Abstract
Background
In southwest Kenya, the prevalence of human immunodeficiency virus (HIV) infection is about 25%. Médecins Sans Frontières has implemented a voluntary community testing (VCT) program, with linkage to care and retention interventions, to achieve the Joint United Nations Program on HIV and AIDS (UNAIDS) 90-90-90 targets by 2017. We assessed the effectiveness and cost-effectiveness of these interventions.
Methods
We developed a time-discrete, dynamic microsimulation model to project HIV incidence over time in the adult population in Kenya. We modeled 4 strategies: VCT, VCT-plus-linkage to care, a retention intervention, and all 3 interventions combined. Effectiveness outcomes included HIV incidence, years of life saved (YLS), cost (2014 €), and cost-effectiveness. We performed sensitivity analyses on key model parameters.
Results
With current care, the projected HIV incidence for 2032 was 1.51/100 person-years (PY); the retention and combined interventions decreased incidence to 1.03/100 PY and 0.75/100 PY, respectively. For 100000 individuals, the retention intervention had an incremental cost-effectiveness ratio (ICER) of €130/YLS compared with current care; the combined intervention incremental cost-effectiveness ratio was €370/YLS compared with the retention intervention. VCT and VCT-plus-linkage interventions cost more and saved fewer life-years than the retention and combined interventions. Baseline HIV prevalence had the greatest impact on the results.
Conclusions
Interventions targeting VCT, linkage to care, and retention would decrease HIV incidence rate over 15 years in rural Kenya if planned targets are achieved. These interventions together would be more effective and cost-effective than targeting a single stage of the HIV care cascade.
Keywords: HIV, voluntary community testing, cascade of care, cost-effectiveness, Kenya
Using a microsimulation model, we assessed testing, linkage, and retention strategies for human immunodeficiency virus (HIV) in Kenya to achieve UNAIDS 90-90-90 treatment targets. Although interventions are cost-effective by international standards, only the retention intervention and combining all 3 strategies decreased HIV incidence.
There continue to be 2.5 million new human immunodeficiency virus (HIV) infections per year worldwide, and HIV remains the leading cause of mortality in sub-Saharan Africa [1, 2]. To achieve an “HIV-free generation,” improvements along the HIV “treatment cascade” are among the most effective interventions [2, 3]. The Joint United Nations Program on HIV and AIDS (UNAIDS) has advocated for the “90-90-90” target by 2020, aiming to reach 90% HIV testing coverage of the infected population, 90% of those infected receiving antiretroviral therapy (ART), and 90% of those receiving ART achieving viral suppression [4].
Ndhiwa is a recently created subcounty, part of Homa Bay County, in southwest Kenya. Its prevalence of HIV is among the highest in the country, at 24.1% [5]. Médecins Sans Frontières (MSF) has recently scaled up its activity within Ndhiwa in partnership with the Kenyan Ministry of Health. This scale-up includes voluntary community testing (VCT), linkage to care using point-of-care CD4 cell count testing, and a retention intervention including improvement in ART coverage and earlier initiation of ART, retention in care, and viral suppression efforts, as well as program monitoring and evaluation. The goal of the MSF program is to meet the UNAIDS 90-90-90 targets in 4 years and then transfer program control to the province. The objective of the current study was to assess the effectiveness, cost, and cost-effectiveness of the overall program and its interventions.
METHODS
Analytic Overview
We developed a dynamic, open cohort, agent-based model of HIV disease progression and transmission to compare 4 strategies: (1) VCT; (2) VCT-plus-linkage to care; (3) a retention intervention including scale-up to treatment for all, improvement of ART coverage, and viral suppression; and (4) the above 3 interventions combined. Effectiveness of interventions on the short-term cascade of care was a model input by calibrating to UNAIDS targets, effectiveness of interventions on long-term cascade of care, years of life saved (YLS), and incidence were model outputs. We also projected the incremental cost-effectiveness ratio (ICER) for each strategy. The ICER is calculated as the difference in cost divided by the difference in YLS for each strategy, compared with the next less costly strategy.
We accounted for both the health gains and medical costs for HIV-infected patients and for the timing, decreased survival, and increased costs associated with projected HIV transmissions. We classified a strategy as “cost-effective” if the ICER was <0.5 times the 2016 per capita gross domestic product (GDP) in Kenya (€515) [6]. For cost-effectiveness calculations, costs and YLS of both the index and transmitted cases were discounted at 3% per year. We conducted a budget impact analysis, evaluating the total undiscounted costs of the interventions in the Ndhiwa subcounty setting [7]. The study time horizon was 15 years; the interventions would be initiated between 2014 and 2017 and maintained. For the budget impact analysis, we also evaluated costs at 2 and 5 years after implementation of the interventions.
Model Structure
The model has 2 modules: the HIV disease and the dynamic transmission modules. The HIV disease module divides the population into 6 health states stratified by sex: no infection, primary HIV infection and not treated, chronic infection and not treated, chronic infection and suppressed with ART, chronic infection and not suppressed with ART, and dead. HIV-infected patients have a monthly probability of HIV diagnosis by routine testing, linkage if tested, and treatment if linked. Patients have a monthly probability of hospitalization related to HIV infection, and a probability of having HIV infection diagnosed when hospitalized. For treated patients, we included 1 ART regimen, as is currently the standard in Ndhiwa subcounty.
The dynamic transmission module divides the population into 2 risk groups. The high-risk group is defined by the number of contacts (Table 1). The probability of transmission is calculated using the methods of McCormick et al [8]. We ran the model for an initialization period of 30 years (roughly the beginning of the HIV epidemic in the region to the first ART distribution in the late 1990s) to obtain steady-state HIV prevalence and incidence, corresponding to epidemiological data from the Kenyan AIDS indicator Survey and the Ndhiwa HIV in Population Survey (NHIPS), a cross-sectional study conducted by MSF [5]. The structure of the model is further detailed in the Supplementary Material.
Table 1.
Input Parameters for a Modeling Analysis of Combination Human Immunodeficiency virus Treatment Strategies in Rural Kenya
| Parameter | Base-Case Value | Reference |
|---|---|---|
| Baseline cohort characteristics | ||
| Age, median (IQR), y | 30 (21–44) | [5] |
| Female/male sex, % | 52/48 | [5] |
| CD4 cell count at diagnosis, mean (SD), cells/µL | 560 (230) | [24] |
| Baseline cascade of care | ||
| Probability of HIV diagnosis if major event | 0.40 | Calibration to [5] |
| Probability of background testing, monthly | 0.008 | Calibration to [5] |
| Probability of linkage to care when tested | 0.65 | Calibration to [5] |
| Probability of retention in care when linked, monthly | 0.97 | Calibration to [5] |
| Probability of treatment, monthly | ||
| CD4 cell count >200/µL | 0.20 | Calibration to [5] |
| CD4 cell count ≤200/µL | 0.70 | Calibration to [5] |
| Viral suppression rate at 1 y (95% CI) | 0.80 (.77–.83) | [5] |
| Costs of current care (2014 €) | ||
| Outpatient, yearlya | 257 | Assumption |
| Consultation | 9 | MSF (unpublished) |
| ART regimen (tenofovir, lamivudine and efavirenz), monthly | 10 | MSF (unpublished) |
| HIV RNA testing | 49 | MSF (unpublished) |
| CD4 count testing | 21 | MSF (unpublished) |
| Inpatient, per stayb | 101 | |
| Hospitalization cost, daily | 24.25 | MSF (unpublished) |
| Admission fee | 4 | MSF (unpublished) |
| Planned interventions | ||
| Voluntary community testing | ||
| Effectiveness target by MSF | ||
| Sensitivity | 1.0 | Assumption |
| Specificity | 1.0 | Assumption |
| Testing coverage (in 2 y) | 0.9 | Assumption |
| Overall start-up cost (2014 €) | ||
| Laboratory | 4763 | MSF (unpublished) |
| Logistics | 31883 | MSF (unpublished) |
| Human resources | 38835 | MSF (unpublished) |
| Fixed cost (2014 €), monthly | ||
| Laboratory | NA | Assumption |
| Logistics | 3310 | MSF (unpublished) |
| Human resources | 4616 | MSF (unpublished) |
| Variable cost (2014 €) | ||
| Laboratory (per kit) | 10 | MSF (unpublished) |
| Logistics (gas, per liter) | 2 | MSF (unpublished) |
| Human resources | NA | Assumption |
| Linkage | ||
| Effectiveness target by MSF | Assumption | |
| Probability of linkage to care | 0.9 | Assumption |
| Start-up cost (2014 €) | ||
| Laboratory | 4763 | MSF (unpublished) |
| Logistics | 31883 | MSF (unpublished) |
| Human resources | 38835 | MSF (unpublished) |
| Fixed cost (2014 €) | ||
| Laboratory | NA | Assumption |
| Logistics | 3310 | MSF (unpublished) |
| Human resources | 9232 | MSF (unpublished) |
| Variable costs (2016 €) | NA | Assumption |
| Retention intervention | ||
| Targeted cascade | ||
| Suppression rate at 1 y | 0.9 | Assumption |
| Start-up cost (2014 €) | ||
| Laboratory | 119942 | MSF (unpublished) |
| Logistics | 1431 | MSF (unpublished) |
| Human resource | 419836 | MSF (unpublished) |
| Fixed cost (2014 €), monthly | ||
| Laboratory | 6644 | MSF (unpublished) |
| Logistics | 3030 | MSF (unpublished) |
| Human resources | 11061 | MSF (unpublished) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile rangea; MSF, Médecins Sans Frontières; NA, not applicable; SD, standard deviation.
aCalculated based on 2 consultations per year with HIV RNA measurement, 1-time CD4 cell count, and 12 months of ART.
bCalculated based on a single upfront admission fee and multiplying the daily hospitalization cost by the average length of stay (4 days).
Input Data
Cohort
The modeled cohort mirrors the adult population of Ndhiwa. Characteristics were drawn from the NHIPS [5].
Natural History
Natural history input data for CD4 cell count decline, non-AIDS, and AIDS mortality rates were obtained from literature on African cohorts (see Supplementary Material, Table 1) [9, 10]. We calibrated the HIV testing probability using the median CD4 cell count at diagnosis and the proportion of patients with a diagnosis of World Health Organization stage 3 or 4 disease as well as MSF data on the cascade of care (Table 1) [5, 11]. We calibrated the cascade of care in the standard-of-care scenario using data from the NHIPS study, by adjusting variables for which we did not have reliable values, that is, the probability of having HIV infection diagnosed during hospitalization and the monthly probability of having HIV infection diagnosed in the community. For MSF data on the cascade of care, we calibrated the model to obtain the effectiveness data for each strategy for the first 4 years, and we then assessed long-term effectiveness by analyzing the projected HIV cascade at 15 years.
VCT Strategy
For the UNAIDS testing target, we increase the base-case background testing rate from 62% of the tested population to 90% of the population tested in 2 years.
VCT-Plus-Linkage Strategy
In addition to increasing the background testing through a VCT intervention, we increase linkage to care from a baseline of 60% to 90%.
Retention Intervention Strategy
The comprehensive retention strategy includes improving retention, increasing ART coverage, and improving viral suppression to achieve overall 90% suppression at 1 year among those receiving ART.
Costs
Standard-of-care costs consist of both outpatient costs and hospital costs. Outpatient costs were estimated at €257 per person per year (Table 1 and Supplementary Material). Hospitalization costs were estimated at €101 per hospitalization (Table 1 and Supplementary Material). Intervention costs include start-up, fixed, and variable costs; each of these include laboratory, logistics, and human resource components (Table 1 and Supplementary Material). All costs were derived from Kenyan national and MSF data.
Budget Impact Analysis
For the budget impact analysis, we considered the total undiscounted costs of interventions for the adult population of Ndhiwa (270000 persons) for each strategy. The variable costs included outpatient costs and hospitalization costs, as described above. We assessed outcomes at 2 and 5 years after implementation of the interventions.
Sensitivity Analyses
In sensitivity analyses we assessed uncertainty in parameter estimates. We first considered uncertainties in input variables, such as the probability of HIV being diagnosed during hospitalization, HIV routine testing coverage, linkage to care, retention in care, and access to ART. Second, we varied the efficacies and cost of the intervention strategies to assess how changes affected the overall results. Finally, we also varied HIV prevalence. In 2-way and multiway sensitivity analysis, we varied all interventions to the upper bound (best-case scenario) or the lower bound (worst-case scenario) of expected efficacies.
RESULTS
Model Validation
The HIV prevalence in the modeled population in Ndhiwa subcounty in 2012 was 23.1%, compared with 24.1% reported in the NHIPS study; the incidence of HIV infection was 1.9/100 PY in the model versus 2.2/100 PY in the study [5]. Testing coverage of the infected population in 2012 was 63.2% in the model versus 61.8% in the NHIPS study; the proportion of patients tested and linked to care was 59.6% versus 57.4%; the proportion tested, linked, and retained in care, 58.3% versus 56.2%; the proportion receiving ART, 38.4% versus 42.2%; and the proportion with virological suppression achieved, 30.8% versus 28.2% (Supplementary Table 2).
Base-Case Scenario
Cascade of Care
Under the VCT strategy alone, over the 15-year time horizon of the analysis (until 2032), HIV testing increased to 93.8% coverage, but ART coverage remained low, with 68.5% of the infected population receiving ART and only 36.2% virologically suppressed (Supplementary Table 2). The VCT-plus-linkage intervention achieved 94.0% testing coverage, with ART coverage and suppression rates of 70.9% and 37.4%, respectively. The retention intervention strategy improved suppression to 49.6%. Finally, the combined intervention led to 95.6% test coverage and 94.2% linked to care, with virological suppression achieved in 56.1% of patients by 2032. Note that if MSF reached its intervention targets, the UNAIDS 90-90-90 target was closest to being met with the combined intervention within 3 years after strategy implementation, with 97.0% tested, 94.2% receiving ART, and 68.8% suppressed (see Supplementary Table 2).
Clinical Outcomes and Cost-effectiveness
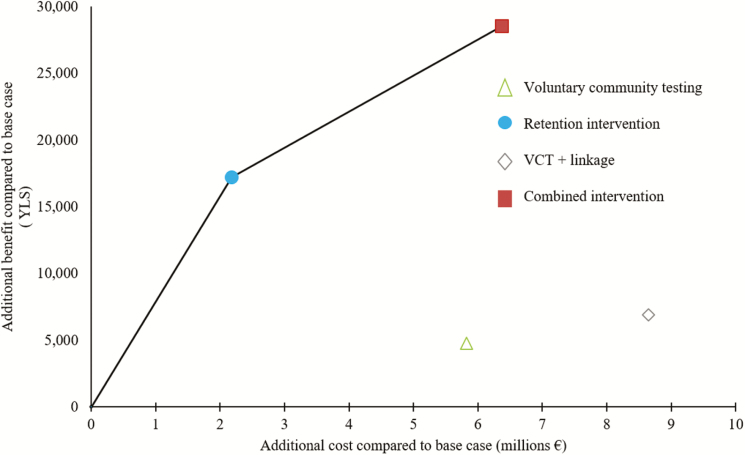
The current cost of care for 100000 persons was €42919500 over 15 years; this increased to €45102400 for the retention intervention, €48745100 for VCT, €49293100 for the combined intervention, and €51573900 for VCT-plus-linkage interventions (Table 2). The VCT-plus-linkage intervention cost more and saved fewer life-years than the combined intervention. The VCT intervention also cost more and was less effective in terms of YLS than the retention intervention. Implementing the retention intervention resulted in an ICER of €130/YLS compared with current care. Implementing the combined intervention saved 11369 YLS compared with the retention intervention (and 28565 YLS compared with current care), with an ICER of €370/YLS (Figure 1). The combined intervention is cost-effective by World Health Organization criteria, well below 0.5 times the 2016 annual per capita GDP of Kenya of €515 [12].
Table 2.
Cost, Effectiveness, and Cost-effectiveness of Human Immunodeficiency Virus Testing and Treatment Strategies 15 Years After the Interventions in Rural Kenyaa
| Strategy | Cost,a € | Years of Life Saveda | ICERb |
|---|---|---|---|
| Current care | 42919500 | … | … |
| Retention | 45102400 | 17200 | 130 |
| VCT | 48745100 | 4750 | Dominatedc |
| Combined | 49293100 | 28570 | 370 |
| VCT-plus-linkage | 51573900 | 6910 | Dominatedc |
Abbreviations: ICER, incremental cost-effectiveness ratio; VCT, voluntary community testing.
aCosts and years of life saved are estimated for 100000 inhabitants and are discounted at 3% per year
bThe ICER is calculated as the difference in the cost of the compared interventions divided by the difference in their life expectancy. Each strategy is compared with the next most effective strategy.
c Dominated means that the strategy costs more and is less effective or is less cost effective than a combination of other strategies.
Figure 1.
Each strategy with the corresponding efficacy in years of life saved (YLS) and cost. The bold line defines the efficiency frontier: the strategies lying on the curve, the retention and the combined intervention, are more economically efficient than those lying to the right of the curve, providing added value for resources. The slope of the bold line represents the incremental cost-effectiveness ratio (€130/YLS for the retention intervention and €370/YLS for the combined intervention). Abbreviation: VCT, voluntary community testing.
HIV Incidence
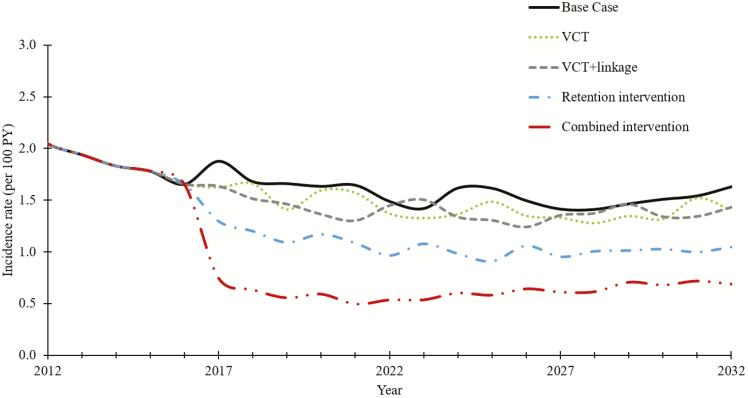
In the base-case scenario, incidence decreased over time and stabilized at 1.51/100 PY (Figure 2). The VCT strategy decreased incidence to 1.39/100 PY, VCT-plus-linkage to 1.32/100 PY, and the retention intervention strategy to 1.03/100 PY. The combined intervention showed the greatest incidence decrease, to 0.75/100 PY.
Figure 2.
Evolution of human immunodeficiency virus incidence over time under 4 strategies compared with the base-case scenario: voluntary community testing (VCT), VCT-plus-linkage, a retention intervention, and the combined intervention. Interventions started in 2014. Current care (base case) is represented by the solid black line. Abbreviation: PY, person-years.
Sensitivity Analyses
One-Way Sensitivity Analyses
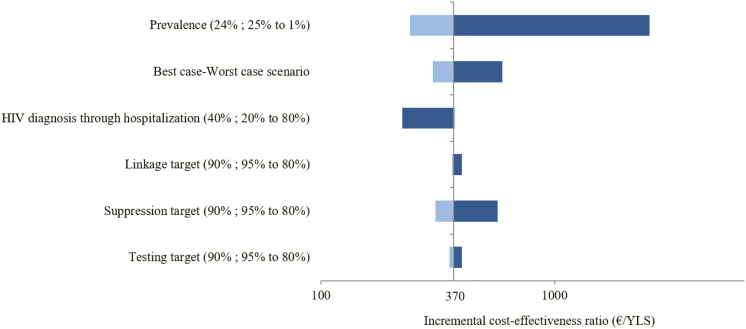
First, we increased the value of key parameters used to calibrate the base case. Decreasing the probability of HIV diagnosis through hospitalization to 0.2 (vs 0.4 in the base-case scenario) increased the proportion of undiagnosed HIV infection in the HIV population, making interventions more cost-effective, decreasing the ICER to €175/YLS (Figure 3). To extrapolate our results, we also varied parameters such as HIV prevalence. Decreasing HIV prevalence to 1% yielded an ICER of €2550/YLS for the combined intervention. Second, we varied efficacies of the intervention strategies. Decreasing efficacies to achieve coverage of 90% versus 80% did not lead to major increases in the ICER (from €370/YLS in the base-case scenario to €570/YLS).
Figure 3.
One-way and scenario sensitivity analysis (tornado diagram). In a 1-way sensitivity analysis, we assess the change in the incremental cost-effectiveness ratio (ICER) of the combined intervention compared with the base case by varying 1 parameter at a time. Each parameter shows the base case value; range tested in parentheses. The worst-case scenario examines the ICER if all interventions achieve the lower efficacy estimate (testing, linkage, and suppression targets: each 0.8), and the best-case scenario examines the ICER if all interventions achieve the higher efficacy estimate (testing, linkage, and suppression targets: each 0.95). Abbreviations: HIV, human immunodeficiency virus; YLS, years of life saved.
Two-Way Sensitivity Analyses
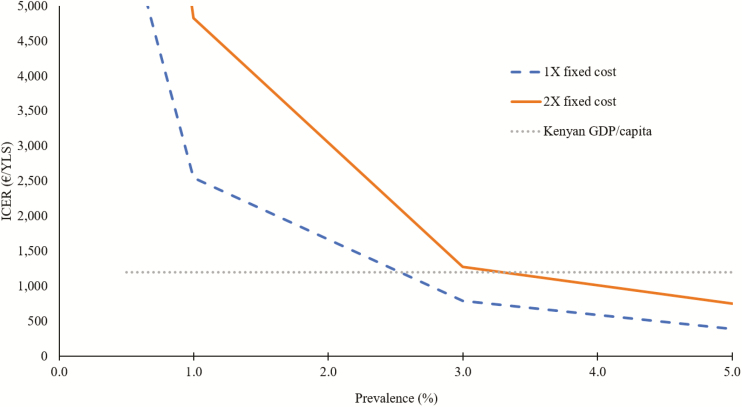
In 2-way sensitivity analysis, we varied the HIV prevalence and fixed costs of the interventions. Even when fixed costs were doubled, the combined intervention remained cost-effective at an HIV prevalence of 3% (the overall Kenyan prevalence); it was no longer cost-effective only when HIV prevalence was <1% (Figure 4).
Figure 4.
Two-way sensitivity analysis on the combined intervention. We represent the variation in the ICER for the combined intervention with the fixed cost and doubling of the fixed cost, as a function of human immunodeficiency virus prevalence. The dotted green line represents the annual Kenyan per capita GDP of €1030. Abbreviations: GDP, gross domestic product; ICER, incremental cost-effectiveness ratio; YLS, years of life saved.
Multiway Sensitivity Analyses
In the best-case scenario (upper bound of intervention efficacies), the combined intervention decreased HIV incidence at 15 years to the same range as the base-case scenario (0.89/100 PY) and resulted in an improved ICER of €110/YLS. HIV incidence was higher under the worst-case scenario (1.27/100 PY), but the outcome remained cost-effective (ICER, €600/YLS) (Figure 3).
Budget Impact
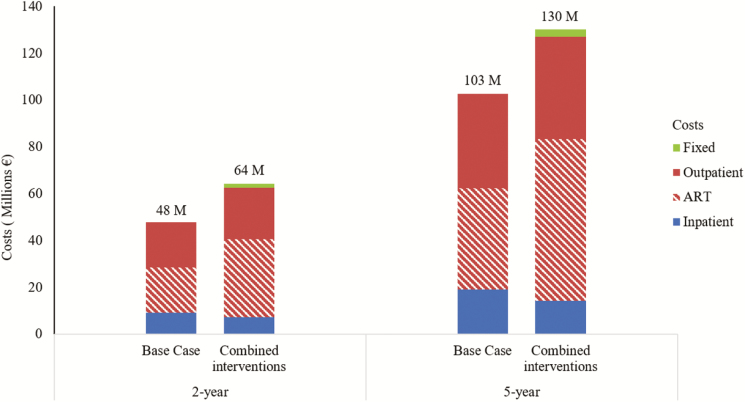
For the 270000 persons projected to be affected by the MSF intervention (24.7% are HIV-infected), the total undiscounted HIV-related costs for the combined intervention were projected to be €64 million over 2 and €130 million over 5 years (Figure 5). Cost increases were primarily driven by outpatient care costs, from €84 million in the base-case scenario to €113 million for the combined intervention at year 5 (including an increase in ART costs from €43 million to €69 million), and a decrease in costs associated with hospitalizations, from €19 million to €4 million (from 18.6% to 10.9% of the total budget).
Figure 5.
Budget impact analysis. This represents the total undiscounted human immunodeficiency virus (HIV)–related cost for the population over 2 years and 5 years under the current care and the combined intervention. HIV-related costs include inpatient costs (mainly hospitalization costs), outpatient costs (costs of consultations, prophylaxis for opportunistic diseases, CD4 cell counts, and HIV RNA laboratory monitoring in patients receiving antiretroviral therapy [ART], and ART costs), and fixed cost (for the combined intervention). Start-up costs are not represented because they are negligible.
DISCUSSION
UNAIDS has set ambitious targets for 2020, which would lead to 73% viral suppression [13]. We used data from an HIV-endemic region of Western Kenya to assess the clinical effectiveness and cost-effectiveness of interventions that aim to these targets.
Beyond the individual clinical benefits to HIV-infected individuals, VCT and VCT-plus-linkage alone did not change HIV incidence, whereas a retention intervention decreased incidence modestly. In contrast, we found that the combination of interventions targeting different stages of the HIV cascade had clear individual and population benefits. Although the absolute number of suppressed patients was higher with VCT, the proportion virologically suppressed was too low to decrease incidence. Adding a linkage to care intervention to the VCT strategy yielded a higher proportion of patients suppressed but was not sufficient to decrease HIV incidence. The retention intervention did not address testing coverage and linkage, which are among the most challenging steps in the cascade of care [5, 14, 15]. Therefore, with these strategies implemented individually, a sizeable proportion of HIV-infected persons remain untreated and contribute to HIV transmission. The combination of all 3 interventions led to the greatest decrease in HIV incidence, about 50% within 15 years. The combined intervention not only improved survival of HIV-infected patients but also averted infections and therefore yielded even more YLS.
The strategies evaluated include a wide range of interventions with plausible targets [16]. Findings from studies in Kenya and Swaziland suggest that it is possible to reach the target of 90% testing coverage of the population in a subcounty [14, 17, 18]. Although current linkage and retention rates are already high in this region of Kenya, MSF aims to further improve linkage and retention while increasing the number of patients tested. This scale-up of linkage to care efforts and ART access expansion has already been done in a similar setting in Malawi [16].
We evaluated the impact of interventions to improve each step in the care cascade. A cost-effectiveness study in South Africa on community-based HIV testing and counseling showed a decrease in incidence from 2.3/100 PY to 1.7/100 PY over 10 years, with an ICER <20% of GDP per capita [19]. Heffernan et al [20] showed that using point-of-care CD4 count testing to improve linkage to care for patients in South Africa was cost-effective, with an estimated ICER of US $4470 per disability-adjusted life-year (DALY) averted. Ying et al [21] evaluated the effect of HIV testing and counseling and expansion of ART in South Africa. They found a decrease in HIV incidence of 51.6%, with an ICER of US $1710 per quality-adjusted life-year (QALY). One study has evaluated the cost and effect of interventions at different stages of HIV care in Kenya [22]. In that model-based study, consistent with our findings, a combination of 5 interventions (including improved linkage, point-of-care CD4 cell counts, voluntary counseling and testing with point-of-care CD4 cell counts, and outreach to improve retention in pre-ART care and on-ART) led to a much larger impact, averting 1.10 million DALYs between 2010 and 2030, 25% of expected new infections, and it was cost-effective (US $571 per DALY averted).
In addition to cascade interventions, alternative strategies are also being examined to decrease incidence. A recent modeling study from Anderson et al [23] examined different prevention strategies in Kenya, mainly outside the cascade of care on targeted populations and locations. These included male circumcision, behavior change communication, early ART, and preexposure prophylaxis. Anderson et al found that a focused approach to these interventions could yield to 33% decrease in incident cases.
We showed that the combined intervention was cost-effective in the context of the Kenyan per capita GDP, although there is debate about the appropriate threshold for cost-effectiveness [6]. Although they are cost-effective, the affordability of these interventions may be challenging. When we considered the budget impact analysis, the total undiscounted additional costs of the combined intervention 2 years after the implementation of the strategy would reach 80% of the total healthcare budget planned by the Province Ministry of Health (Homa Bay County Government, Unpublished).
As with all model-based studies, this analysis is subject to limitations. We derived natural history input parameters from different African cohorts [9, 10]. Moreover, we structured the model based on assumptions including patients who become lost to follow-up (see Supplementary Material). Clinical effectiveness was assessed according to YLS. We were not able to assess cost-effectiveness in terms of QALYs, owing to the lack of adequate data. Because each QALY is less than a full year, if all health states were quality adjusted, the cost-effectiveness ratios would have been slightly increased. We also assumed a uniform cost for scaling up the testing and retention intervention. In sensitivity analysis, however, we found that getting close to the 90-90-90 targets remained cost-effective over a wide range of input parameter values. Furthermore, we did not consider second-line ART in this analysis. Consequently, our model predicts a high number of unsuppressed patients when only a single ART line is available, which is why the overall 73% suppression rate set by the UNAIDS target was not met in this analysis. Second-line treatment is currently not widely accessible to HIV-infected persons in this region; our results suggest that implementation of second-line therapy will be critical to sustain a high rate of virological suppression and to further limit the epidemic.
In conclusion, we developed a dynamic simulation model to assess the HIV epidemic in a district in rural Kenya with high HIV prevalence. Using clinical, epidemiological, and cost data, we found that the combined intervention of testing, linkage, and retention in care with extension of ART scale-up could make it feasible to achieve the UNAIDS 90-90-90 goals and be both effective at an individual and a population level and cost-effective. VCT and VCT-plus-linkage alone were not sufficient to achieve a decrease in incidence, whereas a retention intervention alone had a moderate effect. Combining these interventions will markedly decrease HIV incidence and should be pursued in rural Kenya.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 13–16 February 2017.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. L. B. L. N. had full access to all the data in the study and final responsibility for the decision to submit the manuscript for publication.
Financial support. This work was supported by Médecins Sans Frontières (MSF) France and the Harvard University Center for AIDS Research, a program funded by the National Institutes of Health (grant P30 AI060354) and supported by the following National Institutes of Health cofunding and participating institutes and centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Child Health and Human Development, National Institute of Dental and Craniofacial Research, National Heart, Lung, and Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health, National Institute on Aging, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of General Medical Science, National Institute on Minority Health and Health Disparities, Fogarty International Center, and Office of AIDS Research.
Potential conflicts of interest. S. W., A. V., J. P., W. H., and P. M. are employees of the funder (MSF). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Human Development Network, the World Bank; Institute for Health Metrics and Evaluation. The global burden of disease: generating evidence, guiding policy—sub-Saharan Africa regional edition. The World Bank, 2013. Available at: http://documents.worldbank.org/curated/en/2013/08/18187588/global-burden- disease-generating-evidence-guiding-policy-sub-saharan-africa-regional-edition. Accessed 3 February 2018. [Google Scholar]
- 2. McNairy ML, El-Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: what will it take?Clin Infect Dis 2014; 58:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Granich R, Williams B, Montaner J. Fifteen million people on antiretroviral treatment by 2015: treatment as prevention. Curr Opin HIV AIDS 2013; 8:41–9. [DOI] [PubMed] [Google Scholar]
- 4. UNAIDS. 90–90–90—An ambitious treatment target to help end the AIDS epidemic Available at: http://www.unaids.org/en/resources/documents/2014/90-90-90. Accessed 3 February 2018.
- 5. Maman D, Zeh C, Mukui I, et al. . Cascade of HIV care and population viral suppression in a high-burden region of Kenya. AIDS 2015; 29:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertram MY, Lauer JA, De Joncheere K, et al. . Cost-effectiveness thresholds: pros and cons. Bull World Health Organ 2016; 94:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garattini L, van de Vooren K. Budget impact analysis in economic evaluation: a proposal for a clearer definition. Eur J Health Econ 2011; 12:499–502. [DOI] [PubMed] [Google Scholar]
- 8. McCormick AW, Abuelezam NN, Rhode ER, et al. . Development, calibration and performance of an HIV transmission model incorporating natural history and behavioral patterns: application in South Africa. PLoS One 2014; 9:e98272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anglaret X, Minga A, Gabillard D, et al. ; ANRS 12222 Morbidity/Mortality Study Group AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis 2012; 54:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwena ZA. HIV in fishing communities: prevalence, incidence, risk factors, and interventions [CROI abstract 171]. Top Antivir Med 2016; 24:71. [Google Scholar]
- 11. Amornkul PN, Karita E, Kamali A, et al. ; IAVI Africa HIV Prevention Partnership Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS 2013; 27:2775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Bank. GDP per capita (current US$) Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 3 February 2018.
- 13. Sidibé M, Loures L, Samb B. The UNAIDS 90-90-90 target: a clear choice for ending AIDS and for sustainable health and development. J Int AIDS Soc 2016; 19:21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalal W, Feikin DR, Amolloh M, et al. . Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr 2013; 62:e47–54. [DOI] [PubMed] [Google Scholar]
- 15. Grabbe KL, Menzies N, Taegtmeyer M, et al. . Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. J Acquir Immune Defic Syndr 2010; 54:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maman D, Chilima B, Masiku C, et al. . Closer to 90-90-90: the cascade of care after 10 years of ART scale-up in rural Malawi: a population study. J Int AIDS Soc 2016; 19:20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Genberg BL, Naanyu V, Wachira J, et al. . Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. Lancet HIV 2015; 2:e20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blaizot S, Riche B, Teck R, et al. . Potential impact of implementing pre-exposure prophylaxis (PrEP) among young women in combination with scaling-up antiretroviral therapy and male circumcision on HIV incidence in Shiselweni region, Swaziland: a modelling study [IAS abstract 2646]. Abstracts from the 2017 Ninth IAS Conference on HIV science Paris, France, 2017. Available at: http:// programme.ias2017.org/Abstract/Abstract/2646. Accessed 3 February 2018. [Google Scholar]
- 19. Smith JA, Sharma M, Levin C, et al. . Cost-effectiveness of community-based strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. Lancet HIV 2015; 2:e159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heffernan A, Barber E, Thomas R, Fraser C, Pickles M, Cori A. Impact and cost-effectiveness of point-of-care CD4 testing on the HIV epidemic in South Africa. PLoS One 2016; 11:e0158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ying R, Sharma M, Celum C, et al. . Home testing and counselling to reduce HIV incidence in a generalised epidemic setting: a mathematical modelling analysis. Lancet HIV 2016; 3:e275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olney JJ, Braitstein P, Eaton JW, et al. . Evaluating strategies to improve HIV care outcomes in Kenya: a modelling study. Lancet HIV 2016; 3:e592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson SJ, Cherutich P, Kilonzo N, et al. . Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet 2014; 384:249–56. [DOI] [PubMed] [Google Scholar]
- 24. Lodi S, Phillips A, Touloumi G, et al. ; CASCADE Collaboration in EuroCoord Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 cells/mm³: assessment of need following changes in treatment guidelines. Clin Infect Dis 2011; 53:817–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.