Cytomegalovirus shedding is relatively common in seropositive pregnant women repeatedly assessed in different bodily fluids and is associated with close contact with family members, especially young children. These natural history data serve as reference for planning intervention studies.
Keywords: cytomegalovirus, pregnancy, viral shedding, nonprimary maternal infections
Abstract
Background
Most congenital cytomegalovirus (CMV) infections in highly seropositive populations occur in infants born to women with preexisting CMV seroimmunity. Although essential for developing prevention strategies, CMV shedding patterns in pregnant women with nonprimary infections have not been characterized. We investigated correlates of CMV shedding in a cohort of seropositive pregnant women.
Methods
In a prospective study, saliva, urine, vaginal swabs, and blood were collected from 120 CMV-seropositive women in the first, second, and third trimesters and 1 month postpartum. Specimens were tested for CMV DNA by polymerase chain reaction. We analyzed the contribution of the specific maternal characteristics to viral shedding.
Results
CMV shedding was detected at least once in 42 (35%) women. Mothers living with or providing daily care to young children (3–6 years) were twice as likely to shed CMV at least once compared to women with less exposure to young children (58% vs 26%; adjusted relative risk [aRR], 2.21; 95% confidence interval [CI], 1.37–3.56). Living in crowded households (≥2 people per room) was associated with viral shedding (64% vs 31%; aRR, 1.99; 95% CI, 1.26–3.13). Sexual activity as indicated by the number of sexual partners per year or condom use was not found to be a correlate of viral shedding.
Conclusions
CMV shedding is relatively frequent in seropositive pregnant women. The association between virus shedding and caring for young children as well as crowded living conditions may provide opportunities for increased exposures that could lead to CMV reinfections in seropositive women.
Cytomegalovirus (CMV) is a common cause of intrauterine viral infection, hearing loss, and neurological impairment in children [1]. Birth prevalence of congenital CMV (cCMV) infection has been reported to be high in developing countries despite almost universal CMV seroprevalence in women of childbearing age [1–3]. Therefore, most infants with cCMV infection in these settings are born to women with preconceptional immunity to CMV [4–6].
CMV is usually transmitted through direct contact with bodily fluids. Viral replication can be demonstrated in different compartments (blood, saliva, urine, cervical secretions, milk) during primary infection, reactivation of endogenous latent virus, or reinfection with a new strain of CMV [7]. CMV shedding from mucosal surfaces is the source of virus that results in horizontal transmission within communities.
With the exception of breast milk [8], CMV shedding in different bodily fluids has not been well defined, especially during pregnancy [9–14]. A better understanding of the factors associated with CMV shedding in seropositive pregnant women will help define the risk factors for horizontal as well as vertical transmission, and could provide insight into sources and routes of virus acquisition in maternal populations.
We undertook a longitudinal study of pregnant seropositive women from a highly seropositive population with about 1% birth prevalence of cCMV [2, 4] to characterize CMV shedding patterns and viral loads in saliva, urine, vaginal secretions, and blood at different stages of pregnancy. These specimens and breast milk were tested for CMV DNA 1 month after delivery. In addition, utilizing questionnaires and interviews, we determined if maternal CMV exposure secondary to contact with young children, household crowding, and/or sexual activity were associated with an increased frequency of CMV shedding.
METHODS
Study Design
The overall objectives of the Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study (BraCHS) are to understand the natural history of CMV infections in seropositive pregnant women from a highly CMV-seropositive population and the occurrence and consequences of fetal CMV transmission. All study procedures were approved by the local and national Committees for Ethics in Research (16.928/2013), and written informed consent was obtained from the subjects.
In a cohort design, BraCHS recruited pregnant women at <15 weeks’ gestation in 6 public healthcare centers in Ribeirão Preto city, São Paulo state, Brazil, who agreed to participate. They were enrolled at their first antenatal visit with follow-up evaluations in the second (20–26 weeks) and third (32–36 weeks) trimesters of gestation and at 1 month after delivery. During the study visits, blood, saliva, vaginal swabs, and urine were obtained. At the postpartum visit, a breast milk sample was also collected (median, postpartum day 37; range, 18–93 days). Maternal CMV seropositivity was determined in the first visit. A standardized questionnaire was administered at each study visit by trained research nurses to collect women’s information.
Infants born to study participants were screened for cCMV infection by testing a saliva sample obtained within 1 week of age with a CMV DNA polymerase chain reaction (PCR) assay as previously described [4, 15]. Confirmation of cCMV was done by testing a urine sample collected within the first 3 weeks of age using the same assay.
Sample Size and Derivation of the Study Population
Sample size was calculated based on the estimates of CMV shedding in urine and genital tract during pregnancy that ranged from 10% to 30% [9, 16]. Assuming a 15% frequency of shedding, a precision of 7.5%, and a 95% confidence level, a sample size of 88 women was estimated. To target more precise estimates and accommodate attrition, we studied about 40% more women.
As of March 2014, 359 women had been enrolled into the BraCHS; among these, 114 (31.7%) missed at least 1 visit, 50 (14%) discontinued the study, 31 (8.6%) had pregnancy terminations, and 1 (0.3%) had a stillbirth. The remaining 163 completed the follow-up. However, 13 infants were not screened for cCMV. Among the 150 eligible women, this analysis comprised the first 120 CMV-seropositive participants who were followed from March 2014 to December 2015, were nontransmitters of cCMV infection, and had all planned samples obtained. Overall, 2512 specimens were collected from the 120 study subjects (480 urine, 480 saliva, 475 vaginal secretion, 479 buffy coat, 479 serum, and 119 breast milk samples) and tested.
Sample Collection and Laboratory Assays
Serum, plasma, and buffy coat fractions were obtained from blood. Saliva and vaginal swabs were placed in a sterile tube followed by addition of 750 µL of culture medium. After a 30-minute incubation, swabs were removed, and the medium was stored. Breast milk samples were centrifuged to remove the milk fat, and supernatant and cells were then stored. Aliquots of all specimens were stored at –80ºC until testing.
First-trimester serum samples were tested for anti-CMV using the quantitative immunoglobulin G (IgG) Vidas, bioMérieux, France, according to instructions provided by the manufacturer.
Total DNA was extracted from specimens using the PureLink Genomic DNA Mini Kit (Invitrogen, USA). An in-house qualitative PCR assay was performed using gB1 (5ʹTGGAACTGGAACGTTTGGC3ʹ), gB2 (5ʹGAAACGCGCGGCAATCGG3ʹ), and internal control (PCO3:5ʹACACAACTGTGTTCACTAGC3ʹ; P
CO4:5ʹCAACTTCATCCACGTTCACC3ʹ) primers. Positive samples were then quantified using q-CMV Real Time (Elitech Nanogen, France).
Data Analysis
Standardized forms received clinical, laboratory, and interview data. Trained staff collected and reviewed data. Data were analyzed by using SAS version 9.3 software (Cary, North Carolina).
Women were defined as positive for CMV shedding by the detection of CMV DNA in the specimens. Analyses of CMV shedding in bodily fluids were performed by fitting log-binomial regression models with random effects that consider intraindividual variability of each woman over the trimesters, obtaining prevalence ratios (PRs) and respective 95% confidence intervals (CIs), using the GLIMMIX procedure. To compare the mean viral loads in the bodily fluids, a mixed linear regression model was fitted (MIXED procedure), obtaining multiple comparisons through orthogonal contrasts.
To test the association between CMV shedding and maternal factors, relative risks (RRs) and 95% CIs were estimated by simple and multiple log-binomial regression models (GENMOD procedure). In this analysis, CMV shedding (at least once in any of the specimens throughout gestation and postpartum) was assigned as the dependent variable, and the following independent variables were included in the model: number of persons per room in the household, contact with young children, and sexual activity. Maternal age, ethnicity, and educational level were considered as covariates in the multiple analysis.
Sexual activity was categorized by an index [(number of partners/years of sexual activity since sexual debut) – 1]. Scores representing the exposure to children who may be shedding CMV were applied to categorize the maternal contact with children aged 0–2 and 3–6 years. In this scoring, 4 factors were considered: (1) living with children; (2) living with children attending daycare; (3) providing direct child care, that is, changing diapers, bathing, and feeding a child in the woman’s family and/or a friend’s; (4) taking care of children attending a daycare center. We assigned 1 point to each exposure category, ranging from 0 (no exposure) to 4 (exposure to all factors).
RESULTS
Maternal Characteristics
The median age of participants was 24 years (range, 14–41 years) and the majority of participants (69%) were single. Most of the women were white (52.5%) or black/mixed black (46.7%). The median number of years of formal education was 10 (range, 2–15 years) and half were unemployed. Most mothers (99.2%) belonged to low-income socioeconomic status. The median age of sexual debut was 16 years (range, 12–30 years) with a median of 3 lifetime sex partners (range, 1–20). Most (93.3%) women reported having had 1 sexual partner in the last year before pregnancy. All women were human immunodeficiency virus negative. Coinfections detected during pregnancy included syphilis (1 [0.8%]), hepatitis B (4 [3.3%]), and urinary tract infection (19 [15.8%]). Almost all women (95.0%) delivered at ≥37 weeks’ gestation.
The median number of household rooms (except the bathroom) was 4 (range, 1–7), and the median number of people living in the household was 4 (range, 1–14). Forty-six women (38.3%) reported living with young children (<6 years); 37.0% (17/46) of them were primiparous, and 2 women (4.3%) had older children (>6 years of age).
CMV Shedding
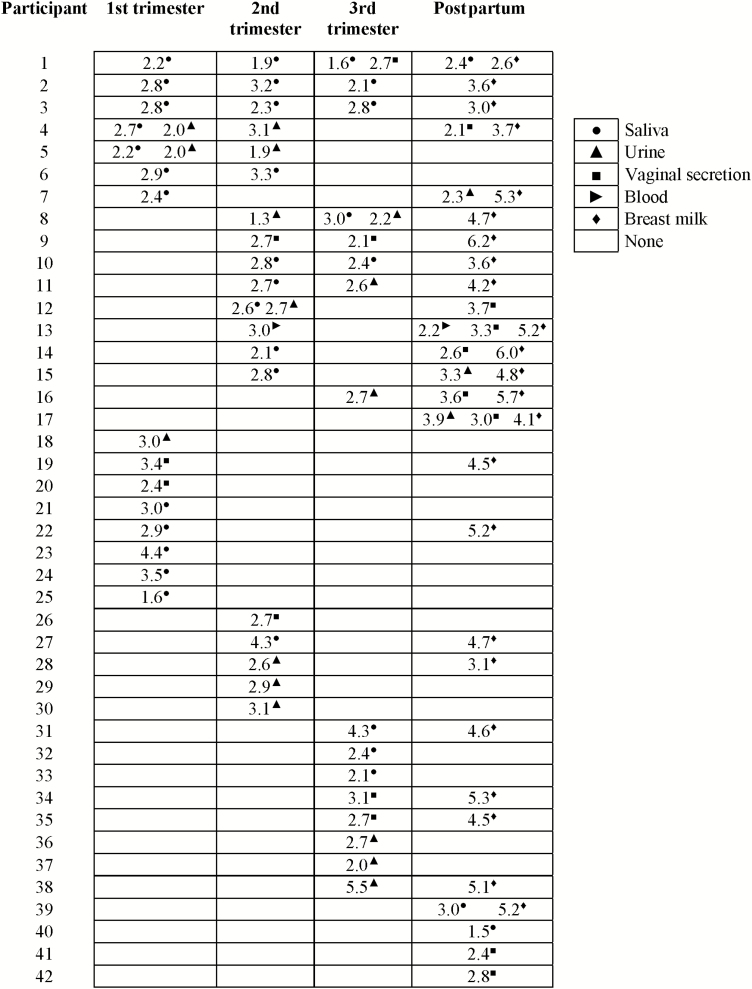
Overall, 35.0% (42/120; 95% CI, 27.0%–44.3%) of women shed CMV at least once in saliva, urine, genital secretions, and/or blood during the study. During gestation, 37 of 120 (30.8%; 95% CI, 23.3%–39.6%) women had at least 1 CMV-positive specimen. CMV DNA was found in saliva (20.0% [24/120]; 95% CI, 13.5%–28.5%), urine (13.3% [16/120]; 95% CI, 8.4%–20.6%), and vaginal secretion (12.5% [15/120]; 95% CI, 7.7%–20.1%). Blood was CMV positive in only 1 woman (0.8%; 95% CI, .15%–5.2%). CMV DNA was detected in 61.3% (73/119) of the breast milk specimens (95% CI, 52.4%–69.6%). As to the timing of milk collection, CMV DNA was detected in 11 of 13 (84.6%), 48 of 74 (64.8%), and 14 of 33 (42.4%) women whose milk specimen was collected at <30 days, 30–45 days, and >45 days after delivery, respectively.
Figure 1 shows CMV shedding in different compartments over time. CMV DNA was detected just once in 26 of 42 (62.0%) women. Among the remaining 16 women, CMV was detected on 2 consecutive visits in 11 women and 5 women shed intermittently.
Figure 1.
Longitudinal cytomegalovirus (CMV) shedding in 42 CMV-seropositive pregnant women who shed virus at least once during pregnancy and/or postpartum in saliva, urine, vaginal secretion, or blood according to the specimen.
The rates of CMV shedding in different specimens and time points are shown in Table 1. Results of DNAemia are not shown due to its detection in only 1 subject. CMV DNA was more frequently detected in saliva in the first trimester than in the postpartum period. No other associations were found over the duration in the study for each specimen type. Among different compartments, CMV DNA was more frequently detected in saliva than in vaginal secretions during the first (10.0% vs 1.7%; PR, 6.0; 95% CI, 1.3–27.0) and second trimesters (8.3% vs 1.6%; PR, 5.0; 95% CI, 1.1–23.0) of gestation. Also, CMV was more commonly detected in saliva than urine, but only in the first trimester (10.0% vs 2.5%; PR, 4.0; 95% CI, 1.1–14.3). CMV shedding was detected 5 times more frequently in breast milk than any other specimens.
Table 1.
Prevalence Ratios of Cytomegalovirus Shedding in Different Bodily Fluids Throughout Pregnancy and Postpartum Periods, According to Specimens and Time Points
| Saliva | Positive, No. (%) |
Negative, No. (%) |
PR | (95% CI) |
|---|---|---|---|---|
| 1st trimester | 12 (10.0) | 108 (90.0) | 4.0 | (1.1–14.2) |
| 2nd trimester | 10 (8.3) | 110 (91.7) | 3.3 | (.9–12.2) |
| 3rd trimester | 8 (6.7) | 112 (93.3) | 2.7 | (.7–10.1) |
| Postpartum | 3 (2.5) | 117 (97.5) | ref | ref |
| Urine | ||||
| 1st trimester | 3 (2.5) | 117 (97.5) | 1.0 | (.2–5.0) |
| 2nd trimester | 7 (5.8) | 113 (94.2) | 2.3 | (.6–9.1) |
| 3rd trimester | 6 (5.0) | 114 (95.0) | 2.0 | (.5–8.0) |
| Postpartum | 3 (2.5) | 117 (97.5) | ref | ref |
| Vaginal secretion | ||||
| 1st trimester | 2 (1.7) | 118 (98.3) | 0.2 | (.1–1.1) |
| 2nd trimester | 2 (1.7) | 118 (98.3) | 0.2 | (.1–1.1) |
| 3rd trimester | 4 (3.3) | 116 (96.7) | 0.5 | (.1–1.6) |
| Postpartum | 8 (7.0) | 107 (93.0) | ref | ref |
| In any bodily fluida | ||||
| 1st trimester | 15 (12.5) | 105 (87.5) | 1.2 | (.5–2.4) |
| 2nd trimester | 19 (15.8) | 101 (84.2) | 1.5 | (.7–2.9) |
| 3rd trimester | 16 (13.3) | 104 (86.7) | 1.2 | (.6–2.6) |
| Postpartum | 13 (10.8) | 107 (89.2) | ref | ref |
| Breast milk | 73 (61.3) | 46 (38.7) | 5.7 | (3.1–10.2) |
Prevalence ratio and 95% confidence intervals were estimated by fitting log-binomial regression models with random effects (SAS software version 9.3, GLIMMIX procedure).
Abbreviations: CI, confidence interval; PR, prevalence ratio; ref, reference category.
aSaliva, urine, vaginal secretion, and/or blood.
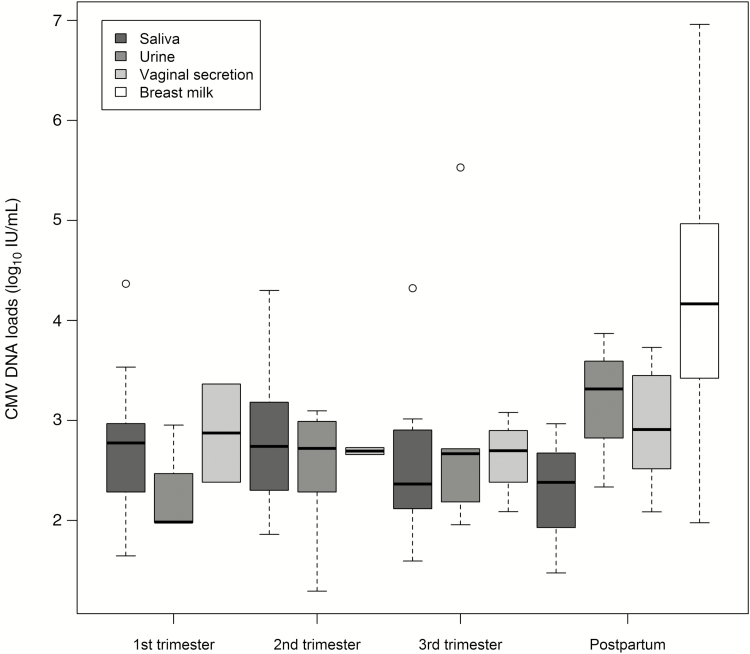
Only 1 woman had DNAemia at 2 visits: in the second trimester (3.01 log10 IU/mL) and postpartum (2.21 log10 IU/mL). Figure 2 shows the viral loads in saliva, urine, vaginal secretions, and breast milk samples with positive PCR. The mean viral loads detected in these bodily fluids during gestation and in the postpartum were, respectively, 2.70 log10 (standard deviation [SD], 0.73) IU/mL, and 2.85 log10 (SD, 0.68) IU/mL, whereas a significantly higher mean viral load of 4.14 log10 (SD, 1.09) IU/mL was found in breast milk compared to other specimens (P < .01 for all multiple comparisons).
Figure 2.
Cytomegalovirus (CMV) DNA loads (log10) in maternal samples collected at first, second, and third trimester and postpartum visits.
Risk Factors for CMV Shedding in Bodily Fluids
The association between maternal characteristics and CMV shedding at least once during pregnancy and postpartum periods are shown in Table 2. CMV shedding was independently associated with household crowding (≥2 persons per room) and exposure (2, 3, or 4 factors) to children aged 3–6 years. Remarkably, 74.3% and 65.4% of women who did not shed CMV during the study reported no contact with children <2 and 3–6 years old, respectively.
Table 2.
Analysis of Maternal Characteristics Associated With Cytomegalovirus Shedding in Seropositive Women
| Variables | CMV Shedding, No. (%) |
Unadjusted RRa | (95% CI) | Adjusted RRa | (95% CI) | |
|---|---|---|---|---|---|---|
| Yes (n = 42) |
No (n = 78) |
|||||
| No. of people per room | ||||||
| <2 | 33 (31.1) | 73 (68.9) | ref | ref | ref | ref |
| ≥2 | 9 (64.3) | 5 (35.7) | 2.1 | (1.3–3.3) | 2.1 | (1.2–3.5) |
| Child contact (0–2 y) | ||||||
| Noneb | 24 (29.3) | 58 (70.7) | ref | ref | ref | ref |
| 1 factorb | 10 (47.6) | 11 (52.4) | 1.6 | (.9–2.8) | 1.6 | (.9–3.1) |
| 2–4 factorsb | 8 (47.6) | 9 (52.4) | 1.6 | (.9–2.9) | 1.6 | (.9–2.9) |
| Child contact (3–6 y) | ||||||
| Noneb | 18 (26.0) | 51 (74.0) | ref | ref | ref | ref |
| 1 factorb | 3 (20.0) | 12 (80.0) | 0.8 | (.2–2.3) | 0.8 | (.3–2.3) |
| 2–4 factorsb | 21 (58.3) | 15 (41.7) | 2.2 | (1.4–3.6) | 2.2 | (1.3–3.7) |
| Sexual activity indexc | ||||||
| ≤0.5 | 28 (33.3) | 56 (66.7) | ref | ref | ref | ref |
| 0.51–0.99 | 8 (47.0) | 9 (53.0) | 1.4 | (.8–2.5) | 1.5 | (.8–2.8) |
| ≥1 | 6 (31.6) | 13 (68.4) | 0.9 | (.5–2.0) | 0.8 | (.3–1.8) |
| Maternal age, y | ||||||
| <18 | 5 (45.4) | 6 (54.6) | 1.3 | (.7–2.7) | … | … |
| ≥18 | 37 (34.0) | 72 (66.0) | ref | ref | … | … |
| Ethnicity | ||||||
| White | 23 (36.5) | 40 (63.5) | 1.1 | (.6–1.7) | … | … |
| Black/Mulatto | 19 (33.9) | 37 (66.1) | ref | ref | … | … |
| Schooling, y | ||||||
| ≤8 | 18 (39.1) | 28 (60.9) | 1.2 | (.7–2.0) | … | … |
| ≥9 | 24 (32.4) | 50 (67.6) | ref | ref | … | … |
| No. of sexual partners in the year before pregnancy | ||||||
| 1 | 41 (36.6) | 71 (63.4) | ref | ref | … | … |
| 2–3 | 1 (12.5) | 7 (81.5) | 0.3 | (.05–2.2) | … | … |
| Use of condoms | ||||||
| Always | 7 (30.4) | 16 (69.6) | ref | ref | … | … |
| Sometimes | 18 (48.7) | 19 (51.3) | 1.6 | (.8–3.2) | … | … |
| Never | 17 (28.3) | 43 (71.7) | 0.9 | (.4–2.0) | … | … |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; ref, reference category; RR, relative risk.
aRelative risks estimated by simple (unadjusted) and multiple (adjusted) log-binomial regression models (SAS software version 9.3, GENMOD procedure). Maternal age, maternal schooling years, and ethnicity were used as covariates.
bExposure to children according to the sum of 4 factors: (1) living with children; (2) living with children who attend daycare; (3) providing child care, ie, changing diapers, bathing, and feeding a child in either the woman’s family or a friend’s; (4) taking care of children in a daycare center.
cSexual activity was categorized by an index of the number of partners per years of sexual activity since sexual debut (≤0.5; 0.51–0.99; ≥1) subtracting 1 to standardize that all women had the first partner [(number of partners / years of sexual activity since sexual debut) – 1].
Mothers living with or providing daily care to young children (3–6 years) were twice as likely to shed the virus than those not exposed to children (58% vs 26%; adjusted RR, 2.21; 95% CI, 1.37–3.56). Living in crowded households (≥2 people per room) was also associated with CMV shedding (64% vs 31%; adjusted RR, 1.99; 95% CI, 1.26–3.13). No other association was found.
DISCUSSION
In a Brazilian cohort of 120 CMV-seropositive pregnant women evaluated longitudinally during pregnancy and in the postpartum period, CMV DNA was detected at least once in saliva, urine, vaginal secretions, and/or blood in 35.0% women whereas it was found only once in 62.0% of them and at ≥2 visits in 16 participants. CMV DNA was more frequently detected in saliva than in vaginal secretions or urine during the first trimester whereas CMV DNAemia was uncommon (0.8%). Median viral load <3 log10 IU/mL was detected in these bodily fluids. In contrast, CMV DNA in breast milk was observed in 63.2% women with higher viral load. Maternal age, ethnicity, education level, parity, and marital status were not associated with viral shedding. Mothers living with or providing daily care to young children (3–6 years) were twice as likely to shed CMV compared to those not providing care or living with children. In addition, living in crowded households (≥2 people per room) was associated with higher frequency of viral shedding. Sexual activity (as indicated by the number of sexual partners over time or in the year before pregnancy) and condom use were not associated with viral shedding.
Although viral shedding has been observed in most subjects after CMV primary infection [17], the data on CMV shedding in seroimmune adults and pregnant women are limited and vary broadly in the literature. This variation could be from the methods used to detect CMV shedding in earlier studies, sociodemographic and sexual activity characteristics, hygienic practices, contact with children, and the CMV seropositivity status of the populations. In general, culture-based studies revealed lower rates of CMV shedding in adults (2.6%–9.4%) [10, 18, 19], including women attending sexually transmitted disease clinics (1.8%–9.4%) [20]. In contrast, higher rates of shedding in healthy adults (7.0%–33.7%) have been reported in studies using PCR-based assays [9, 14, 21–25].
In a study of 205 seropositive North American women from whom 2–7 sequential samples were obtained after delivery, CMV DNA was detected at least once in 83% urine and 52% blood specimens over a median period of 30.8 months [12]. In contrast, we found lower rates of CMV shedding (35%) in our study even though we collected specimens from multiple sites at various time points. The demographic characteristics of the studied women might account for these differences, since they included predominantly low-income, black, young, unmarried women with multiple sexual partners and known to be at increased risk for acquisition of CMV infection and having a child with cCMV [12], likely as a result of a higher frequency of nonprimary CMV infection. In contrast to the mentioned patient population, our study included unselected pregnant women receiving care within the Brazilian public health system.
It has been previously reported that the frequency of CMV shedding in urine and vaginal/cervical fluids of seropositive pregnant women increases with advancing gestation [9, 10, 26]. Rates of CMV shedding ranging from 0.5%–15.2% in the first trimester [9, 10, 16, 26, 27] to 4.6%–37.0% in the last trimester [9, 10, 26] have been reported. Although available data have provided no definitive evidence, it has been suggested that this increase in CMV shedding in later gestation could be from CMV reactivation during pregnancy. Importantly and in contrast to these previous studies, in our prospective study we were unable to show increasing CMV shedding rates during gestation.
Our study is the first to systematically define CMV shedding characteristics in saliva in comparison with other bodily fluids during pregnancy. The findings of our study suggest that contact with saliva is a route of viral spread from seropositive pregnant women. The frequency of CMV shedding in urine and vaginal secretions (13.3% and 12.5%, respectively) was similar. However, previous studies have shown that CMV shedding was more common in vaginal secretions (1.6%–30.0%) than in urine (0.5%–11.1%) in nonpregnant [10, 18, 20], pregnant [9, 10, 18], and postpartum women [28]. Consistent with previously reported findings, the presence of CMV DNA in blood was uncommon (0.8%), even in this population of women in which approximately 31% shed virus from mucosal sites during pregnancy [29, 30]. The low frequency of detection of viremia could be explained by infrequent sampling of study participants and/or a transient viremia in seroimmmune women. Last, as expected, CMV reactivated in the lactating breast in most of the women [8], and maternal milk was the bodily fluid in which CMV was more frequently detected in this maternal population.
Young children have been identified as a major source of CMV exposure [7, 22, 31], and caring for young children is a known risk factor for delivering an infant with cCMV [32]. Young children shed CMV at higher levels [22, 23, 33] in urine and saliva for prolonged periods. We found that exposure to young children attending daycare was associated with maternal CMV shedding. As children attending daycare centers have been shown to shed CMV at increased rates [7, 34], it is likely that children may have been the source of CMV exposure for seropositive mothers, leading to reinfection and subsequent virus shedding. Also, living in crowded households with extended families including young children provides for close contact within family members leading to exposure and transmission of CMV. Our previous data demonstrating serological evidence of reinfection and detection of 2 or more virus strains in up to 35% of seropositive mothers provide support for this possibility [6, 12–14].
We did not observe an association between sexual activity and CMV shedding, as demonstrated by the number of lifetime or recent sexual partners, and condom use. Although we did not have data on recent onset of sexual activity, a variable that has been associated with cCMV [32], maternal age and CMV shedding were not associated in this population when the median age of sexual debut was 16, arguing that recent onset of sexual activity was unlikely a source of virus exposure in these women. However, our study may be limited in its capacity to discriminate the role of sexual activity on viral shedding considering that most (93.3%) women had only 1 sexual partner in the year before pregnancy, and due to the lack of information on the partner(s)’ sexual habits. Additional studies are needed to better define this potential association.
Other limitations include the lack of quantitative serial CMV IgG levels to determine its relationship with virus shedding, and antibody avidity testing to better characterize the study population. Additionally, the small number of shedding events suggests that our study is not powered to compare the shedding patterns between different compartments.
In conclusion, CMV shedding was detected in a third of seropositive pregnant women, mainly in saliva but also in urine and vaginal secretions, albeit at lower frequency, whereas DNAemia was unusual in these women. Our data suggest that CMV acquisition and viral shedding occur during close contact with other family members, especially young children. These results are consistent with a large body of literature that has documented increased CMV acquisition in adults exposed to young children shedding CMV [35, 36].
Thus, our findings provide an alternative explanation for virus acquisition and shedding in seroimumne pregnant women that contrasts with proposed mechanisms that argue that reactivation of persistent infection in seroimmune women leads to infection and virus shedding. Importantly, the virologic information provided in this report adds to the natural history of CMV infection in this highly seropositive maternal population and serves as reference data for designing rational and cost-effective interventions that could significantly decrease the birth prevalence of congenital CMV infection in this population and likely in other maternal populations with high CMV seroprevalence. Moreover, results from such interventions in the maternal population described in this study could potentially distinguish between these 2 mechanisms of virus infection and shedding in seroimmune pregnant women.
Notes
Acknowledgments. We acknowledge Dr Suzi V. Fábio and all of the members from the health centers in the municipality of Ribeirão Preto for their support on subjects’ recruitment; the technicians of the Immunology and Infectious Diseases Laboratory for assaying the specimens; and the Study Center on Maternal, Perinatal and Infant`s Infection staff members for their support.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number 2R01HD061959-07A2 to W. J. B.); São Paulo Research Foundation, Brazil (grant number 2013/06579-0 to M. M. M.-P.), and National Council for Scientific and Technological Development, Brazil (fellowship to N. G. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26:86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamamoto AY, Castellucci RA, Aragon DC, Mussi-Pinhata MM. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol Infect 2013; 141:2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol 2015; 204:263–71. [DOI] [PubMed] [Google Scholar]
- 4. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009; 49:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001; 344:1366–71. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 2010; 202:297 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamprecht K, Goelz R. Postnatal cytomegalovirus infection through human milk in preterm infants: transmission, clinical presentation, and prevention. Clin Perinatol 2017; 44:121–30. [DOI] [PubMed] [Google Scholar]
- 9. Shen CY, Chang SF, Yen MS, Ng HT, Huang ES, Wu CW. Cytomegalovirus excretion in pregnant and nonpregnant women. J Clin Microbiol 1993; 31:1635–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stagno S, Reynolds D, Tsiantos A, et al. Cervical cytomegalovirus excretion in pregnant and nonpregnant women: suppression in early gestation. J Infect Dis 1975; 131:522–7. [DOI] [PubMed] [Google Scholar]
- 11. Chandler SH, Alexander ER, Holmes KK. Epidemiology of cytomegaloviral infection in a heterogeneous population of pregnant women. J Infect Dis 1985; 152:249–56. [DOI] [PubMed] [Google Scholar]
- 12. Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis 2010; 202:1800–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Novak Z, Ross SA, Patro RK, et al. Cytomegalovirus strain diversity in seropositive women. J Clin Microbiol 2008; 46:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ross SA, Novak Z, Ashrith G, et al. Association between genital tract cytomegalovirus infection and bacterial vaginosis. J Infect Dis 2005; 192:1727–30. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto AY, Aquino VH, Figueiredo LT, Mussi-Pinhata MM. Diagnosis of congenital and perinatal infection by cytomegalovirus using polymerase chain reaction [in Portugese]. Rev Soc Bras Med Trop 1998; 31:19–26. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Yamada H, Minami M, et al. Screening for vaginal shedding of cytomegalovirus in healthy pregnant women using real-time PCR: correlation of CMV in the vagina and adverse outcome of pregnancy. J Med Virol 2006; 78:757–9. [DOI] [PubMed] [Google Scholar]
- 17. Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis 1999; 180:702–7. [DOI] [PubMed] [Google Scholar]
- 18. Knox GE, Pass RF, Reynolds DW, Stagno S, Alford CA. Comparative prevalence of subclinical cytomegalovirus and herpes simplex virus infections in the genital and urinary tracts of low-income, urban women. J Infect Dis 1979; 140:419–22. [DOI] [PubMed] [Google Scholar]
- 19. Knowles WA, Gardner SD, Fox H. A comparison of cervical cytomegalovirus (CMV) excretion in gynaecological patients and post-partum women. Arch Virol 1982; 73:25–31. [DOI] [PubMed] [Google Scholar]
- 20. Collier AC, Handsfield HH, Ashley R, et al. Cervical but not urinary excretion of cytomegalovirus is related to sexual activity and contraceptive practices in sexually active women. J Infect Dis 1995; 171:33–8. [DOI] [PubMed] [Google Scholar]
- 21. Yang YS, Ho HN, Chen HF, et al. Cytomegalovirus infection and viral shedding in the genital tract of infertile couples. J Med Virol 1995; 45:179–82. [DOI] [PubMed] [Google Scholar]
- 22. Stowell JD, Mask K, Amin M, et al. Cross-sectional study of cytomegalovirus shedding and immunological markers among seropositive children and their mothers. BMC Infect Dis 2014; 14:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gantt S, Orem J, Krantz EM, et al. Prospective characterization of the risk factors for transmission and symptoms of primary human herpesvirus infections among Ugandan infants. J Infect Dis 2016; 214:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berntsson M, Dubicanac L, Tunbäck P, Ellström A, Löwhagen GB, Bergström T. Frequent detection of cytomegalovirus and Epstein-Barr virus in cervical secretions from healthy young women. Acta Obstet Gynecol Scand 2013; 92:706–10. [DOI] [PubMed] [Google Scholar]
- 25. Silver MI, Paul P, Sowjanya P, et al. Shedding of Epstein-Barr virus and cytomegalovirus from the genital tract of women in a periurban community in Andhra Pradesh, India. J Clin Microbiol 2011; 49:2435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montgomery R, Youngblood L, Medearis DN Jr. Recovery of cytomegalovirus from the cervix in pregnancy. Pediatrics 1972; 49:524–31. [PubMed] [Google Scholar]
- 27. Yan XC, Wang JH, Wang B, et al. Study of human cytomegalovirus replication in body fluids, placental infection, and miscarriage during the first trimester of pregnancy. J Med Virol 2015; 87:1046–53. [DOI] [PubMed] [Google Scholar]
- 28. Kaye S, Miles D, Antoine P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in The Gambia. J Infect Dis 2008; 197:1307–14. [DOI] [PubMed] [Google Scholar]
- 29. Revello MG, Furione M, Rognoni V, Arossa A, Gerna G. Cytomegalovirus DNAemia in pregnant women. J Clin Virol 2014; 61:590–2. [DOI] [PubMed] [Google Scholar]
- 30. Schoenfisch AL, Dollard SC, Amin M, et al. Cytomegalovirus (CMV) shedding is highly correlated with markers of immunosuppression in CMV-seropositive women. J Med Microbiol 2011; 60:768–74. [DOI] [PubMed] [Google Scholar]
- 31. Cannon MJ, Westbrook K, Levis D, Schleiss MR, Thackeray R, Pass RF. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev Med 2012; 54:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 2006; 118:e286–92. [DOI] [PubMed] [Google Scholar]
- 33. Cannon MJ, Stowell JD, Clark R, et al. Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect Dis 2014; 14:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pass RF, Hutto SC, Reynolds DW, Polhill RB. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics 1984; 74:121–6. [PubMed] [Google Scholar]
- 35. Pass RF, Hutto C, Ricks R, Cloud GA. Increased rate of cytomegalovirus infection among parents of children attending day-care centers. N Engl J Med 1986; 314:1414–8. [DOI] [PubMed] [Google Scholar]
- 36. Adler SP. Cytomegalovirus and child day care. Evidence for an increased infection rate among day-care workers. N Engl J Med 1989; 321:1290–6. [DOI] [PubMed] [Google Scholar]