Significance
Inherited pathogenic mutations in genes required for copper delivery to cytochrome c oxidase (CcO) perturb mitochondrial energy metabolism and result in fatal mitochondrial disease. A prior attempt to treat human patients with these mutations by direct copper supplementation was not successful, possibly because of inefficient copper delivery to the mitochondria. We performed a targeted search to identify compounds that can efficiently transport copper across biological membranes and identified elesclomol (ES), an investigational anticancer drug, as the most efficient copper delivery agent. ES rescues CcO function in yeast, zebrafish, and mammalian models of copper deficiency by increasing cellular and mitochondrial copper content. Thus, our study offers a possibility of repurposing this anticancer drug for the treatment of disorders of copper metabolism.
Keywords: copper, mitochondria, elesclomol, cytochrome c oxidase
Abstract
Copper is an essential cofactor of cytochrome c oxidase (CcO), the terminal enzyme of the mitochondrial respiratory chain. Inherited loss-of-function mutations in several genes encoding proteins required for copper delivery to CcO result in diminished CcO activity and severe pathologic conditions in affected infants. Copper supplementation restores CcO function in patient cells with mutations in two of these genes, COA6 and SCO2, suggesting a potential therapeutic approach. However, direct copper supplementation has not been therapeutically effective in human patients, underscoring the need to identify highly efficient copper transporting pharmacological agents. By using a candidate-based approach, we identified an investigational anticancer drug, elesclomol (ES), that rescues respiratory defects of COA6-deficient yeast cells by increasing mitochondrial copper content and restoring CcO activity. ES also rescues respiratory defects in other yeast mutants of copper metabolism, suggesting a broader applicability. Low nanomolar concentrations of ES reinstate copper-containing subunits of CcO in a zebrafish model of copper deficiency and in a series of copper-deficient mammalian cells, including those derived from a patient with SCO2 mutations. These findings reveal that ES can restore intracellular copper homeostasis by mimicking the function of missing transporters and chaperones of copper, and may have potential in treating human disorders of copper metabolism.
Copper is an essential micronutrient required for the assembly and activity of cytochrome c oxidase (CcO), the terminal enzyme of the mitochondrial respiratory chain that catalyzes the reduction of molecular oxygen and drives mitochondrial energy production (1, 2). CcO is a highly conserved, multimeric inner mitochondrial membrane protein complex that has two copper-containing subunits, Cox1 and Cox2, which together form its catalytic core (2). Copper delivery to mitochondria and its insertion into these copper-containing subunits is an intricate process that requires multiple metallochaperones and ancillary proteins (3). Failure to deliver copper to Cox1 and Cox2 disrupts CcO assembly and results in a respiratory deficiency.
Cytosolic copper is delivered to the mitochondrial matrix via the recently identified yeast protein Pic2 (4), where it is stored in a ligand-bound form (5). This mitochondrial matrix copper pool is the main source of copper ions that are inserted into the CcO subunits in the mitochondrial intermembrane space (IMS) (6). Mobilization of copper from the mitochondrial matrix to the IMS for its delivery to copper sites in CcO subunits requires a number of evolutionarily conserved proteins (3). The precise molecular functions of these proteins have remained unsolved, except for the metallochaperones Cox17, Sco1, Sco2, and Cox11, which have been shown to transfer copper to CcO subunits in a bucket-brigade fashion (3). Specifically, Cox17 receives copper from the mitochondrial matrix and transfers it to Cox11 and Sco1/Sco2 (7), which then metallate copper sites on Cox1 and Cox2, respectively (8, 9). Recently, two other proteins, Coa6 and Cox19, have also been shown to be part of this copper delivery pathway in the IMS (10–13).
In humans, inherited partial loss-of-function mutations in SCO1, SCO2, and COA6 result in a CcO deficiency and are associated with hepatopathy, metabolic acidosis, cardiomyopathy, and neurological defects in affected patients (14–16). Copper supplementation rescues CcO deficiency in myoblasts from patients with mutations in SCO2 (17) and restores CcO activity in COA6-deficient yeast and human patient cell lines (16, 18), suggesting that efficient delivery of copper to mitochondria could restore CcO activity by bypassing SCO2 and COA6 functions. In an attempt to translate these observations in a clinical setting, s.c. injections of copper histidine were administered to a patient with an SCO2 mutation. Although copper supplementation improved the patient’s hypertrophic cardiomyopathy, it did not improve other clinical outcomes or survival (19). Thus, a more effective mechanism for restoration of copper homeostasis will be required for human therapeutic agents. The present study employed yeast coa6Δ cells to identify compounds that can efficiently transport copper across biological membranes and restore mitochondrial respiratory chain function over a broad range of concentrations. This approach identified elesclomol (ES), which was shown to reestablish subcellular copper homeostasis in copper-deficient cells, highlighting its therapeutic potential for human diseases of copper metabolism.
Results
A Targeted Search for Copper-Binding Agents Identifies ES as the Most Potent Pharmacological Agent in Rescuing Respiratory Defects of Yeast coa6Δ Cells.
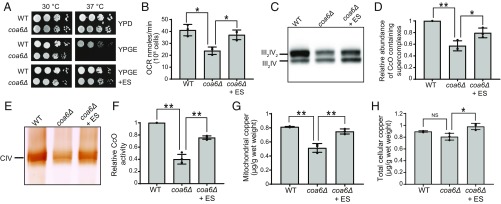
We tested a number of copper-binding pharmacological agents (20) for their ability to rescue respiratory deficient growth of coa6Δ cells. Among all of the compounds tested, ES was unique in that it rescued respiratory growth at low nanomolar concentrations without exhibiting overt toxicity over a broad range of concentrations (SI Appendix, Fig. S1). ES rescued the respiratory growth of coa6Δ cells with an ED50 of 0.8 nM (SI Appendix, Fig. S2). ES-mediated growth rescue of coa6Δ cells was also observed on solid growth medium containing a nonfermentable carbon source (Fig. 1A). Consistent with the rescue of respiratory growth, ES supplementation restored the oxygen consumption rate of coa6Δ cells to that of WT cells (Fig. 1B). To determine the biochemical basis for the observed respiratory rescue of coa6Δ cells, we measured the assembly and activity of CcO-containing mitochondrial respiratory chain supercomplexes by native PAGE blotting and in-gel activity assay, respectively. ES supplementation restored the abundance and activity of CcO-containing supercomplexes to near-WT levels (Fig. 1 C–F). We next tested the efficacy of ES in rescuing COA6 mutations observed in human patients by heterologous expression of yeast–human chimeric proteins with patient mutations (W26C, W33R, and E54X) in yeast coa6Δ cells (SI Appendix, Fig. S3A). Similar to coa6Δ cells, 10 nM ES or 10 μM copper supplementation rescued yeast coa6Δ cells expressing patient mutations (SI Appendix, Fig. S3B). These results show that ES is at least 1,000 times more potent than copper in rescuing the respiratory growth defect of yeast coa6Δ cells.
Fig. 1.
ES supplementation rescues CcO assembly defects by restoring mitochondrial copper levels of coa6Δ cells. (A) Serially diluted WT and coa6Δ cells were seeded on the indicated plates and incubated at 30 °C and 37 °C for 2 d (YPD) or 4 d (YPGE) before imaging. (B–F) WT, coa6Δ and coa6Δ cells supplemented with 20 nM ES were cultured in YP galactose medium until early stationary growth phase followed by (B) oxygen consumption rate (OCR) measurement, (C) BN-PAGE/Western analysis of mitochondrial respiratory chain supercomplexes containing Complex IV (CIV; also called CcO), (D) quantification of supercomplexes, (E) in-gel activity staining for Complex IV, and (F) quantification of CcO activity, (G) mitochondrial copper levels, and (H) total cellular copper content. Error bars represent mean ± SD (n = 3, two-tailed unpaired Student’s t test, *P < 0.05 and **P < 0.005). Data shown in A, C, and E are representative of at least three independent experiments.
A previous study has shown that ES scavenges copper from the culture medium, enters the cell as an ES–copper complex, and selectively accumulates in mitochondria, where it dissociates from copper (21). Consistent with this concept, we observed an almost complete rescue of mitochondrial copper levels in coa6Δ cells supplemented with ES (Fig. 1G). ES supplementation also moderately increased total cellular copper levels (Fig. 1H). To further corroborate that ES increases mitochondrial copper levels by actively transporting extracellular copper into the cells, we decreased copper availability in the extracellular compartment by cotreatment with ES and a known copper chelator, bathocuproine disulfonate (BCS). As expected, BCS treatment resulted in reduced respiratory growth of WT cells, which was rescued by cotreatment with ES, suggesting that ES is also able to overcome pharmacological copper deficiency by outcompeting BCS (SI Appendix, Fig. S4A). Moreover, ES-mediated rescue of coa6Δ was diminished in the presence of BCS (SI Appendix, Fig. S4B). To determine whether ES is able to bypass a mitochondrial copper transporter, Pic2, we performed ES supplementation in pic2Δ and coa6Δpic2Δ cells. Although, under the conditions tested, we did not observe a respiratory growth defect of pic2Δ cells, ES did rescue coa6Δpic2Δ cells, suggesting that this compound can deliver copper to the mitochondria independent of Pic2 function (SI Appendix, Fig. S4C). These results imply that ES-mediated rescue of CcO function is driven by its ability to transport extracellular copper to the mitochondria of coa6Δ cells.
ES Rescues Many Different Yeast Mutants with Impaired Copper Metabolism.
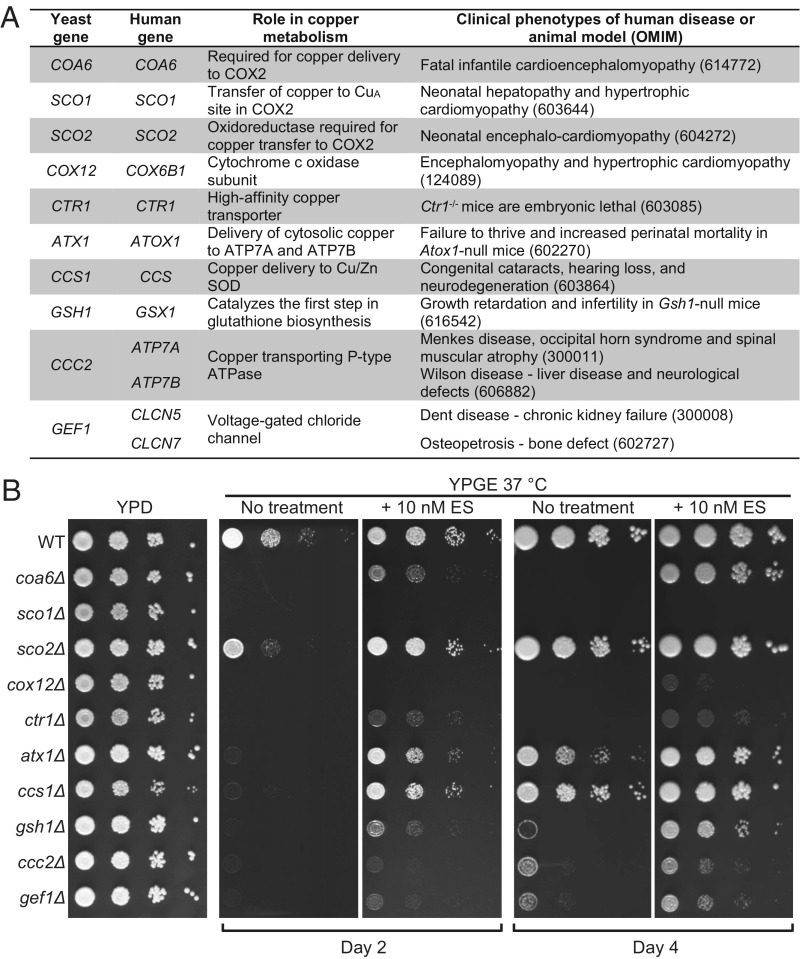
To test the specificity of ES-mediated rescue, we shortlisted a number of yeast mutants of genes required for maintaining cellular and mitochondrial copper homeostasis (1–3). We prioritized genes based on their evolutionary conservation, presence of pathogenic mutations in humans, and/or the existence of a related mouse phenotype (Fig. 2A). These yeast mutants showed a pronounced respiratory-deficient growth phenotype in nonfermentable media at 37 °C after 2 d of growth, which became less evident after 4 d of growth (Fig. 2B). Most of the yeast mutants were rescued with ES supplementation, albeit to different degrees, reflecting their distinct roles in cellular and mitochondrial copper homeostasis (Fig. 2B). ES failed to rescue sco1Δ cells, possibly because of the specific role of Sco1 as a metallochaperone in inserting copper into the Cox2 subunit of CcO (Fig. 2). We noticed that a higher concentration of ES is required to rescue ctr1Δ cells (SI Appendix, Fig. S5), which is consistent with the severe reduction in copper levels in cells lacking Ctr1 (22). Overall, these results suggest the broad applicability of ES in ameliorating defects of cellular and mitochondrial copper homeostasis.
Fig. 2.
ES can rescue the respiratory growth deficiency of yeast mutants of copper metabolism. (A) List of yeast genes and their human orthologs implicated in cellular and mitochondrial copper metabolism and the clinical phenotypes associated with mutations in the human or murine genes. (B) Serially diluted WT cells and the indicated mutants were spotted on YPD, YPGE, and YPGE supplemented with 10 nM of ES. The plates were incubated at 37 °C and allowed to grow for 2 d (YPD) or 2 d and 4 d (YPGE) before imaging.
ES Supplementation Rescues Levels of CcO Subunits in Mammalian Cell Lines with Genetic Defects in Copper Metabolism.
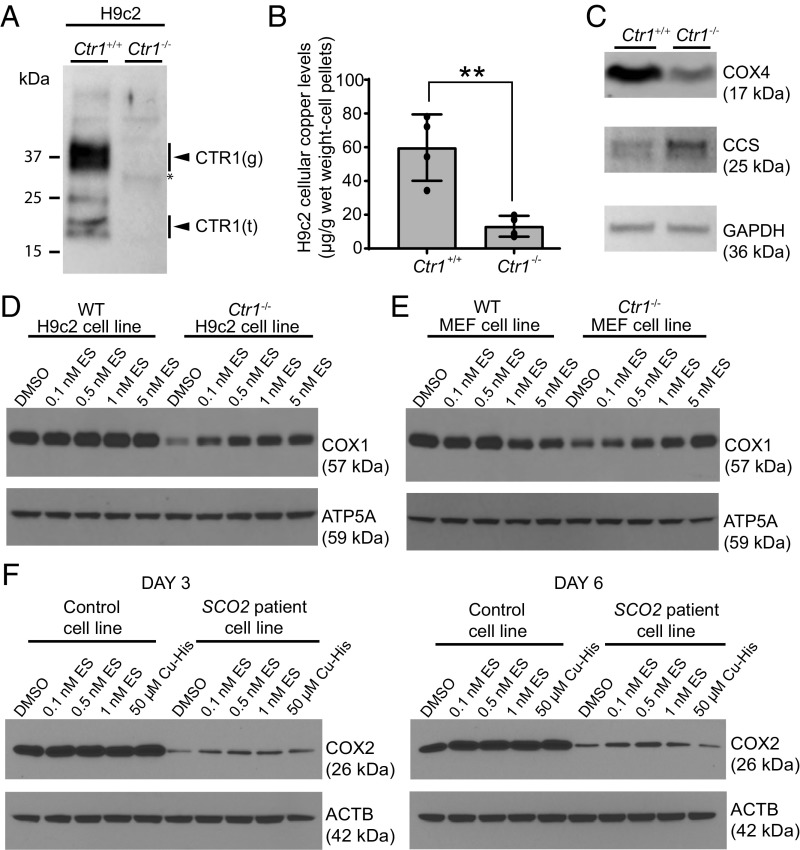
To expand on our findings in yeast and to test the efficacy of ES in mammalian cell culture models of copper deficiency, we constructed a Ctr1-KO rat H9c2 cardiomyocyte cell line by CRISPR/Cas9. The Ctr1−/− cell line was validated by demonstrating the loss of Ctr1 protein (Fig. 3A). As expected, the loss of Ctr1 led to an approximately fourfold decrease in the levels of intracellular copper (Fig. 3B) and a concomitant reduction in the levels of the CcO subunit COX4 (Fig. 3C). Notably, Ctr1−/− cells exhibited elevated levels of CCS, the copper chaperone of SOD1, which is known to increase during copper deficiency (Fig. 3C). We used this validated Ctr1−/− cell line to test the efficacy of ES in rescuing COX1, a copper-containing subunit of CcO. The loss of Ctr1 resulted in reduced levels of COX1 that were rescued by ES in a dose-dependent manner (Fig. 3D). Similarly, we also observed a dose-dependent rescue of COX1 levels in Ctr1−/− mouse embryonic fibroblasts (MEFs; Fig. 3E) (23). However, we noticed ES toxicity at higher doses, which was reflected in reduced COX1 levels in control MEFs (Fig. 3E). Finally, we tested the efficacy of ES in rescuing CcO defects in fibroblasts from a patient with SCO2 mutations that were previously shown to have a COX2 deficiency (17). Unlike copper supplementation, ES treatment was able to partially rescue the steady-state levels of COX2 in a dose- and time-dependent manner (Fig. 3F). Notably, ES toxicity occurs at a concentration 20× higher than the dose that rescued COX2 levels (Fig. 3F and SI Appendix, Fig. S6A). Consistent with the known anticarcinogenic activity of ES, we observed much more pronounced toxicity in immortalized cell lines (SI Appendix, Fig. S6A) compared with primary cultures of the same cell type (SI Appendix, Fig. S6B). Together, these results suggest that a low nanomolar concentration of ES is efficacious in rescuing mitochondrial copper deficiency and restoring CcO levels in mammalian cell lines with genetic defects in copper homeostasis.
Fig. 3.
ES supplementation rescues the steady-state levels of copper-containing subunits of CcO in mammalian cell lines with genetic defects in copper metabolism. (A) Immunoblot analysis of CTR1 in Ctr1+/+ and Ctr1−/− H9c2 cells. The arrowheads labeled “g” and “t” indicate the full-length glycosylated and truncated forms of CTR1, respectively. (B) Total copper levels measured by ICP-MS in H9c2 cells. Data are presented as mean ± SD (n = 4, two-tailed unpaired Student’s t test, **P < 0.001). (C) Immunoblot analysis of CCS, COX4, and GAPDH protein levels in Ctr1+/+ and Ctr1−/− H9c2 cells. GAPDH serves as a loading control. (D) The Ctr1+/+ and Ctr1−/− H9c2 rat cardiomyocytes and (E) MEFs were cultured for 3 d with the indicated doses of ES followed by Western analysis of COX1 protein levels. ATP5A is used as loading control. (F) Control (MCH46) and SCO2 patient cell lines were cultured for 3 or 6 d in the presence of the indicated concentrations of ES or a copper–histidinate complex (Cu-His) in DMEM with 10% FBS. The cellular COX2 levels were detected by SDS/PAGE/Western blot analysis. β-Actin (ACTB) was used as a loading control.
ES Supplementation Rescues Copper-Deficiency Phenotypes in Zebrafish Models.
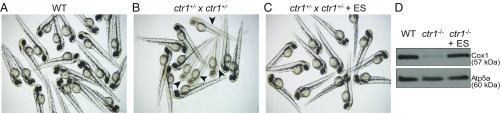
To determine whether ES can rescue phenotypes associated with copper deficiency in an intact developing vertebrate animal model, we utilized zebrafish embryos with a null mutation in the gene encoding the plasma membrane copper importer Ctr1. We chose zebrafish because of the ability to quickly monitor the pigmentation defect that arises as a result of the copper requirement of tyrosinase, an enzyme that catalyzes the critical step in melanin biosynthesis. WT zebrafish embryos have a characteristic melanin pigmentation pattern visible at 48 h post fertilization (hpf; Fig. 4A). To determine if ES can rescue copper-deficiency phenotypes in zebrafish, we incubated zebrafish embryos from heterozygous ctr1 crosses in 10 nM ES and compared these vs. untreated embryos. We found that the expected ∼25% of untreated embryos from ctr1 heterozygous crosses lacked melanin deposition (16 of 59; Fig. 4B), whereas all of the ES-treated embryos from the same crosses were pigmented (49 of 49; Fig. 4C). Similarly, we also observed rescue of the pigmentation defect at 100 nM ES, but the equivalent dose of copper failed to rescue this defect (SI Appendix, Fig. S7). ctr1−/− mutants also exhibited a CcO assembly defect that was likely a result of mitochondrial copper deficiency. To determine whether ES can rescue the observed CcO assembly defect, we grew clutches of embryos from heterozygous ctr1 crosses in the presence of ES until 10 d post fertilization (dpf) and measured levels of Cox1 in their mitochondrial extracts. Compared with WT embryos, the ctr1−/− mutants exhibited a severe reduction in Cox1 levels that was almost completely rescued by treatment with ES (Fig. 4D).
Fig. 4.
ES supplementation rescues pigmentation and the Cox1 deficiency of ctr1-KO zebrafish. (A–C) Clutches of embryos from a WT zebrafish and from a cross of a pair of ctr1+/− heterozygous zebrafish were imaged at 48 hpf following treatment with and without 10 nM ES. Arrows in B indicate homozygous ctr1−/− embryos with a pigmentation defect. (D) Mitochondria were isolated from 10-dpf WT zebrafish and homozygous ctr1−/− zebrafish treated with and without 10 nM ES. Mitochondrial samples were subjected to SDS/PAGE and immunoblotted using the Complex IV-specific antibody anti-Cox1. The mitochondrial protein Atp5a was used as a loading control.
To further establish the potential of ES to treat metabolic diseases involving defective copper delivery to the mitochondrion, we tested the efficacy of ES in rescuing phenotypes associated with coa6 knockdown in zebrafish embryos (18). First, we determined the maximal tolerable dose of ES for zebrafish embryos to be 100 nM (SI Appendix, Fig. S8A). Consistent with the mechanism of action of ES, we observed that cosupplementation of 100 nM ES with 100 nM of copper resulted in 100% lethality (SI Appendix, Fig. S8B). As shown previously (18), zebrafish embryos injected with the zfcoa6 translation-blocking morpholino exhibited pronounced morphological defects characterized by pericardial edema, smaller heads and eyes, and curved tails. The severity of these phenotypes was scored at four different time points (24, 48, 72, and 96 hpf; SI Appendix, Fig. S8C), and we observed rescue with 100 nM ES treatment as early as 48 hpf (SI Appendix, Fig. S8D). Given that one of the most striking features of Coa6 deficiency in this model is a pronounced cardiac edema and a decreased heart rate (18), we next determined whether ES treatment was able to rescue these phenotypes. Indeed, 100 nM ES treatment prevented pericardial edema and significantly increased the heart rate of Coa6-knockdown zebrafish embryos at 72 and 96 hpf without altering the heart rate of control embryos (SI Appendix, Fig. S8 E and F). These results demonstrate the efficacy of ES in rescuing phenotypes associated with copper deficiency in intact living vertebrate animals.
Discussion
Mitochondrial disorders of copper metabolism represent a subset of inborn errors of mitochondrial energy metabolism for which no therapy currently exists (24). A previous attempt to use direct copper supplementation as a therapeutic approach was unsuccessful (19), possibly because of stringent regulation of systemic copper levels. Thus, there is an unmet need to develop better copper delivery agents. With this goal in mind, we tested a number of clinically used pharmacological agents on a yeast mitochondrial disease model of COA6 deficiency and identified ES as the most potent and best tolerated compound capable of restoring mitochondrial function. Subsequent experiments on other yeast, murine, human, and zebrafish models established broad applicability of ES in treating cellular and mitochondrial copper deficiency. ES has undergone multiple human clinical trials, in which it has exhibited a favorable toxicity profile (25, 26); thus, our findings offer an exciting possibility of repurposing this anticancer drug for the treatment of disorders of copper metabolism.
Pharmacological interventions that alter the subcellular concentration and distribution of metals in a targeted manner could be of therapeutic benefit. For example, coadministration of copper with disulfiram, a Food and Drug Administration-approved drug, increased the activity of CcO in the brains of a mouse model of Menkes disease, a genetic disorder characterized by systemic copper deficiency (27). Similarly, CuII-ATSM has been shown to be efficacious in a transgenic mouse model of amyotrophic lateral sclerosis (28). However, a comparative study on the efficacy of these clinically used copper complexes in a model of copper deficiency is lacking. Therefore, our study identifying ES as the most potent pharmacological agent among many of the clinically used copper chelators and ionophores represents an important advance (SI Appendix, Fig. S1). The physicochemical properties of ES, including its binding affinity, its specificity for copper, and the redox potential of the ES–copper complex, allow it to mimic a copper metallochaperone (29). Higher affinity of ES for copper (II) compared with copper (I) allows it to scavenge copper from the extracellular environment, where copper is more likely to exist in an oxidized state (29). ES is unlikely to strip copper from intracellular proteins because of the higher prevalence of copper in the reduced state in the intracellular environment.
Although a previous study shows selective enrichment of the ES–copper complex in the mitochondria (21), the rescue of yeast atx1Δ and ccc2Δ, which have impaired copper homeostasis in the Golgi compartment (1), suggests that ES is also able to deliver copper to other subcellular compartments (Fig. 2). Indeed, the rescue of the pigmentation defect observed in ctr1−/− zebrafish caused by a defective secretory pathway enzyme also indicates that ES could increase copper levels in other organelles (Fig. 4). Finally, the rescue of the respiratory growth defect of ccs1Δ cells, which are deficient in a metallochaperone for the cytosolic protein Sod1, suggests that ES is also able to elevate cytosolic copper levels (Fig. 2). Although the mechanism by which ES is able to deliver copper to different subcellular compartments is not clear, it is possible that some of the ES–copper complexes dissociate before reaching mitochondria, thereby releasing free copper in the cytoplasm. Alternatively, excess mitochondrial copper may “leak” out of the mitochondria and become available to other organelles. Notwithstanding the mechanism, this interesting observation suggests that ES could be efficacious in the treatment of more common disorders of copper deficiency, including Menkes disease.
Materials and Methods
Reagents.
All copper-binding compounds were purchased from Sigma-Aldrich except for ES, which was purchased from Selleckchem. The yeast–human hybrid (hyCOA6) gene construct was codon-optimized for yeast and synthesized by using GeneArt Gene Synthesis (Life Technologies). The hybrid gene hyCOA6 was cloned into pRS416 plasmid under the control of the yeast Coa6 native promoter. COA6 patient mutations were introduced by site-directed mutagenesis (QuikChange Lightning; Agilent Technologies) by using hyCOA6 as a template. All primers used in this study are listed in SI Appendix, Fig. S9. All constructs were sequence-verified.
Yeast Strains and Culture Conditions.
Saccharomyces cerevisiae strains used in this study are listed in SI Appendix, Fig. S10. The authenticity of yeast strains was confirmed by PCR as well as by replica plating on dropout plates. Yeast cells were cultured in standard YP growth media including YPD (1% yeast extract, 2% peptone, and 2% glucose), YPGal (2% galactose), YPGE (3% glycerol + 1% ethanol), or synthetic media (SC glucose). For qualitative growth measurement, 10-fold serial dilutions of overnight cultures were spotted on YPD or YPGE plates and incubated at 30 °C and 37 °C for the indicated period. Growth in liquid media was measured spectrophotometrically at 600 nm.
Mammalian Cell Culture.
The human control MCH46 and SCO2 patient fibroblasts as well as the rat H9c2 control and Ctr1−/− cardiomyocytes were cultured in high-glucose DMEM supplemented with 10% FBS (Sigma) and 1 mM sodium pyruvate (Life Technologies). The MEFs were cultured in DMEM 10% FBS, 1 mM sodium pyruvate, 1× Minimum Essential Medium nonessential amino acids (no. 11140; Life Technologies), 50 μg/mL uridine, and 1× penicillin/streptamycin glutamine (no. 10378; Life Technologies). All cell lines were cultured under 5% CO2 at 37 °C and were treated with indicated concentrations of ES for 3–6 d before harvesting. Whole-cell protein was extracted in lysis buffer (BP-115; Boston BioProducts) supplemented with protease inhibitor mixture (Roche Diagnostics), and the protein concentrations were determined by the bicinchoninic acid assay (Thermo Fisher Scientific).
Construction of a Ctr1-KO Rat H9c2 Cell Line.
A CRISPR/Cas9-mediated Ctr1-KO rat H9c2 cell line was generated by using lentiCRISPR v2 plasmid (no. 52961; Addgene). A guide RNA sequence targeting exon 1 of the Ctr1 gene was identified by using the online CRISPR design tool (crispor.tefor.net). Forward (5′ CACCGTGGTGATGTTGTCGTCCGTG 3′) and reverse (5′ AAACCACGGACGACAACATCACCAC 3′) oligonucleotides were inserted into lentiCRISPR v2 plasmid. The transfection was performed by using PolyJet (SignaGen Laboratories). Two days after transfection, cells were plated on a 96-well plate containing 5 μg/mL puromycin selection media. Each colony formed from single cells was isolated and established in medium without puromycin. Disruption of the Ctr1 gene was confirmed by genomic DNA sequencing.
Oxygen Consumption Measurement.
For measurements of respiration rates, cells were grown to late log phase in YPGal medium and then washed, counted, and resuspended in fresh YPGal medium at 108 cells per milliliter. The rate of oxygen consumption was then measured at 30 °C by using the Oxytherm system (Hansatech). Cyanide-sensitive respiration was calculated after the addition of 1 mM KCN, and the cyanide-insensitive respiration was subtracted from the total respiration.
Immunoblotting and In-Gel Activities.
SDS/PAGE and Blue Native PAGE (BN-PAGE) were performed to separate denatured and native protein complexes, respectively. For SDS/PAGE, mitochondrial lysate (20 μg) was separated on NuPAGE 4–12% Bis-Tris gels (Life Technologies). For BN-PAGE, yeast mitochondria were solubilized in buffer containing 1% digitonin (Life Technologies) by incubating for 15 min at 4 °C. Clear supernatant was collected after a 20,000 × g (30 min, 4 °C) spin, 50× G-250 sample additive was added, and 20 μg of protein was loaded on a 3–12% native PAGE Bis-Tris gel (Life Technologies). Following wet transfer, the membrane was probed with the following primary antibodies: for yeast proteins, Cox2 1:50,000 (no. 110 271; Abcam) and porin 1:50,000 (110 326; Abcam); and for mammalian proteins, COX1 (no. 14705; Abcam), COX2 (no. 110258; Abcam), CTR1, COX4 (A21348; Thermo Fisher Scientific), CCS (FL-274; Santa Cruz Biotechnology), GAPDH (G9545; Sigma), ATP5A (14748; Abcam), and β-actin (A2228; Sigma). Western blots were developed by using Western Lightning Plus-ECL (PerkinElmer). In-gel activity assay for mitochondrial respiratory chain complex IV were performed as described previously (30).
Cellular and Mitochondrial Copper Measurements.
Cellular and mitochondrial copper levels were measured by using a PerkinElmer DRC II inductively coupled plasma (ICP) mass spectrometer. Intact yeast cells and isolated mitochondrial pellets were washed with 100 μM EDTA-containing water, weighed, and digested with 40% nitric acid (TraceSELECT; Sigma) at 90 °C for 18 h. Samples were diluted in ultrapure metal-free water (TraceSELECT; Sigma) and analyzed by ICP-MS. Copper standard solutions were prepared by appropriate dilutions of commercially available mixed metal standards (BDH Aristar Plus). Copper concentrations in mammalian cells were also measured by ICP-MS.
Zebrafish Experiments.
All zebrafish studies were approved by the Marine Biological Laboratory Institutional Animal Care and Use Committee (no. 16–38). WT AB strain and ctr1 heterozygous zebrafish were maintained and crossed by using standard methods. Embryos were staged and raised in egg water at 28.5 °C. For drug treatments, embryos from ctr1 heterozygous crosses were incubated in 10 nM ES diluted in egg water beginning at 3 hpf. For imaging live embryos at 48 hpf, representative embryos of each sample were anesthetized in Tricaine, and imaging was performed on an Olympus SZX12 stereomicroscope. For immunoblots, zebrafish mitochondrial protein was prepared from 10-dpf larvae. Mitochondrial lysate was separated by SDS/PAGE on 4–15% Mini-Protean TGX Gels (Bio-Rad) followed by Western blot analysis using anti-Cox1 at 1:5,000 (anti-MTCO1; ab14705; Abcam) and anti-Atp5a at 1:5,000 (ab110273; Abcam). The morpholino-based experiments were performed as described previously (18).
Supplementary Material
Acknowledgments
We thank members of the laboratory of V.M.G., Dr. Richard Gomer, and Dr. Paul Lindahl for their valuable comments in the preparation of this manuscript. This work was supported by National Institutes of Health Awards R01GM111672 (to V.M.G.), R01 DK110195 (to B.-E.K.), and DK 44464 (to J.D.G.); Welch Foundation Grant A-1810 (to V.M.G.); and Canadian Institutes of Health Research Operating Grant MOP 133562 (to S.C.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: S.S. and V.M.G. are listed as inventors on a provisional patent application filed by Texas A&M University.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806296115/-/DCSupplemental.
References
- 1.Smith AD, Logeman BL, Thiele DJ. Copper acquisition and utilization in fungi. Annu Rev Microbiol. 2017;71:597–623. doi: 10.1146/annurev-micro-030117-020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timón-Gómez A, et al. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin Cell Dev Biol. 2018;76:163–178. doi: 10.1016/j.semcdb.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker ZN, Cobine PA, Leary SC. The mitochondrion: A central architect of copper homeostasis. Metallomics. 2017;9:1501–1512. doi: 10.1039/c7mt00221a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vest KE, Leary SC, Winge DR, Cobine PA. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J Biol Chem. 2013;288:23884–23892. doi: 10.1074/jbc.M113.470674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobine PA, Ojeda LD, Rigby KM, Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem. 2004;279:14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 6.Cobine PA, Pierrel F, Bestwick ML, Winge DR. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem. 2006;281:36552–36559. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 7.Horng Y-C, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J Biol Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 8.Hiser L, Di Valentin M, Hamer AG, Hosler JP. Cox11p is required for stable formation of the Cu(B) and magnesium centers of cytochrome c oxidase. J Biol Chem. 2000;275:619–623. doi: 10.1074/jbc.275.1.619. [DOI] [PubMed] [Google Scholar]
- 9.Leary SC, et al. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet. 2004;13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh A, et al. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum Mol Genet. 2016;25:660–671. doi: 10.1093/hmg/ddv503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheu-Grau D, et al. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metab. 2015;21:823–833. doi: 10.1016/j.cmet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Stroud DA, et al. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA-encoded COX2. Hum Mol Genet. 2015;24:5404–5415. doi: 10.1093/hmg/ddv265. [DOI] [PubMed] [Google Scholar]
- 13.Bode M, et al. Redox-regulated dynamic interplay between Cox19 and the copper-binding protein Cox11 in the intermembrane space of mitochondria facilitates biogenesis of cytochrome c oxidase. Mol Biol Cell. 2015;26:2385–2401. doi: 10.1091/mbc.E14-11-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulou LC, et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 15.Valnot I, et al. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am J Hum Genet. 2000;67:1104–1109. doi: 10.1016/s0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baertling F, et al. Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum Mutat. 2015;36:34–38. doi: 10.1002/humu.22715. [DOI] [PubMed] [Google Scholar]
- 17.Jaksch M, et al. Cytochrome c oxidase deficiency due to mutations in SCO2, encoding a mitochondrial copper-binding protein, is rescued by copper in human myoblasts. Hum Mol Genet. 2001;10:3025–3035. doi: 10.1093/hmg/10.26.3025. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, et al. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum Mol Genet. 2014;23:3596–3606. doi: 10.1093/hmg/ddu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freisinger P, Horvath R, Macmillan C, Peters J, Jaksch M. Reversion of hypertrophic cardiomyopathy in a patient with deficiency of the mitochondrial copper binding protein Sco2: Is there a potential effect of copper? J Inherit Metab Dis. 2004;27:67–79. doi: 10.1023/B:BOLI.0000016614.47380.2f. [DOI] [PubMed] [Google Scholar]
- 20.Helsel ME, Franz KJ. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans. 2015;44:8760–8770. doi: 10.1039/c5dt00634a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai M, et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic Biol Med. 2012;52:2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem. 2002;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 24.Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: What is new and what challenges remain? Science. 2015;349:1494–1499. doi: 10.1126/science.aac7516. [DOI] [PubMed] [Google Scholar]
- 25.Hedley D, et al. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leuk Lymphoma. 2016;57:2437–2440. doi: 10.3109/10428194.2016.1138293. [DOI] [PubMed] [Google Scholar]
- 26.O’Day SJ, et al. Final results of phase IIISYMMETRY study: Randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J Clin Oncol. 2013;31:1211–1218. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- 27.Bhadhprasit W, Kodama H, Fujisawa C, Hiroki T, Ogawa E. Effect of copper and disulfiram combination therapy on the macular mouse, a model of Menkes disease. J Trace Elem Med Biol. 2012;26:105–108. doi: 10.1016/j.jtemb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Roberts BR, et al. Oral treatment with Cu(II)(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2014;34:8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav AA, Patel D, Wu X, Hasinoff BB. Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II) J Inorg Biochem. 2013;126:1–6. doi: 10.1016/j.jinorgbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Wittig I, Karas M, Schägger H. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol Cell Proteomics. 2007;6:1215–1225. doi: 10.1074/mcp.M700076-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.