Abstract
Background
The relationship between concentrations of antituberculosis drugs, sputum culture conversion, and treatment outcome remains unclear. We sought to determine the association between antituberculosis drug concentrations and sputum conversion among patients coinfected with tuberculosis and human immunodeficiency virus (HIV) and receiving first-line antituberculosis drugs.
Methods
We enrolled HIV-infected Ugandans with pulmonary tuberculosis. Estimation of first-line antituberculosis drug concentrations was performed 1, 2, and 4 hours after drug intake at 2, 8, and 24 weeks of tuberculosis treatment. Serial sputum cultures were performed at each visit. Time-to-event analysis was used to determine factors associated with sputum culture conversion.
Results
We enrolled 268 HIV-infected patients. Patients with low isoniazid and rifampicin concentrations were less likely to have sputum culture conversion before the end of tuberculosis treatment (hazard ratio, 0.54; 95% confidence interval, .37–.77; P = .001) or by the end of follow-up (0.61; .44–.85; P = .003). Patients in the highest quartile for area under the rifampicin and isoniazid concentration-time curves for were twice as likely to experience sputum conversion than those in the lowest quartile. Rifampicin and isoniazid concentrations below the thresholds and weight <55 kg were both risk factors for unfavorable tuberculosis treatment outcomes. Only 4.4% of the participants had treatment failure.
Conclusion
Although low antituberculosis drug concentrations did not translate to a high proportion of patients with treatment failure, the association between low concentrations of rifampicin and isoniazid and delayed culture conversion may have implications for tuberculosis transmission.
Clinical Trials Registration: NCT01782950.
Keywords: Tuberculosis, outcomes, pharmacokinetics, anti-tuberculosis drugs
Patients with tuberculosis and human immunodeficiency virus coinfection have low antituberculosis drug concentrations. Although the proportion of patients with unfavorable treatment outcomes is low, those with low rifampicin and isoniazid concentrations are more likely to have delayed sputum culture conversion.
(See the Editorial Commentary by Pasipanodya and Gumbo on pages 717–8.)
Tuberculosis infected >10 million persons in 2015 with approximately 1.5 million deaths, of whom 400000 occurred among persons coinfected with human immunodeficiency virus (HIV) [1]. Clearance of mycobacteria from sputum is necessary to achieve individual cure and decrease tuberculosis transmission; however, up to 50% of patients remain sputum culture positive after 1 month of directly observed therapy, and 20% after 2 months, a fact that has major public health implications for curbing the tuberculosis pandemic [2–4]. Earlier studies reported that a positive sputum culture after 2 months of treatment is a predictor of treatment failure and tuberculosis relapse [5]. However, more recent studies have demonstrated this finding to be a poor predictor of tuberculosis treatment outcome [6, 7]. Despite this, the sputum culture result at month 2 is used to monitor treatment response and as a marker of the sterilizing activity of antituberculosis drugs [7, 8]. Delayed sputum culture conversion has been associated with high bacillary load at treatment initiation, lung cavities, and drug resistance [9, 10].
Rifampicin and isoniazid display concentration-dependent killing of mycobacteria, leading to a decrease in bacillary load within the first few days of treatment [11]. Several studies have demonstrated low antituberculosis drug concentrations in patients receiving tuberculosis treatment [12–14]. At present, there is conflicting evidence about whether there is an association between antituberculosis drug concentrations, time to sputum conversion, and tuberculosis treatment outcome [14–16]. The aim of the current study was to determine the association between antituberculosis drug concentrations and sputum conversion in a cohort of tuberculosis-HIV–coinfected patients receiving first-line antituberculosis drugs at the standard dosage.
METHODS
Setting
We carried out a prospective observational study entitled Study on Outcomes Related to TB and HIV Drug Concentrations among tuberculosis-HIV–coinfected patients from May 2013 to November 2015 at the Infectious Diseases Institute (IDI) in Kampala, Uganda.
Study Design and Population
Tuberculosis was diagnosed using a combination of clinical symptoms (cough for ≥2 weeks, a history of fever as reported by the patient, unintended weight loss of ≥10% in the previous 3 months, and night sweats) and findings of chest radiography, sputum florescent microscopy, cultures, and Xpert MTB/RIF assay. All patients had a documented Uni-Gold test result confirming HIV infection. HIV-infected patients with pulmonary tuberculosis diagnosed were included in the study if they were ≥18 years old, willing to comply with study visits, and had no prior history of tuberculosis treatment. Details of the study methods were published elsewhere [17].
Patients were excluded if they (1) required tuberculosis treatment for >6 months (eg, for tuberculous meningitis); (2) had been previously treated for infections due to mycobacteria other than Mycobacterium tuberculosis; (3) were pregnant; (4) had an elevated alanine aminotransferase level >5 times the upper limit of normal; (5) had a glomerular filtration rate <50 mL/min, which could affect drug elimination; or (6) had comorbid conditions that reduce life expectancy to <1 year (eg, visceral Kaposi sarcoma). Patients were withdrawn from the study on request, based on medical judgment of the study physician, if they became pregnant, experienced a toxic effect necessitating a change or interruption of tuberculosis treatment, had a drug sensitivity test result showing resistance to any first-line antituberculosis drug (which could delay sputum conversion), or had only mycobacteria other than M. tuberculosis identified in the baseline culture.
Tuberculosis treatment was provided according to World Health Organization (WHO) recommendations [14] using fixed-dose combinations, which included 2 months (intensive phase) of rifampicin, isoniazid, ethambutol, and pyrazinamide (RHEZ), followed by 4 months (continuation phase) of isoniazid and rifampicin with dosages based on weight bands: 3 tablets of RHEZ or isoniazid-rifampicin for weight <55 kg, 4 tablets for weight 55–69 kg, and 5 tablets for weight ≥70 kg. Each tablet contained 150 mg of rifampicin, 75 mg of isoniazid for both RHEZ and isoniazid-rifampicin combinations plus 400 mg of pyrazinamide and 275 mg of ethambutol for RHEZ.
Antiretroviral treatment (ART) consisting of tenofovir, lamivudine, and efavirenz was started ≥2 weeks after initiation of tuberculosis treatment, as long as the patient was willing to commence treatment. Patients who were already receiving ART at the time of tuberculosis diagnosis continued their treatment, and patients taking nevirapine were switched to efavirenz to reduce drug-drug interactions. Patients receiving protease inhibitors received rifabutin instead of rifampicin and were therefore excluded from this analysis.
Pharmacokinetic Measurements
Patients were followed up at 2, 8, and 24 weeks after initiation of tuberculosis treatment and fasted for ≥8 hours before the study visit. At each follow-up visit, blood sampling was performed at 0 hours (before drug ingestion) and 1, 2, and 4 hours after witnessed ingestion of antituberculosis drugs. A standardized meal was provided after the 1-hour blood collection. Adherence counseling was performed at every follow-up visit. Adherence was assessed through pill counts and self-report using 7-day and 1-month recall [18].
Blood samples were collected in serum Vacutainer test tubes, and serum was separated by centrifuging within 1 hour of the blood sampling. Serum concentrations of rifampicin, isoniazid, pyrazinamide, and ethambutol were measured at the IDI translational laboratory in Kampala using UV high-performance liquid chromatography, as described elsewhere [19]. Additional information on the methods are available in the Supplementary Material.
Microbiological Assessment
A spot sputum sample was requested at every follow-up visit for fluorescent microscopy, as well as culture using both Löwenstein-Jensen medium and the BACTEC Mycobacteria Growth Indicator Tube 960 system. In accordance with the standard of care, sputum microscopy was also performed after 5 months of tuberculosis treatment. Patients who could not spontaneously provide a sputum sample underwent sputum induction where possible.
After a substudy protocol amendment was approved, the last 108 study participants underwent intensive sputum culture monitoring. This subset of patients provided sputum samples for microscopy and culture every 2 weeks for the first 12 weeks of treatment. All other procedures were identical to those performed in the rest of the study population.
Ethical Considerations
Ethical approval was received from Joint Clinical and Research Centre Institutional Review Board, Uganda National Council for Science and Technology (reference No. HS1303) and the National Drug Authority. This study is registered in Clinicaltrials.gov (NCT01782950). Written informed consent was obtained from all study participants.
Assessment of Outcome
Tuberculosis treatment outcomes were defined according to the WHO treatment guidelines [14]. Sputum culture conversion was defined as conversion of sputum cultures from positive to negative with no subsequent positive cultures in patients with a positive culture at baseline. Participants were considered lost to follow-up if their tuberculosis treatment outcome or clinical status was unknown by the end of the follow-up period (6 months after completion of tuberculosis treatment).
Data Analysis
We included patients who had a positive sputum culture at baseline, were followed up prospectively, and had pharmacokinetic data available on ≥1 occasion. The estimated maximum drug concentration (eCmax) for each drug was defined as the highest concentration of the 1-, 2-, and 4-hour blood samples at each study visit. Population pharmacokinetic models were developed using Monolix software [20] and individual area under the concentration-time curves (AUC) over 24 hours were derived from these models. Details of these models can be found in the Supplementary Material.
We used time-to-event analyses (Kaplan-Meier curves and Cox proportional hazards regression) to determine factors associated with sputum culture conversion. Predictors of low drug concentrations and the status of cured versus not cured were determined with logistic general estimation equations accounting for multiple time points with pharmacokinetic determinations per patient. WHO weight bands were divided into 3 equal tertiles (upper, middle, and lower) as one predictor of low concentrations. Wilcoxon rank-sum tests were used to assess associations between the eCmax of each drug and baseline characteristics. We determined the number of drugs and proportions of patients with an eCmax below the lower limit of the reference ranges for each participant, using thresholds from Peloquin et al [21]: 3 mg/L for isoniazid, 8 mg/L for rifampicin, 20 mg/L for pyrazinamide, and 2 mg/L for ethambutol [21, 22]. We also evaluated the recent maximum drug concentration (Cmax) and AUC thresholds from Pasipanodya et al [23]: 8.8 mg/L and 52 mg · h/L to isoniazid, 6.6 mg/L and 13 mg· h/L for rifampicin, 58.3 mg/L and 363 mg· h/L for pyrazinamide.
A drug was classified as below the cutoff if the eCmax was below the reference range on the study visit when sputum culture conversion was established or below the reference range on ≥1 study visit before sputum culture conversion. For patients without conversion by the end of tuberculosis treatment, we used the eCmax from the last study visit, and for those conversion, we used the lowest eCmax among the values below the threshold. For analysis of AUCs, we used the lowest AUC before or at the time of sputum conversion or the AUC from the last study visit for those who without conversion by the end of tuberculosis treatment. Our hypothesis was that patients with antituberculosis drug concentrations below the reference ranges were more likely to have delayed sputum culture conversions than those with normal concentrations. All analyses were performed using Stata software (version 14.2; StataCorp).
RESULTS
Patient Demographics
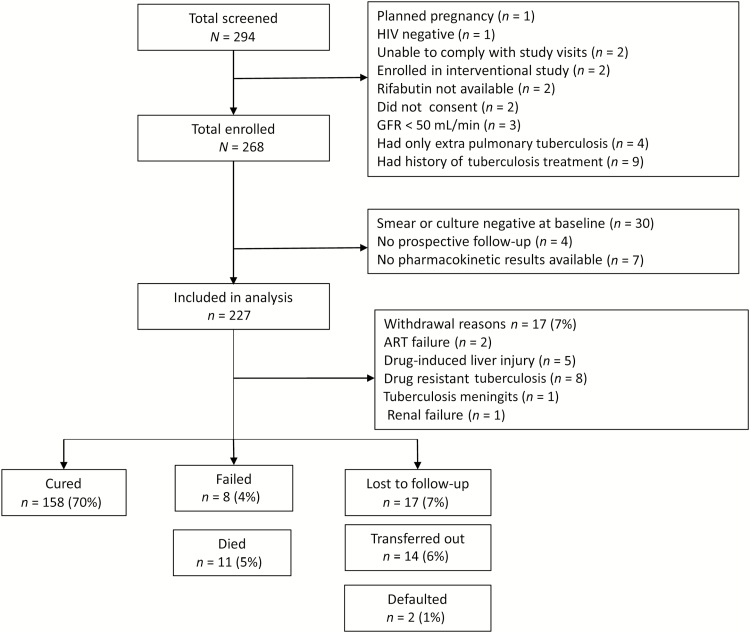
From April 2013 to May 2015, a total of 294 HIV-infected patients were screened for the study, and 268 were enrolled. Forty-one patients (15.3%) were excluded from the analysis: 30 had negative sputum smears and cultures throughout the study, 4 had no prospective follow-up data, and 7 had no pharmacokinetic data (3 of whom were taking rifabutin) (Figure 1). Patients who were excluded had a lower median CD4 cell count (190/µL vs 76/µL; P = .02). The other characteristics of excluded patients are provided in the Supplementary Data.
Figure 1.
Flow diagram shows patients excluded and treatment outcomes in patients followed up. Abbreviations: ART, antiretroviral therapy; GRF, glomerular filtration rate; HIV, human immunodeficiency virus.
We included 227 patients with a median follow-up of 182 person-years in our final analysis; 134 patients (59%) were male, the median age (interquartile range [IQR]) was 34 (29–40) years, the median body mass index (BMI), 19.2 (17.6–21.5) kg/m2, and the median CD4 cell count, 190/µL (IQR, 66–339/µL). Forty-nine patients (21.6%) were receiving ART at baseline (Table 1). Patients weighing >70 kg received a slightly lower dose per kilogram weight for all drugs (Table 2).
Table 1.
Clinical and Demographic Characteristics of Study Population
| Baseline Characteristics | Patients, No. (%)a (N = 227) |
|---|---|
| Male sex | 134 (59) |
| Age, median (IQR), y | 34 (29–40) |
| BMI, median (IQR), kg/m2 | 19.2 (17.7–21.5) |
| BMI <18 kg/m2 | 74 (32.6) |
| Time since HIV diagnosis, median (IQR), mo | 6 (0.5–11) |
| Chest radiographic findings | |
| Cavities | 48 (21.1) |
| Miliary | 29 (12.8) |
| WHO stage III | 200 (88.1) |
| WHO stage IV | 18 (7.9) |
| CD4 cell count, median (IQR), cells/µLb | 190 (66–340) |
| CD4 cell count subgroupb | |
| >200/µL | 91 (48.4) |
| 50–200/µL | 56 (29.8) |
| <50/µL | 41 (21.8) |
| First-line ART | 46 (20.2) |
| Zidovudine, lamivudine, and efavirenz | 23 (10.1) |
| Tenofovir, lamivudine, and efavirenz | 20 (8.8) |
| Zidovudine, lamivudine, and nevirapinec | 3 (1.3) |
| Second-line ART | 3 (1.3) |
| Tenofovir, lamivudine, lopinavir, and ritonavir | 2 (0.9) |
| Tenofovir, lamivudine, atazanavir, and ritonavir | 1 (0.4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; WHO, World Health Organization.
aData represent No. (%) of patients unless otherwise specified.
bCD4 cell counts were available for only 188 patients
cNevirapine was replaced by efavirenz before initiation of tuberculosis treatment.
Table 2.
Dose Given According to Weight Bands
| Weight Band, kg | Dose, mg/kg | ||
|---|---|---|---|
| Rifampicin | Isoniazid | Pyrazinamide | |
| 25–39 | 11.68 | 5.81 | 30.76 |
| 40–54 | 9.38 | 4.69 | 25.13 |
| 55–69 | 10.08 | 5.04 | 27.12 |
| >70 | 8.33 | 4.16 | 22.22 |
Attainment of Therapeutic Targets for Antituberculosis Drugs
The overall median eCmax for isoniazid and rifampicin were below the lower limits of the reference ranges [21] (2.23 [IQR, 1.57–3.03] and 7.07 mg/L [5.22–8.95] mg/L, respectively), whereas the median eCmax values for pyrazinamide and ethambutol were normal (40.9 [35.55–46.4[ and 3.03 [2.12–4.08] mg/L, respectively). Pharmacokinetic results according to tuberculosis treatment outcomes are shown in Table 3. The proportions of participants with concentrations below the reference ranges were 190 of 227 (84%) for isoniazid, 176 of 227 (78%) for rifampicin, 6 of 227 (3%) for pyrazinamide, and 70 of 227 (31%) for ethambutol. Male patients were more likely to have lower eCmax values for rifampicin and isoniazid (hazard ratio [HR], 0.54; 95% confidence interval [CI], .35–.86; P = .009).
Table 3.
Median Concentrations of Isoniazid, Rifampicin, and Pyrazinamide and Tuberculosis Treatment Outcomes
| Treatment Outcome (N = 210) |
Median Value (IQR) | |||||
|---|---|---|---|---|---|---|
| Isoniazid | Rifampicin | Pyrazinamide | ||||
| AUC, mg· h/L | eCmax, mg/L | AUC, mg· h/L |
eCmax, mg/L |
AUC, mg· h/L |
eCmax, mg/L | |
| Cure (n = 158) | 10.70 (6.44–16.46) |
2.42 (1.65–3.43) |
36.36 (27.21–46.67) |
6.98 (5.0–9.60) |
423.22 (369.83–499.28) |
40.93 (35.25–46.85) |
| Death (n = 11) | 7.79 (4.04–10.23) |
1.33 (1–2.47) |
38.45 (31.54–46.21) |
5.73 (1.48–8.48) |
497.48 (283.20–565.67) |
41.96 (31.04–45.13) |
| Failure (n = 8) | 10.32 (6.82–12.71) |
2.73 (1.43–3.79) |
45.70 (32.29–65.19) |
7.55 (6.20–11.08) |
503.54 (386.84–632.87) |
41.1 (35.79–49.85) |
| Default (n = 2) | 8.51 (6.51–10.79) |
1.82 (1.81–2.09) |
40.30 (31.04–74.99) |
6.98 (6.22–11.08) |
478.37 (406.22–550.52) |
41.43 (36.93–42.52) |
| Lost to follow-up (n = 17) | 11.70 (8.27–19.25) |
2.80 (1.96–3.67) |
33.14 (24.51–48.03) |
6.22 (5.11–8.04) |
426.0 (370.47–476.98) |
39.96 (34.75–45.23) |
Abbreviations: AUC, area under the concentration-time curve; eCmax, estimated maximum concentration; IQR, interquartile range.
There was no association between the eCmax of rifampicin and isoniazid and BMI, age, WHO weight bands, or tertiles within the weight bands (Table 4). However, for isoniazid, we observed that patients in the upper tertile of the WHO weight bands had lower median AUCs (9.48 [IQR, 6.09–14.87] mg· h/L) than those in the middle (10.80 [6.83–15.76] mg· h/L) or lower (10.94 [6.98–17.01] mg· h/L) tertiles. The clearance of rifampicin was higher at weeks 8 and 24 than at week 2, with a median drop from week 2 AUC values of 10.07 mg· h/L at week 8 and 5.12 mg· h/mL at week 24.
Table 4.
Predictors of Low Maximum Concentrations of Both Rifampicin and Isoniazid and Unfavorable Treatment Outcomesa
| Characteristic (N = 227) |
Predictors of Low Rifampicin and Isoniazid eCmax | Predictors of Unfavorable Treatment Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | |||||||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age, y | ||||||||||||
| <30 y | Reference | … | … | Reference | … | … | Reference | … | … | Reference | … | … |
| 30–39 y | 1.32 | .85–2.06 | .22 | 1.29 | .82–2.02 | .28 | 0.85 | .44–1.67 | .64 | 0.90 | .45–1.77 | .76 |
| ≥40 y | 1.04 | .62–1.74 | .88 | 1.01 | .59–1.72 | .97 | 0.81 | .37–1.75 | .59 | 0.88 | .41–1.91 | .76 |
| Male sex | 2.03 | 1.38–2.98 | <.001 | 1.82 | 1.18–2.79 | .006 | 0.62 | .35–1.10 | .10 | 1.62 | .90–2.90 | .10 |
| CD4 cell count (per 100 cells/µL)b | 1.00 | .91–1.11 | .87 | … | … | … | 1.10. | .96–1.25. | .17 | … | … | … |
| BMI | ||||||||||||
| Underweight | 1.19 | .81–1.74 | .37 | 1.37 | .86–2.18. | .19 | 0.66 | .36–1.21 | .178 | 0.59 | .32–1.09 | .09 |
| Normal | Reference | … | … | Reference | … | … | Reference | … | … | Reference | … | … |
| Overweight | 0.65 | .25–1.69 | .38 | 0.67 | .26–1.73. | .41. | 1.00 | .24–4.22 | >.99 | 0.89 | .22–3.61 | .87 |
| Obese | 0.35 | .78–1.58 | .17 | 0.32. | .05–1.89 | .21 | 0.51 | .05–4.72 | .55 | 0.43 | .05–4.13 | .47 |
| Weight band | ||||||||||||
| 25–39 kg | 1.04 | .40–2.76 | .92 | 0.62 | .18–2.14 | .45 | 1.28 | 1.03–1.59 | .024 | 1.35 | 1.10–1.65 | .004 |
| 40–54 kg | 1.24 | .83–1.88 | .29 | 0.88 | .54–1.45 | .62 | 1.17 | 1.05–1.31 | .005 | 1.21 | 1.08–1.36 | .001 |
| 55–69 kg | Reference | … | … | … | … | … | Reference | … | … | … | … | … |
| >70 kg | 0.86 | .38–1.95 | .72 | 1.46 | .58–3.70 | .42 | 0.96 | .82–1.12 | .60 | 0.97 | .85–1.10 | .63 |
| Tertile within weight band | ||||||||||||
| Lower | 0.93 | .62–1.38 | .70 | 0.84 | .55–1.29 | .42 | 0.98 | .92–1.05 | .59 | … | … | … |
| Middle | Reference | … | … | Reference | … | … | Reference | … | … | Reference | … | … |
| Upper | 1.33 | .87–2.01 | .18 | 1.39 | .89–2.18 | .15 | 1.00 | .94–1.05 | .90 | … | … | … |
| eCmax below cutoff | ||||||||||||
| None | … | … | … | … | … | … | Reference | … | … | Reference | … | … |
| Isoniazid <3 mg/L | … | … | … | … | … | … | 1.02 | .95–1.10 | .55 | 1.03 | .95–1.11 | .51 |
| Rifampicin <8 mg/L | … | … | … | … | … | … | 1.04 | .97–1.11 | .25 | 1.05 | .98–1.12 | .17 |
| Isoniazid <3 mg/L and rifampicin <8 mg/L | … | … | … | … | … | … | 1.07 | 1.00–1.15 | .06 | 1.09 | 1.01–1.17 | .02 |
Abbreviations: BMI, body mass index; CI, confidence interval; eCmax, estimated maximum concentration; OR, odds ratio.
aTable depicts logistic regression analysis for predictors of low maximum concentrations for both rifampicin and isoniazid, as well as predictors of unfavorable treatment outcomes, which include failure, death, loss to follow-up, and default.
bCD4 cell counts were available for only 188 patients.
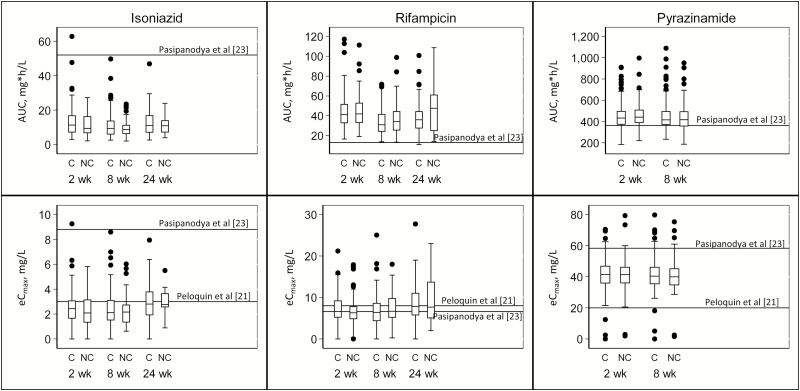
We compared our median eCmax and AUC findings to new targets set by Pasipanodya et al [23]. All but 1 participant had an isoniazid eCmax and AUC below those recommended by Pasipanodya et al. In the case of rifampicin, none of the participants had an AUC below the target recommended by Pasipanodya et al, but 136 of 222 (61.3%) had an eCmax below the new recommended target. The majority of the participants (222 of 224) had a pyrazinamide eCmax less than the new recommended target, and 59 of 224 (26.3%) had a lower pyrazinamide AUC (Figure 2).
Figure 2.
Association between concentrations of rifampicin, isoniazid, and pyrazinamide and tuberculosis treatment outcome. Box plots demonstrating area under the concentration-time curve (AUC) and estimated maximum concentrations (eCmax) for isoniazid, rifampicin, and pyrazinamide at weeks 2, 8 and 24 and stratified by tuberculosis treatment outcome: cured (C) and not cured (NC). The top of each box represents the 75th percentile (Q3); the bottom, the 25th percentile (Q1); and the horizontal line within each box, the 50th percentile (median) concentration. The top whisker represents Q3 + (1.5 × the interquartile range [IQR]), and the bottom whisker, Q1 − (1.5 × IQR); dots represent outliers.
Pharmacokinetics and Sputum Culture Conversion
Using thresholds published by Peloquin et al [21], patients with low eCmax for isoniazid or rifampicin were less likely to have sputum culture conversion before the end of tuberculosis treatment (HR, 0.54; 95% CI, .37–.77; P = .001) or by the end of follow-up (0.61; .44–.85; P = .003) (Table 5). Patients with ≥1 drug below the cutoff were also less likely to have sputum conversion than those who had no drug below the cutoff, after adjustment for age, BMI, and sex (Table 3).
Table 5.
Cox Proportional Hazards Model Showing Predictors of Sputum Culture Conversion
| Characteristic | Unadjusted HRs | Adjusted HRs | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age >40 y | 0.79 | .54–1.16 | .24 | 0.82 | .55–1.21 | .31 |
| BMI | ||||||
| Underweight | Reference | … | … | … | … | … |
| Normal | 1.05 | .79–1.40 | .74 | 1.03 | .76–1.38 | .87 |
| Overweight | 1.05 | .49–2.29 | .90 | 0.97 | .44–2.15 | .93 |
| Obese | 0.75 | .28–2.06 | .58 | 0.75 | .26–2.11 | .58 |
| Male sex | 0.94 | .71–1.24 | .65 | 1.07 | .80–1.45 | .64 |
| No. of drugs below cutoff | ||||||
| 0 | Reference | … | … | … | … | … |
| 1 | 0.42 | .22–0.77 | .006 | 0.40 | .22–0.74 | .003 |
| 2 | 0.27 | .15–0.49 | <.001 | 0.26 | .14–0.47 | <.001 |
| eCmax | ||||||
| Isoniazid <3 mg/L | 0.54 | .37–.77 | .001 | … | … | … |
| Rifampicin <8 mg/L | 0.61 | .44–.84 | .003 | … | … | … |
| Pyrazinamide <20 mg/L | 0.98 | .40–2.38 | .97 | … | … | … |
| Ethambutol <2 mg/L | 0.78 | .58–1.06 | .11 | … | … | … |
| Isoniazid AUC quartiles | ||||||
| <5.7 | Reference | … | … | … | … | … |
| 5.7–8.9 | 1.13 | .75–1.69 | .56 | … | … | … |
| 9.0–13.3 | 1.29 | .87–1.90 | .21 | … | … | … |
| >13.4 | 1.89 | 1.27–2.81 | .002 | … | … | … |
| Rifampicin AUC quartiles | ||||||
| <25.5 | Reference | … | … | … | … | … |
| 25.5–32.3 | 1.25 | .85–1.85 | .25 | … | … | … |
| 32.4–44.5 | 1.53 | 1.02–2.29 | .04 | … | … | … |
| >44.6 | 2.00 | 1.34–3.00 | .001 | … | … | … |
Abbreviations: AUC, area under the concentration-time curve; BMI, body mass index; CI, confidence interval; eCmax, estimated maximum concentration; HR, hazard ratio.
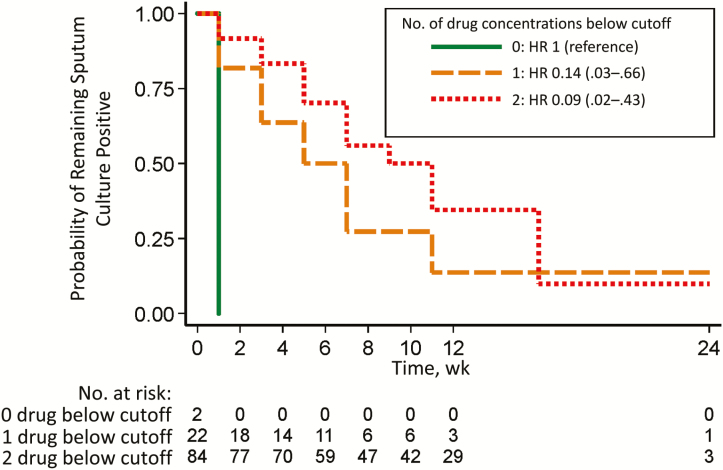
In the subgroup analysis of 108 patients in whom sputum smears and cultures were obtained every 2 weeks, 69% were male, with a median (IQR) age of 35 (30–41) years and a median BMI of 19.03 (17.7–21.4) kg/m2. Patients with low isoniazid and rifampicin concentrations had a <10% probability of remaining culture positive by the end of follow-up (Figure 3). The median times to sputum conversion among those with no drug, 1, or >1 drug below the cutoff were 1 (IQR, 1–1), 5 (I3–7), and 9 (7–11) weeks, respectively.
Figure 3.
Association between number of drugs below the cutoff and time to sputum culture conversion in 108 patients with intensive sputum culture monitoring. Kaplan-Meier curves for these patients demonstrate the probability of remaining sputum culture positive over time, stratified by the number of drug concentrations below the lower limit of the normal reference range for rifampicin (<8 mg/L) and isoniazid (<3 mg/L). Hazard ratios (HRs) are shown with 95% confidence intervals in parentheses.
Every 1-mg· h/L increment in AUC of rifampicin or isoniazid led to a 2%–4% increase in the likelihood of having sputum conversion (HR, 1.02 [95% CI, 1.01–1.02] P = .01] for rifampicin and 1.04 [1.02–1.06; P < .001] for isoniazid). Patients in the highest AUC quartile for rifampicin and for isoniazid were approximately twice as likely to experience sputum conversion by the end of follow-up as those in the lowest AUC quartile (Table 5). Using target concentrations for pyrazinamide described by Pasipanodya et al [23], we found no association between the AUC of pyrazinamide and time to sputum conversion (HR, 0.9; 95% CI, .7–1.32; P = .86) (Table 3).
Tuberculosis Treatment Outcomes
Of the 227 patients, 158 (70%) were cured, 10 (4.4%) had treatment failure, and 17 (7.5%) were withdrawn from the study (Figure 1). All patients lost to follow-up had experienced sputum culture conversion before the end of follow-up. Of those who were withdrawn or transferred out, 20 of 31 (64.5%) had experienced sputum conversion, and 8 (72%) of the 11 patients who died had sputum culture conversion.
Patients with eCmax values below the cutoff for both isoniazid and rifampicin had a moderately increased risk of unfavorable treatment outcomes, including death, failure, loss to follow-up, and default (odds ratio, 1.09; 95% CI, 1.01–1.17; P = .02). Those weighing <55 kg were also more likely to have unfavorable treatment outcomes (Table 4).
DISCUSSION
We found an association between low concentrations of isoniazid and rifampicin and delayed sputum culture conversion. Our findings are consistent with recent studies, which have demonstrated that higher exposures of rifamycins are associated with faster sputum clearance and a higher proportion of patients with negative cultures after 8 weeks of treatment [24, 25]. Delayed sputum culture conversion may have implications for tuberculosis transmission, especially in settings such as ours in sub-Saharan Africa, because patients are not isolated during tuberculosis treatment.
Others have also reported the association between drug concentrations and sputum conversion. In a retrospective study in predominantly HIV-uninfected participants [26] and a prospective study in which >50% of participants were HIV infected [27], patients with a low isoniazid eCmax were more likely to remain culture positive at week 8. Contrary to this finding, Chang and colleagues [15] and van Crevel and colleagues [16] found no association between Cmax of rifampicin and sputum culture conversion. The conflicting evidence may be attributed to the variability in study population and study design, including different sampling time points. In addition, when correlating concentrations with treatment response, the fact that drug concentrations in blood may not be reflective of concentrations at the site of activity (eg, lung tissue) should be taken into account.
Despite the high proportion of patients with low isoniazid and rifampicin concentrations, few patients had tuberculosis treatment failure (4.4%). However, low concentrations of both isoniazid and rifampicin were associated with unfavorable outcomes, which included death, failure, loss to follow-up, and default. Other prospective observational studies reported an association between low concentrations of pyrazinamide [28], isoniazid [27, 29], and rifampicin [29] and unfavorable tuberculosis treatment outcome. Although patients in the lower weight bands were not at risk of lower drug concentrations and actually received a relatively higher dose per kilogram than those in the higher weight bands (who were slightly underdosed), they had worse outcomes. This finding may be attributed to several factors, including the fact that patients in lower weight band may be sicker than those in higher weight bands.
We evaluated other thresholds, including those proposed by Pasipanodya et al [23]. The proposed targets for isoniazid were achieved in almost none of our participants, whereas all of them had AUCs above the proposed targets for rifampicin. Although the target AUC for pyrazinamide was achieved in 74% of our participants, only 21% achieved this target in a study by Alsultan et al [30]. These proposed thresholds still need to be evaluated prospectively. Different thresholds for rifampicin (<3.01 mg/L) and pyrazinamide (<38.1 mg/L) were associated with treatment failure and death in children with mostly extrapulmonary tuberculosis. Therefore, the site of tuberculosis may contribute to the difference in study findings and high variability in pharmacokinetic parameters [31].
The limitations of our study include our use of the total drug concentrations, and yet it is the free drug that is pharmacologically active. The AUC/minimum inhibitory concentration (MIC) and Cmax/MIC have been shown by others to be useful predictors of culture conversion [32, 33]. Lack of MIC data limits our ability to further evaluate pharmacokinetic/pharmacodynamic markers of sputum conversion and tuberculosis treatment outcomes. Sturkenboom and colleagues [34] described variability in the Cmax of rifampicin (0.4–5.7 hours), and several studies have demonstrated variability in the Cmax of isoniazid based on N-acetyltransferase polymorphisms; therefore, sampling up to 4 hours after dosing may have limited the accuracy of the pharmacokinetic parameter estimates [34, 35]. We did not evaluate the effect of drug concentrations on relapse, which has been associated with low pyrazinamide concentrations [23, 28].
The merits of our study include its large sample size, its prospective design with a large body of clinical data, and the pharmacokinetic sampling done on 3 occasions (at 2, 8, and 24 weeks) and with 3 time points (1, 2, and 4 hours), which enabled us to have a close approximation of the eCmax and develop models for AUCs. With intensive monitoring of sputum cultures we were able to give a more accurate estimate of the time to sputum conversion in a subset of the population. In conclusion, although low isoniazid and rifampicin concentrations in this HIV-tuberculosis–coinfected population did not translate into high proportions of patients with treatment failure, the association between low concentrations of isoniazid and rifampicin and prolonged sputum culture positivity has implications for tuberculosis transmission and warrants further investigation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the contributions of the study participants, staff, and management of the Infectious Diseases Institute (IDI) and the University of Zurich.
Disclaimer. The funders had no role in the design, data collection, analysis, or reporting of this study. C. S. W. had access to all the data in the study and had final responsibility for the decision to submit it for publication.
Author contributions M. L., P. B. K., M. J., Y. C. M., N. C., C. S. W., and J. S. F. contributed to the conceptualization of this protocol. C. S. W. and A. v. B. contributed equally to writing this article. C. S. W., A. v. B., A. B., L. H., J. Ma., U. G., N. E., and D. M. contributed to the data collection, C. S. W., B. L., P. D., A. J., J. Mu., and M. R. K. contributed to the data management and analysis, M. d. K., A. K., B. C., and J. S. F. supervised the writing of this article, and all authors read and approved the final version.
Financial support. This work was supported by the collaboration between the IDI and the University of Zurich (supported by AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Lunge Zürich, Merck, Shimadzu, Swiss HIV Cohort Study, and ViiV Healthcare); the Medical Education Partnership Initiative (grant 5R24TW008886) from the Office of the US Global AIDS Coordinator, National Institutes of Health and Health Resources and Services Administration.
Potential conflicts of interest. J. S. F. has received grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Merck, ViiV Healthcare, and Roche Diagnostics, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 2 March 2017.
- 2. Nandawula J. Prevalence and factors associated with non-conversion of positive sputum smears at 8 weeks of treatment among new pulmonary tuberculosis patients in Kampala [master’s thesis]. Kampala, Uganda: College of Health Sciences, Makerere University, 2013. [Google Scholar]
- 3. Bawri S, Ali S, Phukan C, Tayal B, Baruwa P. A study of sputum conversion in new smear positive pulmonary tuberculosis cases at the monthly intervals of 1, 2 & 3 month under directly observed treatment, short course (DOTS) regimen. Lung India 2008; 25:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pajankar S, Khandekar R, Al Amri MA, Al Lawati MR. Factors influencing sputum smear conversion at one and two months of tuberculosis treatment. Oman Med J 2008; 23:263–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao FZ, Levy MH, Wen S. Sputum microscopy results at two and three months predict outcome of tuberculosis treatment. Int J Tuberc Lung Dis 1997; 1:570–2. [PubMed] [Google Scholar]
- 6. Wallis RS, Doherty TM, Onyebujoh P, et al. . Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 2009; 9:162–72. [DOI] [PubMed] [Google Scholar]
- 7. Horne DJ, Royce SE, Gooze L, et al. . Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010; 10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis 1993; 147:1062–3. [DOI] [PubMed] [Google Scholar]
- 9. Fitzwater SP, Caviedes L, Gilman RH, et al. . Prolonged infectiousness of tuberculosis patients in a directly observed therapy short-course program with standardized therapy. Clin Infect Dis 2010; 51:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parikh R, Nataraj G, Kanade S, Khatri V, Mehta P. Time to sputum conversion in smear positive pulmonary TB patients on category I DOTS and factors delaying it. J Assoc Physicians India 2012; 60:22–6. [PubMed] [Google Scholar]
- 11. Gumbo T, Louie A, Deziel MR, et al. . Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 2007; 51:3781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McIlleron H, Rustomjee R, Vahedi M, et al. . Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother 2012; 56:3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peloquin CA, Nitta AT, Burman WJ, et al. . Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother 1996; 30:919–25. [DOI] [PubMed] [Google Scholar]
- 14. Burhan E, Ruesen C, Ruslami R, et al. . Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother 2013; 57:3614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang KC, Leung CC, Yew WW, et al. . Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur J Clin Microbiol Infect Dis 2008; 27:467–72. [DOI] [PubMed] [Google Scholar]
- 16. van Crevel R, Alisjahbana B, de Lange WC, et al. . Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int J Tuberc Lung Dis 2002; 6:497–502. [DOI] [PubMed] [Google Scholar]
- 17. Sekaggya-Wiltshire C, Castelnuovo B, von Braun A, et al. . Cohort profile of a study on outcomes related to tuberculosis and antiretroviral drug concentrations in Uganda: design, methods and patient characteristics of the SOUTH study. BMJ Open 2017. Sep 18; 7(9):e014679. doi:10.1136/bmjopen-2016-014679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006; 10:227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sekaggya-Wiltshire C, von Braun A, Scherrer AU, et al. . Anti-TB drug concentrations and drug-associated toxicities among TB/HIV-coinfected patients. J Antimicrob Chemother 2017; 72:1172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monolix. Built for model based drug development computing Available at: http://lixoft.com/products/monolix/. Accessed 3 March 2017.
- 21. Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 2002; 62:2169–83. [DOI] [PubMed] [Google Scholar]
- 22. Peloquin C. The role of therapeutic drug monitoring in mycobacterial infections. Microbiol Spectr 2017. Jan; 5(1). doi:10.1128/microbiolspec.TNMI7-0029-2016. [DOI] [PubMed] [Google Scholar]
- 23. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boeree MJ, Heinrich N, Aarnoutse R, et al. ; PanACEA consortium High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dorman SE, Savic RM, Goldberg S, et al. ; Tuberculosis Trials Consortium Daily rifapentine for treatment of pulmonary tuberculosis: a randomized, dose-ranging trial. Am J Respir Crit Care Med 2015; 191:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mah A, Kharrat H, Ahmed R, et al. . Serum drug concentrations of INH and RMP predict 2-month sputum culture results in tuberculosis patients. Int J Tuberc Lung Dis 2015; 19:210–5. [DOI] [PubMed] [Google Scholar]
- 27. Sloan D. Pharmacokinetic variability in TB therapy: associations with HIV and effect on outcome. Presented at: Conference on Retroviruses and Opportunistic Infections (3–6 March 2014; Boston, Massachusetts); 2014. [Google Scholar]
- 28. Chideya S, Winston CA, Peloquin CA, et al. . Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 2009; 48:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prahl JB, Johansen IS, Cohen AS, Frimodt-Møller N, Andersen ÅB. Clinical significance of 2 h plasma concentrations of first-line anti-tuberculosis drugs: a prospective observational study. J Antimicrob Chemother 2014; 69:2841–7. [DOI] [PubMed] [Google Scholar]
- 30. Alsultan A, Savic R, Dooley KE, et al. . Population pharmacokinetics of pyrazinamide in patients with tuberculosis. Antimicrob Agents Chemother 2017; 61(6). doi:10.1128/AAC.02625-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swaminathan S, Pasipanodya JG, Ramachandran G, et al. . Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rockwood N, Pasipanodya JG, Denti P, et al. . Concentration-dependent antagonism and culture conversion in pulmonary tuberculosis. Clin Infect Dis 2017; 64:1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chigutsa E, Pasipanodya JG, Visser ME, et al. . Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sturkenboom MG, Mulder LW, de Jager A, et al. . Pharmacokinetic modeling and optimal sampling strategies for therapeutic drug monitoring of rifampin in patients with tuberculosis. Antimicrob Agents Chemother 2015; 59:4907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verbeeck RK, Günther G, Kibuule D, Hunter C, Rennie TW. Optimizing treatment outcome of first-line anti-tuberculosis drugs: the role of therapeutic drug monitoring. Eur J Clin Pharmacol 2016; 72:905–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.