HIV-infected adults are commonly coinfected with cytomegalovirus (CMV). In this cross-sectional, observational, exploratory study, higher CMV immunoglobulin G level in blood was associated with worse neurocognitive performance in adults living with HIV and taking suppressive antiretroviral therapy.
Keywords: HIV, cytomegalovirus, neurocognitive disorders, cerebrospinal fluid
Abstract
Background
Cytomegalovirus (CMV) has been linked to higher risk of cardiovascular disease and mortality. We aimed to determine if CMV is associated with neurocognitive performance in adults infected with human immunodeficiency virus (HIV).
Methods
In this cross-sectional analysis, anti-CMV immunoglobulin G (IgG) concentrations in blood and CMV DNA copies in blood and cerebrospinal fluid (CSF) were measured in stored specimens of 80 HIV-infected adults who were previously assessed with a comprehensive neurocognitive test battery. Thirty-eight were taking suppressive antiretroviral therapy (ART) and 42 were not taking ART. A panel of 7 soluble biomarkers was measured by immunoassay in CSF.
Results
Anti-CMV IgG concentrations ranged from 5.2 to 46.1 IU/mL. CMV DNA was detected in 7 (8.8%) plasma specimens but in no CSF specimens. Higher anti-CMV IgG levels were associated with older age (P = .0017), lower nadir CD4+ T-cell count (P < .001), AIDS (P < .001), and higher soluble CD163 (P = .009). Higher anti-CMV IgG levels trended toward an association with worse neurocognitive performance overall (P = .059). This correlation was only present in those taking suppressive ART (P = .0049). Worse neurocognitive performance remained associated with higher anti-CMV IgG levels after accounting for other covariates in multivariate models (model P = .0038). Detectable plasma CMV DNA was associated with AIDS (P = .05) but not with neurocognitive performance.
Conclusions
CMV may influence neurocognitive performance in HIV-infected adults taking suppressive ART. Future clinical trials of anti-CMV therapy should help to determine whether the observed relationships are causal.
Immune responses decline with age, a condition termed “immunosenescence” [1]. All components of innate and adaptive immunity decline, although the clinical impact of these changes has not been well characterized. Several cross-sectional reports have identified that age-associated changes in adaptive immune responses are influenced by infection with cytomegalovirus (CMV). A smaller number of longitudinal studies identified a cluster of immune indicators associated with survival, termed the “immune risk profile.” This profile includes CMV seropositivity as well as the presence of differentiated CD8+ T cells, many of which are specific for CMV antigens [2].
In the Sacramento Area Latino Study on Aging (SALSA) cohort study of 1204 older adults from the general population, those who had the highest anti-CMV immunoglobulin G (IgG) concentrations had the worst neurocognitive decline over 4 years [3]. In an independent analysis of adults aged >65 years, higher CMV IgG levels and larger numbers of CMV-specific CD4+ T cells were associated with worse neurocognitive performance and worse functional status [4].
HIV-seropositive (HIV+) adults are more commonly infected with CMV than the general population [5]. As a result, CMV may account for a relatively greater degree of immune changes and mortality in this group, especially as they survive into their sixth and seventh decades [2]. Supporting this theory is the observation that adults treated with antiretroviral therapy (ART) have higher levels of CMV-specific CD8+ T cells than do untreated adults [6]. This could increase the risk of cardiovascular disease, and perhaps cerebrovascular disease as higher CMV-specific T-cell responses are associated with thicker intima media of the carotids [7].
CMV-related changes in immune responses may contribute to the accelerated aging that can occur in HIV+ adults. This can affect multiple organs, including the brain, and occurs more frequently than in the general population [8]. Neurocognitive decline occurs particularly commonly among aging individuals [9]. The repeatedly observed association between low CD4+ T-cell count and HIV-associated neurocognitive disorder (HAND) [10, 11] is incompletely understood but would be consistent with reactivation of a latent pathogen, such as CMV. Although CMV reactivation may not progress to overt end-organ disease after ART-induced immune recovery, repeated reactivation could increase immune activation and hasten progression to immunosenescence. Consistent with the CMV hypothesis, Brunt et al reported that higher anti-CMV IgG levels in blood correlated with lower nadir CD4+ T-cell count and worse neurocognitive performance in 91 HIV+ adults, although the latter relationship weakened after adjusting for age [12].
To confirm and expand these findings, we measured anti-CMV IgG in serum and CMV DNA in cerebrospinal fluid (CSF) and blood and assessed associations with demographic, disease, and neurocognitive data in 80 HIV+ adults from a well-characterized US cohort. Our primary hypothesis was that higher anti-CMV IgG levels would be associated with worse neurocognitive performance.
MATERIALS AND METHODS
Design
We performed a cross-sectional analysis of 80 HIV+ CMV-seropositive adults who were assessed in the CHARTER (CNS HIV Antiretroviral Therapy Effects Research) cohort. CSF, blood plasma, and serum were analyzed along with linked demographic, disease, treatment, and neurocognitive data from participants who were either taking suppressive ART (n = 38, a group that should be more generalizable to current clinical populations) or no ART (n = 42, a group that should reflect the influence of CMV during the natural history of HIV disease). CHARTER is an observational research cohort that, between 2003 and 2008, enrolled participants who were in medical care. Clinical decisions, such as initiation of ART, were determined by each patient in consultation with his or her healthcare provider. None of the participants had severe neuropsychiatric comorbid diagnoses (eg, schizophrenia). CHARTER is a National Institutes of Health–funded cohort study based in 6 US cities. The research was performed in accordance with the Declaration of Helsinki; the institutional review board at each site approved the study protocol; and all participants provided written informed consent.
Neurocognitive Testing
Participants completed a comprehensive battery of neurocognitive tests that conformed to published guidelines [13]. The best available normative standards were used, which correct for effects of age, education, sex, and ethnicity. Test scores were converted to demographically corrected standard scores (T scores). Seven cognitive abilities were assessed: motor functioning, executive functioning, speed of information processing, learning, delayed recall, working memory, and verbal fluency. Global performance was analyzed using global mean T scores (continuous). Global neurocognitive impairment (NCI) was determined by the Global Deficit Score (GDS) method (binary: GDS ≥0.5). Analyses of specific cognitive abilities used domain mean T scores. Neuropsychiatric comorbid conditions were categorized following published guidelines [13]. Participants who had severe neuropsychiatric comorbid conditions were excluded.
Neuromedical Assessment
Standardized assessments were performed to collect data on HIV disease characteristics, use of ART and other prescribed medications, medical diagnoses, and other characteristics. All participants underwent standardized physical examination. Distribution of ART drugs into the central nervous system (CNS) was estimated by the CNS penetration-effectiveness (CPE) approach [14]. Blood and CSF were collected, processed, and placed into storage at –80°C within 1 hour of collection.
Laboratory Procedures
Anti-CMV IgG was measured in serum (diluted 1:80) using the Cytomegalovirus IgG Enzyme Immunoassay kit (GenWay Biotech, San Diego, California). Samples with results below the assay sensitivity were repeated at a 1:40 dilution. All results were quantified in international units per milliliter (IU/mL). CMV DNA was quantified in CSF and plasma using a validated, in-house, real-time polymerase chain reaction (PCR) assay. No other herpesviruses were characterized in this analysis, but total serum globulins were measured. HIV RNA was quantified in plasma and CSF by reverse-transcription PCR (Roche Amplicor, lower limit of quantitation 50 copies/mL). Soluble CD14 (R&D Systems, Minneapolis, Minnesota) and soluble CD163 (Trilium Diagnostics, Brewer, Maine) were measured using enzyme-linked immunosorbent assays. CXCL10, CCL2, interleukin 6, tumor necrosis factor alpha (TNF-α), and interferon-γ were measured using bead suspension arrays (Millipore, Billerica, Massachusetts).
Statistical Analysis
CMV variables were compared to demographic (eg, age), disease (eg, HIV RNA), treatment (eg, ART duration), and neurocognitive variables. As an indirect indicator of the specificity of the anti-CMV immune response, serum globulin levels were compared to serum anti-CMV IgG levels and were included as a candidate covariate in multivariate models. Analyses were performed using standard statistical methods, including correlation coefficients, t tests, and linear and logistic regression. Stepwise selection in multivariate regression was performed using the Akaike information criterion. Data distributions were transformed when needed to improve symmetry. Effect sizes were estimated by Cohen d (continuous data) and relative risk (categorical data). Because the study group varied by use of suppressive ART by design, stratified analyses were performed (no ART vs suppressive ART). The false discovery rate (FDR) method was used to account for type I error in multivariate models (JMP Pro, version 13.1.0, Cary, North Carolina).
RESULTS
Demographics and Disease Characteristics
As summarized in Table 1, the 80 participants were mostly middle-aged (mean, 42.1 years) men (87.5%) without AIDS (62.5%). Compared with those not taking ART, those taking suppressive ART were older and had longer duration of HIV disease, higher current CD4+ T-cell count, and lower serum globulins and CSF CXCL10 as well as trends toward an AIDS diagnosis and higher anti-CMV IgG.
Table 1.
Participant Characteristics
| Characteristic | Entire Cohort (N = 80) |
Off ART (n = 42) |
On ART (n = 38) |
P Value |
|---|---|---|---|---|
| Age, y, mean | 42.1 | 39.6 | 44.8 | .005 |
| Sex, female, No. (%) | 10 (12.5) | 6 (14.3) | 4 (10.5) | .61 |
| Ethnicity/ancestry, No. (%) | .70 | |||
| European, non-Hispanic | 40 (50.0) | 20 (47.6) | 20 (52.6) | |
| African | 30 (37.5) | 16 (38.1) | 14 (36.9) | |
| Hispanic | 9 (11.3) | 5 (11.9) | 4 (10.5) | |
| Other | 1 (1.2) | 1 (2.4) | 0 (0) | |
| Education, y, mean | 12.9 | 12.9 | 12.9 | .95 |
| Neuropsychiatric comorbidities (moderate), No. (%) | 34 (42.5) | 16 (38.1) | 18 (47.4) | .27 |
| Global T score | 45.5 | 45.4 | 45.6 | .90 |
| Global neurocognitive impairment, No. (%) | 42 (52.5) | 20 (47.6) | 18 (47.4) | .98 |
| HAND diagnosis, No. (%) | .70 | |||
| Unimpaired | 28 (35.0) | 13 (31.0) | 15 (39.5) | |
| ANI | 36 (45.0) | 20 (40.6) | 16 (42.1) | |
| MND | 13 (16.25) | 8 (19.0) | 5 (13.2) | |
| HAD | 3 (3.75) | 1 (2.4) | 2 (5.2) | |
| HCV serostatus (seropositive), No. (%) | 17 (21.2) | 8 (19.0) | 9 (23.7) | .61 |
| Estimated duration of HIV, mo, mean | 99.7 | 82.3 | 119.0 | .003 |
| Current CD4+ count, cells/µL, mean | 520.0 | 453.4 | 591.8 | .04 |
| Nadir CD4+ count, cells/µL, mean | 302.8 | 338.4 | 263.4 | .11 |
| AIDS diagnosis, No. (%) | 30 (37.5) | 12 (28.6) | 18 (47.4) | .08 |
| CPE value, mean | … | … | 7.9 | … |
| HIV RNA, plasma | ||||
| Off ART, log10 copies/mL, mean | … | 4.39 | … | … |
| On ART, ≤50 copies/mL, No. (%) | … | … | 38 (100) | … |
| HIV RNA, CSF | ||||
| Off ART, log10 copies/mL, mean | … | 2.83 | … | … |
| On ART, ≤50 copies/mL, No. (%) | … | … | 38 (100) | … |
| Anti-CMV IgG, serum, IU/mL, mean | 21.0 | 19.6 | 23.8 | .07 |
| Detectable CMV DNA, plasma, No. (%) | 7 (8.8) | 5 (11.9) | 5 (2.3) | .28 |
| Globulins, serum, mg/dL, mean | 3.50 | 3.71 | 3.28 | .02 |
| Leukocytes, CSF, cells/µL, median | 2.0 | 3.0 | 1.0 | .003 |
| Total protein, CSF, mg/dL, mean | 43.2 | 43.1 | 43.4 | .96 |
| CCL2, CSF, log10 pg/mL, mean | 2.96 | 2.97 | 2.95 | .48 |
| CXCL10, CSF, log10 pg/mL, mean | 3.31 | 3.49 | 3.10 | < .001 |
| IFN-γ, CSF, log10 pg/mL, mean | 0.42 | 0.42 | 0.43 | .71 |
| IL-6, CSF, log10 pg/mL, mean | 0.27 | 0.26 | 0.28 | .77 |
| Soluble CD14, CSF, log10 ng/mL, mean | 2.02 | 2.04 | 2.00 | .29 |
| Soluble CD163, CSF, sq root ng/mL, mean | 7.47 | 7.58 | 7.35 | .41 |
| TNF-α, CSF, log10 pg/mL, mean | 0.38 | 0.39 | 0.38 | .73 |
P values <.10 are bolded.
Abbreviations: ANI, asymptomatic neurocognitive impairment; ART, antiretroviral therapy; CCL2, C-C motif Chemokine Ligand 2; CMV, Cytomegalovirus; CPE, central nervous system penetration-effectiveness; CSF, cerebrospinal fluid; CXCL10, C-X-C motif Chemokine Ligand 10; HAD, HIV-associated dementia; HAND, HIV-associated neurocognitive disorder; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN-γ, interferon gamma; IgG, immunoglobulin G; IL-6, interleukin 6; MND, mild neurocognitive disorder; TNF-α, tumor necrosis factor alpha.
Anti-CMV IgG and CMV DNA Concentrations
Anti-CMV IgG concentrations ranged from 5.2 to 46.1 IU/mL (median, 21.1 IU/mL; interquartile range [IQR], 11.5–28.7 IU/mL). Seven participants (8.8%) had detectable CMV DNA in plasma and none had detectable CMV DNA in CSF. Among those who had detectable CMV DNA in plasma, concentrations ranged from 2.4 to 138 copies/mL (median 6.5 copies/mL; IQR 3.3–28.7 copies/mL). Anti-CMV IgG concentrations were similar in those with or without detectable CMV DNA in plasma (P = .81).
Associations With Disease and Treatment Characteristics
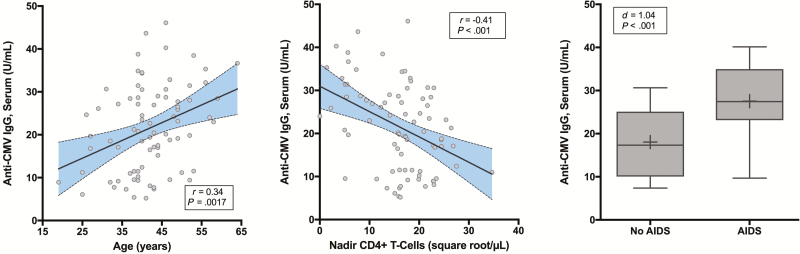
Higher anti-CMV IgG levels were associated with older age (Figure 1A), lower nadir CD4+ count (Figure 1B), and AIDS (Figure 1C). Multivariate linear regression with Akaike information criterion selection identified that higher anti-CMV IgG levels were independently associated with older age (P = .036; FDR P = .036) and AIDS (P = .003; FDR P = .006) (model R2 = 0.22, P < .001). Detectable CMV DNA in plasma trended toward associations with longer duration of HIV (P = .061) and with AIDS (P = .056).
Figure 1.
Correlates of anti–cytomegalovirus (CMV) immunoglobulin G (IgG). Higher anti-CMV IgG concentrations in serum correlated with older age (A), lower nadir CD4+ T-cell counts (B), and AIDS (C). “+” indicates the subgroup mean.
Associations With Neurocognitive Performance
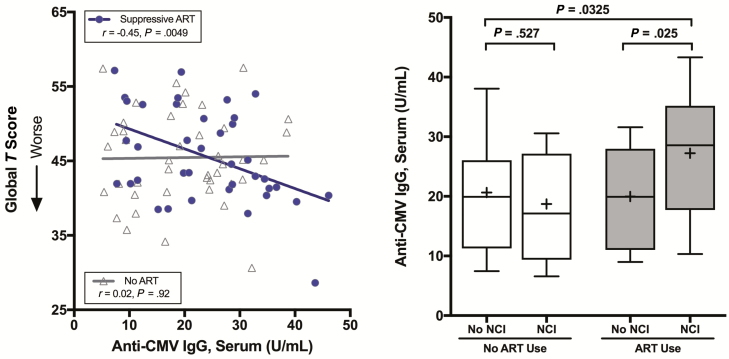
Higher anti-CMV IgG levels trended toward an association with worse neurocognitive performance in the entire group (n = 80; r = –0.21, P = .059). This relationship was stronger in those taking suppressive ART (r = –0.45, P = .0049) than in those not taking ART (r = 0.02, P = .92). This difference between ART users and nonusers was statistically significant when tested by an interaction term between anti-CMV IgG and the ART group (P = .045; Figure 2A).
Figure 2.
Associations between anti–cytomegalovirus (CMV) immunoglobulin G (IgG) levels and neurocognitive functioning. A, Higher anti-CMV IgG concentrations in serum correlated with worse global T scores in participants who were taking suppressive antiretroviral therapy (ART). The P value for the interaction between anti-CMV IgG and ART use in predicting global T score was .045. B, During suppressive ART, higher anti-CMV IgG levels were associated with neurocognitive impairment (NCI).
Table 2 presents a summary regression table for global T scores. Candidate variables for multivariate linear regression included those that were associated with global T scores or anti-CMV IgG concentrations in univariable analyses with P values ≤.10. The best multivariate model included anti-CMV IgG levels and sex (model R2 = 0.27, P = .0038).
Table 2.
Regression Table of Global T Scores in Participants Taking Suppressive Antiretroviral Therapy
| Variable | Risk | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Direction | β | P Value | β | P Value | FDR-Adjusted P Value | |
| Anti-CMV IgG | Higher | –.27 | .0049 | –.24 | .008 | .016 |
| Sex | Women | –6.69 | .043 | –5.54 | .068 | .068 |
| Nadir CD4+ count | Lower | .29 | .043 | … | … | … |
| Soluble CD163, CSF | Higher | –1.94 | .044 | … | … | … |
| AIDS status | AIDS | –1.81 | .075 | … | … | … |
| CXCL10, CSF | Higher | –5.79 | .101 | … | … | … |
| Model R2 = 0.27 | .0038 | |||||
Abbreviations: CMV, cytomegalovirus; CSF, cerebrospinal fluid; CXCL10, C-X-C motif Chemokine Ligand 10; FDR, false discovery rate; IgG, immunoglobulin G.
Examining specific cognitive abilities in participants taking suppressive ART, higher anti-CMV IgG concentrations were associated with worse performance in 3 of 7 domains (delayed recall [β = –.38, P = .0064], executive functioning [β = –.36, P = .0019], and working memory [β = –.30, P = .011]) with trends in 3 others (learning [β = –.25, P = .067], speed of information processing [β = –.21, P = .078], and verbal fluency [β = –.24, P = .093]).
Similar associations were observed with global NCI, a binary outcome measure. Similar to global T scores, higher anti-CMV IgG levels were associated with NCI in those taking suppressive ART (P = .025; Figure 2B) but not among ART nonusers (P = .527). The interaction term between ART status and anti-CMV IgG was again statistically significant (P = .0445). Higher anti-CMV IgG levels were specifically associated with impairment in delayed recall (β = –.092, P = .0077) and executive functioning (β = –.11, P = .0037) with a trend toward impaired verbal fluency (β = –.084, P = .066).
Serum globulins concentration, an indicator of nonspecific B-cell activation, was not associated with neurocognitive performance (overall: r = –0.02, P = .86; suppressive ART: r = –0.17, P = .28) or impairment (overall: β = –.17, P = .53; suppressive ART: β = –.51, P = .23). As CMV has been linked to vascular disease, which can also affect neurocognitive performance, we also examined several vascular disease risk factors (systolic and diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and body mass index), but these did not weaken the relationship between anti-CMV IgG and neurocognitive performance.
Associations With Biomarkers in CSF
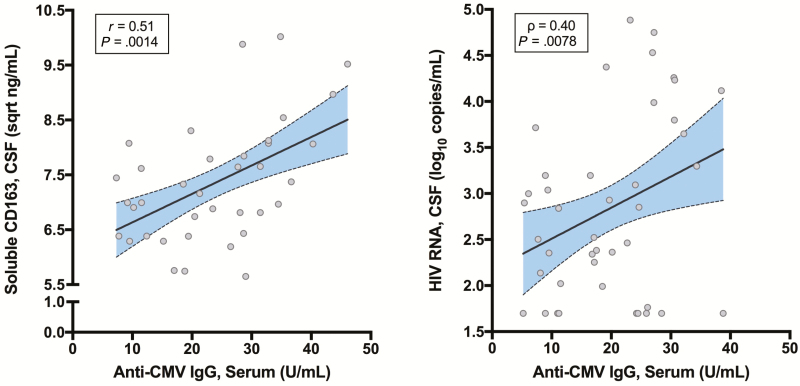
Overall, higher anti-CMV IgG levels correlated with higher soluble CD163 (r = 0.29, P = .009) but not the other 6 biomarkers. In those taking suppressive ART, higher anti-CMV IgG levels remained correlated with higher soluble CD163 (r = 0.51, P = .0014; Figure 3A) and trended toward correlations with higher CXCL10 (r = 0.30, P = .072) and lower TNF-α (r = –0.30, P = .066). In those not taking ART, higher anti-CMV IgG concentrations correlated with higher HIV RNA levels in CSF (ρ = 0.40, P = .0078; Figure 3B) but not in plasma (ρ = 0.089, P = .58). In this group, trends were also present between anti-CMV IgG levels and 2 other CSF biomarkers: CXCL10 (r = 0.26, P = .097) and TNF-α (r = 0.27, P = .081).
Figure 3.
Higher anti–cytomegalovirus (CMV) immunoglobulin G (IgG) levels in serum correlated with cerebrospinal fluid (CSF) biomarkers in subgroups. A, Higher anti-CMV IgG in serum correlated with higher soluble CD163 in CSF in participants taking suppressive antiretroviral therapy (ART). B, Higher anti-CMV IgG in serum correlated with higher human immunodeficiency virus (HIV) RNA levels in CSF in participants not taking ART.
DISCUSSION
This analysis identifies that CMV may influence neurocognitive performance in HIV+ adults who are taking suppressive ART. This is particularly notable as participants did not have past or current clinical CMV meningoencephalitis and had achieved at least moderate immune recovery. The findings are consistent with reports that have identified correlations between CMV and reduced neurocognitive performance in older adults without HIV disease [15], as well as reports from the HIV literature linking CMV to immune activation [16], worse carotid intima media thickness [7], and worse neurocognitive performance [12]. The strongest findings from our sample were for anti-CMV IgG levels in serum, not CMV DNA, which was undetectable in most specimens. Anti-CMV IgG concentrations in serum reflect a stronger immune response to CMV [17], which likely both results from CMV reactivation and helps control subsequent CMV replication. In our analysis, anti-CMV IgG concentration in serum did not correlate with CMV DNA in plasma, although our cross-sectional analysis was not well powered to perform these comparisons.
With a persistently high prevalence of cognitive problems in HIV+ adults taking suppressive ART [18], the most important connection with CMV that we observed was with neurocognitive performance. Similar to Parrinello et al, most associations were strongest in participants who had suppressed HIV RNA levels. In participants who were not using ART, the effects of HIV on neurocognitive performance may overshadow those of CMV since HIV replication is not controlled [19]. The link between higher anti-CMV IgG concentrations and worse neurocognitive performance is also consistent with data from HIV-negative elders, such as those from the SALSA study [15]. At a population level, CMV may be a relatively more important risk for morbidity in HIV+ adults than in HIV-uninfected adults, as a higher proportion of HIV+ adults is infected with this virus. Higher anti-CMV IgG concentrations were also associated with older age in our analyses, lending further support to the idea that CMV could contribute to accelerated aging of the brain [8]. Unlike the report from Brunt et al [12], however, age did not appear to confound our comparisons, possibly because the effects of typical aging were controlled with demographically corrected norms and the corrected scores were not associated with age.
In addition to the neurocognitive findings, our cross-sectional analysis supports several other inferences about the effects of CMV on the CNS. The most important may be that CMV may lead to greater dissemination of HIV into the CNS by increasing migration of activated HIV replication-competent monocytes. This inference is supported by the observations that higher anti-CMV IgG concentrations correlated with both higher soluble CD163 in CSF (a soluble biomarker of monocyte/macrophage activation [20–22]) and higher HIV RNA in CSF, although they did not correlate with CSF leukocyte count (r = 0.25, P = .135; data not shown). Higher anti-CMV IgG levels were also associated with worse immunosuppression in the past (AIDS or lower nadir CD4+ T-cell counts), which has been linked to worse neurocognitive performance in multiple reports [10, 11]. CMV and worse past immunosuppression have also been linked to greater risk for vascular disease, which also increases the risk for neurocognitive impairment in HIV+ adults [23, 24]. To assess whether vascular disease might be responsible for the observed associations between CMV and neurocognitive performance, we examined several vascular disease risk factors, but these did not account for the relationship between anti-CMV IgG and neurocognitive performance. CMV could also influence the CNS by upregulating systemic inflammation outside the CNS, but we did not directly assess this, as our project focused on inflammation biomarkers in CSF.
Taken together, our data support that CMV may play an independent role in the neurocognitive impairment that can occur in successfully treated HIV+ adults [23, 24]. In this group, a receiver-operating characteristic curve identified that an anti-CMV IgG level ≥28.1 IU/mL had a positive predictive value of 100% for neurocognitive impairment and a negative predictive value of 48% (data not shown). If CMV does play a role in HIV pathogenesis in the CNS, then CMV-targeted interventions might treat or prevent HAND in patients who are taking suppressive ART and have elevated anti-CMV IgG concentrations. For example, a small clinical trial indicated that valganciclovir might benefit people with HIV and CMV-related immune activation [25]. In this trial, 30 subjects were randomized to either placebo or valganciclovir for 8 weeks. Subjects who were treated with valganciclovir better reduced CD8+ T-cell activation.
An alternative explanation for our findings is that high anti-CMV IgG levels reflect a robust immune response to CMV that controls replication but injures infected CNS cells and bystander cells in the process [17]. If this is the dominant mechanism of injury, then the best approach may be curbing the immune response [2, 5, 26]. A third possible mechanism is that viral proteins that might be produced in the absence of intact virions could injure CNS cells [27]. Larger observational and interventional studies are needed to confirm our findings and to determine how CMV may cause CNS injury in adults taking suppressive ART. Future studies should evaluate the relative contributions of CMV reactivation over time, CMV-specific T-cell responses, and immune dysregulation to determine the best targets for intervention.
The chief limitations of this analysis are its relatively small sample size and its cross-sectional design, which is prone to bias and cannot establish causality. In particular, relatively few participants were taking suppressive ART, which reduced power and the generalizability of our overall findings. Despite the limited power, we found statistically significant associations in this clinically important group. Because our analyses involved many statistical tests, we must also caution against type I error, although we attempted to account for this using the FDR method.
Another important limitation is that the CHARTER study (and thus this analysis) did not include an HIV-uninfected control group. As a result, we cannot comment on whether the findings are uniquely associated with the HIV+ population. Since the seroprevalence of CMV in HIV+ populations approaches 100%—compared with 50% in the general population [28]—and since most HIV+ adults have experienced some degree of immunosuppression, the data provide at least indirect support for the importance of CMV in the health trajectory of HIV+ adults. We did not measure anti-CMV IgG in CSF as a prior analysis from our group detected it in only a minority of CSF specimens collected from patients with Alzheimer’s disease [29]. We also did not measure soluble biomarkers of immune activation in blood because our focus was the CNS. Finally, because other herpesviruses, such as Epstein-Barr virus and human herpesvirus type 6, may infect the same people as CMV and may also periodically reactivate, anti-CMV IgG may have been a surrogate for another virus. We attempted to assess the specificity of the CMV response by including serum globulins in our analyses but this is likely inferior, for instance, to measuring anti–herpes simplex virus type 1 IgG.
Together, our analyses support that the CMV immune response, as indexed by anti-CMV IgG, may cause greater monocyte activation (soluble CD163), greater HIV dissemination into the CNS (HIV RNA in CSF), and worse neurocognitive performance. Because anti-CMV IgG levels were associated with worse immunosuppression in the past, the findings also support that earlier ART initiation may reduce CMV-associated neurocognitive impairment. Finally, since CMV is treatable, our observations may be valuable if they inform a therapeutic or preventive strategy for HAND.
Notes
Acknowledgments. Participating CHARTER sites are Johns Hopkins University (J. McArthur), Mount Sinai School of Medicine (S. Morgello and D. Simpson), University of California, San Diego (J. A. McCutchan), University of Texas Medical Branch, Galveston (B. Gelman), University of Washington (A. Collier and C. Marra), and Washington University (D. Clifford).
Financial support. This work was supported by the National Institutes of Health (award numbers N01 AI35172 [principal investigator {PI}: J. Bremer], N01 MH22005 and HHSN271201000036C [PI: I. Grant], AI 06701 ACTG Immunology Specialty Laboratory [PI: A. Landay], and K24 MH097673 [PI: S. Letendre]).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 19th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 5–8 March 2012.
References
- 1. Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res 2011; 157:175–9. [DOI] [PubMed] [Google Scholar]
- 2. Gianella S, Letendre S. Cytomegalovirus and HIV: a dangerous pas de deux. J Infect Dis 2016; 214(Suppl 2):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vescovini R, Biasini C, Telera AR, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol 2010; 184:3242–9. [DOI] [PubMed] [Google Scholar]
- 5. Gianella S, Massanella M, Wertheim JO, Smith DM. The sordid affair between human herpesvirus and HIV. J Infect Dis 2015; 212:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006; 20:2275–83. [DOI] [PubMed] [Google Scholar]
- 8. High KP, Brennan-Ing M, Clifford DB, et al. ; OAR Working Group on HIV and Aging HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60(Suppl 1):S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii aging with HIV-1 cohort. Neurology 2004; 63:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellis RJ, Badiee J, Vaida F, et al. ; CHARTER Group CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses 2008; 24:1301–7. [DOI] [PubMed] [Google Scholar]
- 12. Brunt SJ, Cysique LA, Lee S, Burrows S, Brew BJ, Price P. Short communication: do cytomegalovirus antibody levels associate with age-related syndromes in HIV patients stable on antiretroviral therapy?AIDS Res Hum Retroviruses 2016; 32:567–72. [DOI] [PubMed] [Google Scholar]
- 13. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med 2010; 18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 15. Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc 2006; 54:1046–54. [DOI] [PubMed] [Google Scholar]
- 16. Freeman ML, Mudd JC, Shive CL, et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis 2016; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gianella S, Morris SR, Tatro E, et al. Virologic correlates of anti-CMV IgG levels in HIV-1-infected men. J Infect Dis 2014; 209:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parrinello CM, Sinclair E, Landay AL, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 2012; 205:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Becker JT, Kingsley L, Mullen J, et al. ; Multicenter AIDS Cohort Study Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright EJ, Grund B, Robertson K, et al. ; INSIGHT SMART Study Group Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 2010; 75:864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeman ML, Lederman MM, Gianella S. Partners in crime: the role of CMV in immune dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep 2016; 13:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Penkert RR, Kalejta RF. Tale of a tegument transactivator: the past, present and future of human CMV pp71. Future Virol 2012; 7:855–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin Infect Dis 2010; 50:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lurain NS, Hanson BA, Martinson J, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 2013; 208:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]