Abstract
Tissue fibrosis, an accumulation of extracellular matrix proteins such as collagen, accompanies cardiac ageing in humans and this is linked to an increased risk of cardiac failure. The mechanisms driving age-related tissue fibrosis and cardiac dysfunction are unclear, yet clinically important. Drosophila is amenable to the study of cardiac ageing as well as collagen deposition; however it is unclear whether collagen accumulates in the ageing Drosophila heart.
This work examined collagen deposition and cardiac function in ageing Drosophila, in the context of reduced expression of collagen-interacting protein SPARC (Secreted Protein Acidic and Rich in Cysteine) an evolutionarily conserved protein linked with fibrosis. Heart function was measured using high frame rate videomicroscopy. Collagen deposition was monitored using a fluorescently-tagged collagen IV reporter (encoded by the Viking gene) and staining of the cardiac collagen, Pericardin.
The Drosophila heart accumulated collagen IV and Pericardin as flies aged. Associated with this was a decline in cardiac function. SPARC heterozygous flies lived longer than controls and showed little to no age-related cardiac dysfunction. As flies of both genotypes aged, cardiac levels of collagen IV (Viking) and Pericardin increased similarly. Over-expression of SPARC caused cardiomyopathy and increased Pericardin deposition.
The findings demonstrate that, like humans, the Drosophila heart develops a fibrosis-like phenotype as it ages. Although having no gross impact on collagen accumulation, reduced SPARC expression extended Drosophila lifespan and cardiac health span. It is proposed that cardiac fibrosis in humans may develop due to the activation of conserved mechanisms and that SPARC may mediate cardiac ageing by mechanisms more subtle than gross accumulation of collagen.
Keywords: Ageing, Collagen, Fibrosis, Heart, Drosophila, SPARC
Highlights
-
•
The ageing Drosophila heart accumulates collagens (Viking and Pericardin).
-
•
This fibrosis-like phenotype accompanied age-dependent cardiac dysfunction.
-
•
Reduced expression of the collagen-interacting protein SPARC prevented the age-related cardiac dysfunction.
-
•
SPARC overexpression in nephrocytes drives cardiomyopathy and Pericardin deposition.
-
•
Reduced SPARC did not prevent collagen accumulation in the ageing heart.
1. Introduction
Collagens are crucial for the development of form and function in multicellular animals. In mammals, increased collagen deposition occurs in response to injury as part of the tissue repair process (Wynn, 2008). Collagen accumulation in humans, typically referred to as tissue fibrosis, is a clinically important feature of chronic disease states as well as ageing. In both humans and mammalian models, tissue fibrosis is linked to organ dysfunction and an increased risk of mortality. Understanding the molecular genetics of collagen deposition and fibrosis is therefore an important step towards developing anti-fibrotic therapies. Fibrosis-related proteins and the signalling pathways leading to fibrosis show a high degree of genetic and functional conservation in the animal kingdom (Volk et al., 2014, Hartley et al., 2016, Sessions et al., 2016). The fruit or vinegar fly, Drosophila melanogaster expresses several collagen genes, as well as matricellular proteins required for the assembly of extracellular matrices (Yasothornsrikul et al., 1997, Martinek et al., 2008). Collagens and associated matricellular proteins are important mediators of cardiac development in Drosophila (Hartley et al., 2016, Chartier et al., 2002, Drechsler et al., 2013). Despite Drosophila being highly amenable to studies of age-related cardiac decline (Wessells et al., 2004, Cannon et al., 2017, Klassen et al., 2017, Lee et al., 2010, Nishimura et al., 2014, Monnier et al., 2012), there are currently no studies examining collagen deposition in the ageing heart.
SPARC (Secreted Protein Acidic and Rich in Cysteine) is a well-characterised collagen binding matricellular protein involved in tissue fibrosis (Weaver et al., 2008, Bradshaw, 2012). SPARC is evolutionarily and functionally conserved and known to mediate collagen deposition in Drosophila embryos (Martinek et al., 2008). SPARC expression is increased in a number of clinically important settings and accumulates (along with other extracellular matrix (ECM) proteins) in the ageing mammalian heart (Bradshaw et al., 2010, de Castro Bras et al., 2014), suggesting it may play a role in cardiac dysfunction in human ageing. Recent findings indicate that reduced SPARC expression can correct cardiomyopathy in Drosophila (Hartley et al., 2016). In addition, reduced expression of the ECM proteins Laminin, Viking and Pericardin in the Drosophila heart can impede age-related cardiac dysfunction (Sessions et al., 2016). Despite this knowledge, there is no data on whether ECM proteins accumulate within the ageing Drosophila heart.
Embryonic and larval development of Drosophila is dependent on the expression of type-IV collagen α2 and α1 chains encoded by Viking and Cg25C, as well as Multiplexin (an ortholog of human COL18A1) and the heart specific type-IV-like collagen, Pericardin; the latter two proteins being integral to cardiac development (Chartier et al., 2002, Harpaz et al., 2013). Although Pericardin was initially described as a matrix forming type-IV-like collagen, it also adopts prominent fibre-like structures and acts as a ‘tendenous’ bridge between the heart and alary muscles. How these collagenous fibres are formed and maintained remains largely uncharacterised.
In humans and mammalian models, fibrosis is regarded as an accumulation of extracellular matrix in a given tissue. Most attention has focused on fibril forming collagens, however fibrosis is complex and outcomes in terms of organ function are affected not only by the gross accumulation of collagen subtypes and other ECM proteins but also by the ratios of different collagens and ECM proteins (Karsdal et al., 2017). Experimental evidence indicates that Drosophila may be a tractable model with which to study the mechanisms leading to tissue fibrosis in humans. For example, an accumulation of collagen in and around Drosophila adipocytes alters innate immunity (Zang et al., 2015) whereas diet-dependent changes to Drosophila heart function are associated with cardiac fibrosis (Na et al., 2013). These findings make Drosophila a valuable tool with which to understand and identify mechanisms regulating collagen deposition and its impact on organ function. Given that collagen turnover (i.e. the expression, deposition and degradation of collagen) as well as the ageing process are evolutionarily conserved, it was predicted that collagen may accumulate as part of the ageing process in the Drosophila heart model and that SPARC may mediate this process.
In this report we describe the accumulation of collagen in the ageing Drosophila heart and show that this accompanies the well-described age-dependent functional decline of the fly's heart. It is also shown that SPARC heterozygous flies have a longer lifespan as well as extended cardiac health span. Despite this, the accumulation of collagen around the heart does not seem to be affected by reduced SPARC expression; whereas, SPARC over-expression led to cardiomyopathy and Pericardin accumulation. The findings support the idea that age-related fibrosis in mammals is an evolutionarily conserved process which can be studied in simpler, genetically tractable models.
2. Materials and methods
2.1. Stock chemicals and fly husbandry
Picrosirius red and all stock chemicals were from Sigma (Poole, Dorset, UK). The Canton Special, w1118, yw, SPARCMI00329 (with a MiMIC insertion in the 5-prime region of the SPARC locus; described in (Venken et al., 2011)) and Viking-GFP strains were obtained from the Bloomington Drosophila Stock Centre. The Dorothy-Gal4 line was described previously (Kimbrell et al., 2002) and is used to drive the expression of genes downstream of a UAS (upstream activation sequence) element. The UAS-SPARC line was described in (Martinek et al., 2008). Flies were reared and maintained on a standard cornmeal-yeast-agar diet under 12 h:12 h light:dark cycles at 25 °C. SPARCMI00329 were backcrossed to a yw background for > 6 generations and then outcrossed once to the w1118 line to generate heterozygous flies for phenotyping and lifespan studies.
2.2. Lifespan studies
Ten male or female flies within 1 day of eclosion were collected and transferred to fresh vials with standard diet. Five to ten vials per trial were prepared. At least two trials were conducted for each genotype. Vials were maintained on their side and flies tipped to fresh food twice per week for the duration of the lifespan studies. All vials were maintained under 12 h:12 h light:dark cycles at 25 °C. Lifespan data is presented as Kaplan-Meier plots as well as the mean of the median (the point at which 50% of the population had died) and maximum lifespan (± SEM).
2.3. Histochemistry, imaging and qPCR
Adult flies were anaesthetised by brief exposure to CO2, hearts were exposed by dissection and fixed using 1% formaldehyde for 15 min. Picrosirius red dye was then added for 5 min at ambient temperature and rinsed from dissected hearts using acidified water (1% acetic acid in water). Picrosirius red comprises sulphonic acid and stains the basic amine residues of most proteins but preferentially stains collagens because of their abundance and the large number of glycine residues. Colour phase images were captured on a Leica DMLB fitted with a Leica DC300 colour camera, images were taken using μManager software (Edelstein et al., 2010). Pericardin was imaged after staining dissected hearts with the mouse-anti Pericardin monoclonal antibody clone EC11 (Zaffran et al., 1995) (Developmental Studies Hybridoma Bank, University of Iowa) followed by a fluorescently-tagged secondary antibody. Fluorescence images were taken using a Zeiss LSM 710 confocal microscope and images quantified using ImageJ by drawing a line across a region of interest and recoding the maximum fluorescence signal.
2.4. Analysis of cardiac Pericardin
Hearts stained with anti-Pericardin antibodies were imaged by confocal microscopy and z-projections of stacks analysed in ImageJ. The same number of z-projected stacks was sued for each genotype and age. All images were of the distal region of the heart comprising approximately 2/3 of the total heart, where the majority of anti-Pericardin staining is found. Images were thresholded and the percentage of Pericardin positive signal quantified as a percentage of the total image. For measurement of Pericardin fibre thickness, single confocal image stacks were used and at least six fibre measurements were taken from four different flies. Data are presented as the mean fibre thickness (± SEM).
2.5. Heart function analysis
Adult flies were anaesthetised by brief exposure to Triethylamine vapour (20 μL of a 50% solution made in 50% ethanol/water, placed into the underside of a fly vial's cotton cap). Flies were dissected as described previously (Catterson et al., 2013) and imaged using a Zeiss Axiolab microscope fitted with a water immersion 10 × objective, linked to a Myocam S (Ionoptix Ltd., Ireland) high speed video camera. The beating heart was imaged for 10–20 s at 120 frames per second (fps) and heart function analysed using SOHA (Fink et al., 2009). For heart analysis involving SPARC over-expression, video capture was performed with a Ximea XDi camera and a frame rate of 25 fps.
2.6. Western Blotting
For each lane 10 female hearts were harvested and directly lysed in 10 μL Ripa buffer (Sigma, Poole, UK). Polypeptides were separated on a 4–20% SDS-page gel and transferred to a PVDF membrane. The membrane was then probed with anti-pericardin (DSHB, EC11) and anti-actin (DSHB JLA20) antibodies followed by secondary antibodies conjugated to alkaline phosphatase. Secondary antibodies were detected using NBT (nitro-blue tetrazolium chloride) with BCIP (5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt).
2.7. Statistics
When two means were compared, an unpaired two-tailed student's t-Test was applied to establish the P-value. When mean data for genotype and age were compared in an experiment, two-way ANOVA was used. Graphs show mean ± SEM.
3. Results
3.1. Overall collagen and collagen-IV deposition increases in older flies
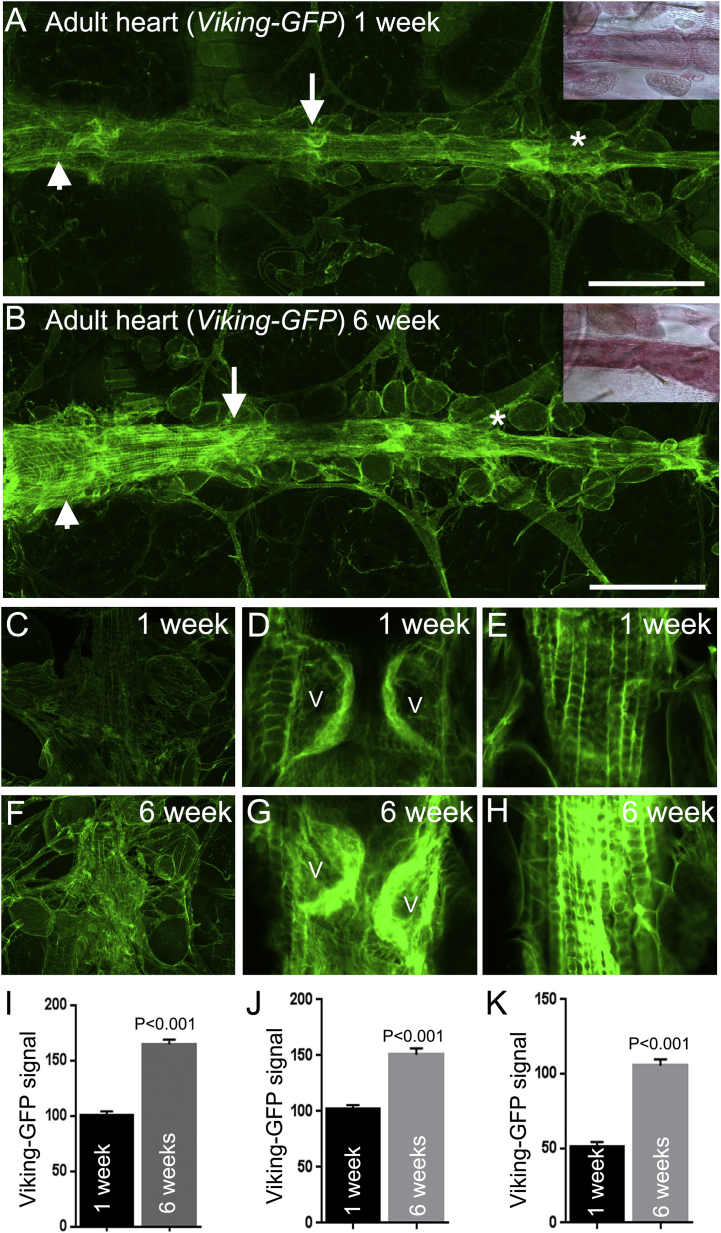
To assess collagen deposition in the heart two approaches were initially used. Firstly picrosirius red, a histochemical dye used widely to quantify collagen in mammalian tissue section was used to stain the heart of one-week and six-week-old wild type (Canton S) Drosophila. The adult Drosophila heart exhibited extensive pink-red staining that became more intense in older flies (inset Fig. 1A and B). Quantification of the signal was not possible due to older flies developing a red-brown pigmentation in their cuticle which affected colour-based image analysis. The second approach used flies expressing a fluorescently tagged collagen-IV protein encoded by the viking gene (Morin et al., 2001). This reporter system has been used to visualise collagen-IV during embryogenesis and egg morphogenesis and reliably reports Viking protein distribution in basement membranes (Pastor-Pareja and Xu, 2011, Haigo and Bilder, 2011). In accordance with the picrosirius red results, Viking-GFP fluorescence was abundant along the heart of adult flies and particularly intense at the 1st, 2nd and 3rd valve pairs (arrow denotes the 2nd valve pair in Fig. 1A), the distal heart (asterisks Fig. 1A and B) and the longitudinal muscles, especially those overlying the conical chamber (arrowheads, Fig. 1A and B). As for the picrosirius red result, the Viking-GFP signal at the distal heart, valve and longitudinal muscles (Fig. 1C–E) was markedly increased in older flies (Figs. F–H). Unlike the picrosirius red signal, the Viking-GFP fluorescence signal was amenable to quantification and recorded as significantly increasing at the distal heart, valves and longitudinal muscles of one and six-week-old flies (Fig. 1I–K; P < 0.01). Additionally, valve cells exhibited age-dependent remodelling (Supplemental Fig. S1). This change to valve morphology in Drosophila does not appear to have been documented before but may reflect the senescence-related changes known to occur to human aortic valve leaflets (Collins et al., 2014).
Fig. 1.
The Viking-GFP signal accumulates at all regions of the ageing heart.
(A and B) The heart of adult flies expressing a Viking-GFP fusion protein shows an increase of signal over six weeks. This insets show the signal from the histological stain picrosirius red of similarly aged Canton S wild type flies. The Viking-GFP signal is increased at the valve (arrows), conical chamber (arrowheads) and distal heart (asterisk); scale bar = 150 μm. Confocal images and quantified data of the Viking-GFP signal at the distal heart (C, F and I), valves (D, G and J) and longitudinal muscles of the conical chamber (E, H and K) of one and six week old flies. V = valves. Quantified data was obtained from four to six individual flies at each age. Mean fluorescence is expressed in arbitrary units ± SEM. P values were determined by t-Test.
3.2. Cardiac Pericardin accumulation
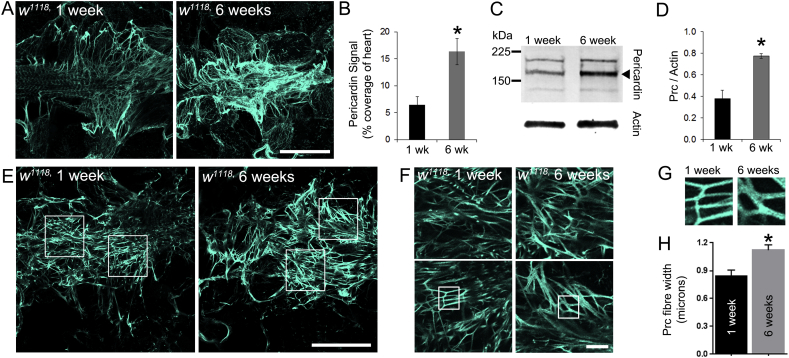
In addition to Collagen-IV quantification, the level of Pericardin was quantified in one and six-week old flies (Fig. 2). Pericardin is a type-IV-like collagen associated exclusively with the insect dorsal vessel (Chartier et al., 2002). Hearts of w1118 flies stained with antisera to Pericardin exhibited a complex network of overlapping Pericardin fibres, which were especially concentrated at the distal heart region (Fig. 2A) and between adjacent nephrocytes and the heart tube (not shown). The immunofluorescence signal for Pericardin in six week old flies was increased relative to younger, one week old flies (Fig. 2B; P < 0.01). Western blot analysis of total cell lysates from isolated hearts confirmed an increased level of cardiac Pericardin on older flies (Fig. 2C and D; P < 0.05). In addition, Pericardin fibre thickness was analysed in single confocal sections and also found to be greater in older flies (Fig. 2E–H; P < 0.05).
Fig. 2.
Pericardin levels increase in the ageing heart.
(A) Confocal micrographs of z-projected stacks through the distal region of the hearts in flies of different ages stained with antisera to Pericardin. (B) The amount of Pericardin fluorescence signal in 1 wk and 6 wk-old flies; n = 3; *P < 0.01. (C) Western blot of whole cell lysate of hearts from young (1 week) and older flies (6 weeks) stained with antibodies raised to Pericardin. Actin was used as a loading control. (D) Quantification of polypeptide band intensity relative to the control (Actin). Data are the mean (± SEM) of measurements from three independent experiments; *P < 0.05. (E–G) A single confocal slice through the distal region of the heart of young (1 week) and older flies (6 weeks); scale bars = 100 μm in E; 10 μm in F. Boxes depict examples of regions used for quantifying Pericardin fibre thickness. (H) Pericardin fibre thickness in young (1 week) and older flies (6 weeks). Data are the mean (± SEM) of fibres from at least 6 fibre measurements from four individual flies at each age. *P < 0.01.
3.3. Reduced SPARC expression is associated with extended lifespan
It is well established that, like the mammalian heart, there is an age-dependent decline in cardiac function in Drosophila (Wessells et al., 2004). In addition, there is an association between increased Pericardin deposition and abnormal cardiac function in a diet-induced Drosophila cardiomyopathy model (Na et al., 2013). Recent evidence from the Drosophila heart model indicates that SPARC mediates the development of a cardiomyopathy caused by disruption of the fly's kidney-like nephrocytes (Hartley et al., 2016). As Drosophila and mammalian SPARC are both involved in collagen deposition, we asked whether a hypomorphic allele of Drosophila SPARC may alter the age-dependent decline in cardiac function, age-dependent collagen deposition in the fly heart or both.
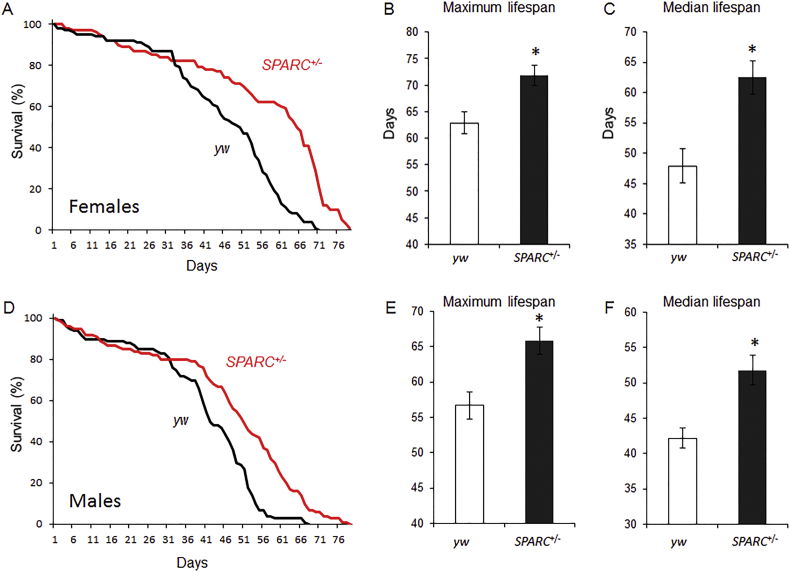
The mutant SPARC allele is caused by a transgene insertion that reduces the gene's expression by 60% in heterozygous flies (Hartley et al., 2016). Both female and male SPARC heterozygous flies had extended lifespan compared to controls (Fig. 3). Maximum and median lifespan for female SPARC heterozygotes were 72 days and 63 days, nine and fifteen days longer than control's (Fig. 3B & C; P < 0.01). Similarly, the maximum and median lifespan of male SPARC heterozygous flies were 66 days and 52 days; nine and ten days longer than controls (Fig. 3E & F; P < 0.01).
Fig. 3.
Extended lifespan in SPARC heterozygous flies.
(A) Representative Kaplan-Meier plot of survival over time for flies of the control (w1118, black line) and SPARC heterozygous genotypes (red line). Data is from females for each genotype. (B & C) Quantified data for maximum and median lifespan. Data represents mean of two independent trials using five groups of 15 flies of each genotype per trial. Data are presented as the mean (± SEM) for each genotype. *P < 0.01.
3.4. Impact of reduced SPARC expression on the age-dependent decline of heart function
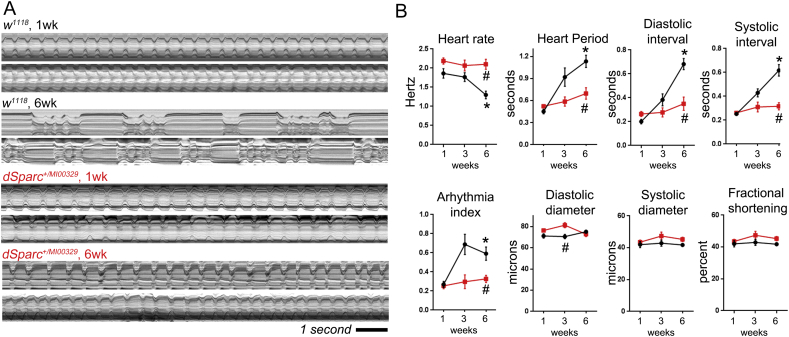
To establish if SPARC mediated the age-dependent decline of cardiac function, heart function was monitored at one, three and six weeks in control and SPARC heterozygous flies (Fig. 4). The cardiac function of control flies (w1118) exhibited an age-dependent decline as expected, whereas SPARC heterozygous flies showed no apparent change to cardiac function. Example m-modes (two-dimensional images of the heart's contractions over time) show regular beating of the heart in young (one week old control and SPARC heterozygous flies; Fig. 4A). There was a significant change to the rhythmicity of cardiac contractions in six week old controls, characterised by a longer cardiac cycle and periods of arrhythmia. This age-dependent change was not as apparent in older SPARC heterozygous flies (lower m-modes in Fig. 4A), which exhibited cardiac function comparable to that of younger flies. Quantification of heart rate, heart period, diastolic and systolic intervals as well as the arrhythmia index (a measure of beat-to-beat variability) confirmed there was an age-dependent change to all parameters in controls but not SPARC heterozygous flies (Fig. 4B; P < 0.01 for age-dependent decline in controls; whereas there was no significant difference in SPARC heterozygous flies as they aged). In terms of cardiac morphology and contraction distance (FS, fractional shortening), there was a small but significant difference between the genotypes' heart diameter at diastole (EDD) but not systole (ESD; Fig. 4B; P < 0.01). However, neither genotype showed any age-related changes to these three parameters (P < 0.05).
Fig. 4.
Extended cardiac health span in SPARC heterozygous flies.
(A) The adult beating heart was imaged in semi-intact preparations and functional parameters measured from videos captured at ~ 120 frames per second. The m-modes show heart contractions over a 12 s period. M-modes from two different flies of each genotype, control (w1118) and mutant (SPARCMI00329 heterozygotes) are show for each age (1 and 6 weeks old). Older wild type flies exhibit a disrupted heart rhythm, however this does not occur in SPARCMI00329 heterozygotes. (B) Quantified data for heart function showing that the age-dependent decline in heart function recorded in the wild types (black lines) does not develop in SPARC+/− flies (red lines). Graphs show mean ± SEM for each parameter. *P < 0.001, there is an age-related change in the parameter; #P < 0.01 there is a difference between genotypes by 2-way-ANOVA, n = 20–50 flies per genotype, per time-point.
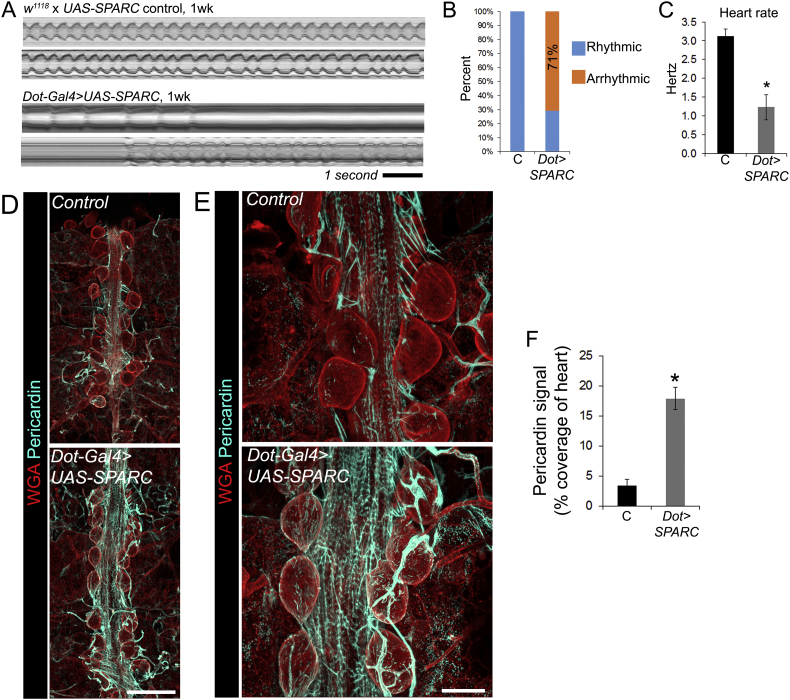
3.5. Over-expression of SPARC is sufficient to cause cardiomyopathy and increase Pericardin deposition
To assess whether SPARC expression is sufficient to cause cardiac dysfunction SPARC was over-expressed using Dorothy-Gal4 in pericardial nephrocytes (large, kidney-like cells that lie adjacent to the heart). Within one week of eclosion, cardiac function was severely disrupted in flies over-expressing SPARC. Heart dysfunction mirrored the phenotype of older flies, with the majority showing periods of arrhythmia (71% of experimental flies versus 0% of controls; Fig. 5A and B) and a very slow heart rate (Fig. 5C; P < 0.01). Pericardial nephrocytes are also a major source of Pericardin, and over-expression of SPARC in the nephrocytes caused a significant (~ 4-fold) increase to Pericardin deposition around nephrocytes and the heart (Fig. 5D–F; P < 0.01).
Fig. 5.
Over-expression of SPARC causes cardiomyopathy and increased Pericardin deposition.
(A) M-modes showing the impact of SPARC over-expression on heart function at one week of age (from videos captured at 25 frames per second). (B) The percentage of hearts that were arrhythmic in controls (‘C’, the UAS-SPARC parent line outcrossed to w1118) versus SPARC overexpression flies (driven by the Dorothy enhancer; ‘UAS-SPARC’); n = 7–10 flies for each genotype. (C) Mean heart rate (± SEM) for the control and SPARC over-expression flies at one week. n = 7 flies for each genotype; *P < 0.01. (D) Confocal micrographs of adult 1 week old hearts stained with wheat germ agglutinin (red, to highlight cell surfaces) and antibodies to Pericardin (cyan); scale bar = 100 μm. (E) Higher magnification of hearts stained with WGA and Pericardin antibodies; scale bar = 50 μm. (F) Percentage coverage of heart staining positive for Pericardin (mean, ± SEM); n = 5; *P < 0.01.
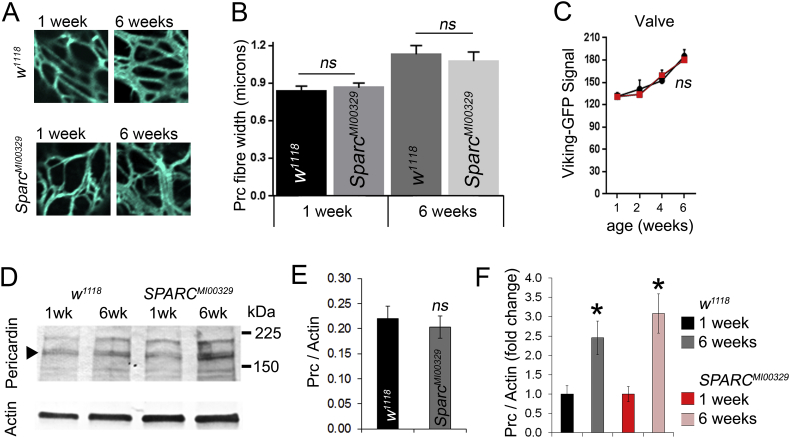
3.6. No evidence for reduced age-dependent collagen accumulation in SPARC heterozygous flies
The impact of SPARC modulation on heart function was followed up by an analysis of collagen deposition in one and six-week-old control and SPARC heterozygous flies. There was no apparent abnormality to the fibrous Pericardin network in SPARC heterozygous flies compared to controls, at either one or six weeks of age (Fig. 6A). Quantification of fibre width in controls corroborated earlier findings, with older flies having thicker fibres than younger flies (Fig. 6B); however the same was true for SPARC heterozygous flies which also exhibited thicker Pericardin fibres in older flies. Additionally, the Viking-GFP signal in the heart valves of control and SPARC heterozygous flies was similar at all ages (1, 2, 4 and 6 weeks; Fig. 6C; P > 0.05). Finally, Pericardin was quantified in young and old flies by western blot and found to be similar in young flies of each genotype and to increase comparably in both genotypes (Fig. 6D–F).
Fig. 6.
The levels of Viking and Pericardin in aged SPARC heterozygous hearts.
(A) Confocal slices through the distal region of the hearts of young (1 week) and older (6 week) control (w1118) and SPARC heterozygous flies (SPARCMI00329) stained with antibodies to Pericardin. (B) Mean (± SEM) Pericardin fibre thickness in flies of different ages and genotype as described in the figure. ns = P > 0.05. n = 6 measurement from 4 four individual flies. (C) Mean (± SEM) of the Viking-GFP signal at the valves of flies of different ages and genotype (w1118, black lines and SPARCMI00329, red lines). ns = P > 0.05. n = 6–10 flies of each age and genotype. (D) Western blot of heart tissue stained with Pericardin antibodies and actin as a loading control. (E) Quantification of anti-Pericardin western blot band intensity using at one week of age; ns = not statistically different from control. (F) Quantified data showing fold change in Pericardin band intensity; n = three blots; *P < 0.05 for there being a difference in Pericardin at 6 weeks compared to 1 week.
4. Discussion
This study examined collagen deposition in the ageing Drosophila heart. This was done using a well-known histological stain that detects collagen (picrosirius red), a collagen-IV reporter fusion protein (Viking-GFP) and immunological detection of the type-IV-like collagen, Pericardin. The work tested the hypothesis that age-related changes to either cardiac function or cardiac collagen accumulation may be mediated by the matricellular protein, SPARC. There was evidence for increased collagen deposition in the heart that accompanied cardiac dysfunction. Age-dependent cardiac dysfunction was prevented in SPARC heterozygous flies, however there was no evidence of reduced collagen accumulation. SPARC over-expression was sufficient to cause cardiomyopathy in young flies and this accompanied increased Pericardin deposition. We conclude that the Drosophila heart develops a fibrosis-like phenotype as it ages and that SPARC mediates cardiac ageing and Pericardin accumulation. These findings suggest the existence of evolutionarily-conserved mechanisms of age-related collagen deposition and that the Drosophila model may contribute to the study of age-related fibrosis in humans. However, it remains unclear how SPARC and the accumulation of collagen are linked to cardiac dysfunction in Drosophila.
Fibrosis can be regarded as an accumulation of extracellular matrix (ECM) in a given tissue. In humans and mammalian models, fibrosis typically develops as part of a tissue repair process, chronic inflammatory disease or ageing. Fibrotic outcomes in terms of organ function appear to be determined not only by the gross accumulation of ECM but also by the ratios of different ECM proteins (Karsdal et al., 2017). The fact that we observed thickening of Pericardin fibres and an accumulation of type-IV collagen is consistent with the development of a fibrosis-like phenotype in the ageing Drosophila heart.
Accompanying the fibrosis-like phenotype was a decline of cardiac function. Cardiac dysfunction in ageing was expected and reported previously by others (Wessells et al., 2004, Kaushik et al., 2015). It is known that collagen accumulates in the ageing mouse myocardium and that this correlates with increased SPARC protein levels, as well as cardiac stiffness (Bradshaw et al., 2010). These age-related changes to collagen and cardiac stiffness are blunted in SPARC-null animals (Bradshaw et al., 2010). Similarly, in mice heterozygous for SPARC, collagen IV levels do not increase in ageing ventricles (de Castro Bras et al., 2014). There is recent evidence that SPARC mediates the development of cardiomyopathy in Drosophila (Hartley et al., 2016) and that reduced expression of extracellular matrix (ECM) proteins can prevent cardiac ageing in flies (Sessions et al., 2016). In accordance with these findings it was hypothesised that reduced SPARC expression may prevent the age-related decline of cardiac function in Drosophila, possibly by modulating ECM deposition.
Using video microscopy of semi-intact heart preparations, it was found that reduced SPARC expression prevented the age-dependent decline of cardiac function. In addition, SPARC heterozygous flies had extended median and maximal lifespan compared to controls. Hence, reduced SPARC contributes to a longer life with an accompanying extension of cardiac health-span in Drosophila. In contrast, over-expression of SPARC in pericardial nephrocytes was sufficient to cause a severe a cardiomyopathy in young flies that reflected the phenotype of the aged heart. This cardiomyopathy accompanied the accumulation of Pericardin, indicating that increased SPARC expression can promote Pericardin deposition in Drosophila.
In contrast to the prediction that reduced SPARC may mediate the accumulation of cardiac collagen in ageing flies and the over-expression result, reduced SPARC expression was recorded as not having an impact on the quantity of Viking or Pericardin deposited around the heart at any age. Both control and SPARC heterozygous flies had similar levels of cardiac Viking or Pericardin at one and six weeks of age, hence both genotypes accumulated the collagens at a similar rate as they aged. This suggests that SPARC's impact on cardiac health-span may depend on mechanisms distinct to collagen accumulation. However, this conclusion is offered tentatively because there is a wealth of data indicating that SPARC mediates collagen assembly in other models (Camino et al., 2008). Hence, it is not possible to conclude that gross accumulation of collagen around the ageing heart was the reason for the decline in cardiac function. One mechanism by which SPARC may affect cardiac ageing is by activation of the Integrin-Linked Kinase pathway (ILK). It is known that mammalian SPARC directly interacts with β-1 integrin and activates ILK signalling (Weaver et al., 2008). A recent study in Drosophila established that ILK regulates cardiac ageing (Nishimura et al., 2014). It is therefore plausible that reduced SPARC may prevent cardiac ageing by reducing cardiac ILK signalling. However, this problem may be somewhat intractable because earlier work demonstrated that cardiac-restricted knock-down of SPARC has deleterious effects on heart development and function (Hartley et al., 2016). Additionally, the glycation of collagen (the formation of cross-linkages between collagen fibres) increases as organisms age and this affects basement membrane flexibility (Paul and Bailey, 1996). Future work will examine whether such qualitative changes to collagens occur in ageing Drosophila model and whether they impact cardiac function.
In summary, the ageing Drosophila heart develops a fibrosis-like phenotype typified by an accumulation of collagen-IV (Viking) and the type-IV-like collagen, Pericardin. This is associated with age-dependent decline in cardiac function. Whilst reduced SPARC can ameliorate the decline of cardiac function it does so by a mechanism that appears independent of the gross changes to collagen accumulation measured in this study. The findings imply that the Drosophila heart can be used to model the genetic pathways contributing to age-dependent fibrosis in higher organisms, including humans.
The following is the supplementary data related to this article.
Morphometric analysis of the valve cells in ageing flies. Confocal micrographs of cardiac valves were used to measure valve width and length in young (1 week old) and older (6 week old) flies. Both parameters were reduced in older flies (*P < 0.01), n = 12–14 valves per age.
Funding
This work was funded by a British Heart Foundation Intermediate Basic Science Fellowship to PSH (FS/13/17/29905). The funders played no role in the experimental design, data analysis or writing-up of the experimental findings.
Acknowledgments
Acknowledgements
The authors would like to acknowledge the British Heart Foundation for supporting the work. We are also indebted to Dr. Wei-Jun Liang (Bournemouth University) for helpful comments on the manuscript. The anti-Pericardin monoclonal antibody (clone EC11) was developed by the Sémériva research group was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. We would also like to thank the Bournemouth University Executive and department of Life and Environmental Science for providing important resources and equipment.
References
- Bradshaw A.D. Diverse biological functions of the SPARC family of proteins. Int. J. Biochem. Cell Biol. 2012;44:480–488. doi: 10.1016/j.biocel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw A.D., Baicu C.F., Rentz T.J., Van Laer A.O., Bonnema D.D. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H614–622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino A.M., Atorrasagasti C., Maccio D., Prada F., Salvatierra E. Adenovirus-mediated inhibition of SPARC attenuates liver fibrosis in rats. J. Gene Med. 2008;10:993–1004. doi: 10.1002/jgm.1228. [DOI] [PubMed] [Google Scholar]
- Cannon L., Zambon A.C., Cammarato A., Zhang Z., Vogler G. Expression patterns of cardiac aging in Drosophila. Aging Cell. 2017;16:82–92. doi: 10.1111/acel.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro Bras L.E., Toba H., Baicu C.F., Zile M.R., Weintraub S.T. Age and SPARC change the extracellular matrix composition of the left ventricle. Biomed. Res. Int. 2014;2014(810562) doi: 10.1155/2014/810562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterson J.H., Heck M.M., Hartley P.S. Fermitins, the orthologs of mammalian Kindlins, regulate the development of a functional cardiac syncytium in Drosophila melanogaster. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier A., Zaffran S., Astier M., Semeriva M., Gratecos D. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development. 2002;129:3241–3253. doi: 10.1242/dev.129.13.3241. [DOI] [PubMed] [Google Scholar]
- Collins J.A., Munoz J.V., Patel T.R., Loukas M., Tubbs R.S. The anatomy of the aging aorta. Clin. Anat. 2014;27:463–466. doi: 10.1002/ca.22384. [DOI] [PubMed] [Google Scholar]
- Drechsler M., Schmidt A.C., Meyer H., Paululat A. The conserved ADAMTS-like protein lonely heart mediates matrix formation and cardiac tissue integrity. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A., Amodaj N., Hoover K., Vale R., Stuurman N. John Wiley & Sons, Inc; 2010. Computer Control of Microscopes Using μManager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M., Callol-Massot C., Chu A., Ruiz-Lozano P., Izpisua Belmonte J.C. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo S.L., Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz N., Ordan E., Ocorr K., Bodmer R., Volk T. Multiplexin promotes heart but not aorta morphogenesis by polarized enhancement of slit/robo activity at the heart lumen. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley P.S., Motamedchaboki K., Bodmer R., Ocorr K. SPARC-dependent cardiomyopathy in Drosophila. Circ. Cardiovasc. Genet. 2016;9:119–129. doi: 10.1161/CIRCGENETICS.115.001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M.A., Nielsen S.H., Leeming D.J., Langholm L.L., Nielsen M.J. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017 doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Kaushik G., Spenlehauer A., Sessions A.O., Trujillo A.S., Fuhrmann A. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci. Transl. Med. 2015;7:292ra299. doi: 10.1126/scitranslmed.aaa5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell D.A., Hice C., Bolduc C., Kleinhesselink K., Beckingham K. The Dorothy enhancer has Tinman binding sites and drives hopscotch-induced tumor formation. Genesis. 2002;34:23–28. doi: 10.1002/gene.10134. [DOI] [PubMed] [Google Scholar]
- Klassen M.P., Peters C.J., Zhou S., Williams H.H., Jan L.Y. Age-dependent diastolic heart failure in an in vivo Drosophila model. elife. 2017;6 doi: 10.7554/eLife.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Budanov A.V., Park E.J., Birse R., Kim T.E. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek N., Shahab J., Saathoff M., Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J. Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- Monnier V., Iche-Torres M., Rera M., Contremoulins V., Guichard C. dJun and Vri/dNFIL3 are major regulators of cardiac aging in Drosophila. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J., Musselman L.P., Pendse J., Baranski T.J., Bodmer R. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Kumsta C., Kaushik G., Diop S.B., Ding Y. A dual role for integrin-linked kinase and beta1-integrin in modulating cardiac aging. Aging Cell. 2014;13:431–440. doi: 10.1111/acel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja J.C., Xu T. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted collagen IV and perlecan. Dev. Cell. 2011;21:245–256. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R.G., Bailey A.J. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int. J. Biochem. Cell Biol. 1996;28:1297–1310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- Sessions A.O., Kaushik G., Parker S., Raedschelders K., Bodmer R. Extracellular matrix downregulation in the Drosophila heart preserves contractile function and improves lifespan. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K.J., Schulze K.L., Haelterman N.A., Pan H., He Y. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., Wang S., Rotstein B., Paululat A. Matricellular proteins in development: perspectives from the Drosophila heart. Matrix Biol. 2014;37:162–166. doi: 10.1016/j.matbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Weaver M.S., Workman G., Sage E.H. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J. Biol. Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells R.J., Fitzgerald E., Cypser J.R., Tatar M., Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S., Davis W.J., Cramer G., Kimbrell D.A., Dearolf C.R. viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997;198:17–25. doi: 10.1016/s0378-1119(97)00274-6. [DOI] [PubMed] [Google Scholar]
- Zaffran S., Astier M., Gratecos D., Guillen A., Semeriva M. Cellular interactions during heart morphogenesis in the Drosophila embryo. Biol. Cell. 1995;84:13–24. doi: 10.1016/0248-4900(96)81314-1. [DOI] [PubMed] [Google Scholar]
- Zang Y., Wan M., Liu M., Ke H., Ma S. Plasma membrane overgrowth causes fibrotic collagen accumulation and immune activation in Drosophila adipocytes. elife. 2015;4 doi: 10.7554/eLife.07187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphometric analysis of the valve cells in ageing flies. Confocal micrographs of cardiac valves were used to measure valve width and length in young (1 week old) and older (6 week old) flies. Both parameters were reduced in older flies (*P < 0.01), n = 12–14 valves per age.