought about by the absence of retinal input, relay neurons still retain a sufficient degree of dendritic complexity, cell class identity, and functional integrity (El-Danaf et al. 2015). Thus additional sources of trophic support, possibly arising from descending cortical projections, could contribute to the maintenance and stabilization of relay neuron structure and function (see Corticogeniculate projections).
While morphologically distinct, relay neurons in dLGN show little variation in their intrinsic membrane properties and firing characteristics (Fig. 6; Krahe et al. 2011). Each relay neuron is equipped with the same complement of voltage-gated conductances, which when activated, can lead to inward and outward membrane rectification and a nonlinear filtering of spike firing. These include a mixed cation conductance (H) that leads to strong inward rectification and a depolarizing sag during membrane hyperpolarization; a transient A-type K+ conductance activated by membrane depolarization that delays the onset of spiking; at least three different K+ conductances that produce outward rectification that become active during tonic spike firing, producing after hyperpolarizing potentials (AHPs) between spikes and frequency accommodation; and a low-threshold (LT) Ca2+ conductance that gives rise to a large triangular depolarization (LT spike) and burst firing during hyperpolarized membrane states (El-Danaf et al. 2015; Jaubert-Miazza et al. 2005; Krahe et al. 2011). The spike firing characteristics of dLGN neurons have been well documented, responding to excitatory input in one of two modes known as tonic and burst (Sherman 2001). Tonic mode, which prevails during the waking state when thalamic neurons are relatively depolarized, comprise trains of Na+ spikes that fire at rates that reflect a faithful linear transfer of retinally evoked events. Burst mode, which is activated by a LT Ca2+ conductance during hyperpolarized states, is a high-frequency burst of Na+ spikes that ride the crest of the triangular-shaped LT spike, and introduces a form of nonlinear signal distortion to retinogeniculate transmission. In general, burst firing prevails during quiescent states, especially during slow-wave sleep where its rhythmic firing pattern seems to serve as an interrupt signal, preventing the emergence of visually evoked activity (Llinás and Steriade 2006). However, arrhythmic bursting also occurs during the waking state, driven by visually evoked activity, and it serves to amplify the salience of visual objects during early stages of visual attention (Guido et al. 1995; Guido and Weyand 1995; Sherman and Guillery 2002; Swadlow and Gusev 2001; Wang et al. 2007; Weyand et al. 2001). Finally, it is important to note that burst and tonic response modes are controlled by voltage-gated conductances that rely on dc shifts in membrane potential, activity that is under the extrinsic control of nonretinal modulatory input to dLGN (see nonretinal projections to dlgn).
Recordings conducted at young postnatal ages indicate that developing relay neurons are remarkably mature, possessing many of the spike firing properties and voltage-gated conductances observed in adults (Jaubert-Miazza et al. 2005; Krahe et al. 2011; MacLeod et al. 1997). However, there are a number of age-related changes in their synaptic properties and patterns of retinal convergence (Fig. 2; Guido 2008; Hong and Chen 2011). For example, the inhibitory aspects of dLGN circuitry are not fully developed at birth. Feedforward inhibition materializes sometime during the second postnatal week (Bickford et al. 2010). During the first postnatal week, the synaptic responses are purely excitatory, with many neurons exhibiting binocular responses (Jaubert-Miazza et al. 2005; Ziburkus and Guido 2006). Estimates based on the size and strength of EPSP/Cs evoked by electrical stimulation of optic tract reveals that relay neurons receive as many as one or two dozen functional inputs (Chen and Regehr 2000). Over the course of the next 2 wk, monocular responses prevail and the number of functional inputs decline to just a few. Synaptic pruning is also accompanied by changes in synaptic strength and glutamate receptor composition (Liu and Chen 2008). Initially, excitatory currents are small and dominated by NMDA receptor activation. However, at late postnatal ages synaptic responses are substantially larger and show a much higher AMPA-to-NMDA current ratio.
The degree of retinal convergence onto adult dLGN relay neurons has been a topic of debate, with electrophysiological methods yielding much lower estimates than those based on an anatomical means of synapse identification (Hammer et al. 2015; Morgan et al. 2016; Rompani et al. 2017) A recent transsynaptic labeling study suggests that some adult dLGN relay neurons receive input from a diverse and rather large cohort of RGC types (Rompani et al. 2017). However, it is unclear whether this is a general feature of dLGN organization, since widely different patterns of convergence were found, and only a limited sample of dLGN neurons were studied. Nevertheless, other studies using serial section electron microscopy also show that individual dLGN neurons receive far more retinal inputs than estimated using electrophysiological techniques (Hammer et al. 2015; Morgan et al. 2016). One explanation for this discrepancy is that relay neurons receive several inputs, but most are weak and undetectable, and that only a select few are capable of driving measurable evoked postsynaptic activity (Chen et al. 2016; Litvina and Chen 2017). In fact, a recent in vitro recording study that used electrical and optogenetic techniques to stimulate optic tract, along with computational modeling to estimate functional convergence, concluded that relay neurons receive many weak subthreshold inputs, but only a few are strong enough to drive spiking activity (Litvina and Chen 2017). This notion is consistent with in vivo recordings showing that the visual responses of mouse dLGN neurons appear driven by a single RGC type (see visual response properties of dlgn neurons), and in vitro recordings illustrating that the strength and number of retinal inputs can be modified by visual experience (Hong and Chen 2011).
One of the more compelling aspects of dLGN remodeling is the transient expression of a voltage-gated, high-threshold, L-type Ca2+ channels among relay neurons (Dilger et al. 2011). When activated by strong membrane depolarization or retinally evoked EPSPs, these channels give rise to long-lasting, slow-decaying, plateau potentials (Fig. 2; Dilger et al. 2011, 2015). These events are encountered far more frequently during the early phases of retinogeniculate remodeling, and then decline with age, so one week after natural eye opening (P21), they are rarely evoked. At least three factors have been identified that contribute to the high incidence of plateau activity during early postnatal life: the high expression levels L-type channels, the paucity of inhibition, and the high degree of retinal convergence coupled with heightened NMDA activity. Interestingly, the episodic nature of retinal activity brought about by spontaneous retinal waves (i.e., sustained activation of many neighboring RGCs) is also well suited to activate L-type plateau potentials (Lo et al. 2002). These events can lead to long-term changes in synaptic strength (Ziburkus et al. 2009), as well as the activation of Ca2+-dependent signaling cascades implicated in developmental plasticity (Greer and Greenberg 2008). In fact, L-type plateau activity is required for normal retinogeniculate refinement (Dilger et al. 2015). Experiments in mutant mice showing reduced levels of L-type channels lack plateau potentials and experience a breakdown in refinement. Retinogeniculate projections in these mice fail to segregate properly, and dLGN cells retain a high degree of retinal convergence even at late postnatal ages. These defects are also accompanied by a reduction in CREB phosphorylation, a signaling event that has been shown to be essential for retinogeniculate axon segregation (Pham et al. 1999, 2001).
Intrinsic interneurons.
The discovery of a transgenic mouse line (GAD67-GFP) that provides unambiguous visualization of dLGN interneurons (Tamamaki et al. 2003) has led to a number of recent discoveries about their development, structure, and function. Developmentally, interneurons destined for dLGN arise from the neuroepithelium of the third ventricle (Golding et al. 2014), although an additional path has been identified that originates from the SC (Jager et al. 2016). Those from the ventricle begin to migrate toward the dLGN at prenatal ages, following a path through the ventral portion of the vLGN, and entering the dorsolateral sector of dLGN at birth. Initially, they populate the dorsolateral region, and then by the end of first postnatal week, they are distributed throughout all of dLGN (Fig. 5; Charalambakis et al. 2016; Golding et al. 2014). Loss-of-function studies also reveal that retinal signaling controls interneuron positioning within the dLGN, as well as some aspects of their synapse formation with relay neurons (Golding et al. 2014).
Targeted recordings and morphological reconstructions of biocytin-filled dLGN interneurons do not reveal any obvious groupings (Fig. 6; Seabrook et al., 2013b). However, two classes have been identified on the basis of differences in their intrinsic membrane properties, with one group showing smaller soma, higher input resistance, and an increased density of HCN channels (Leist et al. 2016). Interneurons exhibit a type II or Class B morphology, having a few long sinuous primary dendritic processes that exit from opposing poles of a fusiform-shaped soma (Fig. 6). Although they lack a conventional axonal projection, embedded in their dendritic trees are thin processes with terminal swellings, the presumed site of F2 profiles (Fig. 5). Their growth and dendritic elaboration are also more protracted than relay neurons. Before obtaining their adult-like appearance, interneurons go through a distinctive pruning phase, where branch number and complexity are reduced (Charalambakis et al. 2016).
Unlike the regional preferences of relay neurons, interneurons are evenly dispersed throughout dLGN and have dendritic processes that frequently cross eye-specific borders (Seabrook et al., 2013b). Their intrinsic membrane properties and spike firing characteristics also differ from relay neurons (Fig. 6; Leist et al. 2016; Perreault et al. 2003; Seabrook et al. 2013b; Williams et al. 1996). In general, interneurons are more excitable, have a higher input resistance and resting membrane levels, and show weaker LT spiking. Interneurons also exhibit high rates of firing, showing a large linear operating range and little spike frequency adaptation. Most notable is a prominent high threshold L-type Ca2+ conductance within their dendrites. When activated by retinally evoked EPSPs, they trigger long plateau-like depolarizations that give rise to sustained levels of GABA release from F2 profiles, and a powerful disynaptic inhibitory response in relay neurons (Acuna-Goycolea et al. 2008; Pressler and Regehr 2013). Unlike relay neurons, which experience a 4- to 6-fold decrease in retinal input during development, interneurons retain a high degree of retinal convergence throughout postnatal development (Seabrook et al. 2013b). Estimates reveal that some adult interneurons receive as many as 10–12 retinal inputs. This feature is consistent with their unique electronic and morphological structure, and provides the sufficient excitatory drive to mediate both widespread and local inhibitory interactions with relay neurons (see Neuronal composition and intrinsic circuitry).
VISUAL RESPONSE PROPERTIES OF dLGN NEURONS
Conventional properties.
Although the degree of retinal convergence onto mouse dLGN neurons might be higher than the estimates found in carnivores and primates, their receptive field properties appear to reflect input from a single, albeit diverse, RGC type. In vivo recording experiments indicate that most dLGN neurons have large receptive fields (10–20°) and center-surround receptive field organization, with a center that responds in a sustained or transient manner to stimulus onset or offset (Denman and Contreras 2016; Durand et al. 2016; Grubb and Thompson 2003; Piscopo et al. 2013; Suresh et al. 2016; Tang et al. 2016). The responses to drifting gratings indicate that dLGN neurons summate information in a linear manner, exhibit a monotonic increase in firing with increasing levels of contrast but have relatively poor spatial resolution (0.01–0.05 c/d) and low temporal frequency cutoffs (1–4 Hz). Although there is a general agreement that dLGN neurons are monocularly driven, one study finds a high incidence of binocular responses evoked by very bright visual stimuli (Howarth et al. 2014). These responses are stronger than the weak disynaptic, binocular interactions reported in cats and some primates (Guido et al. 1989; Marrocco and McClurkin 1979), and perhaps reflect direct monosynaptic input from the two eyes (Rompani et al. 2017).
Unconventional properties.
While not necessarily unique to the mouse (Masland and Martin 2007), a substantial proportion of dLGN neurons in this species have unusual receptive field properties that can encode for a diverse assortment of stimulus features. Most notable are the visual responses that show selectivity for one direction (DS) or two opposing directions (orientation selective, OS) of a moving stimulus (Marshel et al. 2012; Piscopo et al. 2013; Scholl et al. 2013; Zhao et al. 2013). These DS/OS responses have broad tuning functions along the four cardinal axes, are present in the absence of descending cortical input, and are found among neurons that reside largely in the shell. While rare, some dLGN neurons can detect the absence of contrast in a visual scene (Piscopo et al. 2013; Suresh et al. 2016). This profile is similar to suppressed-by-contrast RGCs where firing rates are reduced by increasing levels of contrast (Tien et al. 2015). The use of specially designed chromatic stimuli that activate intrinsically photosensitive RGCs, reveals that some dLGN neurons act as irradiance detectors, responding to whole-field light steps (Brown et al. 2010). These irradiant responses are likely to reflect input from the M4 (ON-α) subtype of intrinsically photosensitive RGCs (Ecker et al. 2010; Schmidt et al. 2014). While the mouse visual system is considered dichromatic, they have an unusual chromatic opsin wavelength sensitivity that is expressed in a spatially nonuniform manner across the retina (Applebury et al. 2000; Wang et al. 2011). In dLGN, this gives rise to UV-dominated responses in the upper visual fields and green-dominated responses in lower and nasal visual fields (Denman et al. 2017).
Parallel visual pathways.
As discussed throughout this review, there is mounting evidence to support the existence of separable visual channels within the mouse retinogeniculate pathway. The strongest indication pertains to the retinal projections that terminate in the shell of dLGN and form part of a specialized channel designed to convey information about stimulus motion (Bickford et al. 2015; Cruz-Martín et al. 2014). This region is the recipient domain for many types of DSGCs, receives driver-like input from SC, has the highest concentration of DS/OS visual responses, and houses an exclusive class of dLGN relay neurons (W-cells) that project to the superficial layers of visual cortex. The case for other separable visual channels is more difficult to make. For example, X- and Y-cells exhibit regional preferences within the core, an area that receives input from a rather large and diverse set of RGCs. Nonetheless, it is conceivable that multiple types of RGCs that share common visual properties converge onto these dLGN cell classes. Several types of RGCs with a conventional center-surround organization that respond to light increments or decrements are known to project to the core, thus making X- and/or Y-cells part of a channel(s) that serve the more conventional aspects of spatial vision (Dhande et al. 2015; Seabrook et al. 2017). Of course, these scenarios remain to be tested, and perhaps by adopting large-scale methods similar to the recent studies conducted in the mouse SC, where the visual response properties of morphologically identified cell types were obtained, will resolve this issue (Gale and Murphy 2014, 2016; see also Suresh et al. 2016). Lastly, it is important to consider whether parallel channels extend beyond dLGN and project in a systematic way to the visual cortex. Within the visual cortex, there seems to be some laminar segregation for thalamocortical afferents that convey information about visual motion (layers 1–2/3) and spatial vision (layer IV) (Bickford et al. 2015; Cruz-Martín et al. 2014; Lien and Scanziani 2013; but see Kondo and Ohki 2016; Sun et al. 2016) The regional preferences of X- and Y-cells also seem linked to monocular and binocular segments of dLGN, with X-cells residing in the ventral monocular region, and Y-cells occupying a large sector in the central core, which contains projections from both eyes (Krahe et al. 2011). Such separation may have implications for visual cortical organization, since thalamocortical axons arising from these regions project in a parallel manner to monocular and binocular regions of the visual cortex (Caviness and Frost 1980; Coleman et al. 2009). Although the response properties of visual cortical neurons suggest they receive thalamocortical input from separate parallel channels, at least in the spatiotemporal domain (Gao et al. 2010), it is unclear how such organization relates to projections streams of X- and Y-cells, or to the monocular and visual zones of visual cortex. Viral tracing studies that target the afferent and efferent projections of specific cell types offer the best chance to evaluate whether the mouse retino-geniculo-cortical system possesses a system-wide parallel organization (Cruz Martín et al. 2014; Rompani et al. 2017; Zingg et al. 2017).
GRANTS
Support was provided by the National Eye Institute (EY12716).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.G. conceived and designed research; W.G. prepared figures; W.G. drafted manuscript; W.G. edited and revised manuscript; W.G. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank my past (E. Dilger, R. El-Danaf, G. Govindaiah, C. Jurgens, T. Krahe, T. Seabrook, J. Ziburkus) and present (P. Campbell, N. Charalambakis, G. Sokhadze, B. O’Steen) laboratory members, as well as my long-standing collaborators (M. Bickford, M. Fox) for their invaluable scientific contributions. I especially thank T. Seabrook who brought an artistic flair to many of the figures.
REFERENCES
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490: 219–225, 2012. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron 57: 420–431, 2008. doi: 10.1016/j.neuron.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the rat thalamus: VI. The posterior lobule of the thalamic neuroepithelium and the time and site of origin and settling pattern of neurons of the lateral geniculate and lateral posterior nuclei. J Comp Neurol 284: 581–601, 1989. doi: 10.1002/cne.902840407. [DOI] [PubMed] [Google Scholar]
- Angevine JB., Jr Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol 139: 129–187, 1970. doi: 10.1002/cne.901390202. [DOI] [PubMed] [Google Scholar]
- Antal M, Acuna-Goycolea C, Pressler RT, Blitz DM, Regehr WG. Cholinergic activation of M2 receptors leads to context-dependent modulation of feedforward inhibition in the visual thalamus. PLoS Biol 8: e1000348, 2010. doi: 10.1371/journal.pbio.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27: 513–523, 2000. doi: 10.1016/S0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature 529: 345–350, 2016. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros JM, Van der List DA, Chalupa LM. Formation of eye-specific retinogeniculate projections occurs prior to the innervation of the dorsal lateral geniculate nucleus by cholinergic fibers. Thalamus Relat Syst 3: 157–163, 2005. doi: 10.1017/S1472928807000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME. Synaptic organization of the dorsal lateral geniculate nucleus. Eur J Neurosci. In Press. doi: 10.1111/ejn.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, Guido W. Synaptic development of the mouse dorsal lateral geniculate nucleus. J Comp Neurol 518: 622–635, 2010. doi: 10.1002/cne.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Zhou N, Krahe TE, Govindaiah G, Guido W. Retinal and tectal “driver-like” inputs converge in the shell of the mouse dorsal lateral geniculate nucleus. J Neurosci 35: 10523–10534, 2015. doi: 10.1523/JNEUROSCI.3375-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Regehr WG. Timing and specificity of feed-forward inhibition within the LGN. Neuron 45: 917–928, 2005. doi: 10.1016/j.neuron.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Brooks JM, Su J, Levy C, Wang JS, Seabrook TA, Guido W, Fox MA. A molecular mechanism regulating the timing of corticogeniculate innervation. Cell Reports 5: 573–581, 2013. doi: 10.1016/j.celrep.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 8: e1000558, 2010. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caleo M, Medini P, von Bartheld CS, Maffei L. Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J Neurosci 23: 287–296, 2003. doi: 10.1523/JNEUROSCI.23-01-00287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P, Guido W. Development of feedforward and feedback connections between dLGN and TRN (Abstract 529.21/WW9). Society for Neuroscience 2016 Annual Meeting San Diego, CA, November 12–16, 2016. [Google Scholar]
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci 36: 51–77, 2013. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron 57: 511–523, 2008. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS Jr, Frost DO. Tangential organization of thalamic projections to the neocortex in the mouse. J Comp Neurol 194: 335–367, 1980. doi: 10.1002/cne.901940205. [DOI] [PubMed] [Google Scholar]
- Charalambakis N, Govindaiah G, Guido W. The absence of retinal input affects the targeting and morphological development of intrinsic interneurons of dLGN (Abstract 529.23/WW11). Society for Neuroscience 2016 Annual Meeting San Diego, CA, November 12–16, 2016. [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955–966, 2000. doi: 10.1016/S0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Chen C, Bickford ME, Hirsch JA. Untangling the web between eye and brain. Cell 165: 20–21, 2016. doi: 10.1016/j.cell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. J Neurosci 33: 6864–6876, 2013. doi: 10.1523/JNEUROSCI.5208-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Lom B. Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses. Int J Dev Biol 48: 947–956, 2004. doi: 10.1387/ijdb.041883sc. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Law K, Bear MF. Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience 161: 561–571, 2009. doi: 10.1016/j.neuroscience.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Beatty JA. The multifaceted role of inhibitory interneurons in the dorsal lateral geniculate nucleus. Vis Neurosci 34: E017, 2017. doi: 10.1017/S0952523817000141. [DOI] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507: 358–361, 2014. doi: 10.1038/nature12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Malpeli JG. Activity in the parabigeminal nucleus during eye movements directed at moving and stationary targets. J Neurophysiol 89: 3128–3142, 2003. doi: 10.1152/jn.01067.2002. [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci 23: 2851–2860, 2003. doi: 10.1523/JNEUROSCI.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeulemeester H, Arckens L, Vandesande F, Orban GA, Heizmann CW, Pochet R. Calcium binding proteins as molecular markers for cat geniculate neurons. Exp Brain Res 83: 513–520, 1991. doi: 10.1007/BF00229828. [DOI] [PubMed] [Google Scholar]
- Denman DJ, Contreras D. Complex effects on in vivo visual responses by specific projections from mouse cortical layer 6 to dorsal lateral geniculate nucleus. J Neurosci 35: 9265–9280, 2015. doi: 10.1523/JNEUROSCI.0027-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman DJ, Contreras D. On parallel streams through the mouse dorsal lateral geniculate nucleus. Front Neural Circuits 10: 20, 2016. doi: 10.3389/fncir.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman DJ, Siegle JH, Koch C, Reid RC, Blanche TJ. Spatial organization of chromatic pathways in the mouse dorsal lateral geniculate nucleus. J Neurosci 37: 1102–1116, 2017. doi: 10.1523/JNEUROSCI.1742-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB, Crair MC. Development of single retinofugal axon arbors in normal and β2 knock-out mice. J Neurosci 31: 3384–3399, 2011. doi: 10.1523/JNEUROSCI.4899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Stafford BK, Lim JA, Huberman AD. Contributions of retinal ganglion cells to subcortical visual processing and behaviors. Annu Rev Vis Sci 1: 291–328, 2015. doi: 10.1146/annurev-vision-082114-035502. [DOI] [PubMed] [Google Scholar]
- Diao Y, Cui L, Chen Y, Burbridge TJ, Han W, Wirth B, Sestan N, Crair MC, Zhang J. Reciprocal connections between cortex and thalamus contribute to retinal axon targeting to dorsal lateral geniculate nucleus. Cereb Cortex 28: 1168–1182, 2018. doi: 10.1093/cercor/bhx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger EK, Morhardt D, Krahe TE, Shin H-S, Guido W. Absence of synaptically evoked plateau potentials leads to a breakdown in retinogeniculate refinement. J Neurosci 35: 3652–3662, 2015. doi: 10.1523/JNEUROSCI.2343-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger EK, Shin HS, Guido W. Requirements for synaptically evoked plateau potentials in relay cells of the dorsal lateral geniculate nucleus of the mouse. J Physiol 589: 919–937, 2011. doi: 10.1113/jphysiol.2010.202499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger UC, Olsen JF. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol 191: 383–412, 1980. doi: 10.1002/cne.901910306. [DOI] [PubMed] [Google Scholar]
- Durand S, Iyer R, Mizuseki K, de Vries S, Mihalas S, Reid RC. A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. J Neurosci 36: 12,144–12,156, 2016. doi: 10.1523/JNEUROSCI.1741-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67: 49–60, 2010. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Danaf RN, Krahe TE, Dilger EK, Bickford ME, Fox MA, Guido W. Developmental remodeling of relay cells in the dorsal lateral geniculate nucleus in the absence of retinal input. Neural Dev 10: 19, 2015. doi: 10.1186/s13064-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EM, Gauvain G, Sivyer B, Murphy GJ. Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. J Neurophysiol 116: 602–610, 2016. doi: 10.1152/jn.00227.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerson PM, Huguenard JR. Tapping the brakes: cellular and synaptic mechanisms that regulate thalamic oscillations. Neuron 92: 687–704, 2016. doi: 10.1016/j.neuron.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander MJ, Lin CS, Stanford LR, Sherman SM. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol 46: 80–129, 1981. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Gale SD, Murphy GJ. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J Neurosci 34: 13458–13471, 2014. doi: 10.1523/JNEUROSCI.2768-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Murphy GJ. Active dendritic properties and local inhibitory input enable selectivity for object motion in mouse superior colliculus neurons. J Neurosci 36: 9111–9123, 2016. doi: 10.1523/JNEUROSCI.0645-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, DeAngelis GC, Burkhalter A. Parallel input channels to mouse primary visual cortex. J Neurosci 30: 5912–5926, 2010. doi: 10.1523/JNEUROSCI.6456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaün J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol 230: 552–575, 1984. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Golding B, Pouchelon G, Bellone C, Murthy S, Di Nardo A, Govindan S, Ogawa M, Shimogori T, Luscher C, Dayer A, Jabaudon D. Retinal input directs the recruitment of inhibitory interneurons into thalamic visual circuits. Neuron 81: 1057–1069, 2014. doi: 10.1016/j.neuron.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Grant E, Hoerder-Suabedissen A, Molnár Z. The regulation of corticofugal fiber targeting by retinal inputs. Cereb Cortex 26: 1336–1348, 2016. doi: 10.1093/cercor/bhv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59: 846–860, 2008. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the β2 subunit of the nicotinic acetylcholine receptor. Neuron 40: 1161–1172, 2003. doi: 10.1016/S0896-6273(03)00789-X. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J Neurophysiol 90: 3594–3607, 2003. doi: 10.1152/jn.00699.2003. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Visual response properties of burst and tonic firing in the mouse dorsal lateral geniculate nucleus. J Neurophysiol 93: 3224–3247, 2005. doi: 10.1152/jn.00445.2004. [DOI] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol 586: 4357–4362, 2008. doi: 10.1113/jphysiol.2008.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Lu SM, Vaughan JW, Godwin DW, Sherman SM. Receiver operating characteristic (ROC) analysis of neurons in the cat’s lateral geniculate nucleus during tonic and burst response mode. Vis Neurosci 12: 723–741, 1995. doi: 10.1017/S0952523800008993. [DOI] [PubMed] [Google Scholar]
- Guido W, Tumosa N, Spear PD. Binocular interactions in the cat’s dorsal lateral geniculate nucleus. I. Spatial-frequency analysis of responses of X, Y, and W cells to nondominant-eye stimulation. J Neurophysiol 62: 526–543, 1989. doi: 10.1152/jn.1989.62.2.526. [DOI] [PubMed] [Google Scholar]
- Guido W, Weyand T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol 74: 1782–1786, 1995. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z Zellforsch Mikrosk Anat 96: 1–38, 1969. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsádi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci 21: 28–32, 1998. doi: 10.1016/S0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Gut NK, Winn P. The pedunculopontine tegmental nucleus—A functional hypothesis from the comparative literature. Mov Disord 31: 615–624, 2016. doi: 10.1002/mds.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Acsády L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci 39: 680–693, 2016. doi: 10.1016/j.tins.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol 262: 105–124, 1987. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hammer S, Monavarfeshani A, Lemon T, Su J, Fox MA. Multiple retinal axons converge onto relay cells in the adult mouse thalamus. Cell Reports 12: 1575–1583, 2015. doi: 10.1016/j.celrep.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ, Sherman SM. Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature 317: 618–621, 1985. doi: 10.1038/317618a0. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, van Lieshout DP. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J Comp Neurol 304: 275–306, 1991a. doi: 10.1002/cne.903040210. [DOI] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout DP, Hashikawa T, Weber JT. The parabigeminogeniculate projection: connectional studies in eight mammals. J Comp Neurol 305: 559–581, 1991b. doi: 10.1002/cne.903050404. [DOI] [PubMed] [Google Scholar]
- Hasse JM, Briggs F. A cross species comparison of corticogeniculate structure and function. Vis Neurosci 34: E016, 2017. doi: 10.1017/S095252381700013X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Wang X, Sommer FT, Martinez LM. How inhibitory circuits in the thalamus serve vision. Annu Rev Neurosci 38: 309–329, 2015. doi: 10.1146/annurev-neuro-071013-014229. [DOI] [PubMed] [Google Scholar]
- Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol 21: 228–237, 2011. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Park S, Litvina EY, Morales J, Sanes JR, Chen C. Refinement of the retinogeniculate synapse by bouton clustering. Neuron 84: 332–339, 2014. doi: 10.1016/j.neuron.2014.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52: 281–291, 2006. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci 28: 4807–4817, 2008. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Walmsley L, Brown TM. Binocular integration in the mouse lateral geniculate nuclei. Curr Biol 24: 1241–1247, 2014. doi: 10.1016/j.cub.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31: 479–509, 2008a. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59: 425–438, 2008b. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62: 327–334, 2009. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie YY, Hong-Hu Y, Spreur V, Fisher RS, Campagnoni AT. Visualization of corticofugal projections during early cortical development in a tau-GFP-transgenic mouse. Eur J Neurosci 25: 17–30, 2007. doi: 10.1111/j.1460-9568.2006.05258.x. [DOI] [PubMed] [Google Scholar]
- Jager P, Ye Z, Yu X, Zagoraiou L, Prekop HT, Partanen J, Jessell TM, Wisden W, Brickley SG, Delogu A. Tectal-derived interneurons contribute to phasic and tonic inhibition in the visual thalamus. Nat Commun 7: 13579, 2016. doi: 10.1038/ncomms13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22: 661–676, 2005. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jurgens CW, Bell KA, McQuiston AR, Guido W. Optogenetic stimulation of the corticothalamic pathway affects relay cells and GABAergic neurons differently in the mouse visual thalamus. PLoS One 7: e45717, 2012. doi: 10.1371/journal.pone.0045717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31: 7753–7762, 2011. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Guido W. Organization of the dorsal lateral geniculate nucleus in the mouse. Vis Neurosci 34: E008, 2017. doi: 10.1017/S0952523817000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci 30: 1452–1462, 2010. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Ohki K. Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nat Neurosci 19: 316–319, 2016. doi: 10.1038/nn.4215. [DOI] [PubMed] [Google Scholar]
- Krahe TE, El-Danaf RN, Dilger EK, Henderson SC, Guido W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J Neurosci 31: 17437–17448, 2011. doi: 10.1523/JNEUROSCI.4370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Datunashvilli M, Kanyshkova T, Zobeiri M, Aissaoui A, Cerina M, Romanelli MN, Pape HC, Budde T. Two types of interneurons in the mouse lateral geniculate nucleus are characterized by different h-current density. Sci Rep 6: 24904, 2016. doi: 10.1038/srep24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien AD, Scanziani M. Tuned thalamic excitation is amplified by visual cortical circuits. Nat Neurosci 16: 1315–1323, 2013. doi: 10.1038/nn.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvina EY, Chen C. Functional convergence at the retinogeniculate synapse. Neuron 96: 330–338.e5, 2017. doi: 10.1016/j.neuron.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen C. Different roles for AMPA and NMDA receptors in transmission at the immature retinogeniculate synapse. J Neurophysiol 99: 629–643, 2008. doi: 10.1152/jn.01171.2007. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95: 3297–3308, 2006. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Lo FS, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca2+-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol 87: 1175–1185, 2002. doi: 10.1152/jn.00715.1999. [DOI] [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM. The brain-stem parabrachial region controls mode of response to visual stimulation of neurons in the cat’s lateral geniculate nucleus. Vis Neurosci 10: 631–642, 1993. doi: 10.1017/S0952523800005332. [DOI] [PubMed] [Google Scholar]
- MacLeod N, Turner C, Edgar J. Properties of developing lateral geniculate neurones in the mouse. Int J Dev Neurosci 15: 205–224, 1997. doi: 10.1016/S0736-5748(96)00088-3. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, McClurkin JW. Binocular interaction in the lateral geniculate nucleus of the monkey. Brain Res 168: 633–637, 1979. doi: 10.1016/0006-8993(79)90319-6. [DOI] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I, Callaway EM. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76: 713–720, 2012. doi: 10.1016/j.neuron.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martersteck EM, Hirokawa KE, Evarts M, Bernard A, Duan X, Li Y, Ng L, Oh SW, Ouellette B, Royall JJ, Stoecklin M, Wang Q, Zeng H, Sanes JR, Harris JA. Diverse central projection patterns of retinal ganglion cells. Cell Reports 18: 2058–2072, 2017. doi: 10.1016/j.celrep.2017.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR. The projection of different retinal ganglion cell classes to the dorsal lateral geniculate nucleus in the hooded rat. Exp Brain Res 62: 77–88, 1986. doi: 10.1007/BF00237404. [DOI] [PubMed] [Google Scholar]
- Masland RH, Martin PR. The unsolved mystery of vision. Curr Biol 17: R577–R582, 2007. doi: 10.1016/j.cub.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP. Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron 94: 7–18, 2017. doi: 10.1016/j.neuron.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Menna E, Cenni MC, Naska S, Maffei L. The anterogradely transported BDNF promotes retinal axon remodeling during eye-specific segregation within the LGN. Mol Cell Neurosci 24: 972–983, 2003. doi: 10.1016/S1044-7431(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Berger DR, Wetzel AW, Lichtman JW. The fuzzy logic of network connectivity in mouse visual thalamus. Cell 165: 192–206, 2016. doi: 10.1016/j.cell.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J Neurosci 22: 5259–5264, 2002. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Mounty EJ, Bradford R, Lieberman AR. The postnatal development of neurons in the dorsal lateral geniculate nucleus of the rat: a Golgi study. J Comp Neurol 171: 481–499, 1977. doi: 10.1002/cne.901710405. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Qin Y, Heggelund P, Zhu JJ. Postnatal development of GABAergic signalling in the rat lateral geniculate nucleus: presynaptic dendritic mechanisms. J Physiol 546: 137–148, 2003. doi: 10.1113/jphysiol.2002.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof I, Sherman SM. Functional significance of synaptic terminal size in glutamatergic sensory pathways in thalamus and cortex. J Physiol 591: 3125–3131, 2013. doi: 10.1113/jphysiol.2012.247619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason CA. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci 31: 295–315, 2008. doi: 10.1146/annurev.neuro.31.060407.125609. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Rentería RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci 8: 1022–1027, 2005. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci 26: 12873–12884, 2006. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TA, Impey S, Storm DR, Stryker MP. CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron 22: 63–72, 1999. doi: 10.1016/S0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JL, Silva AJ, Storm DR, Stryker MP. The CRE/CREB pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron 31: 409–420, 2001. doi: 10.1016/S0896-6273(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 46: 1–31, 2004. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J Neurosci 33: 4642–4656, 2013. doi: 10.1523/JNEUROSCI.5187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, Regehr WG. Metabotropic glutamate receptors drive global persistent inhibition in the visual thalamus. J Neurosci 33: 2494–2506, 2013. doi: 10.1523/JNEUROSCI.3458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafols JA, Valverde F. The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study. J Comp Neurol 150: 303–331, 1973. doi: 10.1002/cne.901500305. [DOI] [PubMed] [Google Scholar]
- Reese BE. ‘Hidden lamination’ in the dorsal lateral geniculate nucleus: the functional organization of this thalamic region in the rat. Brain Res 13: 119–137, 1988. doi: 10.1016/0165-0173(88)90017-3. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci 31: 8760–8769, 2011. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani SB, Müllner FE, Wanner A, Zhang C, Roth CN, Yonehara K, Roska B. Different modes of visual integration in the lateral geniculate nucleus revealed by single-cell-initiated transsynaptic tracing. Neuron 93: 767–776.e6, 2017. [Erratum in Neuron 93: 1519, 2017.] doi: 10.1016/j.neuron.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso DL, Qiao M, Kagan RD, Yamagata M, Palmiter RD, Sanes JR. Two pairs of ON and OFF retinal ganglion cells are defined by intersectional patterns of transcription factor expression. Cell Reports 15: 1930–1944, 2016. doi: 10.1016/j.celrep.2016.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci 38: 221–246, 2015. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT, Hattar S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron 82: 781–788, 2014. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Tan AY, Corey J, Priebe NJ. Emergence of orientation selectivity in the Mammalian visual pathway. J Neurosci 33: 10616–10624, 2013. doi: 10.1523/JNEUROSCI.0404-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. Architecture, function, and assembly of the mouse visual system. Annu Rev Neurosci 40: 499–538, 2017. doi: 10.1146/annurev-neuro-071714-033842. [DOI] [PubMed] [Google Scholar]
- Seabrook TA, El-Danaf RN, Krahe TE, Fox MA, Guido W. Retinal input regulates the timing of corticogeniculate innervation. J Neurosci 33: 10085–10097, 2013a. doi: 10.1523/JNEUROSCI.5271-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Krahe TE, Govindaiah G, Guido W. Interneurons in the mouse visual thalamus maintain a high degree of retinal convergence throughout postnatal development. Neural Dev 8: 24, 2013b. doi: 10.1186/1749-8104-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks JA, Ito S, Schaevitz L, Yamada J, Chen B, Litke A, Feldheim DA. Corticothalamic axons are essential for retinal ganglion cell axon targeting to the mouse dorsal lateral geniculate nucleus. J Neurosci 36: 5252–5263, 2016. doi: 10.1523/JNEUROSCI.4599-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Kirkwood PA. Prenatal development of functional connections in the cat’s retinogeniculate pathway. J Neurosci 4: 1378–1397, 1984. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24: 122–126, 2001. doi: 10.1016/S0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends Neurosci 27: 670–675, 2004. doi: 10.1016/j.tins.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357: 1695–1708, 2002. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol 106: 1068–1077, 2011. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- Sokhadze G, Zhou N, Bickford ME, Guido W. The organization and development of cholinergic brainstem input to the mouse visual thalamus (Abstract 817.02/EE8). Society for Neuroscience 2014 Annual Meeting Chicago, IL, October 17–21, 2014. [Google Scholar]
- Stanford LR, Friedlander MJ, Sherman SM. Morphology of physiologically identified W-cells in the C laminae of the cat’s lateral geniculate nucleus. J Neurosci 1: 578–584, 1981. doi: 10.1523/JNEUROSCI.01-06-00578.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford LR, Friedlander MJ, Sherman SM. Morphological and physiological properties of geniculate W-cells of the cat: a comparison with X- and Y-cells. J Neurophysiol 50: 582–608, 1983. doi: 10.1152/jn.1983.50.3.582. [DOI] [PubMed] [Google Scholar]
- Sun W, Tan Z, Mensh BD, Ji N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat Neurosci 19: 308–315, 2016. doi: 10.1038/nn.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh V, Çiftçioğlu UM, Wang X, Lala BM, Ding KR, Smith WA, Sommer FT, Hirsch JA. Synaptic contributions to receptive field structure and response properties in the rodent lateral geniculate nucleus of the thalamus. J Neurosci 36: 10949–10963, 2016. doi: 10.1523/JNEUROSCI.1045-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci 4: 402–408, 2001. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467: 60–79, 2003. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tang J, Ardila Jimenez SC, Chakraborty S, Schultz SR. Visual receptive field properties of neurons in the mouse lateral geniculate nucleus. PLoS One 11: e0146017, 2016. doi: 10.1371/journal.pone.0146017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nat Neurosci 3: 608–616, 2000. doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]
- Thompson AD, Picard N, Min L, Fagiolini M, Chen C. Cortical feedback regulates feedforward retinogeniculate refinement. Neuron 91: 1021–1033, 2016. doi: 10.1016/j.neuron.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien NW, Pearson JT, Heller CR, Demas J, Kerschensteiner D. Genetically identified suppressed-by-contrast retinal ganglion cells reliably signal self-generated visual stimuli. J Neurosci 35: 10815–10820, 2015. doi: 10.1523/JNEUROSCI.1521-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol 76: 213–235, 2005. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Tschetter WW, Govindaiah G, Etherington IM, Guido W, Niell CM. Refinement of spatial receptive fields in the developing mouse LGN is coordinated with excitatory and inhibitory remodeling. J Neurosci. In press. doi: 10.1523/JNEUROSCI.2857-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vue TY, Aaker J, Taniguchi A, Kazemzadeh C, Skidmore JM, Martin DM, Martin JF, Treier M, Nakagawa Y. Characterization of progenitor domains in the developing mouse thalamus. J Comp Neurol 505: 73–91, 2007. doi: 10.1002/cne.21467. [DOI] [PubMed] [Google Scholar]
- Wang X, Wei Y, Vaingankar V, Wang Q, Koepsell K, Sommer FT, Hirsch JA. Feedforward excitation and inhibition evoke dual modes of firing in the cat’s visual thalamus during naturalistic viewing. Neuron 55: 465–478, 2007. doi: 10.1016/j.neuron.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci 31: 7670–7681, 2011. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand TG, Boudreaux M, Guido W. Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J Neurophysiol 85: 1107–1118, 2001. doi: 10.1152/jn.2001.85.3.1107. [DOI] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Anderson CM, Crunelli V. Electrophysiological and morphological properties of interneurones in the rat dorsal lateral geniculate nucleus in vitro. J Physiol 490: 129–147, 1996. doi: 10.1113/jphysiol.1996.sp021131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen H, Liu X, Cang J. Orientation-selective responses in the mouse lateral geniculate nucleus. J Neurosci 33: 12751–12763, 2013. doi: 10.1523/JNEUROSCI.0095-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Heggelund P. Muscarinic regulation of dendritic and axonal outputs of rat thalamic interneurons: a new cellular mechanism for uncoupling distal dendrites. J Neurosci 21: 1148–1159, 2001. doi: 10.1523/JNEUROSCI.21-04-01148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Uhlrich DJ. Nicotinic receptor-mediated responses in relay cells and interneurons in the rat lateral geniculate nucleus. Neuroscience 80: 191–202, 1997. doi: 10.1016/S0306-4522(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Uhlrich DJ. Cellular mechanisms underlying two muscarinic receptor-mediated depolarizing responses in relay cells of the rat lateral geniculate nucleus. Neuroscience 87: 767–781, 1998. doi: 10.1016/S0306-4522(98)00209-7. [DOI] [PubMed] [Google Scholar]
- Žiburkus J, Dilger EK, Lo FS, Guido W. LTD and LTP at the developing retinogeniculate synapse. J Neurophysiol 102: 3082–3090, 2009. doi: 10.1152/jn.90618.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol 96: 2775–2784, 2006. doi: 10.1152/jn.01321.2004. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Lo FS, Guido W. Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. J Neurophysiol 90: 1063–1070, 2003. doi: 10.1152/jn.00178.2003. [DOI] [PubMed] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, Zhang LI. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93: 33–47, 2017. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]