We conducted an observational study in Malawi to understand the patient impact of implementing point-of-care early infant diagnosis (POC EID). Antiretroviral treatment initiation rates were significantly improved with the implementation of same-day POC EID testing compared with referred, longer-turnaround laboratory-based testing.
Keywords: point-of-care, early infant diagnosis, HIV, ART initiation
Abstract
Background
In Malawi in 2014, <20% of human immunodeficiency virus (HIV)–exposed infants received an early infant diagnosis (EID) test in the first 2 months of life and only 30% of HIV-infected children were on antiretroviral therapy (ART). We sought to understand the potential patient impact of improving timely infant diagnosis and treatment initiation through implementation of point-of-care (POC) EID technologies in Malawi.
Methods
In this observational study, POC EID technologies were introduced into routine services at 7 health facilities across Malawi in September 2015. The primary outcome was the proportion of HIV-infected infants initiating ART within 60 days of sample collection in the POC arm compared to the baseline arm with conventional laboratory-based EID testing.
Results
The time from sample collection to result received by the patient decreased significantly from 56 days (interquartile range [IQR], 30–81 days) in the baseline arm to <1 day in the POC arm (P < .001). Of the HIV-infected infants, the time between sample collection and ART initiation was reduced from 38 days (IQR, 30–54 days) in the baseline arm to <1 day (IQR, 0–1 day) in the POC arm (P = .019). Furthermore, the proportion of HIV-infected infants initiated on ART within 60 days of sample collection increased significantly from 41.9% to 91.1% after the introduction of POC (adjusted risk ratio, 2.28; P < .001).
Conclusions
ART initiation rates were significantly improved with the implementation of same-day POC EID testing compared with referred, longer-turnaround laboratory-based testing.
Despite progress in reducing the number of children newly infected with human immunodeficiency virus (HIV) annually from 490000 to 150000 in the last 15 years, an estimated 1.8 million children under the age of 15 are living with HIV in 2015 [1, 2]. Compared to older populations, HIV-infected infants have high rates of mortality and rapid disease progression [3–5]. Among untreated HIV-infected infants, mortality peaks at 2–3 months, and approximately 35% die by their first birthday and 52% die by 2 years of age [3, 5]. However, studies show that access to antiretroviral therapy (ART) can dramatically reduce infant mortality and HIV progression, especially if initiated early before immunological and clinical disease progression [6–8].
Providing HIV-infected infants with life-saving access to ART is a public health priority. The World Health Organization (WHO) recommends that all HIV-exposed infants have a virological test at 4–6 weeks of age and that all HIV-infected infants initiate ART immediately after diagnosis [8]. Early infant diagnosis (EID) of HIV is a critical step for prompt linkage to care and initiation of ART to improve childhood survival and development [7–9]. Although access to EID has increased in recent years, only 43% of HIV-exposed children received virological testing within the first 2 months of life in 2015 [10], and <45% of HIV-infected infants are effectively linked to care and on ART [8, 10, 11].
HIV testing in infant populations is complicated by the presence of maternal HIV antibodies that can circulate in children up to 18 months after birth [8]. To accurately diagnose HIV infection in infants, virological detection of HIV nucleic acid–based assays are required, as opposed to the common antibody-based HIV tests used to test older children and adults [8]. Nucleic acid testing in the laboratory requires sophisticated infrastructure, equipment, and skilled technicians that are often only available in central facilities [12, 13]. This system presents a major bottleneck for timely EID, as sample transport from decentralized and rural clinics to central laboratories results in long test turnaround times of up to several months and families may not always return to receive test results [13]. Novel point-of-care (POC) technologies for EID address these programmatic challenges by allowing for on-site, same-day testing that provides rapid results within 1–2 hours of sample collection, thus potentially reducing test turnaround times and enabling prompt clinical decision making [14]. In 2016, WHO guidelines recommended the use of POC for EID, and the WHO prequalification program has thus far approved the use of 2 POC assays [8, 15].
There is limited evidence to demonstrate whether implementation of POC EID testing translates into significant patient impact in low-resource settings. To address this paucity of evidence, the Ministry of Health in Malawi implemented POC EID testing in several facilities across the national healthcare system to compare against standard laboratory-based EID. This pilot study investigated the impact of POC testing on routine patient management along the testing and treatment cascade. It was hypothesized that POC EID would reduce patient attrition and delays across the EID cascade, particularly between sample collection and result receipt by the patient and ART initiation of HIV-infected infants, while also improving timely infant diagnosis and treatment initiation.
METHODS
Study Design
This was an observational study conducted between January 2015 and June 2016. Seven healthcare facilities were selected on the basis of on having an HIV prevalence among pregnant/antenatal care attendee women of >10%, minimum EID testing volumes of ≥3 per week, access to a sample transport system and EID referral testing laboratory, and provision of pediatric ART services. The health facilities included were Kamuzu Central Hospital, Mzuzu Central Hospital, Ntcheu District Hospital, Machinga District Hospital, Migowi Health Centre, Nsanama Health Centre, and Ntaja Health Centre. Data on infants included in the baseline control arm with laboratory-based testing were retrospectively collected for the period between January and August 2015, while the intervention arm with POC testing had prospective data collection between September 2015 and March 2016. Data registers, patient records, EID algorithms, and clinical training and practices were the same in both arms and across the period of the study.
Population
A total of 1752 infants were included in the study, with 963 enrolled in the baseline arm and 789 in the POC arm. Infants were included consecutively based on the routine clinical indication for an EID test per national guidelines, including infants suspected of HIV exposure or infection in infants >4 weeks and <12 months of age. Infants already diagnosed with HIV or already initiated on ART for management of HIV infection were excluded from the study. After an initial positive test result, all infants in the POC arm received a second (confirmatory) POC test with a new sample. All test results were concordant.
Testing
POC testing was performed using the Alere q device (Alere Technologies, Jena, Germany). These technologies had national regulatory approval for in vitro diagnostic use within the standard of clinical care in Malawi. Implementation began at each of the 7 healthcare facilities in September 2015. Patients were tested using 25 μL of blood collected from a lancet heel-prick and placed directly into the test cartridges, which were immediately inserted into the device. Testing time in the analyzer took approximately 60 minutes per test. Healthcare providers were trained on sample collection and testing per the manufacturer’s instructions.
For the baseline control arm using conventional laboratory-based testing, 2–5 drops of whole blood were collected using a lancet heel-prick and applied to a filter paper card (Whatman 903, GE Healthcare Biosciences, Pittsburgh, Pennsylvania). Cards were stored overnight at room temperature to dry and shipped to the central laboratory for processing and testing using the Abbott RealTime HIV-1 Qualitative test on the m2000sp system (Abbott Molecular, Des Plaines, Illinois) according to the manufacturer’s instructions.
Outcomes
The primary outcome for this study was the proportion of HIV-infected infants initiating ART within 60 days of sample collection in the POC arm compared to the baseline arm with conventional laboratory-based EID testing. WHO recommendations currently strongly recommend that test results from virological testing be returned to the clinic and child/mother/carer as soon as possible, but at the very latest within 4 weeks of specimen collection [16]. We selected the timing for the primary outcome because of this recommendation and to allow for some potential delays in ART initiation upon result receipt for consultations and preparation of the mother or caregiver to support and deliver treatment to the HIV-infected infant. The secondary outcomes were times to complete different stages of the cascade of care, from sample collection, to result received by the patient, to ART initiation.
Sample Size
The sample size calculation was calculated based on an expected improvement from POC testing on ART initiation from the current rate of 40% to 75%, a choice of significance level (α = .05), variability between facilities, and the expected power of this pilot to detect outcome differences between baseline control and intervention arms (80%) [17]. The minimum number of HIV-infected infants needed was 70 and 35 in each arm, respectively. The estimated mother-to-child HIV transmission rate in Malawi was 5%; therefore, it was expected that a minimum of 1400 enrolled infants would be needed overall.
Data Collection and Analysis
Demographic and clinical data were collected for each infant using national clinic logbooks and patient charts, when necessary. The standard national routine EID testing logbook provided the majority of data for both arms, including date of birth, EID sampling, testing and result received dates, HIV diagnosis, and ART initiation date. HIV-infected infants without a date of ART initiation or listed treatment regimen were considered as having failed to initiate treatment. Furthermore, patients with dates relevant to each data metric calculated were included in the analysis and infants with some missing dates were not excluded from all analyses.
Statistical analysis was performed with the R statistical software (version 3.3.2, Free Software Foundation, Boston, Massachusetts). Two-samples comparison was done using the nonparametric rank-based Wilcoxon-Mann-Whitney test, and Fisher exact tests were used to determine the independence of 2 × 2 tables. Confidence intervals of binomial probabilities were computed by the Wilson method. Relative risk regression was implemented using a modified Poisson regression approach [18].
Protocol Approval
This study was approved by Malawi’s National Health Sciences Research Committee and the Chesapeake Institutional Review Board in the United States.
RESULTS
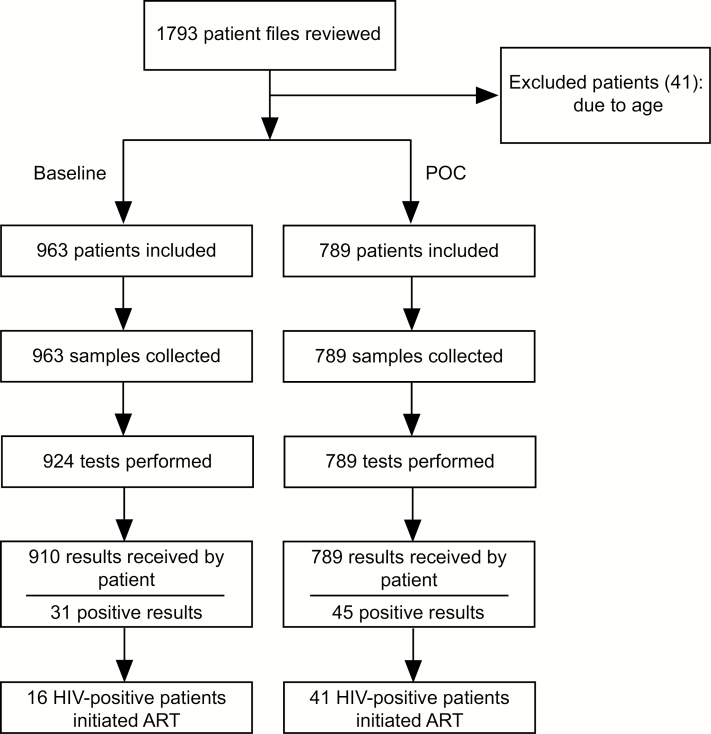
A total of 1793 patient files were reviewed, with 41 infants being excluded because they had been tested before 4 weeks of age (Figure 1). None of the infants had previously tested positive for HIV or already initiated ART. Approximately 50% of included infants were female and the majority (56.3%) had a sample collected at <2 months of age (Table 1). A third of infants were between 2 and 6 months of age at sample collection. The median age at sample collection was similar between the 2 arms, at approximately 55 days.
Figure 1.
Flowchart for patient cohorts in the intervention point-of-care and laboratory (baseline) study arms. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; POC, point of care.
Table 1.
Demographic Data of the Point-of-Care and Laboratory (Baseline) Study Populations Undergoing Human Immunodeficiency Virus Early Infant Diagnosis Testing and Antiretroviral Therapy
| Characteristic | Total | Point-of-Care | Baseline | P value | |||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | ||
| Total | 1752 | (100) | 789 | (45.0) | 963 | (55.0) | |
| Sex | |||||||
| Female | 870 | (49.7) | 349 | (44.2) | 521 | (54.1) | .033 |
| Male | 799 | (45.6) | 362 | (45.9) | 437 | (45.4) | |
| Data not available | 83 | (4.7) | 78 | (9.9) | 5 | (0.5) | |
| Age at sample collection | |||||||
| ≤2 mo | 987 | (56.3) | 441 | (55.9) | 546 | (56.7) | .042 |
| >2 mo–≤6 mo | 536 | (30.6) | 235 | (29.8) | 301 | (31.3) | |
| >6 mo–≤12 mo | 191 | (10.9) | 105 | (13.3) | 86 | (8.9) | |
| >12 mo | 21 | (1.2) | 8 | (1.0) | 13 | (1.3) | |
| Proportion positive, no./No. (%) | 76/1752 | (4.3) | 45/789 | (5.7) | 31/963 | (3.2) | .034 |
The proportion of HIV-infected infants was higher in the POC arm (5.7%) compared with the baseline arm (3.2%) (P = .034). The majority of HIV-infected infants came from inpatient wards in both arms (64.5% and 48.9% in the baseline and POC arms, respectively), followed by prevention of mother-to-child transmission (PMTCT; 22.6% and 37.8% in the baseline and POC arms, respectively). In both arms, routine records were consistently well completed: >75% of patient records had ≤2 missing fields.
The median age at result received was significantly reduced in the POC arm to 55 days (interquartile range [IQR], 45–111 days) compared with the baseline arm of 126 days (IQR, 93–183 days) (P < .001; Table 2). The time between sample collection and results received by the patient decreased significantly from 56 days (IQR, 30–81 days) in the baseline arm to 0 days (IQR, 0–0 days) in the POC arm (P < .001). Nearly all infants tested in the POC arm received their results on the same day as sample collection (99.5%) compared with none in the baseline arm (P < .001; Table 3). All infants tested in the POC arm had received their results within 30 days of sample collection, yet only 18.1% had in the baseline arm (adjusted risk ratio [aRR], 5.48; P < .001). By 60 days after sample collection, only 41.0% of infants in the baseline arm had received their results (aRR, 2.41; P < .001). Furthermore, the time between sample collection and results received by the patient among HIV-infected infants was significantly reduced from 40 days (IQR, 27–57 days) to 0 days (IQR, 0–0 days) (P < .001) with the introduction of POC.
Table 2.
Median Age at and Days Between Steps Along the Testing and Treatment Cascade
| Outcomes measured | POC | Baseline | P value |
|---|---|---|---|
| Age at sample collection | 55 (45–111) | 53 (45–95) | .030 |
| Age at result received | 55 (45–111) | 126 (93–183) | <.001 |
| Age at ART initiation | 148 (74–225) | 164 (104–230) | NS |
| Time between sample collection and results received by patient | 0 (0–0) | 56 (30–81) | <.001 |
| Time between sample collection and results received by patient (among HIV-infected infants) | 0 (0–0) | 40 (27–57) | <.001 |
| Time between sample collection and ART initiation | 0 (0–1) | 38 (30–54) | .019 |
| Time between results received by patient and ART initiation | 0 (0–1) | 1 (0–5) | .047 |
Data are presented as median days (interquartile range).
Abbreviations: ART, antiretroviral treatment; HIV, human immunodeficiency virus; NS, not significant; POC, point-of-care.
Table 3.
Proportions and Risk Ratios for Achieving Steps Toward Pediatric Testing and Treatment for Infants Undergoing Point-of-Care and Laboratory Baseline Early Infant Diagnosis Testing
| Outcomes measured | POC | Baseline | Unadjusted RR (95% CI) | Adjusteda RR (95% CI) | P Value (Adjusted) |
|---|---|---|---|---|---|
| Results received by patient | |||||
| Same day as sample collection | 785 (99.5) | 0 (0.00) | ∞ | ∞ | <.001 |
| Within 30 d of sample collection | 789 (100) | 171 (18.1) | 5.54 (4.84–6.34) | 5.48 (4.79–6.28) | <.001 |
| Within 60 d of sample collection | 789 (100) | 388 (41.0) | 2.44 (2.26–2.63) | 2.41 (2.24–2.60) | <.001 |
| ART initiation | |||||
| Within 60 d of sample collection | 41 (91.1) | 13 (41.9) | 2.17 (1.42–3.32) | 2.28 (1.50–3.48) | <.001 |
| Within 180 d of sample collection | 41 (91.1) | 15 (48.4) | 1.88 (1.29–2.74) | 2.01 (1.39–2.91) | <.001 |
| Overall | 41 (91.1) | 16 (51.6) | 1.77 (1.24–2.51) | 1.90 (1.34–2.69) | .001 |
| ART same day as results received by patient | 29 (70.7) | 7 (43.8) | 1.62 (.90–2.91) | 1.60 (.88–2.89) | .120 |
| ART within 30 d of results received by patient | 41 (100) | 15 (93.8) | 1.07 (.94–1.21) | 1.06 (.95–1.19) | .319 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; POC. point-of-care; RR, risk ratio.
aAdjusted for age and sex.
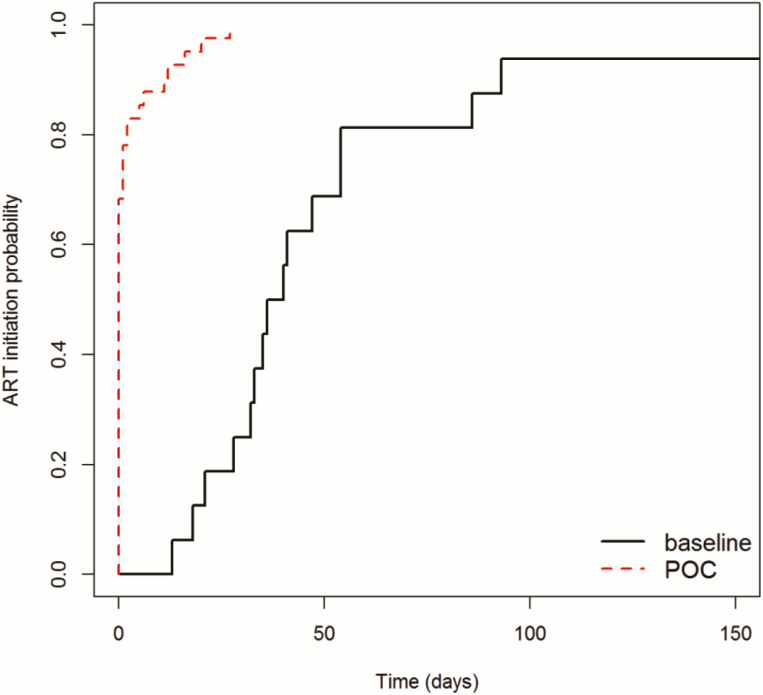
The median age at ART initiation was not significantly different between the POC arm at 148 days (IQR, 74–225 days) compared with the baseline arm at 164 days (104–230 days) (Table 2). The time between sample collection and ART initiation for HIV-infected infants was significantly reduced from 38 days (IQR, 30–54 days) to 0 days (IQR, 0–0 days) (P = .019). Significantly higher rates of ART initiation within 60 days of sample collection were observed in the POC arm (91.1%) compared with the baseline arm (41.9%) (aRR, 2.28; P < .001) (Table 3; Figure 2). Few additional ART initiations occurred >60 days after sample collection: none in the POC arm, 2 in the baseline arm within 180 days of sample collection, and 1 in the baseline arm after 180 days from sample collection. There was no significant difference between arms in the proportion of HIV-infected infants who initiated ART on the same day results were received by the patient (70.7% in the POC arm vs 43.8% in the baseline arm; P = .120).
Figure 2.
Kaplan-Meier estimate of time from sample collection to antiretroviral therapy (ART) initiation for infants receiving point-of-care (POC) or laboratory (baseline) early infant diagnosis testing. Unadjusted log-rank test (P < .001).
DISCUSSION
Implementation of POC EID significantly increased the proportion of HIV-infected infants initiated on life-saving ART and shortened the time along the testing and treatment cascade. Two times more HIV-infected infants were initiated on ART in the POC arm compared with the baseline arm. Additionally, the time from sample collection to results received was reduced by >50 days, while the time from sample collection to results received and ART initiation for HIV-infected infants were both reduced by >1 month to the same day. These time reductions are a critical improvement in care considering the high early mortality rates observed in HIV-infected untreated infants [3, 4, 7]. In fact, nearly half of the HIV-infected infants in the baseline arm were lost and did not initiate treatment.
The results observed in this study were consistent to those recently presented from a cluster randomized controlled trial in Mozambique [19]. The laboratory system in Mozambique had a longer median time from sample collection to results received of 125 days compared with 56 days in the present study; however, the effect of POC EID was consistent. In both studies, >98% of infants in the POC arms received test results and the majority of HIV-infected infants initiated ART on the same day as sample collection. In the Mozambique study, ART initiation rates within 60 days of sample collection improved from 12.8% to 89.7% in the POC arm, whereas in Malawi it improved from 41.9% to 91.1%. The consistencies in these data suggest that the results from both studies may represent the impact of POC EID in wider, generalizable settings. Interestingly, both studies found a phenomenon of HIV-infected infants presenting to care at older ages. HIV-infected infants were a median of 60 and 128 days old at sample collection in the baseline and POC arms, respectively, in Mozambique and 127 and 148 days old in the baseline and POC arms, respectively, in Malawi. While extensive PMTCT efforts have considerably reduced maternal-to-child transmission rates across sub-Saharan Africa, HIV-infected infants seem to continue to present to care and EID testing later than the currently recommended 6-week time point. It is thus imperative to understand why HIV-infected infants present for testing late instead of at 6 weeks of age and to encourage all HIV-affected families of the benefits of earlier identification.
Though in the POC arm, sample collection and testing occurred on the same day and nearly all results were provided to patients and caregivers on the same day, 4 HIV-infected infants (9%) did not initiate ART. Unfortunately, these 4 infants were lost to follow-up due to a delay in ART initiation caused by a serious medical condition and/or referral to a treatment center. It is possible that they were initiated on ART at a different healthcare facility. Point-of-care testing and same-day result return may significantly improve patient outcomes; however, it is critical to ensure that clinical systems are accessible and robust to initiate and retain all HIV-infected infants. Nonetheless, nearly all HIV-infected infants in this study were initiated on ART within 60 days of sample collection. Three HIV-infected infants in the baseline arm were initiated on ART >60 days after sample collection, while 15 HIV-infected infants in the baseline arm did not initiate ART, likely in part due to the long delay from sample collection to results available for clinical decision making. Long test turnaround times may be a factor in loss to follow-up, highlighting the need to ensure rapid sample transport, testing, and result return in settings where referral testing is the principal test option available.
An increased proportion of HIV-infected infants were found in the POC arm compared with the baseline arm. During the period of this study, no significant changes to the PMTCT program occurred and the WHO Option B+ recommendation has been implemented in Malawi since 2011; therefore, we do not expect that this represents a generalizable increase in transmission rates. Alternatively, we suspect that fewer HIV-infected test results were returned to the healthcare facility due to the need to confirm the positive test result using a second dried blood spot from the same sample. Either these samples were delayed further than expected and outside the study window, or perhaps they were more likely to be lost than negative samples within the laboratory system.
Several studies have highlighted the strong performance and high accuracy of POC EID technologies compared with laboratory-based technologies [13, 20–22]. Furthermore, a consortium of scientists, laboratorians, and academics has pooled data together to further support this finding [23]. Currently, 2 POC or near-POC technologies have been included on the WHO list of prequalified in vitro diagnostic products [15]. It is important to note, however, that regardless of technology, POC or laboratory-based, it remains critical to conduct confirmatory testing of infants with initial positive results. With continually decreasing maternal-to-child transmission rates, the positive predictive value of a single test to determine diagnosis is poor [24]. In the current study, all positive EID tests in the POC arm were confirmed with a second POC test prior to starting treatment and all were concordant.
This study has several limitations. The study design was observational for both the retrospective data collection in the baseline arm and the prospective data collection in the intervention arm. Although randomization of facilities or patients for study inclusion did not take place, we expected limited facility bias due to the criteria used in the purposeful selection process and consecutive patient enrollment. Within-facility temporal bias may exist; however, national guidelines, trainings, and algorithms did not change over the course of the study. Furthermore, we did not include ART retention outcomes, nor morbidity or mortality outcomes, due to the costs and complexity of such a study. Though the sample size of HIV-infected infants was relatively small (76 total), it was adequate to observe high statistical significance across study outcomes. Finally, observational and retrospective studies often suffer from poor routine record keeping and data storage conditions in public healthcare facilities. While this may also be an issue in the current study, the standard national routine EID testing logbook provided nearly all needed data and had few missing data points in either arm.
Implementing POC EID testing in Malawi resulted in significant improvements in patient impact over conventional laboratory-based testing. Large reductions in turnaround times along the testing and treatment cascade led to substantially more HIV-infected infants being initiated on life-saving treatment. This novel technology could, therefore, considerably support 90-90-90 global pediatric targets; however, maximizing their use will require strong tracking systems for referrals, good documentation, training and mentorship, robust supply chain systems, and clear patient linkages. Thoughtful and creative placement of POC EID technologies within the national system and healthcare facilities will allow for expanded access to testing for infants presenting at traditional PMTCT entry points as well as additional healthcare facility entry points, such as maternity, nutrition, and inpatient wards.
Notes
Acknowledgments. We acknowledge the healthcare facility staff involved for their dedication and work carried out in support of their patients and this study. We also acknowledge the Diagnostics Division of the Ministry of Health Malawi for their continued mentorship and the Department of HIV/AIDS for their policy guidance and site supervision.
Financial support. We are grateful to Unitaid and the United Nations Children’s Fund for financial support of devices, commodities, and study operational costs.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. AIDS by the numbers. Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. Children and HIV fact sheet. Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 3. Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS 2009; 23:101–6. [DOI] [PubMed] [Google Scholar]
- 4. Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol 2011; 40:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F; Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43. [DOI] [PubMed] [Google Scholar]
- 6. Penazzato M, Prendergast A, Muhe L, Tindyebwa D, Abrams E. Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age. Cochrane Database Syst Rev 2014; CD004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Violari A, Cotton MF, Gibb DM, et al. CHER Study Team Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 9. Cromwell EA, Dow AE, Low D, et al. Barriers to successful early infant diagnosis of HIV infection at primary care level in Malawi. Pediatr Infect Dis J 2015; 34:273–5. [DOI] [PubMed] [Google Scholar]
- 10. Joint United Nations Programme on HIV/AIDS. Ending AIDS: progress towards the 90-90-90 targets. Geneva, Switzerland: UNAIDS, 2017. [Google Scholar]
- 11. Chatterjee A, Tripathi S, Gass R, et al. Implementing services for early infant diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health 2011; 11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chibwesha CJ, Ford CE, Mollan KR, Stringer JS. Point-of-care virologic testing to improve outcomes of hiv-infected children in Zambia: a clinical trial protocol. J Acquir Immune Defic Syndr 2016; 72(Suppl 2):S197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jani IV, Meggi B, Mabunda N, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr 2014; 67:e1–4. [DOI] [PubMed] [Google Scholar]
- 14. Unitaid. HIV/AIDS diagnostic technology landscape. 5th ed Geneva, Switzerland: Unitaid, 2015. [Google Scholar]
- 15. World Health Organization. List of prequalified in vitro diagnostic products. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 16. World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children. Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 17. Hayes RJ, Moulton LH.. Cluster randomised trials. Chapman & Hall/CRC Biostatistics Series. Abingdon, UK: Taylor & Francis, 2009. [Google Scholar]
- 18. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 19. Jani IV, Meggi B, Loquiha O, et al. Effect of point-of-care testing on antiretroviral-therapy initiation rates in infants [abstract 26]. In: Special issue: abstracts from the 2017 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med 2017; 25:983. [Google Scholar]
- 20. Dunning L, Kroon M, Hsiao NY, Myer L. Field evaluation of HIV point-of-care testing for early infant diagnosis in Cape Town, South Africa. PLoS One 2017; 12:e0189226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsiao NY, Dunning L, Kroon M, Myer L. Laboratory evaluation of the Alere q point-of-care system for early infant HIV diagnosis. PLoS One 2016; 11:e0152672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray TY, Sherman GG, Nakwa F, et al. Field evaluation of performance of Alere and Cepheid qualitative HIV assays for pediatric point-of-care testing in an academic hospital in Soweto, South Africa. J Clin Microbiol 2017; 55:3227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carmona S, Wedderburn C, Macleod W. Field performance of point-of-care HIV testing for early infant diagnosis: pooled analysis from six countries from the EID Consortium. In: 21st International AIDS Conference, Durban, South Africa,2016. [Google Scholar]
- 24. Dunning L, Francke JA, Mallampati D, et al. The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: a cost-effectiveness analysis. PLoS Med 2017; 14:e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]