Abstract
Management of the left subclavian artery (SCA) during aortic arch surgery is associated with several challenges, including preserving distal perfusion, achieving hemostasis and preventing posterior circulation stroke and spinal cord injury. The most common challenge remains its deep position in the chest, often exacerbated by posterior and apical displacement from an arch aneurysm. We discuss several management options consisting of pre-, intra- and post-operative strategies and their respective advantages, disadvantages and clinical outcomes. A clinical algorithm is proposed to help guide decision-making in managing the difficult left SCA during aortic arch repair.
Keywords: Aortic arch surgery, left subclavian artery (SCA), revascularization
Introduction
Background
Contemporary surgical techniques for aortic arch repair have evolved significantly (1); however, reconstruction of the left subclavian artery (SCA) still can be a difficult challenge. It often remains deep to a sternotomy-based approach, sometimes exacerbated when the left SCA ostium is displaced superiorly or posteriorly by the aneurysmal aorta. Added challenge occurs when the left SCA itself is aneurysmal, dissected, calcified, fragile or adherent from previous surgery. Circulatory arrest can be required to gain reasonable exposure for reconstruction and direct manipulation of the SCA is associated with a higher risk of injury to the recurrent laryngeal nerve (2). While coverage of the left SCA was initially popularized with thoracic endovascular stent grafting, posterior circulation stroke, spinal cord ischemia, compromised left internal thoracic artery (LITA) flow and left arm ischemia remain important concerns (3-5). In this paper, we review several options for addressing the difficult left SCA and propose a clinical algorithm for use.
General indications for left SCA revascularization
In general, we prefer to preserve the left SCA flow. In order of importance, revascularization of the left SCA might be indicated in various conditions. First, if the right SCA is absent, has diminished flow, or the patient has a dominant left vertebral artery, the left SCA is essential to ensure adequate perfusion of the vertebral arteries. Second, if the LITA has been previously used for coronary bypass, revascularization of the left SCA is warranted to avoid myocardial ischemia. Third, if a patient has a patent left axillo-femoral bypass, left SCA flow should be maintained to prevent lower limb ischemia. Fourth, if a patient is left handed, left SCA flow should be preserved as to maintain normal function of the left limb. Additionally, in the setting of patients at higher risk for spinal cord injury, such as in the case of previous descending thoracic or abdominal aortic replacement, left SCA ligation or coverage may compromise spinal cord perfusion and potentiate ischemia (3). Moreover, many of the patients presenting with aortic arch pathologies have pan-aortic disease and will often require multiple aortic procedures in their lifetime; thus, preservation of perfusion to the spinal cord is paramount in preventing catastrophic paraplegia.
The Society for Vascular Surgery has provided recommendations for the management of the left SCA in the context of endovascular repair of the thoracic aorta. These guidelines recommend pre-operative revascularization of the left SCA in various conditions, mainly when the LITA has been used in coronary artery bypass grafting (CABG), in the setting of a dominant left vertebral artery, in the presence of a left arm fistula, or when multiple intercostal arteries could be jeopardized by the coverage of a long segment of the descending thoracic aorta (≥20 cm) (6). The Society for Vascular Surgery has also commented on the management of the left SCA in the setting of endovascular repair for traumatic thoracic aortic injury. These recommendations indicate that given that the left SCA is frequently covered by an endovascular device, it should be revascularized selectively, depending on the patency of the right vertebral artery. The latter may be assessed by the pre-operative computed tomography (CT) scan or intra-operatively by angiography, and will permit the evaluation of the patency of the posterior cerebral circulation. In terms of the timing to revascularize the left SCA, this could be done either before or after the endovascular intervention (7).
General strategies
In general, the left SCA could be managed in three broad approaches, consisting of pre-operative, intra-operative, or post-operative management. Pre-operative management is performed in elective cases of aortic arch repair, and consists of either left carotid-SCA transposition, or left carotid-SCA bypass, with a proximal SCA plug/ligation. In patients with acute aortic syndromes requiring emergency surgery, intra-operative management consists of either direct anatomic reconstruction of the left SCA through an end-to-end anastomosis of the left SCA with the aortic arch graft, extra-anatomic reconstruction by bypassing the left SCA with the left axillary artery, or primary ligation. Lastly, post-operative options for the left SCA consist of intraoperative ligation with careful monitoring and potentially delayed reconstruction if necessary. We summarize the published evidence supporting these different approaches in Table 1.
Table 1. Summarized description of each revascularization technique.
| Technique | Follow-up duration | Patency rates | Advantages | Disadvantages |
|---|---|---|---|---|
| Carotid-Subclavian transposition | 31.5 months (8); 61 months (9) | 100% (8); 99% (9) | Done electively. Relatively simple procedure. Does not require a prosthetic conduit | Requires planning. Requires adequate exposure of proximal SCA. Cannot be used if previous CABG with LITA |
| Carotid-Subclavian bypass | 58 months (9) | 84% (9) | Done electively. Only requires exposing the mid SCA | Requires planning and a prosthetic conduit |
| Anatomic reconstruction | Mean of 51.3 months (10) | 100% (10) | Re-establishes the normal anatomy of the aortic arch | Technically challenging, mainly if deep SCA |
| Extra-anatomic reconstruction | Mean 27.2 months (11) | 92.6% freedom from re-operation (11) | Avoids mobilization of the proximal SCA | Requires additional reconstruction and mobilization of the axillary artery. Potential thoracic compression of the graft |
| Hybrid vascular graft | 17.8 stent-years (12) | 100% (12) | Rapid and simple deployment of the graft | Potential risk of endoleak. Additional follow-up is required for this technique |
| SCA ligation without revascularization | 16.6 months (13) | 100% freedom from re-operation (13) | Shortens surgical procedure | Requires thorough assessment of collateral circulation and monitoring of post-operative complications |
SCA, subclavian artery; CABG, coronary artery bypass grafting; LITA, left internal thoracic artery.
Pre-operative carotid-subclavian transposition or bypass with proximal ligation
These two techniques are ideal for patients undergoing elective aortic arch repair. The carotid-subclavian transposition consists of proximal division of the left SCA before the vertebral artery take-off and transposing the artery to the left carotid artery (Figure 1). This technique preserves all the branches of the left SCA with proximal ligation but can be challenging in patients with large or fragile aortic arch/descending aortic aneurysms, or those with previous distal arch surgery. The carotid-SCA bypass consists of a left carotid-SCA bypass using a prosthetic conduit and proximal ligation through the same supraclavicular incision when feasible. However, in some patients where the proximal portion of the left SCA is not safely accessible through the supraclavicular incision, an alternative to the proximal ligation of the SCA consists of the staged-coil embolization or placement of a vascular plug in the proximal SCA, in an attempt to avoid the hazard of exposing and ligating the proximal SCA (Figure 2) (3).
Figure 1.
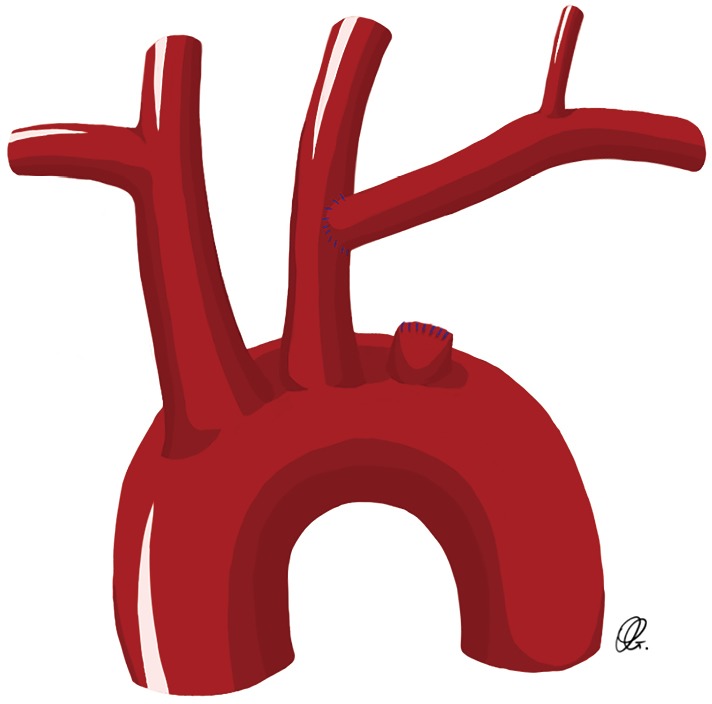
Drawing of a carotid-subclavian transposition with ligation of the proximal subclavian artery.
Figure 2.
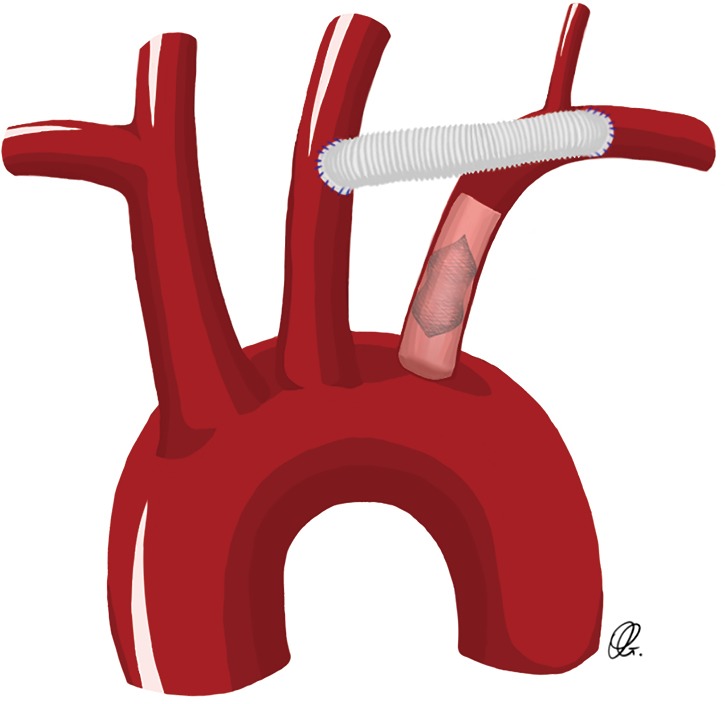
Drawing of a carotid-subclavian bypass with coil embolization of the proximal subclavian artery.
In a cohort study comparing carotid-subclavian transposition to carotid-subclavian bypass in the setting of occlusive disease of the SCA, Cinà et al. reported a 99% patency of SCA transposition at 61 months, as compared to an 84% patency of SCA bypasses at 58 months, suggesting a superiority of SCA transposition to SCA bypasses (9).
Peterson and colleagues reported their experience with 70 patients who underwent left SCA revascularization in the setting of endoluminal repair of acute and chronic thoracic aortic pathology. They concluded that both techniques of revascularization are relatively safe (14). In terms of complications, with a mean follow-up of 18 months, of the six patients who had the SCA covered with a stent without revascularization, four patients experienced strokes (mostly to the posterior circulation) and one patient developed subclavian-vertebral steal. As for the 22 patients who underwent transposition, two patients developed left-sided vocal cord palsy (15). They recommended the bypass technique to be used only in patients with previous CABG with a patent LITA conduit, because this technique avoids clamping of the left SCA proximal to the take-off of the LITA. When the bypass technique is used, control and ligation of the proximal SCA is indicated, especially when the SCA originates within the aneurysm, to prevent potential type II endoleak (14,15). A stent directly apposed on the origin of the SCA may promote proximal thrombosis and obviate proximal ligation or embolization.
In a study with longer follow-up by Canaud and colleagues looking at the outcomes of SCA revascularization with the transposition technique, excellent long-term outcomes were observed. In fact, 30-day mortality related to SCA revascularization was 0% and patency rates, at a mean follow-up of 31.5 months, were 100% (8).
A recent study by Kamman and colleagues looked at the cerebrovascular hemodynamic effects of pre-operative left SCA revascularization using either the transposition or the bypass technique. Among other findings, they reported that the antegrade flow to the left vertebral artery decreased significantly and that in contrast, the retrograde flow increased. Flows in the right vertebral artery remained the same. At a mean follow-up of 36.6±26.8 months, patency rates were 100%. Despite revascularization of the left SCA, stroke rate was 6.9%. Strokes were entirely embolic in nature, and mostly affected the posterior circulation of the brain (16).
We see that both carotid-subclavian transposition and carotid-subclavian bypass remain excellent pre-operative options to preserve the left SCA in elective cases and both yield excellent long-term results with acceptable complication risks.
Intraoperative anatomic/extra-anatomic reconstruction
In patients presenting urgently or emergently for aortic arch repair, pre-operative SCA reconstruction is often not feasible. Primary reconstruction of the left SCA with a trifurcated arch graft, a separate Dacron graft (Figure 3) or an island technique remain good options but can be technically difficult to reach. Exposure of the left SCA may be enhanced by extending the skin incision along the left neck or by transecting the innominate vein. The success of this technique remains dependent on the aortic arch anatomy and shape, and the deep location of the left SCA in the surgical field, thus increasing the difficulty of the anastomosis and potentially inducing injury to the phrenic and recurrent laryngeal nerves. Thus, different intra-operative strategies have been developed to facilitate the left SCA anastomosis (10,11,17).
Figure 3.
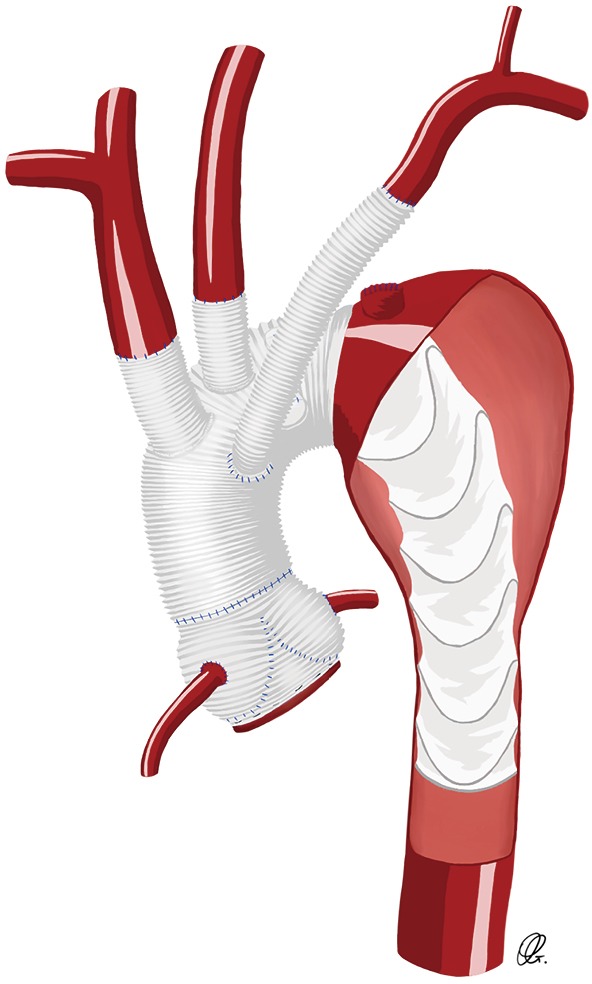
Drawing of an anatomic reconstruction of the subclavian artery using a Dacron graft between the subclavian artery and the aortic arch graft.
Liu and colleagues reported a surgical technique to skeletonize the elephant trunk in the setting of type A aortic dissection. They described an operative technique where all the aortic arch vessels, including the left SCA, are de-branched, and then re-anastomosed back to the aortic stent graft after its deployment in the native aorta. In terms of complications, in-hospital mortality was 3.3%. Three patients had to be re-operated for bleeding at the aortic stump and three patients exhibited strokes. The authors reported that in 12 patients, the left SCA was too deep, and they had to ligate it proximally and bypass it with the left axillary artery (17).
Another approach has been described by Tang and colleagues to revascularize the left SCA through a fenestration technique in the setting of type A aortic dissection. The surgical procedure involved performing an elephant trunk procedure that included the complete coverage of the left SCA with the aortic graft. If the left SCA was intact and was not involved in the dissection, a patch of the stent graft was removed just proximal to the origin of the SCA and an end-to-end anastomosis was carried between the SCA and the aortic graft. If the left SCA was involved in the dissection, it was then reconstructed using one branch of the aortic graft. In-hospital mortality was 7.8%, none of which was secondary to stroke. Left limb ischemia was 0%. Follow-up was between 12 and 145 months, with a mean of 51.3 months. Survival rate was 100% at 1 year, 90.8% at 5 years and 70.2% at 10 years. All survivors maintained a well perfused left SCA, without evidence of endovascular leak or dissection when assessed by CT scan (10).
Xiao and colleagues described a novel strategy in the setting of type A dissection to facilitate the left SCA anastomosis and to bring the distal aortic arch anastomosis more proximally, between the left common carotid artery (LCCA) and the left SCA. In their technique, the aortic arch was transected between the LCCA and the left SCA. A 4-branch vascular graft was used to reconstruct the aortic arch and to anastomose the arch branches. The left SCA was ligated and one of the branches of the vascular graft was passed through the left first intercostal space and anastomosed with the left axillary artery. With a mean follow-up of 27.2 months, survival was 96.3% and the freedom from re-operation was 92.6%. There were no cases of recurrent laryngeal nerve injury. No cases of left-arm ischemia were noted, demonstrating the effectiveness of the SCA revascularization by the left axillary artery (Figure 4) (11).
Figure 4.
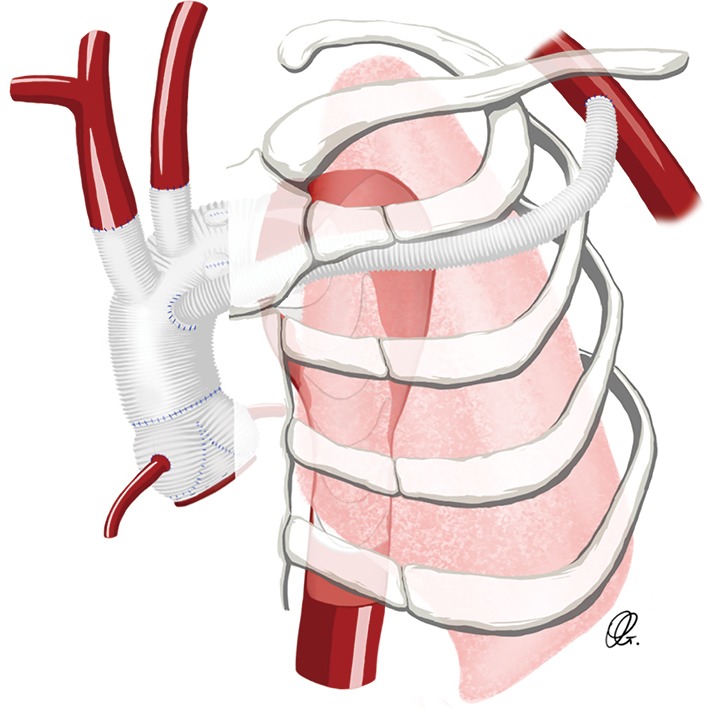
Drawing of an extra-anatomic reconstruction of the subclavian artery bypassed to the left axillary artery.
Laurin and colleagues reported their experience with the use of transthoracic aorto-axillary extra-anatomical bypass to revascularize the difficult SCA. After exposing the axillary artery using a sub-clavicular incision, an 8-mm Dacron graft originating from the aortic graft was tunnelled over the second rib and anastomosed to the axillary artery (Figure 4). A total of 77 grafts were evaluated, with 25 cases involving the right axillary artery and 52 cases involving the left axillary artery. At a mean imaging follow-up of about 3 years, graft patency was 95.3%, demonstrating the effectiveness of this technique (18).
Alternatively, rather than tunnelling between ribs, the graft fashioned to the SCA or the axillary artery can be directed over the clavicle and posterior to the sternum, into the mediastinum. Using finger dissection, a tract is developed over the clavicle, directed medially toward the sternotomy (Figure 5). The sternohyoid muscle is divided and the proximal end of the graft is delivered into the mediastinum to be attached centrally as the opportunity presents during the course of the operation.
Figure 5.
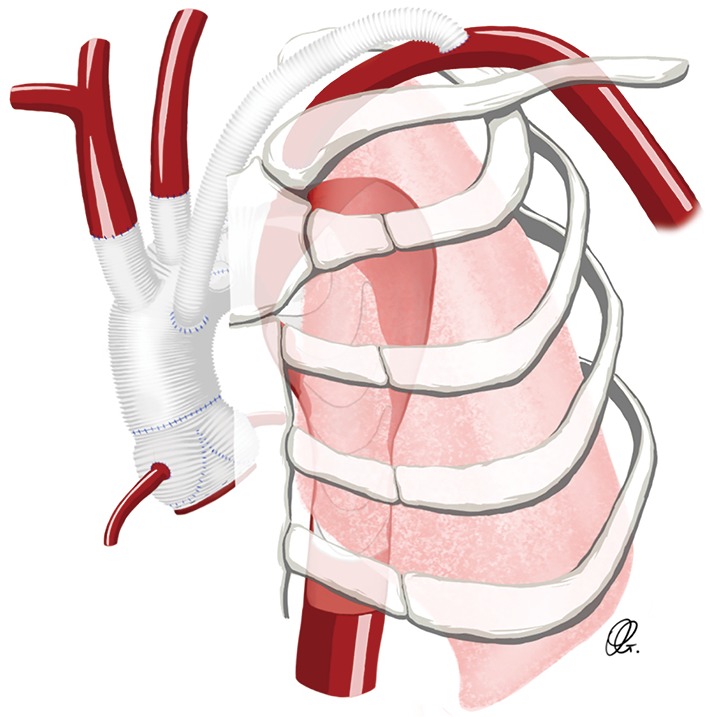
Drawing of an extra-anatomic reconstruction using a Dacron graft between the aortic arch graft and the distal left subclavian artery.
In summary, direct anastomosis of the left SCA to the aortic arch graft is often challenging due to its deep location in the surgical field, hindering the control of the proximal left SCA. Multiple techniques are available to mitigate for this challenge, including bypassing the left SCA to the left axillary artery.
Direct cannulation of the SCA ostium with hybrid vascular graft
A hybrid vascular graft technique has been recently described to facilitate the anastomosis of the aortic arch vessels—notably the left SCA when it is deep in the surgical field or calcified. This is to mitigate risk around the fragility of the branching vessels, to reduce circulatory arrest time by reducing the anastomosis time and to minimise operative bleeding (12,19,20). Pichlmaier and colleagues published their experience with the self-expanding covered stents in order to bridge, in an end-to-end fashion, the aortic graft and the supra-aortic vessels (12). The surgical technique consists of the introduction of a stent graft (VIABAHN endoprostheses; W.L. Gore & Assoc, Flagstaff, AZ, USA) in an antegrade fashion within the proximal origin of the SCA, without compromising the take-off of the vertebral artery (Figure 6). The proximal end of the stent graft is anastomosed to the aortic arch graft (12). In their series of 31 patients who underwent this hybrid technique, the left SCA was bridged in 26 patients and yielded excellent results. In fact, there was only one perioperative complication, which consisted of an over-stenting of the vertebral artery take-off. With a total follow-up of 17.8 stent-years, there was no issue with SCA occlusion, stent migration or failure, embolism or bleeding (12).
Figure 6.
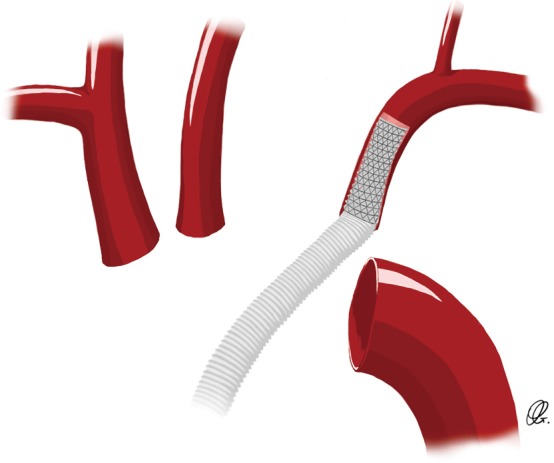
Drawing of a direct cannulation of the subclavian artery ostium with a hybrid vascular graft.
Chen and colleagues described a hybrid endovascular technique to mitigate for the challenging anastomosis of the left SCA, using a home-made single-branched stent-graft. This innovative graft is composed of two sections, one of which is the frozen elephant trunk, and the other is the sidearm stent-graft to be positioned inside of the left SCA, deployed simultaneously under transesophageal echocardiography guidance. The authors used this device in ten consecutive patients and reported relative procedural ease with deployment of the single-branched stent-graft with good hemostasis and no patients experiencing recurrent laryngeal nerve injury. All the grafts were patent when assessed by enhanced computed tomographic scan at 3 months of follow-up (20). Commercial single branch endoprostheses have emerged to enable complete endovascular zone 2 distal arch repair (21,22), which in the future, could potentially be used in a hybrid approach to facilitate easier SCA reconstruction during total arch replacement.
Intraoperative ligation of the SCA with post-operative reconstruction
Another approach used is direct ligation of the left SCA, relying upon collateral circulation for left arm perfusion. Expectant management would then determine the need for late extra-thoracic revascularization. Cui and colleagues described their experience with 28 patients that underwent selective ligation of the left SCA in total aortic arch replacement (13). In fact, when the left SCA is difficult to expose and manipulate, mainly in the setting of chronic aortic dissection that displaces the aortic arch branches deeply posteriorly, selective ligation of the left SCA was employed. To avoid post-operative complications, thorough pre-operative and intra-operative evaluation of the collateral flow was completed. In their series, they used at least one of these three criterions to determine collaterals patency: (I) pre-operative magnetic resonance imaging (MRI) of the head and neck vessels was used to assess for the completeness of the circle of Willis and for the patency of bilateral vertebral arteries; (II) in acute dissection when MRI angiography was not feasible, intra-operative evaluation was conducted by perfusing the right axillary artery, snaring of the three brachiocephalic branches and assessing the left radial artery pressure; (III) after weaning from cardiopulmonary bypass, the left SCA was temporarily clamped and oxygen saturation and waveform were assessed from the left hand. Using these assessments, the authors had excellent post-operative results. In fact, with a follow-up of 16.6 months, no patients had any symptoms of left arm ischemia or left SCA steal syndrome (13).
We see that careful evaluation of the collateral circulation of the left SCA is extremely important when considering ligation of the left SCA without further bypass. In fact, when adequate collateral circulation has been determined to be present, this technique yields good results with low risk of complications.
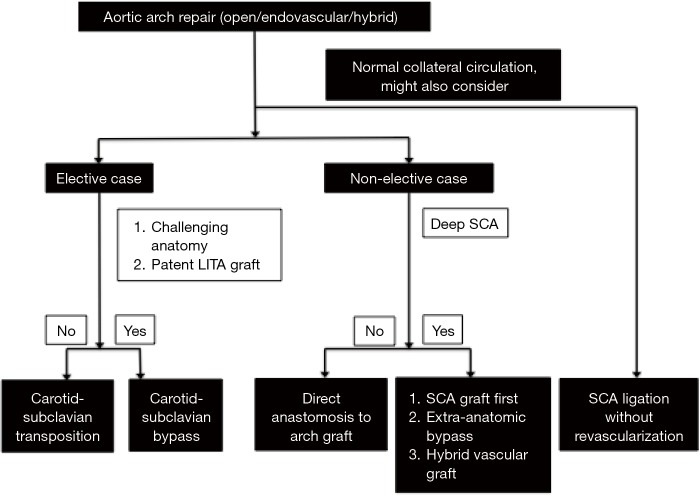
Proposed clinical algorithm
We propose a multifactorial framework to help guide decision-making in managing the difficult left SCA during aortic arch repair (Figure 7). In the setting of elective aortic arch repair, carotid-subclavian transposition appears optimal, unless the proximal subclavian is difficult to reach through the neck, in which case carotid-subclavian bypass may be more appropriate. In the setting of an urgent/emergent aortic arch repair, direct anastomosis of the left SCA to the arch graft is appropriate, if the left SCA is accessible. If the left SCA is deep in the chest, reconstruction of the left SCA using a separate SCA graft created during circulatory arrest, an extra-anatomic bypass or a hybrid vascular graft is suggested. In both settings (elective and urgent/emergent cases), direct ligation of the left SCA and relying upon collateral circulation may be considered. In this case, expectant management would then determine the need for late extra-thoracic revascularization.
Figure 7.
Approach to the selection of the left SCA revascularization technique. SCA, subclavian artery; LITA, left internal thoracic artery.
Conclusions
Aortic arch surgery requires mobilization and manipulation of the epiaortic vessels, with the left SCA often remaining the most challenging due to its deep location in the surgical field. We propose an algorithm to help with clinical decision making in choosing the optimal SCA reconstructive strategy to optimize patient outcomes. Future options may also include hybrid techniques utilizing single branched endoprostheses that obviate the need for direct surgical manipulation of difficult to reach subclavian arteries with conventional proximal arch repair.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Appoo JJ, Bozinovski J, Chu MW, et al. Canadian Cardiovascular Society/Canadian Society of Cardiac Surgeons/Canadian Society for Vascular Surgery joint position statement on open and endovascular surgery for thoracic aortic disease. Can J Cardiol 2016;32:703-13. 10.1016/j.cjca.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 2.Heinemann MK, Buehner B, Jurmann MJ, et al. Use of the “elephant trunk technique” in aortic surgery. Ann Thorac Surg 1995;60:2-6; discussion 7. 10.1016/S0003-4975(95)00319-3 [DOI] [PubMed] [Google Scholar]
- 3.Feezor RJ, Lee WA. Management of the left subclavian artery during TEVAR. Semin Vasc Surg 2009;22:159-64. 10.1053/j.semvascsurg.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 4.Waterford SD, Chou D, Bombien R, et al. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thorac Surg 2016;101:381-9. 10.1016/j.athoracsur.2015.05.138 [DOI] [PubMed] [Google Scholar]
- 5.Sobocinski J, Patterson BO, Karthikesalingam A, et al. The effect of left subclavian artery coverage in thoracic endovascular aortic repair. Ann Thorac Surg 2016;101:810-7. 10.1016/j.athoracsur.2015.08.069 [DOI] [PubMed] [Google Scholar]
- 6.Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009;50:1155-8. 10.1016/j.jvs.2009.08.090 [DOI] [PubMed] [Google Scholar]
- 7.Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg 2011;53:187-92. 10.1016/j.jvs.2010.08.027 [DOI] [PubMed] [Google Scholar]
- 8.Canaud L, Ziza V, Ozdemir BA, et al. Outcomes of left subclavian artery transposition for hybrid aortic arch debranching. Ann Vasc Surg 2017;40:94-7. 10.1016/j.avsg.2016.06.037 [DOI] [PubMed] [Google Scholar]
- 9.Cinà CS, Safar HA, Laganà A, et al. Subclavian carotid transposition and bypass grafting: consecutive cohort study and systematic review. J Vasc Surg 2002;35:422-9. 10.1067/mva.2002.120035 [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Liao Z, Han L, et al. Left Subclavian Artery Fenestration: A Novel Treatment Strategy for Acute Type A Aortic Dissection. Ann Thorac Surg 2016;101:95-9. 10.1016/j.athoracsur.2015.06.069 [DOI] [PubMed] [Google Scholar]
- 11.Xiao Z, Meng W, Zhu D, et al. Treatment strategies for left subclavian artery during total arch replacement combined with stented elephant trunk implantation. J Thorac Cardiovasc Surg 2014;147:639-43. 10.1016/j.jtcvs.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 12.Pichlmaier M, Luehr M, Rutkowski S, et al. Aortic Arch Hybrid Repair: Stent-Bridging of the Supra-Aortic Vessel Anastomoses (SAVSTEB). Ann Thorac Surg 2017;104:e463-5. 10.1016/j.athoracsur.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Lu F, Han L, et al. Selective left subclavian ligation in total aortic arch replacement. Ann Thorac Surg 2012;93:110-4. 10.1016/j.athoracsur.2011.08.032 [DOI] [PubMed] [Google Scholar]
- 14.Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433-9. 10.1016/j.jvs.2005.11.049 [DOI] [PubMed] [Google Scholar]
- 15.Morasch MD, Peterson B. Subclavian artery transposition and bypass techniques for use with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43 Suppl A:73-7A. [DOI] [PubMed]
- 16.Kamman AV, Eliason JL, Williams DM, et al. Impact of Left Subclavian Artery Revascularization before Thoracic Endovascular Aortic Repair on Postoperative Cerebrovascular Hemodynamics. Ann Vasc Surg 2018;46:307-13. 10.1016/j.avsg.2017.06.046 [DOI] [PubMed] [Google Scholar]
- 17.Liu ZG, Sun LZ, Chang Q, et al. Should the “elephant trunk” be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:107-13. 10.1016/j.jtcvs.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 18.Laurin C, Chu M, Appoo J, et al. Transthoracic aorto-axillary extra-anatomical bypass for management of difficult subclavian artery revascularization: a multicenter patency study. Can J Cardiol 2017;33:S3 10.1016/j.cjca.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levack MM, Bavaria JE, Gorman RC, et al. Rapid aortic arch debranching using the Gore hybrid vascular graft. Ann Thorac Surg 2013;95:e163-5. 10.1016/j.athoracsur.2013.01.078 [DOI] [PubMed] [Google Scholar]
- 20.Chen LW, Dai XF, Yang GF, et al. Open-branched stent graft placement makes total arch replacement easier for acute type a aortic dissection. Ann Thorac Surg 2010;89:1688-90. 10.1016/j.athoracsur.2009.07.041 [DOI] [PubMed] [Google Scholar]
- 21.Appoo JJ, Gregory AJ, Fichadiya A, et al. Zone 2 Arch Replacement and Staged Thoracic Endovascular Aortic Repair for Acute Type A Aortic Dissection. Ann Thorac Surg 2017;104:e299-301. 10.1016/j.athoracsur.2017.05.039 [DOI] [PubMed] [Google Scholar]
- 22.Patel HJ, Dake MD, Bavaria JE, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the gore thoracic branch endoprosthesis. Ann Thorac Surg 2016;102:1190-8. 10.1016/j.athoracsur.2016.03.091 [DOI] [PubMed] [Google Scholar]