The primary visual cortex of mammals contains a systematic map of visual stimulus space that changes with the distinct visual requirements of each species. For example, the cat visual cortex has a map that represents predominantly the retinotopy of the binocular field and includes other additional stimulus dimensions such as light/dark polarity, orientation, direction, and spatial frequency (Kremkow et al. 2016). As the number of stimulus dimensions increases within the cortical map, it becomes more difficult to represent equally all the possible combinations of dimensions. Consequently, carnivores and primates have a biased cortical representation of stimulus dimensions that include central vision, the contralateral eye, dark stimuli, and binocular high spatial frequencies (Jin et al. 2008; Kremkow et al. 2016; Nauhaus et al. 2016; Yeh et al. 2009). Such biased cortical representation would seem unnecessary in the mouse visual cortex because it has only been found to have a retinotopic map despite the fact that neurons can be highly selective for other features such as orientation and spatial frequency (Niell and Stryker 2008). However, this notion is rapidly changing. A paper by Jimenez et al. (2018) in the Journal of Neurophysiology demonstrates a weak but significant bias in the combined representation of retinotopy and stimulus tuning in mouse visual cortex as well as a cortical bias for dark stimuli similar to that found in larger brains (Jin et al. 2008; Kremkow et al. 2016; Lee et al. 2016; Yeh et al. 2009).
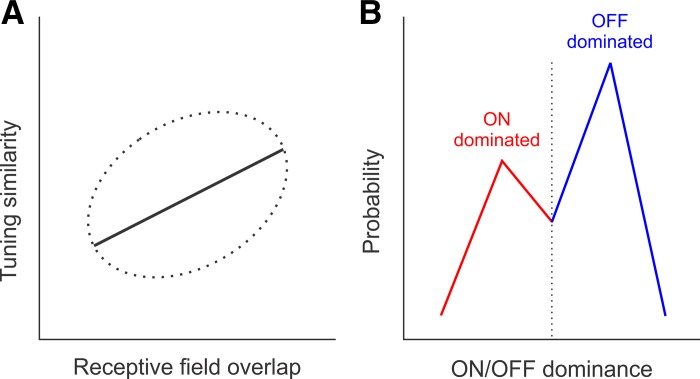
The authors combined viral expression of the calcium indicator GCaMP6f with two-photon imaging to measure the tuning properties of large populations of individual neurons in the primary visual cortex of awake mice. They used sinusoidal patterns with different orientations and spatial frequencies to measure stimulus tuning and light/dark small stimuli to map the cortical receptive fields and their dominant contrast polarity (ON-dominated: preference for lights; OFF-dominated: preference for darks). When the authors compared the tuning similarity and receptive field overlap of pairs of neurons, they found a weak but significant positive correlation: as the receptive field overlap increased, the tuning similarity also increased (Fig. 1A). In addition, they found that OFF-dominated neurons were more numerous than ON-dominated neurons (Fig. 1B) and that ON receptive field subregions were more scattered in visual space than OFF receptive field subregions. Taken together with previous studies (Jin et al. 2008; Kremkow et al. 2016; Lee et al. 2016; Nauhaus et al. 2016; Yeh et al. 2009), these results demonstrate that the visual cortex of rodents, carnivores, and primates do not represent all combinations of stimulus dimensions equally and that dark stimuli dominate the cortical representation.
Fig. 1.
Cortical biases in the combined representation of retinotopy with stimulus tuning and dark/light contrast polarity in mouse primary visual cortex (Jimenez et al. 2018). A: cartoon representing a weak but significant positive correlation between tuning similarity and receptive field overlap (the dotted ellipse represents data spread, and the solid line represents the data trend). B: cartoon representing the bias of cortical responses toward dark stimuli. Blue histogram represents the probability of finding a cortical neuron dominated by the OFF pathway (responds stronger to dark stimuli). Red histogram represents the probability of finding a cortical neuron dominated by the ON pathway (responds stronger to light stimuli). Dotted line represents neurons with balanced ON/OFF responses.
The results of Jimenez et al. (2018) may also shed some light on the development of visual cortical maps for stimulus orientation in carnivores and primates and the lack of these maps in mice. Although the precise developmental mechanisms remain unknown, an interesting possibility is that orientation maps emerge from the tiling of visual space by ON and OFF ganglion cells in the retina (Paik and Ringach 2011; Soodak 1987; Wässle et al. 1981). According to this model, the position of ON and OFF retinal ganglion cells determines not only the cortical retinotopy, but also the cortical preference for stimulus orientation. Closely spaced ON and OFF retinal ganglion cells bias each cortical region toward a specific stimulus orientation. In the large cat visual cortex, this bias leads to the development of orientation maps. In the smaller mouse visual cortex, it leads to small clusters of cortical neurons with similar orientation at a given retinotopic location. A prediction from this model is that stimulus tuning should be more similar among cortical neurons with overlapping receptive fields than those with distant receptive fields. The reasoning behind this prediction is that both stimulus tuning and receptive field geometry originate from the same mechanism: the ON and OFF receptive field positions inherited from the retina. The positive correlation between stimulus tuning and receptive field overlap that the authors demonstrate is certainly consistent with this prediction. However, providing support for this model will require testing other predictions that more directly rule out alternative models. A crucial test to the model would be to demonstrate that the organization of ON and OFF retinal ganglion cells can be used to predict the organization of the cortical orientation map in the same animal.
A main conclusion from Jimenez et al. (2018) is that the primary visual cortex may not need to represent equally all combinations of retinotopy, orientation, and spatial frequency to extract visual information efficiently. The authors provide a helpful analogy to explain this point. The photoreceptor array can sense a limited set of wavelength combinations at each spatial location of the visual field, but the brain is still able to extract color information efficiently. Similarly, a biased set of stimulus-tuning combinations for orientation and spatial frequency in visual cortex can be also enough to extract shape information.
The number of combined stimulus dimensions within a cortical map depends on many factors, including the size of the cortex, the size of the visual field, and the visual resolution of the eye. The cortex does not need to represent spatial frequencies that the eye cannot see or orientation differences that the eye cannot discriminate. Therefore, because visual acuity is more than one order of magnitude lower in mice than cats, mice need less cortical resources to process the visual scene.
Regardless of brain size, all mammals with eyes need a systematic representation of stimulus location within a cortical retinotopic map. However, the retinotopy gradient within this map (how fast retinotopy moves with cortical distance) varies greatly across animals. For example, in cats, a movement of 500 μm within the visual cortical map only changes retinotopy by a quarter of a receptive field center (<0.3° in central vision). In contrast, the same movement in the mouse visual cortical map changes retinotopy by over a full receptive field center (Bonin et al. 2011), a displacement in visual space two orders of magnitude larger than in cats. Cats use >1 mm2 of visual cortex to represent the same retinotopy, which allows accommodating multiple combinations of stimulus dimensions for the same location of visual space and even sorting the dimension combinations by eye input and light/dark polarity (Kremkow et al. 2016). In contrast, the cortical allocation is at least one order of magnitude smaller in the mouse. Since retinotopy changes so rapidly across mouse visual cortex, there is potentially less space to accommodate the multiple combinations of stimulus dimensions for each location of visual space. Therefore, the bias in the combined representation of retinotopy and stimulus tuning that Jimenez et al. (2018) found may reflect either the statistics of ON and OFF retinal wiring or simply a compromise to represent the most relevant stimulus combinations in the available cortical space (similar to the bias for central vision and OFF dominance in carnivores and primates). Whatever the reasons for the cortical biases are, the work of Jimenez et al. (2018) clearly indicates that the available cortical space in the mouse does not represent all combinations of retinotopy and stimulus tuning equally. However, the consequences of this bias for visual function remain unclear.
GRANTS
We were supported by National Eye Institute Grants EY-027157 (to R. Mazade), EY-023190 (to C. M. Niell), and EY-05253 (to J. M. Alonso).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M., C.M.N., and J.M.A. drafted manuscript; R.M., C.M.N., and J.M.A. edited and revised manuscript; R.M., C.M.N., and J.M.A. approved final version of manuscript.
REFERENCES
- Bonin V, Histed MH, Yurgenson S, Reid RC. Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J Neurosci 31: 18506–18521, 2011. doi: 10.1523/JNEUROSCI.2974-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez LO, Tring E, Trachtenberg JT, Ringach DL. Local tuning biases in mouse primary visual cortex. J Neurophysiol. First published April 18, 2018. doi: 10.1152/jn.00150.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JZ, Weng C, Yeh CI, Gordon JA, Ruthazer ES, Stryker MP, Swadlow HA, Alonso JM. On and off domains of geniculate afferents in cat primary visual cortex. Nat Neurosci 11: 88–94, 2008. doi: 10.1038/nn2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremkow J, Jin J, Wang Y, Alonso JM. Principles underlying sensory map topography in primary visual cortex. Nature 533: 52–57, 2016. doi: 10.1038/nature17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Huang X, Fitzpatrick D. Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature 533: 90–94, 2016. doi: 10.1038/nature17941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauhaus I, Nielsen KJ, Callaway EM. Efficient receptive field tiling in primate V1. Neuron 91: 893–904, 2016. doi: 10.1016/j.neuron.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci 28: 7520–7536, 2008. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SB, Ringach DL. Retinal origin of orientation maps in visual cortex. Nat Neurosci 14: 919–925, 2011. doi: 10.1038/nn.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soodak RE. The retinal ganglion cell mosaic defines orientation columns in striate cortex. Proc Natl Acad Sci USA 84: 3936–3940, 1987. doi: 10.1073/pnas.84.11.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Boycott BB, Illing RB. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B Biol Sci 212: 177–195, 1981. doi: 10.1098/rspb.1981.0033. [DOI] [PubMed] [Google Scholar]
- Yeh CI, Xing D, Shapley RM. “Black” responses dominate macaque primary visual cortex V1. J Neurosci 29: 11753–11760, 2009. doi: 10.1523/JNEUROSCI.1991-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]