Abstract
Tenascins are a family of large extracellular matrix (ECM) glycoproteins. Four family members (tenascin-C, -R, -X, and -W) have been identified to date. Each member consists of the same types of structural domains and exhibits time- and tissue-specific expression patterns, suggesting their specific roles in embryonic development and tissue remodeling. Among them, the significant involvement of tenascin-C (TNC) and tenascin-X (TNX) in the progression of vascular diseases has been examined in detail. TNC is strongly up-regulated under pathological conditions, induced by a number of inflammatory mediators and mechanical stress. TNC has diverse functions, particularly in the regulation of inflammatory responses. Recent studies suggest that TNC is involved in the pathophysiology of aneurysmal and dissecting lesions, in part by protecting the vascular wall from destructive mechanical stress. TNX is strongly expressed in vascular walls, and its distribution is often reciprocal to that of TNC. TNX is involved in the stability and maintenance of the collagen network and elastin fibers. A deficiency in TNX results in a form of Ehlers–Danlos syndrome (EDS). Although their exact roles in vascular diseases have not yet been elucidated, TNC and TNX are now being recognized as promising biomarkers for diagnosis and risk stratification of vascular diseases.
Keywords: tenascin-C, tenascin-X, inflammation, aortic aneurysm, aortic dissection
Introduction
Tenascins are a family of large extracellular matrix (ECM) glycoproteins. There are four tenascins in vertebrates: tenascin-C (TNC), tenascin-R (TNR), tenascin-X (TNX) (known as tenascin-Y in birds), and tenascin-W (TNW, also tenascin-N).1,2) Each of the four tenascin family members consists of the same types of structural domains, including a cysteine-rich segment at the amino terminus, epidermal growth factor (EGF)-like repeats, fibronectin type III (FNIII)-like repeats, and a fibrinogen (FG)-like domain at the carboxyl terminus.
TNC is the original “tenascin.” It was discovered independently by several laboratories, given different names, and was shown to be highly expressed in tendons, embryos, and cancer stroma. TNR is the second member and is predominantly expressed in the central and peripheral nervous systems. TNW is primarily found in pre-osteogenic areas, the periosteum, kidney, smooth muscle, and most prominently, in cancer stroma. TNX is expressed in loose connective tissue such as the dermis, epimysium, and blood vessels.
Tenascin family members exhibit time- and tissue-specific expression patterns in the embryo and adult and have been categorized as “matricellular proteins,” which are unique ECM proteins.3,4) Although each matricellular protein is unrelated based on the primary structure, they share a number of characteristics: 1) secreted by diverse types of cells, 2) associated with, but not necessarily a part of the insoluble/fibrillar ECM, 3) counter-adhesive for cells under various conditions, 4) prevalent in areas of tissue remodeling associated with normal and pathological processes, and 5) featured prominently in embryogenesis.5)
Tenascin-C is an original matricellular protein in addition to thrombospondin-1 (TSP-1) and SPARC.3,6) TNX, TSP2, osteopontin, CCN1, and CTGF (CCN2) were subsequently added.7) The term “matricellular protein” is becoming more widely used and new members, such as galectins, periostin, and fibulin-5, have joined this group.5,8)
An increasing number of reviews have described the roles of tenascin family members, particularly TNC, in cancer invasion and neurological and inflammatory diseases.9–18) Therefore, we herein focus on the roles of TNC and TNX in development, homeostasis, and aortic diseases.
TNC
TNC is a large molecule of approximately 220–400 kDa as an intact monomer and assembles as a hexamer. It exists in a number of different isoforms that are created by the alternative splicing of FNIII-like repeats and post-translational modifications through glycosylation and citrullination (reviewed in refs. 16, 18).
One of the characteristics of TNC is its spatiotemporally and tightly regulated expression. TNC is highly and transiently expressed in embryos at restricted sites often associated with cell migration, epithelial-mesenchymal transition (EMT)/mesenchymal-epithelial transition (MET). It is sparsely expressed in normal adults, except in a few tissues that bear high mechanical stress are the locations of high cell turnover and are a specific niche for immune cells.
However, TNC is strongly up-regulated during injury, inflammation, repair/regeneration, and cancer invasion (reviewed in refs. 14, 18). Conversely, the prominent de novo expression of TNC in adults is a hallmark of injury and inflammation, which makes TNC applicable to clinical diagnoses. An increasing number of studies have reported the utility of the serum level of TNC as a biomarker for assessing disease activity and predicting the prognosis of patients, for example, with myocardial diseases (reviewed by refs. 19–21), aortic diseases22,23), and coronary aneurysms in Kawasaki disease.24) Furthermore, in vivo inflammatory lesions in the cardiovascular system have been successfully imaged using Fab’ or the single chain Fv fragment of a monoclonal anti-TNC antibody.25–29)
Regulation of TNC expression
The specific expression of TNC suggests the existence of an accurately tuned regulatory system. The promoter of the TNC gene (Tnc) contains a TAT A box and a number of signaling pathways and transcription factors, such as Prx1, Notch, and Sox4, might contribute to specific expression in embryonic development (reviewed in refs. 16, 18). Furthermore, a number of cytokines, growth factors, inflammatory mediators, angiotensin II, endothelin I, and signaling cascades, including transforming growth factor (TGF)/Smad 3/4, TLR4/NFκB, c-Jun/NFκB, platelet-derived growth factor (PDGF)/phosphoinositide 3-kinase/Akt, and PDGF/MAPK, are involved in the control of TNC expression during inflammatory/repair responses after injury.
Among the multiple regulatory mechanisms of expression, it is important to note that mechanical stress is a strong inducer of TNC.30,31) TNC is known to be highly expressed at sites subjected to mechanical stress such as myotendinous and osteotendinous junctions as well as the branching points of arteries.32,33) Chiquet and co-workers reported a mechanism by which a mechano-signal up-regulates TNC expression.34) Mechanical stimuli activate the RhoA/ROCK signaling pathway via integrin β1,35) which, in turn, induces the assembly of G-actin and stress fiber formation. The depletion of the cytoplasmic G-actin pool results in the translocation of megakaryoblastic leukemia 1 (MKL1)/myocardin-related transcription factor A (MRTFA) into the nucleus, which is predominantly localized in the cytoplasm through interaction with G-actin. MLK1/MRTFA induces TNC transcription partly as a co-activator of serum response factor (SRF).36,37) This RhoA-dependent mechanotransduction requires pericellular fibronectin.38) TNC binds fibronectin and negatively affects the activation of RhoA.39) TNC also affects tissue stiffness, modeling the mechanical effects of its microenvironment.40,41) Moreover, TNC itself is an elastic molecule that may be stretched to several-fold its original size.42,43) Therefore, TNC may modulate tissue elasticity and stiffness and may protect cells against mechanical stress.
Function of TNC
TNC directly affects cell behavior by binding to various cell surface receptors including integrins, which are their main receptor.15,44) TNC also indirectly acts by binding to other ECM molecules such as fibronectin or proteoglycans (reviewed in refs. 16, 18). In vitro, TNC might be effective in cell migration, inhibiting focal adhesion assembly, promoting proliferation, playing an inductive or protective role in apoptosis, and changing gene expression in a manner that is dependent on the cellular context. In addition to many other “important genes,” TNC knockout (TNC KO) mice show an almost normal phenotype,45) which initially suggested that TNC was a superfluous molecule. However, subsequent investigations on various disease models using TNC KO demonstrated the significant roles of TNC, particularly in inflammation, fibrosis, and tissue repair.
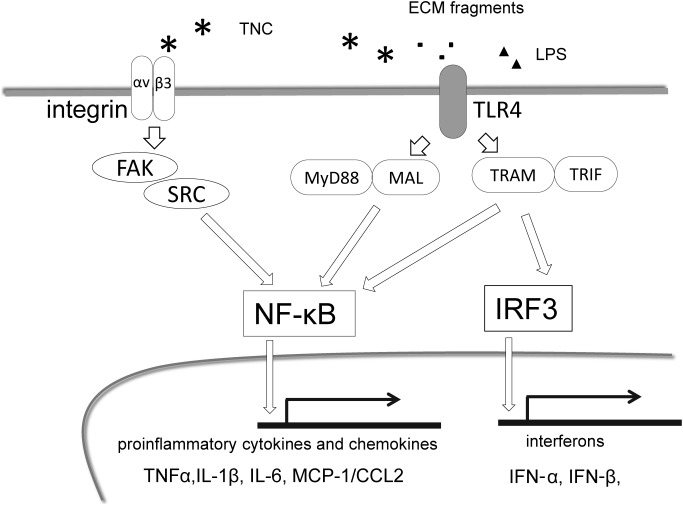
Accumulating evidence has highlighted the promotion of inflammation by TNC through its induction of innate immunity and up-regulation of proinflammatory cytokines, chemokines, and proteases via many mechanisms.14) The most studied cascade is TNC activating Toll-like receptor 4 (TLR4) as a damage-associated molecular pattern (DAMP) in macrophages, fibroblasts, chondrocytes, and neutrophils,11,46,47) thereby inducing two downstream signaling pathways: the NFκB pathway and interferon regulatory factor (IRF) pathway (Fig. 1). TLR4 activation, coupled with the signaling adaptor, myeloid differentiation factor 88 (MyD88) adaptor-like protein (MAL), results in the nuclear translocation of NFκB and translation of proinflammatory cytokines. MyD88-independent signaling activates NFκB and IRF, inducing interferon (IFN) synthesis.47) TNC also stimulates inflammatory cells via several integrins. It stimulates the migration and proinflammatory cytokine synthesis of macrophages through integrin αvβ3/ FAK/Src/NFκB48) and also activates macrophages, dendritic cells, and fibroblasts via α9-integrin.49,50) Furthermore, TNC may bind and interact with a range of growth factors including fibroblast growth factor (FGF), PDGF, and TGF-β,51) forming a positive feedback loop to augment inflammation. In support of this proinflammatory effect, in vivo studies demonstrated that a TNC deficiency attenuated experimental inflammatory diseases such as rheumatic arthritis,49,50) hepatitis,52) myocarditis,53) and angiotensin II-induced myocardial fibrosis.48)
Fig. 1 Major proinflammatory signaling pathways by TNC. Activated TLR4 by TNC, ECM fragments as well as LPS induce two downstream signaling pathways. Nuclear translocation of NFκB and translation of proinflammatory cytokines/chemokines are induced mediated by MyD88/MAL. MyD88-independent signaling activates NFκB and IRF, inducing interferon synthesis. TNC also stimulates inflammatory cells through integrin αvβ3/FAK/Src/NFκB. MAL: myeloid differentiation factor 88 (MyD88) adaptor-like protein; TRAM: TLR-4 signaling adaptor; TRIF: Toll/IL-IR domain-containing adaptor-inducing IFN-β; IRF: Interferon regulatory factor.
In contrast, aggravated inflammation in dermatitis,54) nephritis,55) and pressure-overloaded hearts56) have also been reported in TNC KO mice. TNC promotes T-lymphocyte recruitment, activation, and polarization, whereas it inhibits the proliferation and transmigration of T cells.57) TNC appears to play pro- and anti-inflammatory roles in a context-dependent manner, and thus, may orchestrate immune responses that protect the living body from the dangers posed by tissue injury and pathogen invasion.
TNC in the aorta
In the aorta, TNC is highly expressed during embryonic development, weakly expressed in normal adults, and its expression is up-regulated under pathological conditions as well as in other tissues. However, its expression pattern is slightly complex. In mouse embryos, the expression of TNC in the wall of the ascending aorta becomes detectable by embryonic day (ED) 12–13; its expression is up-regulated around ED 14–15 when the systemic circulatory system is established, and its expression is stronger after birth.30) These changes in expression might reflect increased hemodynamic stress on the aortic wall. In adult mice, the constitutive expression of TNC is observed in the abdominal aorta but not in the thoracic aorta.58) TNC-producing cells of the abdominal aorta are medial smooth muscle cells as well as during embryonic development. Since the origin of smooth muscle cells of the aorta is heterogeneous (reviewed in ref. 59), the characteristics of cells of the thoracic aorta and abdominal aorta may differ. However, the distribution of TNC in the aortic wall is distinct in the mouse but ambiguous in humans (our unpublished data).
TNC and atherosclerosis
Atherosclerosis underlies the pathogenesis of vascular diseases including those of the aorta.
Atherosclerosis is an inflammatory disease that begins in the intima of large- and medium-sized arteries and is caused by endothelial injury, hemodynamic disturbances, or the accumulation of low-density lipoproteins. Atherosclerotic plaques involve intimal thickening (neointima) and liquid accumulation, which are formed by proliferation and migration into the intima and ECM synthesis by vascular smooth muscle cells and accompanied by the infiltration of a wide range of inflammatory cells.60)
Previous studies reported that the deletion of TNC exacerbated the atherosclerotic intimal lesions of TNC in Apo-E-deficient mice,61,62) whereas neointimal hyperplasia was found to be reduced in a vascular injury model and artery graft model in TNC KO mice.63,64) Therefore, TNC might have harmful and protective effects on neointimal lesions.
TNC and abdominal aortic aneurysms
Abdominal aortic aneurysms (AAA) are a common and often fatal disease in elderly men. The central pathogenesis of AAA is considered to be chronic inflammation of the medial to adventitial layers of the aortic wall on the background of atherosclerosis.60,65) Inflammation and various up-regulated proteinases including elastin-degrading enzymes possibly induced by hemodynamic stress and an over-activated renin-angiotensin-aldosterone system trigger the fragmentation of elastic fibers. The fragment products of elastin and other ECM recruit more inflammatory cells to the aortic wall, causing an innate immune response that attempts to resolve damaged tissue followed by adaptive immune responses.
These inflammatory responses are normally controlled with an acute phase and subsequent resolution; however, poorly regulated and ongoing inflammation causes progressive damage to and vulnerability in the aortic wall, resulting in the progression of aneurysms.
The histopathological findings of AAA lesions are characterized by the destruction and fragmentation of elastic fibers and apoptosis of smooth muscle cells associated with chronic inflammatory cell infiltration. However, these pathological changes are not distributed homogeneously throughout the aneurysmal wall.65,66) In the longitudinal strip of a human aortic aneurysm, the region with a normal diameter shows some intimal thickening but also well-preserved structures of the elastic lamella and smooth muscle layers with few inflammatory cells. The transitional region from the normal diameter area to the dilated area exhibits straightened or fragmented elastic fibers, a mild decrease in smooth muscle cells, and the infiltration of macrophages. The maximally dilated region shows the marked loss of smooth muscle cells and elastic fibers replaced by collagen fibers. The strong expression of TNC is associated with inflammation and tissue destruction,67–69) mainly in the transitional/inflammatory region.70) In a CaCl2-induced mouse AAA model, the expression level of TNC was found to correlate with the expansion rate of the AAA diameter and destruction of the aortic wall.70) These findings suggest that TNC reflects inflammation and disease activity; however, the exact role of TNC in the progression of AAA remains unclear.
TNC and acute aortic dissection
Acute aortic dissection (AAD) is another major aortic disease.57) AAD starts with tearing of the intimal layer, and this is followed by tearing of the medial wall due to blood flow into the pseudolumen formed between the inner and outer layers of the medial layer of the aorta.
The pathogenesis of AAD is a multifactorial process. In addition to aortic stiffness and hypertension, several genetic disorders including Marfan syndrome (MFS), Loeys–Dietz syndrome (LDS), and vascular Ehlers–Danlos syndrome (EDS) are known to predispose individuals to the development of AAD. MFS is a connective tissue disease caused by mutations in FBN1 encoding fibrillin-1.71) Fibrillin-1 molecules assemble into microfibrils, which are a physically supporting structure and play an important role in the modulation of TGF-β signaling.72)
The identification of mutations in the TGF-β receptors 1 and 2 (TGFBR1/2) gene in patients with LDS, a Marfan-related syndrome, implicated the involvement of excessive TGF-β signaling in the development of AAD73); however, the molecular mechanisms responsible for tissue destruction remain largely unknown.
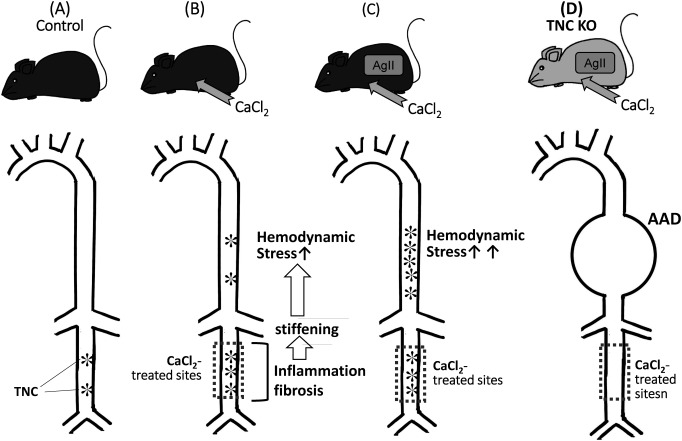
We recently reported a protective role for TNC in the progression of AAD.58) As discussed above, TNC is constitutively expressed in the abdominal aorta of normal adult mice. The biomechanically measured flexibility of the abdominal aorta of TNC KO mice was found to be significantly less than that of wild-type (WT) mice; however, TNC KO mice did not show a distinct phenotype in the normal state.
When we applied CaCl2 to the infrarenal aorta of a mouse, it caused inflammation that induced the destruction of the elastic lamina and periaortic fibrosis (Fig. 2). As a result, stiffness in treated sites increased, which, in turn, enhanced hemodynamic stress on the suprarenal aorta. The infusion of angiotensin II into this mouse model increased blood pressure and strongly augmented hemodynamic stress in the upper aorta, which resulted in the expression of TNC being strongly up-regulated. When we applied this strong hemodynamic stress to the TNC KO mouse using a combination of a CaCl2 treatment and AgII infusion, it caused marked enlargement of the upper aorta, in which TNC was highly up-regulated in the WT mouse, and this was associated with the disruption of the elastic lamella of the vascular wall.
Fig. 2 A protective role of TNC in the aorta from destructive mechanical stress. (A) TNC is constitutively expressed in the abdominal aorta of normal adult mice. (B) Application of CaCl2 to the infrarenal aorta causes inflammation and fibrosis, resulting in stiffness in the treated sites and enhancement of hemodynamic stress on the suprarenal aorta, which induces TNC expression. (C) Infusion of angiotensin II into (B) increases blood pressure and augments hemodynamic stress in the upper aorta, which strongly up-regulates TNC. (D) Application of the strong hemodynamic stress using a combination of a CaCl2 treatment and AgII infusion to the TNC KO causes AAD.
Transcriptome analysis of the suprarenal aorta of TNC KO mice before the development of AAD revealed the enhanced induction of proinflammatory genes and impaired induction of ECM protein genes over that in the WT mouse. Correspondingly, the TNC KO aorta showed greater infiltration of CD45-positive inflammatory cells and stronger activation of NFκB and STAT3 but reduced activation of the downstream molecule of TGF-β signaling, SMAD2.
One possible explanation is that TNC induced by mechanical stress may protect tissue from destructive mechanical stress, thus reducing the inflammatory reaction evoked by tissue injury. In vitro, exogenous TNC suppressed proinflammatory gene expression by smooth muscle cells from the thoracic aorta elicited by TNFα and partially rescued the down-regulated expression of the collagen gene family by TNFα. These findings suggest that TNC promotes matrix synthesis as a repair response to tissue injury and suppresses the inflammatory responses of vascular smooth muscle cells, which is in contrast to its proinflammatory effects exhibited in most settings. Although the impact of inflammatory responses under pathological conditions on injury is often emphasized, inflammation is also a necessary response for the restoration of the tissue architecture. TNC may delicately adjust inflammatory reactions during tissue injury and repair depending on the context.
TNC may have many other roles in the progression of AAD such as protecting smooth muscle cells from apoptosis.74) The abnormal expression of TNC has been reported in the aneurysm tissue of patients with MFS associated with smooth muscle cell apoptosis.75)
This functional diversity may reconcile complex clinical data showing that a high serum TNC level on admission is a predictor of high mortality,23) whereas a high serum TNC level on hospital day 7 may be a predictive marker for a low risk of enlargement of the aortic lesions during the chronic stage.22)
TNX
TNX is the largest member of the tenascin family. It was identified as one of the causative genes of EDS, a clinically and genetically heterogeneous connective tissue disease.76) Approximately 1 in 5,000 individuals are affected. EDS has been classified into six subtypes based on major and minor diagnostic criteria.77) A complete deficiency of TNX is associated with a recessive form of the classical type of EDS,78) whereas TNX haploinsufficiency leads to the hypermobility type of EDS,79,80) which is a major clinical feature of EDS. Thus, a TNX deficiency is the first example of a causative mutation for the classic type of EDS caused by factors other than fibrillar collagen or collagen-modifying enzymes.
Discovery of TNX
The human TNX gene (TNXB) was initially identified as an unknown gene termed gene X in 3′overlapping the opposite strand of the steroid 21-hydroxylase gene (CYP21A2, formerly CYP21B), a steroidogenic enzyme that is crucial for the synthesis of cortisol and mineralocorticoids in the class III region of the major histocompatibility complex (MHC).81) Extensive genomic analysis of an upstream portion of gene X identified a cluster of FNIII-like repeats that are highly homologous to those of TNC.82) Complete human and mouse genes were subsequently reported.83,84) Bovine TNX was also discovered as a new ECM glycoprotein that localized with fibril-associated collagens such as type XII and XIV collagens and was initially termed flexilin.85) Avian tenascin-Y (TNY) is an orthologous protein of TNX.86) Trimers are the main unit of the assembly of TNX.87)
Tissue expression of TNX
TNX is ubiquitously expressed in most tissues and is strongly detected in the heart, skin, and skeletal muscle.88) TNX is also frequently reported to be associated with blood vessels in all of the tissues that have been analyzed. In cardiac tissue, TNX localizes along muscle cells with a typical pattern of ECM glycoproteins.89) During the late stages of embryonic heart development, TNX expression starts in migrating cells of the epicardium and later in association with developing sub-epicardial blood vessels, and then by a non-myocyte, possibly interstitial fibroblasts.90,91) TNX is also strongly expressed in peripheral nerves, for example, in the perineurium and endoneurium of sciatic nerves.92,93)
Under physiological conditions, the distribution of TNX is distinct and often reciprocal to that of TNC in the skin and the digestive tract, particularly the esophagus, gut, and stomach.88) Under pathological conditions such as during tumorigenesis, TNX expression is opposite rather than reciprocal to that of TNC.94,95) In xenographed experiments on tumor cells, TNC was found to be prominent in the host fibrous stroma, which is adjacent to the tumor nest composed of transplanted tumor cells. In contrast, TNX expression was shown to be down-regulated in transplanted tumor cells and the host fibrous stroma. Another study showed that the expression of TNX negatively correlated with the grade of astrocytomas, being lower in the perivascular stroma around tumor vessels in high-grade astrocytomas than in low-grade astrocytomas. In contrast to the expression of TNX, TNC was more strongly expressed in intercellular spaces in high-grade astrocytomas than in low-grade astrocytomas.96)
In in vitro analyses, the expression of TNX is significantly reduced in fibroblasts by glucocorticoids, similar to that of TNC; however, its expression is not affected by growth factors such as TGF-β, EGF, TGF-α, PDGF, or basic FGF (bFGF), which have been shown to alter the expression level of TNC.94) Brain-derived neurotrophic factor (BDNF) induces the expression of TNX as well as that of vascular endothelial growth factor-B (VEGF-B)97) because TNX interacts with VEGF-B, and collectively, they enhance endothelial proliferation.98) Furthermore, short peptides derived from TNX show proangiogenic properties.99)
Limited information is currently available on the transcription factors of TNX expression. However, a previous study demonstrated that the ubiquitously expressed transcription factor Sp1 was involved in the expression of TNX.100)
Cell surface receptors for TNX
Evidence for TNX possibly interacting with integrin came from initial reports on a bovine TNX sequence.89) FNIII-like repeat 10 in bovine TNX has the integrin-binding tripeptide motif RGD. Elefteriou et al.101) showed that osteosarcoma (MG63) and bladder carcinoma (ECV304) cells attached the FNIII-like repeats 9 and 10 but not FNIII-like repeat 10 itself via αvβ3 integrin. They also found that MG63 cells adhere their FG-like domain via β1-containing integrin. Alcaraz et al.102) reported that fibrosarcoma (HT-1080) cells adhere to the FG-like domain in bovine TNX via α11β1 integrin, which is required for the activation of latent TGF-β.
Function of TNX
Several lines of evidence support TNX regulating the ECM architecture and tissue integrity. Flexilin (bovine TNX) was identified as a collagen fibril-associated protein and localizes at the surface of and between collagen fibrils.85) A solid-phase interaction assay demonstrated that TNX interacted with types I, III, and V fibrillar collagens, types XII and XIV fibril-associated collagens,103) and decorin.104) In contrast, TNX very weakly binds to laminin and does not bind to fibronectin.103,105) The interfibrillar distance of collagen fibrils was found to be increased in the TNX-deficient skin of an EDS patient.106) Other studies suggested that TNX is involved in collagen fibrillogenesis and increases both the rate and extent of fibril formation in vitro.105,107) Further analyses showed that the presence of TNX in collagen gels in vitro augmented collagen gel stiffness. This finding indicates that TNX participates in the mechanical properties of the extracellular environment.108) TNX-deficient mice exhibit several features, similar to patients with EDS, such as progressive skin hyperextensibility.109) The density of collagen fibrils in the skin of TNX-deficient mice was shown to be significantly reduced. Fibroblasts derived from TNX-deficient mice failed to deposit type I collagen around their cells. These findings indicated that TNX is involved in collagen synthesis or deposition.
Apart from the role of TNX as a regulator of the collagenous network in ECM, it is also involved in the stability and maintenance of elastin fibers. Patients with TNX-deficient EDS showed fragmented immature elastic fibers with irregular shapes in the skin dermis.110,111)
Similar to TNC with antiadhesive properties that promote the detachment of cells, TNX also has antiadhesive properties. The antiadhesive properties of TNX are mediated by the activation of p38 mitogen-activated protein (MAP) kinase.112) TNX-deficient mice showed greater tumor invasion and the metastasis of inoculated melanoma cells than WT mice.113) Matrix metalloproteinase (MMP)-2 and MMP-9 activities were increased in TNX-deficient mice in vivo. Increased MMP activities may promote tumor invasion and metastasis in TNX-deficient mice. Furthermore, since TNX exhibits antiadhesive activity, the adhesion of cancer cells in a metastatic focus may be facilitated by a TNX deficiency.
Alcaraz et al.102) showed that the FG-like domain of TNX interacts with the small latent TGF-β in vivo and in vitro and activates the latent form of TGF-β into the mature form, leading to EMT in epithelial cells.
EDS caused by a TNX deficiency and haploinsufficiency
EDS is a heterogeneous group of connective tissue diseases that are characterized by skin hyperextensibility, joint hypermobility, and tissue fragility.114) Most forms of EDS result from mutations in one of the genes encoding fibrillar collagen or collagen-processing enzymes. However, a complete deficiency of TNX results in an autosomal recessive form of EDS.78) Patients with a complete deficiency of TNX have marked joint hypermobility, skin hyperextensibility, and easy bruising without atrophic scars or poor wound healing, which distinguish the TNX-deficient type from another classical type of EDS caused by defects in type I and V collagen. Since TNX is weakly expressed in the early phases of wound healing87) and is not involved in matrix deposition, the lack of atrophic scars indicates that TNX is involved in the later phases at which matrix assembly and maturation occur.115) Previous studies reported a high prevalence of cardiovascular abnormalities in patients with EDS, particularly in the vascular type of EDS.116) Among all known EDS subtypes, the vascular type of EDS has the most severe clinical findings, including the rupture of arteries, excessive bruising, a characteristic facial appearance, and thin and translucent skin, but without skin hyperextensibility.114)
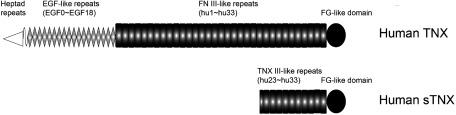
Regarding the clinical findings of TNX-deficient EDS in cardiovascular diseases, a previous study investigated cardiac abnormalities in seven patients with TNX-deficient EDS.117) Two patients from the same family showed slight billowing of the mitral valve and another patient had mitral valve prolapse. Although the serum form of TNX is present with 140 kDa (Fig. 3), which is detected at a molecular weight of 140 kDa with the C-terminal part (10.5 fibronectin type III-like repeats (hu23–hu33) and a C-terminal fibrinogen-like domain) of full-length TNX,84,118) coagulation properties were not impaired in these patients. Although the patient group is small, these findings indicate that severe cardiovascular impairment is not present in TNX-deficient patients.
Fig. 3 Structure of human TNX and serum form of TNX (sTNX). Human TNX consists of a cysteine-rich segment (heptad repeats) at the amino terminus, 18.5 EGF-like repeats (EGF0–EGF18), 33 FNIII-like repeats (hu1–hu33), and a FG-like domain at the carboxyl terminus. Human sTNX consists of the C-terminal part of full-length TNX, namely, 10.5 FNIII-like repeats (hu23–hu33) and a FG-like domain.
On the other hand, the clinical findings of the hypermobility type of EDS are hyperextensible smooth and velvety skin, joint hypermobility, recurring joint dislocations, tissue fragility, and chronic pain. The hypermobility type of EDS shows similar clinical features to joint hypermobility syndrome (JHMS), which is also called benign joint hypermobility syndrome (BJHS).119) The causative genes of the hypermobility type of EDS had not been identified for a long time. However, Zweers et al.79,80) showed that TNX haploinsufficiency was associated with the hypermobility type of EDS. The incidence of TNX haploinsufficiency in patients diagnosed with the hypermobility type of EDS was 7.5%, and all patients were female.79) Patients with the TNX-haploinsufficient hypermobility type of EDS had hypermobile joints, joint subluxations, and chronic musculoskeletal pain.79) However, skin abnormalities such as hyperextensibility were less severe than in those with the TNX-deficient type of EDS.114) Furthermore, patients with the TNX-haploinsufficient hypermobility type of EDS lack the easy bruising observed in patients with the TNX-deficient type of EDS.79) Cardiovascular anomalies have not yet been reported in patients with the TNX-haploinsufficient hypermobility type of EDS. However, Atzinger et al.120) demonstrated that although there is an increased risk of aortic dilation and mitral valve prolapse in patients with the classical and hypermobility types of EDS, they are generally not severe.
Furthermore, CAH-X syndrome patients with TNX-haploinsufficient EDS and congenital adrenal hyperplasia (CAH) due to a 21-hydroxylase deficiency not only had EDS clinical features such as joint hypermobility and chronic joint pain but also structural cardiovascular abnormalities including a cardiac valve abnormality and chamber enlargement.121,122)
Petersen and Douglas123) hypothesized that a deficiency in or haploinsufficiency of TNX reduced risk factors for adverse cardiovascular events by the laxity of arterial stiffness. However, this hypothesis does not appear to be supported by existing data.122)
TNX in cardiovascular disease
TNX is strongly expressed in aortas and is present throughout the entire vascular wall, colocalizing with elastic lamellae in the aortic media.124) However, immunofluorescent staining showed that the expression of TNX strongly decreased in AAA tissues without an association with elastin.124) In contrast, AAA is associated with a high level of serum TNX (sTNX).124) Furthermore, Zweers et al.124) suggested a relationship between sTNX levels and peripheral vascular disease but not between sTNX levels and cerebrovascular or coronary artery disease. These findings suggest that systemically increased TNX expression or the increased breakdown of TNX in AAA tissues may occur.
Decreased levels of TNX in calcific aortic valves
Calcific aortic valves (CAVs) result in valvular aortic stenosis, which leads to heart failure and death. The process of calcification of the aortic valves is similar to that of bone formation in valves.4) There were several significant advances related to our understanding of the basic mechanisms of dystrophic vascular calcification. The identification of potential biomarkers associated with the development and progression of CAVs is important for its diagnosis and risk stratification. Therefore, we comprehensively identified proteins in CAVs that were differentially expressed from those in adjacent normal valvular (NV) tissues using a quantitative proteomic approach with the isobaric tag for relative and absolute quantitation (iTRAQ) labeling followed by nano-liquid chromatography (nano-LC)-matrix-assisted laser desorption ionization (MALDI)-time of flight (TOF/TOF)-tandem MS (MS/MS).125) Consequently, we identified 105 proteins that were differentially expressed in CAVs than in adjacent NV tissues. Among them, fetuin-A (α-2-HS-glycoprotein), a potent calcification inhibitor,126) showed maximum increase in expression (6.54-fold).125) This may have been due to the feedback mechanism of increased fetuin-A to prevent further ectopic calcification in the aortic valves. On the other hand, we identified TNX as the protein showing the greatest decrease in its expression (0.37-fold) in CAVs from that in adjacent NV tissues.125) Furthermore, the expression of many ECM proteins that are important for the modulation of the collagen structure and deposition, such as type I collagen, mimecan, decorin, fibromodulin, and type VI collagen, was also weaker in CAVs than in NV tissues. These findings indicate that the large-scale destruction of ECM involved in the modulation of collagen fibrils occurs during the onset of valvular calcification.
Quantification of sTNX by nano-LC/MS/MS
The quantification of sTNX is necessary for the diagnosis and risk stratification of EDS caused by TNX deficiency and haploinsufficiency. Furthermore, it is of interest to measure sTNX in patients with cardiovascular diseases such as AAA and aortic valve sclerosis and stenosis. Therefore, we attempted to develop a method for the quantification of sTNX using nano-LC/MS/MS with selected/multiple reaction monitoring (SRM/MRM).127) We initially selected the candidate peptide, AVAVSGLDPAR, which is located in the 26th fibronectin type III-like repeat (hu26) in sTNX,84) for the quantification of sTNX concentrations in silico using Pinpoint software. The limit of quantification (LOQ) of sTNX by the nano-LC/MS/MS method was 2.8 pg. Using this analysis, the sTNX concentration in the sera of healthy controls was found to be 144 ng/ml.127)
Conclusion
TNC and TNX may play significant roles in the pathophysiology of cardiovascular diseases such as aortic aneurysms and dissection.
Many have shown that various inflammatory mediators and mechanical stress induce TNC expression and also that TNC has diverse functions that regulate cell behavior, particularly inflammatory responses in tissue repair after injury. Since TNC may exert harmful and beneficial effects in a context-dependent manner, understanding the clinical significance of TNC may not be straightforward. Few studies have documented mutations in TNC genes in human patients with cardiovascular disease. Carefully designed experiments using TNC KO mice will provide insights for understanding its significant role in vascular diseases.
In contrast, a complete TNX deficiency results in EDS; however, patients with TNX-deficient or TNX-haploinsufficient EDS do not show severe cardiovascular impairment. The exact roles of cardiovascular abnormalities in many of these patients need to be elucidated in more detail.
Furthermore, in view of practical clinical applications, TNC and TNX are both promising biomarkers for the diagnosis and risk stratification of various cardiovascular diseases.
Acknowledgments
The authors thank D. Yamamoto for drawing the illustration for this manuscript. This work was supported in part by a grant from JSPS KAKENHI (Grant Number JP16H05351 to KIY) and grants for intractable disease from the Ministry of Health, Labour and Welfare of Japan (201128212, 201324121, 20145052 to KIY).
Disclosure Statement
KM and KIY have no conflict of interest.
Author Contributions
Study conception: KM, KIY
Data collection: KM, KIY
Analysis: KM, KIY
Writing: KM, KIY
Funding acquisition: KM, KIY
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Critical review and decision: all authors
Accountability for all aspects of the work: all authors
References
- 1).Tucker RP, Drabikowski K, Hess JF, et al. Phylogenetic analysis of the tenascin gene family: evidence of origin early in the chordate lineage. BMC Evol Biol 2006; 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Adams JC, Chiquet-Ehrismann R, Tucker RP. The evolution of tenascins and fibronectin. Cell Adh Migr 2015; 9: 22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 1995; 130: 503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Mohler ER 3rd. Mechanisms of aortic valve calcification. Am J Cardiol 2004; 94: 1396-402. [DOI] [PubMed] [Google Scholar]
- 5).Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol 2014; 37: 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem 1991; 266: 14831-4. [PubMed] [Google Scholar]
- 7).Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002; 14: 608-16. [DOI] [PubMed] [Google Scholar]
- 8).Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal 2009; 3: 163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal 2009; 3: 287-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Midwood KS, Hussenet T, Langlois B, et al. Advances in tenascin-C biology. Cell Mol Life Sci 2011; 68: 3175-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Udalova IA, Ruhmann M, Thomson SJ, et al. Expression and immune function of tenascin-C. Crit Rev Immunol 2011; 31: 115-45. [DOI] [PubMed] [Google Scholar]
- 12).Van Obberghen-Schilling E, Tucker RP, Saupe F, et al. Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. Int J Dev Biol 2011; 55: 511-25. [DOI] [PubMed] [Google Scholar]
- 13).Brellier F, Chiquet-Ehrismann R. How do tenascins influence the birth and life of a malignant cell? J Cell Mol Med 2012; 16: 32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Chiquet-Ehrismann R, Orend G, Chiquet M, et al. Tenascins in stem cell niches. Matrix Biol 2014; 37: 112-23. [DOI] [PubMed] [Google Scholar]
- 15).Tucker RP, Chiquet-Ehrismann R. Tenascin-C: its functions as an integrin ligand. Int J Biochem Cell Biol 2015; 65: 165-8. [DOI] [PubMed] [Google Scholar]
- 16).Giblin SP, Midwood KS. Tenascin-C: form versus function. Cell Adh Migr 2015; 9: 48-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Brösicke N, Faissner A. Role of tenascins in the ECM of gliomas. Cell Adh Migr 2015; 9: 131-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Midwood KS, Chiquet M, Tucker RP, et al. Tenascin-C at a glance. J Cell Sci 2016; 129: 4321-7. [DOI] [PubMed] [Google Scholar]
- 19).Imanaka-Yoshida K. Tenascin-C in cardiovascular tissue remodeling: from development to inflammation and repair. Circ J 2012; 76: 2513-20. [DOI] [PubMed] [Google Scholar]
- 20).Okamoto H, Imanaka-Yoshida K. Matricellular proteins: new molecular targets to prevent heart failure. Cardiovasc Ther 2012; 30: e198-209. [DOI] [PubMed] [Google Scholar]
- 21).Franz M, Jung C, Lauten A, et al. Tenascin-C in cardiovascular remodeling: potential impact for diagnosis, prognosis estimation and targeted therapy. Cell Adh Migr 2015; 9: 90-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Nozato T, Sato A, Hikita H, et al. Impact of serum tenascin-C on the aortic healing process during the chronic stage of type B acute aortic dissection. Int J Cardiol 2015; 191: 97-9. [DOI] [PubMed] [Google Scholar]
- 23).Nozato T, Sato A, Hirose S, et al. Preliminary study of serum tenascin-C levels as a diagnostic or prognostic biomarker of type B acute aortic dissection. Int J Cardiol 2013; 168: 4267-9. [DOI] [PubMed] [Google Scholar]
- 24).Okuma Y, Suda K, Nakaoka H, et al. Serum tenascin-C as a novel predictor for risk of coronary artery lesion and resistance to intravenous immunoglobulin in Kawasaki disease—a multicenter retrospective study. Circ J 2016; 80: 2376-81. [DOI] [PubMed] [Google Scholar]
- 25).Sato M, Toyozaki T, Odaka K, et al. Detection of experimental autoimmune myocarditis in rats by 111In monoclonal antibody specific for tenascin-C. Circulation 2002; 106: 1397-402. [DOI] [PubMed] [Google Scholar]
- 26).Odaka K, Uehara T, Arano Y, et al. Noninvasive detection of cardiac repair after acute myocardial infarction in rats by 111In Fab fragment of monoclonal antibody specific for tenascin-C. Int Heart J 2008; 49: 481-92. [DOI] [PubMed] [Google Scholar]
- 27).Taki J, Inaki A, Wakabayashi H, et al. Dynamic expression of tenascin-C after myocardial ischemia and reperfusion: assessment by 125I-anti–tenascin-C antibody imaging. J Nucl Med 2010; 51: 1116-22. [DOI] [PubMed] [Google Scholar]
- 28).Kobayashi N, Odaka K, Uehara T, et al. Toward in vivo imaging of heart disease using a radiolabeled single-chain Fv fragment targeting tenascin-C. Anal Chem 2011; 83: 9123-30. [DOI] [PubMed] [Google Scholar]
- 29).Taki J, Wakabayashi H, Inaki A, et al. 14C-Methionine uptake as a potential marker of inflammatory processes after myocardial ischemia and reperfusion. J Nucl Med 2013; 54: 431-6. [DOI] [PubMed] [Google Scholar]
- 30).Imanaka-Yoshida K, Yoshida T, Miyagawa-Tomita S. Tenascin-C in development and disease of blood vessels. Anat Rec (Hoboken) 2014; 297: 1747-57. [DOI] [PubMed] [Google Scholar]
- 31).Imanaka-Yoshida K, Aoki H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol 2014; 5: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Järvinen TA, Kannus P, Jarvinen TL, et al. Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand J Med Sci Sports 2000; 10: 376-82. [DOI] [PubMed] [Google Scholar]
- 33).Mackie EJ, Scott-Burden T, Hahn AW, et al. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol 1992; 141: 377-88. [PMC free article] [PubMed] [Google Scholar]
- 34).Chiquet M, Sarasa-Renedo A, Tunc-Civelek V. Induction of tenascin-C by cyclic tensile strain versus growth factors: distinct contributions by Rho/ROCK and MAPK signaling pathways. Biochim Biophys Acta 2004; 1693: 193-204. [DOI] [PubMed] [Google Scholar]
- 35).Chiquet M, Tunc-Civelek V, Sarasa-Renedo A. Gene regulation by mechanotransduction in fibroblasts. Appl Physiol Nutr Metab 2007; 32: 967-73. [DOI] [PubMed] [Google Scholar]
- 36).Asparuhova MB, Gelman L, Chiquet M. Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand J Med Sci Sports 2009; 19: 490-9. [DOI] [PubMed] [Google Scholar]
- 37).Asparuhova MB, Ferralli J, Chiquet M, et al. The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. FASEB J 2011; 25: 3477-88. [DOI] [PubMed] [Google Scholar]
- 38).Lutz R, Sakai T, Chiquet M. Pericellular fibronectin is required for RhoA-dependent responses to cyclic strain in fibroblasts. J Cell Sci 2010; 123: 1511-21. [DOI] [PubMed] [Google Scholar]
- 39).Midwood KS, Schwarzbauer JE. Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Mol Biol Cell 2002; 13: 3601-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Bhattacharyya S, Wang WX, Morales-Nebreda L, et al. Tenascin-C drives persistence of organ fibrosis. Nat Commun 2016; 7: 11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Herum KM, Choppe J, Kumar A, et al. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell 2017; 28: 1871-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Oberhauser AF, Marszalek PE, Erickson HP, et al. The molecular elasticity of the extracellular matrix protein tenascin. Nature 1998; 393: 181-5. [DOI] [PubMed] [Google Scholar]
- 43).Marín JL, Muñiz J, Huerta M, et al. Folding–unfolding of FN-III domains in tenascin: an elastically coupled two-state system. J Biomech 2003; 36: 1733-7. [DOI] [PubMed] [Google Scholar]
- 44).Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adh Migr 2015; 9: 96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Saga Y, Yagi T, Ikawa Y, et al. Mice develop normally without tenascin. Genes Dev 1992; 6: 1821-31. [DOI] [PubMed] [Google Scholar]
- 46).Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009; 15: 774-80. [DOI] [PubMed] [Google Scholar]
- 47).Monaco C, Terrando N, Midwood KS. Toll-like receptor signaling: common pathways that drive cardiovascular disease and rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011; 63: 500-11. [DOI] [PubMed] [Google Scholar]
- 48).Shimojo N, Hashizume R, Kanayama K, et al. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin alphaVbeta3/nuclear factor-kappaB/interleukin-6 axis. Hypertension 2015; 66: 757-66. [DOI] [PubMed] [Google Scholar]
- 49).Kanayama M, Kurotaki D, Morimoto J, et al. Alpha9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J Immunol 2009; 182: 8015-25. [DOI] [PubMed] [Google Scholar]
- 50).Kanayama M, Morimoto J, Matsui Y, et al. alpha9beta1 integrin-mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J Immunol 2011; 187: 5851-64. [DOI] [PubMed] [Google Scholar]
- 51).De Laporte L, Rice JJ, Tortelli F, et al. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS ONE 2013; 8: e62076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).El-Karef A, Yoshida T, Gabazza EC, et al. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 2007; 211: 86-94. [DOI] [PubMed] [Google Scholar]
- 53).Machino-Ohtsuka T, Tajiri K, Kimura T, et al. Tenascin-C aggravates autoimmune myocarditis via dendritic cell activation and Th17 cell differentiation. J Am Heart Assoc 2014; 3: e001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Koyama Y, Kusubata M, Yoshiki A, et al. Effect of tenascin-C deficiency on chemically induced dermatitis in the mouse. J Invest Dermatol 1998; 111: 930-5. [DOI] [PubMed] [Google Scholar]
- 55).Nakao N, Hiraiwa N, Yoshiki A, et al. Tenascin-C promotes healing of Habu-snake venom-induced glomerulonephritis: studies in knockout congenic mice and in culture. Am J Pathol 1998; 152: 1237-45. [PMC free article] [PubMed] [Google Scholar]
- 56).Song L, Wang L, Li F, et al. Bone marrow-derived tenascin-C attenuates cardiac hypertrophy by controlling inflammation. J Am Coll Cardiol 2017; 70: 1601-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Huang JY, Cheng YJ, Lin YP, et al. Extracellular matrix of glioblastoma inhibits polarization and transmigration of T cells: the role of tenascin-C in immune suppression. J Immunol 2010; 185: 1450-9. [DOI] [PubMed] [Google Scholar]
- 58).Kimura T, Shiraishi K, Furusho A, et al. Tenascin C protects aorta from acute dissection in mice. Sci Rep 2014; 4: 4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 2007; 27: 1248-58. [DOI] [PubMed] [Google Scholar]
- 60).Dale MA, Ruhlman MK, Baxter BT. Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol 2015; 35: 1746-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Wang L, Shah PK, Wang W, et al. Tenascin-C deficiency in apo E−/− mouse increases eotaxin levels: implications for atherosclerosis. Atherosclerosis 2013; 227: 267-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Wang L, Wang W, Shah PK, et al. Deletion of tenascin-C gene exacerbates atherosclerosis and induces intraplaque hemorrhage in Apo-E-deficient mice. Cardiovasc Pathol 2012; 21: 398-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Sawada Y, Onoda K, Imanaka-Yoshida K, et al. Tenascin-C synthesized in both donor grafts and recipients accelerates artery graft stenosis. Cardiovasc Res 2007; 74: 366-76. [DOI] [PubMed] [Google Scholar]
- 64).Yamamoto K, Onoda K, Sawada Y, et al. Tenascin-C is an essential factor for neointimal hyperplasia after aortotomy in mice. Cardiovasc Res 2005; 65: 737-42. [DOI] [PubMed] [Google Scholar]
- 65).Yoshimura K, Aoki H. Recent advances in pharmacotherapy development for abdominal aortic aneurysm. Int J Vasc Med 2012; 2012: 648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Curci JA, Baxter BT, Thompson RW. Arterial aneurysms: etiologic considerations. In: Rutherford RB ed. Vascular Surgery. Philadelphia: Saunders/Elsevier, 2005: 475-92.
- 67).Satta J, Soini Y, Pollanen R, et al. Tenascin expression is associated with a chronic inflammatory process in abdominal aortic aneurysms. J Vasc Surg 1997; 26: 670-5. [DOI] [PubMed] [Google Scholar]
- 68).Paik DC, Fu C, Bhattacharya J, et al. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med 2004; 36: 524-33. [DOI] [PubMed] [Google Scholar]
- 69).Didangelos A, Yin X, Mandal K, et al. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics 2011; 10: M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Kimura T, Yoshimura K, Aoki H, et al. Tenascin-C is expressed in abdominal aortic aneurysm tissue with an active degradation process. Pathol Int 2011; 61: 559-64. [DOI] [PubMed] [Google Scholar]
- 71).Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991; 352: 337-9. [DOI] [PubMed] [Google Scholar]
- 72).Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312: 117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355: 788-98. [DOI] [PubMed] [Google Scholar]
- 74).Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol 1997; 139: 279-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation 2003; 108 Suppl 1: II-329-34. [DOI] [PubMed] [Google Scholar]
- 76).Burch GH, Gong Y, Liu W, et al. Tenascin-X deficiency is associated with Ehlers–Danlos syndrome. Nat Genet 1997; 17: 104-8. [DOI] [PubMed] [Google Scholar]
- 77).Beighton P, De Paepe A, Steinmann B, et al. Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet 1998; 77: 31-7. [DOI] [PubMed] [Google Scholar]
- 78).Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers–Danlos syndrome caused by tenascin-X deficiency. N Engl J Med 2001; 345: 1167-75. [DOI] [PubMed] [Google Scholar]
- 79).Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers–Danlos syndrome. Am J Hum Genet 2003; 73: 214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Zweers MC, Hakim AJ, Grahame R, et al. Joint hypermobility syndromes: the pathophysiologic role of tenascin-X gene defects. Arthritis Rheum 2004; 50: 2742-9. [DOI] [PubMed] [Google Scholar]
- 81).Morel Y, Bristow J, Gitelman SE, et al. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci USA 1989; 86: 6582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Matsumoto K, Arai M, Ishihara N, et al. Cluster of fibronectin type III repeats found in the human major histocompatibility complex class III region shows the highest homology with the repeats in an extracellular matrix protein, tenascin. Genomics 1992; 12: 485-91. [DOI] [PubMed] [Google Scholar]
- 83).Bristow J, Tee MK, Gitelman SE, et al. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol 1993; 122: 265-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Ikuta T, Sogawa N, Ariga H, et al. Structural analysis of mouse tenascin-X: evolutionary aspects of reduplication of FNIII repeats in the tenascin gene family. Gene 1998; 217: 1-13. [DOI] [PubMed] [Google Scholar]
- 85).Lethias C, Descollonges Y, Botillon MM, et al. Flexilin: a new extracellular matrix glycoprotein localized on collagen fibrils. Matrix Biol 1996; 15: 11-9. [DOI] [PubMed] [Google Scholar]
- 86).Hagios C, Koch M, Spring J, et al. Tenascin-Y: a protein of novel domain structure is secreted by differentiated fibroblasts of muscle connective tissue. J Cell Biol 1996; 134: 1499-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Valcourt U, Alcaraz LB, Exposito JY, et al. Tenascin-X: beyond the architectural function. Cell Adh Migr 2015; 9: 154-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Matsumoto K, Saga Y, Ikemura T, et al. The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C. J Cell Biol 1994; 125: 483-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Elefteriou F, Exposito JY, Garrone R, et al. Characterization of the bovine tenascin-X. J Biol Chem 1997; 272: 22866-74. [DOI] [PubMed] [Google Scholar]
- 90).Burch GH, Bedolli MA, McDonough S, et al. Embryonic expression of tenascin-X suggests a role in limb, muscle, and heart development. Dev Dyn 1995; 203: 491-504. [DOI] [PubMed] [Google Scholar]
- 91).Imanaka-Yoshida K, Matsumoto K, Hara M, et al. The dynamic expression of tenascin-C and tenascin-X during early heart development in the mouse. Differentiation 2003; 71: 291-8. [DOI] [PubMed] [Google Scholar]
- 92).Geffrotin C, Garrido JJ, Tremet L, et al. Distinct tissue distribution in pigs of tenascin-X and tenascin-C transcripts. Eur J Biochem 1995; 231: 83-92. [DOI] [PubMed] [Google Scholar]
- 93).Matsumoto K, Sawa H, Sato M, et al. Distribution of extracellular matrix tenascin-X in sciatic nerves. Acta Neuropathol 2002; 104: 448-54. [DOI] [PubMed] [Google Scholar]
- 94).Sakai T, Furukawa Y, Chiquet-Ehrismann R, et al. Tenascin-X expression in tumor cells and fibroblasts: glucocorticoids as negative regulators in fibroblasts. J Cell Sci 1996; 109: 2069-77. [DOI] [PubMed] [Google Scholar]
- 95).Geffrotin C, Horak V, Crechet F, et al. Opposite regulation of tenascin-C and tenascin-X in MeLiM swine heritable cutaneous malignant melanoma. Biochim Biophys Acta 2000; 1524: 196-202. [DOI] [PubMed] [Google Scholar]
- 96).Hasegawa K, Yoshida T, Matsumoto K, et al. Differential expression of tenascin-C and tenascin-X in human astrocytomas. Acta Neuropathol 1997; 93: 431-7. [DOI] [PubMed] [Google Scholar]
- 97).Takeda K, Shiba H, Mizuno N, et al. Brain-derived neurotrophic factor enhances periodontal tissue regeneration. Tissue Eng 2005; 11: 1618-29. [DOI] [PubMed] [Google Scholar]
- 98).Ikuta T, Ariga H, Matsumoto K. Extracellular matrix tenascin-X in combination with vascular endothelial growth factor B enhances endothelial cell proliferation. Genes Cells 2000; 5: 913-27. [DOI] [PubMed] [Google Scholar]
- 99).Demidova-Rice TN, Geevarghese A, Herman IM. Bioactive peptides derived from vascular endothelial cell extracellular matrices promote microvascular morphogenesis and wound healing in vitro. Wound Repair Regen 2011; 19: 59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Minamitani T, Ariga H, Matsumoto K. Transcription factor Sp1 activates the expression of the mouse tenascin-X gene. Biochem Biophys Res Commun 2000; 267: 626-31. [DOI] [PubMed] [Google Scholar]
- 101).Elefteriou F, Exposito JY, Garrone R, et al. Cell adhesion to tenascin-X mapping of cell adhesion sites and identification of integrin receptors. Eur J Biochem 1999; 263: 840-8. [DOI] [PubMed] [Google Scholar]
- 102).Alcaraz LB, Exposito JY, Chuvin N, et al. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J Cell Biol 2014; 205: 409-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).Lethias C, Carisey A, Comte J, et al. A model of tenascin-X integration within the collagenous network. FEBS Lett 2006; 580: 6281-5. [DOI] [PubMed] [Google Scholar]
- 104).Elefteriou F, Exposito JY, Garrone R, et al. Binding of tenascin-X to decorin. FEBS Lett 2001; 495: 44-7. [DOI] [PubMed] [Google Scholar]
- 105).Minamitani T, Ikuta T, Saito Y, et al. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp Cell Res 2004; 298: 305-15. [DOI] [PubMed] [Google Scholar]
- 106).Bristow J, Carey W, Egging D, et al. Tenascin-X, collagen, elastin, and the Ehlers–Danlos syndrome. Am J Med Genet C Semin Med Genet 2005; 139C: 24-30. [DOI] [PubMed] [Google Scholar]
- 107).Egging D, van den Berkmortel F, Taylor G, et al. Interactions of human tenascin-X domains with dermal extracellular matrix molecules. Arch Dermatol Res 2007; 298: 389-96. [DOI] [PubMed] [Google Scholar]
- 108).Margaron Y, Bostan L, Exposito JY, et al. Tenascin-X increases the stiffness of collagen gels without affecting fibrillogenesis. Biophys Chem 2010; 147: 87-91. [DOI] [PubMed] [Google Scholar]
- 109).Mao JR, Taylor G, Dean WB, et al. Tenascin-X deficiency mimics Ehlers–Danlos syndrome in mice through alteration of collagen deposition. Nat Genet 2002; 30: 421-5. [DOI] [PubMed] [Google Scholar]
- 110).Zweers MC, Schalkwijk J, van Kuppevelt TH, et al. Transplantation of reconstructed human skin on nude mice: a model system to study expression of human tenascin-X and elastic fiber components. Cell Tissue Res 2005; 319: 279-87. [DOI] [PubMed] [Google Scholar]
- 111).Zweers MC, van Vlijmen-Willems IM, van Kuppevelt TH, et al. Deficiency of tenascin-X causes abnormalities in dermal elastic fiber morphology. J Invest Dermatol 2004; 122: 885-91. [DOI] [PubMed] [Google Scholar]
- 112).Fujie S, Maita H, Ariga H, et al. Tenascin-X induces cell detachment through p38 mitogen-activated protein kinase activation. Biol Pharm Bull 2009; 32: 1795-9. [DOI] [PubMed] [Google Scholar]
- 113).Matsumoto K, Takayama N, Ohnishi J, et al. Tumour invasion and metastasis are promoted in mice deficient in tenascin-X. Genes Cells 2001; 6: 1101-11. [DOI] [PubMed] [Google Scholar]
- 114).De Paepe A, Malfait F. The Ehlers–Danlos syndrome, a disorder with many faces. Clin Genet 2012; 82: 1-11. [DOI] [PubMed] [Google Scholar]
- 115).Egging D, van Vlijmen-Willems I, van Tongeren T, et al. Wound healing in tenascin-X deficient mice suggests that tenascin-X is involved in matrix maturation rather than matrix deposition. Connect Tissue Res 2007; 48: 93-8. [DOI] [PubMed] [Google Scholar]
- 116).Germain DP. Ehlers–Danlos syndrome type IV. Orphanet J Rare Dis 2007; 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117).Peeters AC, Kucharekova M, Timmermans J, et al. A clinical and cardiovascular survey of Ehlers–Danlos syndrome patients with complete deficiency of tenascin-X. Neth J Med 2004; 62: 160-2. [PubMed] [Google Scholar]
- 118).Egging DF, Peeters AC, Grebenchtchikov N, et al. Identification and characterization of multiple species of tenascin-X in human serum. FEBS J 2007; 274: 1280-9. [DOI] [PubMed] [Google Scholar]
- 119).Grahame R. Joint hypermobility and genetic collagen disorders: are they related? Arch Dis Child 1999; 80: 188-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120).Atzinger CL, Meyer RA, Khoury PR, et al. Cross-sectional and longitudinal assessment of aortic root dilation and valvular anomalies in hypermobile and classic Ehlers–Danlos syndrome. J Pediatr 2011; 158: 826-30.e1. [DOI] [PubMed] [Google Scholar]
- 121).Merke DP, Chen W, Morissette R, et al. Tenascin-X haploinsufficiency associated with Ehlers–Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2013; 98: E379-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Morissette R, McDonnell NB, Merke DP. Tenascin-X gene defects and cardiovascular disease. Med Hypotheses 2014; 83: 844. [DOI] [PubMed] [Google Scholar]
- 123).Petersen JW, Douglas JY. Tenascin-X, collagen, and Ehlers–Danlos syndrome: tenascin-X gene defects can protect against adverse cardiovascular events. Med Hypotheses 2013; 81: 443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124).Zweers MC, Peeters AC, Graafsma S, et al. Abdominal aortic aneurysm is associated with high serum levels of tenascin-X and decreased aneurysmal tissue tenascin-X. Circulation 2006; 113: 1702-7. [DOI] [PubMed] [Google Scholar]
- 125).Matsumoto K, Satoh K, Maniwa T, et al. Noticeable decreased expression of tenascin-X in calcific aortic valves. Connect Tissue Res 2012; 53: 460-8. [DOI] [PubMed] [Google Scholar]
- 126).Evrard S, Delanaye P, Kamel S, et al. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta 2015; 438: 401-14. [DOI] [PubMed] [Google Scholar]
- 127).Yamada K, Watanabe A, Takeshita H, et al. A method for quantification of serum tenascin-X by nano-LC/MS/MS. Clin Chim Acta 2016; 459: 94-100. [DOI] [PubMed] [Google Scholar]