Significance
Organisms’ responses to climate change can result in altered species interactions, with cascading effects on communities and ecosystems. Understanding these processes is especially relevant in the rapidly warming Arctic, where faster decomposition of stored soil carbon is expected to result in positive carbon feedbacks to the atmosphere. We provide evidence that warmer temperatures alter the cascading effects of wolf spiders, an abundant and widespread predator, on ecosystem functioning. Specifically, we find that warming tends to reverse the effect of high spider densities on fungal-feeding Collembola and ultimately leads to slower decomposition rates. Our work demonstrates that climate change can alter the nature of predator effects on decomposition, resulting in unexpected changes in ecosystem function with potentially important global implications.
Keywords: Arctic, predator, decomposition, aboveground–belowground, trophic interactions
Abstract
Predators can disproportionately impact the structure and function of ecosystems relative to their biomass. These effects may be exacerbated under warming in ecosystems like the Arctic, where the number and diversity of predators are low and small shifts in community interactions can alter carbon cycle feedbacks. Here, we show that warming alters the effects of wolf spiders, a dominant tundra predator, on belowground litter decomposition. Specifically, while high densities of wolf spiders result in faster litter decomposition under ambient temperatures, they result, instead, in slower decomposition under warming. Higher spider densities are also associated with elevated levels of available soil nitrogen, potentially benefiting plant production. Changes in decomposition rates under increased wolf spider densities are accompanied by trends toward fewer fungivorous Collembola under ambient temperatures and more Collembola under warming, suggesting that Collembola mediate the indirect effects of wolf spiders on decomposition. The unexpected reversal of wolf spider effects on Collembola and decomposition suggest that in some cases, warming does not simply alter the strength of top-down effects but, instead, induces a different trophic cascade altogether. Our results indicate that climate change-induced effects on predators can cascade through other trophic levels, alter critical ecosystem functions, and potentially lead to climate feedbacks with important global implications. Moreover, given the expected increase in wolf spider densities with climate change, our findings suggest that the observed cascading effects of this common predator on detrital processes could potentially buffer concurrent changes in decomposition rates.
Despite general agreement that species interactions will be affected by climate change (1, 2), surprisingly few studies have explored the ways in which climate-modified trophic cascades might affect ecosystem functioning (reviewed in ref. 3). Individual and population-level responses to altered climatic conditions could have cascading effects that either amplify or dampen the impacts of existing trophic cascades on ecosystems. However, interactive effects between climate change and these population-level responses could also lead to completely new community structures with subsequent impacts on function. It is increasingly apparent that changes in community-scale dynamics can influence important ecosystem processes, such as productivity, nutrient cycling, and decomposition (4, 5). Thus, understanding how global change will shape both community interactions and function is important when predicting the effects of future climate change.
Shifts in aboveground–belowground trophic interactions due to climate change are particularly relevant in the Arctic, a region that is warming rapidly (6) and stores a large amount of soil organic carbon (C) (7, 8). Rapid warming is facilitating increased belowground microbial respiration from thawing permafrost more than it is increasing plant uptake of C, thereby causing the Arctic to become a source of atmospheric C and accelerating the rate of global climate change (9). Prior studies have assessed the feedbacks between the Arctic terrestrial ecosystem and climate change by measuring the balance between plant community and microbial responses to warmer temperatures (reviewed in ref. 10). The extent to which these responses may be affected by interactions with organisms at other trophic levels, which are also responding to warming (e.g., detritivores, herbivores, pollinators, predators), has been largely overlooked (but see refs. 11–13 for discussion of effects of vertebrate herbivores on plant communities under warming). In particular, there has been little consideration of how changes in trophic interactions between belowground microbes and their associated consumers, which are both sensitive to warming (14–19), will affect ecosystem functioning in the Arctic (but see refs. 14, 20).
The composition and activity of microbial communities are often modified by soil micro- and macroinvertebrates (5), which are themselves regulated by higher level predators, such as surface-dwelling spiders (21). At our study site in the Alaskan Arctic, wolf spiders are extremely abundant at the soil surface (22, 23) and outweigh larger mammalian predators, such as wolves, by several orders of magnitude (SI Appendix), making them one of the dominant predators of the tundra community. In temperate forests and agroecosystems, wolf spider predation has indirect effects on key ecosystem processes, including plant productivity (24), nutrient cycling (25, 26), and decomposition (27–29). As in temperate systems, Arctic wolf spiders are generalist feeders that prey upon herbivores, detritivores, and other predators (30–32), suggesting that they could indirectly influence these processes in the Arctic as well. While experimental work has failed to detect changes in plant performance due to Arctic wolf spider predation on herbivores (33), more recent work suggests that these predators may obtain the majority of their energy from detritivorous prey [e.g., Collembola (22)], and therefore are more likely to have measurable indirect effects on processes such as decomposition and nutrient cycling. Currently, Arctic wolf spiders are responding to earlier snowmelt dates brought on by climate change by becoming larger (34) and hence more fecund (35). Because bigger and more abundant spiders could exert stronger predation pressure on belowground prey, these changes could have important consequences for microbial activity and nutrient cycling in the tundra.
Arctic wolf spiders and the food web to which they belong provide us with an ideal system to study how trophic interactions might alter the predicted effects of climate change on ecosystem functioning. Here, we explore the role of Arctic wolf spiders in mediating the response of the belowground community to warming. Specifically, using a fully factorial experimental design, we investigated the extent to which warming and the expected associated increase in wolf spider densities can impact lower trophic levels, decomposition rates, and soil nutrients over two summers in a well-studied area of moist acidic tundra in northern Alaska. Over the second summer, we measured densities of Collembola and oribatid mites, two of the dominant detritivore groups (22); microbial biomass (fungal and bacterial); and abundances of surface-active and soil-dwelling intermediate predators (i.e., other than wolf spiders) that could either serve as alternative prey for wolf spiders or influence detritivore densities. To assess belowground function, we measured decomposition and nitrogen (N) loss from litter. We also measured soil nutrient availability to identify the potential indirect effects that wolf spiders may have on plant nutrient uptake.
Although we measured various components of the belowground arthropod food web, our hypotheses of potential cascading effects of wolf spiders were based upon the premise that Collembola, in particular, are an important prey item of wolf spiders (31). Thus, we expected to see a direct negative effect of wolf spiders on Collembola such that Collembola densities would decrease when more wolf spiders were present (hypothesis 1). Collembola activity can affect decomposition rates directly through consumption of detritus or indirectly through consumption of the microbial community. At our site, Collembola likely consume primarily fungi, which have a larger role in decomposition and nutrient cycling in comparison to bacteria (36). Therefore, we expected that lower Collembola densities would release fungi from predation and would indirectly result in faster microbial decomposition of litter (hypothesis 2). Because cascading effects of top predators on soil nutrient availability can be context-dependent with positive, neutral, or negative effects (e.g., refs. 37–40), we did not have a priori expectations about soil nutrient responses to higher densities of wolf spiders. Based upon previous reports that warmer temperatures increased wolf spider activity (41) and likely increased overall predation rates (42), we expected that the overall effects of wolf spiders on the structure and function of the detrital community would be strengthened by warming (hypothesis 3).
Results
We maintained 1.5-m-diameter experimental mesocosms over two consecutive summers in the northern Alaska tundra to measure the effects of wolf spider densities on belowground community structure and function. We manipulated wolf spider densities in the mesocosms at the beginning of each summer and actively maintained density levels throughout the season. Half of the plots were warmed using International Tundra Experiment (ITEX) open-topped passive warming chambers (OTCs) (43). Wolf spider density treatments included low density, control density, and high density; high-density treatments had approximately twice the densities of the average early season densities from the control treatments. Warming treatments were either ambient temperature or warmed.
Given the pace at which the Arctic is warming and the fact that wolf spider densities may be higher in the future, our primary interest for this experiment was in capturing potential interactions between the wolf spider density and warming treatments. We used a series of mixed effects models to investigate these potential interactive treatment effects on belowground community structure and function. In each model, either average soil moisture or litter moisture content was included as a fixed effect and experimental block was included as a random effect. Post hoc tests (e.g., Tukey) are not available for general linear mixed models with interaction terms; thus, for those models that showed significant interactive effects on the response variable, we split the models by ambient temperature and warming treatments to investigate pairwise differences under the three spider density treatments (low, control, or high).
Effects of Wolf Spider Density and Warming on Community Structure.
To explore treatment effects on community structure, we focused on several important groups within the belowground food web, including Collembola, oribatid mites, predatory mites, and other soil-dwelling and surface-active intermediate predators (including centipedes; unidentifiable juvenile spiders; and other small spiders from the Dysderidae, Thomisidae, Linyphiidae, and Gnaphosidae families). Among these groups, Collembola, in particular, are key prey for wolf spiders (31). Through their consumption of detritus and fungi, Collembola and oribatid mites alter soil processes, such as decomposition and nutrient cycling (44); therefore, both have important functional roles within the tundra ecosystem (22). Our analyses of treatment effects on these organisms relied on individual mixed effects models, where the response variable was the average density of a given microarthropod group (i.e., Collembola, oribatid mites, predatory mites, soil-dwelling intermediate predators) from samples taken from the upper organic layer of soil in June and July of the second experimental summer. In the Collembola model only, Collembola order (Entomobrymorpha, Poduromorpha, and Symphypleona) was included as a fixed effect and random effects included study plot nested within experimental block.
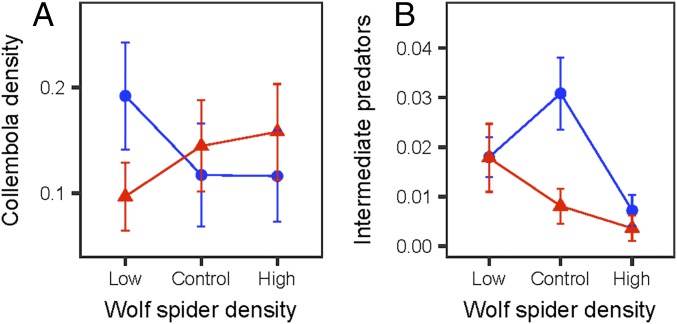
We found that there was a significant interactive effect of the wolf spider density and warming treatments on Collembola densities (Fig. 1A and Table 1). Plots across all treatments also contained significantly fewer Collembola from the order Symphypleona than from the orders Entomobryomorpha (P < 0.0001; Table 1) and Poduromorpha (P < 0.0001). Average soil moisture was a marginally significant predictor of Collembola densities, whereby plots with lower soil moisture trended toward having more Collembola (P = 0.063; Table 1). SI Appendix, Table S2 contains the mean ± SE of Collembola density by treatment.
Fig. 1.
Interactive treatment effects of altered wolf spider densities and warming on Collembola densities (A; individuals per cubic centimeter) and surface-active intermediate predators (B; log average total abundance per plot) within the experimental plots. Blue circles are from ambient temperature plots, and red triangles are from the experimentally warmed plots. Points are mean treatment effects, and error bars are SEs. Full model results are shown in Table 1 and SI Appendix, Table S5.
Table 1.
Effects of wolf spider density and warming treatments on community structure
| Response | Fixed effects terms | Estimate | SE | df | t | P |
| Collembola | Intercept | 0.243 | 0.029 | 77 | 8.225 | 0.000 |
| Soil moisture | −0.001 | 0.001 | 77 | −1.887 | 0.063 | |
| Control SD | −0.050 | 0.033 | 77 | −1.520 | 0.133 | |
| High SD | −0.048 | 0.033 | 77 | −1.454 | 0.150 | |
| Warming | −0.064 | 0.033 | 77 | −1.930 | 0.057 | |
| Poduromorpha | 0.006 | 0.023 | 77 | 0.249 | 0.804 | |
| Symphypleona | −0.155 | 0.023 | 77 | −6.832 | 0.000 | |
| Control SD × warming | 0.085 | 0.046 | 77 | 1.843 | 0.069 | |
| High SD × warming | 0.098 | 0.046 | 77 | 2.132 | 0.036 | |
| Oribatid mites | Intercept | 0.982 | 0.202 | 19 | 4.863 | 0.000 |
| Soil moisture | −0.008 | 0.004 | 19 | −1.936 | 0.068 | |
| Control SD | 0.107 | 0.251 | 19 | 0.425 | 0.675 | |
| High SD | −0.203 | 0.253 | 19 | −0.802 | 0.433 | |
| Warming | −0.186 | 0.252 | 19 | −0.739 | 0.469 | |
| Control SD × warming | 0.180 | 0.355 | 19 | 0.509 | 0.617 | |
| High SD × warming | 0.286 | 0.353 | 19 | 0.810 | 0.428 | |
| Predatory mites | Intercept | 0.134 | 0.044 | 19 | 3.036 | 0.007 |
| Soil moisture | 0.001 | 0.001 | 19 | 1.118 | 0.278 | |
| Control SD | −0.095 | 0.055 | 19 | −1.730 | 0.100 | |
| High SD | −0.051 | 0.055 | 19 | −0.924 | 0.367 | |
| Warming | −0.069 | 0.055 | 19 | −1.253 | 0.226 | |
| Control SD × warming | 0.093 | 0.077 | 19 | 1.202 | 0.244 | |
| High SD × warming | 0.106 | 0.077 | 19 | 1.380 | 0.184 | |
| Intermediate predators | Intercept | 0.005 | 0.002 | 19 | 2.786 | 0.012 |
| (soil-dwelling) | Soil moisture | 0.000 | 0.000 | 19 | −0.505 | 0.620 |
| Control SD | 0.000 | 0.002 | 19 | 0.035 | 0.972 | |
| High SD | 0.001 | 0.002 | 19 | 0.247 | 0.808 | |
| Warming | −0.001 | 0.002 | 19 | −0.343 | 0.735 | |
| Control SD × warming | −0.002 | 0.003 | 19 | −0.677 | 0.506 | |
| High SD × warming | 0.001 | 0.003 | 19 | 0.409 | 0.687 | |
| Intermediate predators | Intercept | 0.026 | 0.008 | 19 | 3.384 | 0.003 |
| (surface-active) | Soil moisture | 0.000 | 0.000 | 19 | −2.003 | 0.060 |
| Control SD | 0.017 | 0.009 | 19 | 1.806 | 0.087 | |
| High SD | −0.006 | 0.010 | 19 | −0.627 | 0.538 | |
| Warming | 0.004 | 0.010 | 19 | 0.465 | 0.647 | |
| Control SD × warming | −0.028 | 0.013 | 19 | −2.113 | 0.048 | |
| High SD × warming | −0.009 | 0.013 | 19 | −0.641 | 0.529 | |
| Fungal biomass | Intercept | 1.668 | 1.881 | 16 | 0.886 | 0.389 |
| Soil moisture | 0.121 | 0.047 | 16 | 2.563 | 0.021 | |
| Control SD | −0.513 | 2.210 | 16 | −0.232 | 0.819 | |
| High SD | 0.673 | 2.228 | 16 | 0.302 | 0.767 | |
| Warming | 0.677 | 2.187 | 16 | 0.309 | 0.761 | |
| Control SD × warming | −2.266 | 3.051 | 16 | −0.743 | 0.468 | |
| High SD × warming | −4.330 | 3.160 | 16 | −1.370 | 0.190 | |
| Bacterial biomass | Intercept | −0.009 | 0.017 | 18 | −0.495 | 0.626 |
| Soil moisture | 0.001 | 0.000 | 18 | 1.838 | 0.083 | |
| Control SD | −0.008 | 0.021 | 18 | −0.379 | 0.709 | |
| High SD | −0.002 | 0.022 | 18 | −0.091 | 0.929 | |
| Warming | −0.009 | 0.021 | 18 | −0.416 | 0.683 | |
| Control SD × warming | 0.009 | 0.030 | 18 | 0.295 | 0.772 | |
| High SD × warming | 0.058 | 0.030 | 18 | 1.924 | 0.070 |
Mixed effects model results of community response variables, as predicted by wolf spider density (SD; reference category: low SD), warming (reference category: ambient temperature), and average soil moisture. All community response variables were measured in the field during the second summer of experimental treatment. Collembola, oribatid mites, predatory mites, and soil-dwelling intermediate predators were extracted from the upper organic soil layer; densities (individuals per cubic centimeter of soil) are expressed as the average from the June and July sampling periods. Surface-active intermediate predator abundance is the average total abundance from several bouts of live pitfall trapping. Fungal and bacterial biomass (mg per gram of soil) were sampled from the upper organic soil layer. Model results with the reference category for the wolf SD treatment as the control SD are shown in SI Appendix, Table S5.
As a post hoc test to investigate the pairwise interactive treatment effects on Collembola, we ran separate models for the Collembola data under ambient temperatures and under warming using a Bonferroni correction (Pcritical = 0.01; Materials and Methods). At this level, there was not enough power to detect differences in Collembola densities between the three wolf spider density treatments (SI Appendix, Table S4). However, we did observe that the addition of wolf spiders tended to reduce Collembola densities under ambient conditions, as might be expected if wolf spiders were consuming Collembola (Fig. 1A and SI Appendix, Table S4). In experimentally warmed plots, there was the opposite trend: Plots with high wolf spider densities tended to have more Collembola than plots with lower wolf spider densities (Fig. 1A and SI Appendix, Table S4).
We did not find significant interactions between the wolf spider density and warming treatments on densities of any of the other measured groups of soil-dwelling microarthropods, including oribatid mites, predatory mites, and intermediate predators (Table 1 and SI Appendix, Tables S2 and S5). Moreover, none of these groups showed significant variation in association with either of the experimental treatments when models were simplified by eliminating nonsignificant terms on a one-by-one basis. Soil moisture had a marginally negative effect on oribatid mites but was not related to densities of predatory mites or soil-dwelling intermediate predators (Table 1).
Several other surface-active, intermediate predators within this community (including crab spiders, linyphiid spiders, unidentifiable juvenile spiders, and centipedes; hereafter referred to as intermediate predators) could serve as either alternative prey for wolf spiders or predators of Collembola. We therefore fitted a separate mixed effects model to explore the treatment effects on the average total abundance of these surface intermediate predators throughout the second summer season. We found that the average number of surface intermediate predators observed in our plots was also subject to interactive treatment effects in our experiment (Fig. 1B and Table 1; mean abundances by treatment are shown in SI Appendix, Table S2). Again, we ran separate models using a Bonferroni correction (Pcritical = 0.01) to explore the pairwise effects of the wolf spider density treatments in ambient temperature and warmed plots. Although there was not enough power to detect treatment differences here either, we found that regardless of warming treatment, as wolf spider density increased, there tended to be fewer intermediate predators (all P values >0.0167; Fig. 1B and SI Appendix, Table S4). The exception to this was in ambient temperature plots with control densities of wolf spiders, which tended to have more intermediate predators than those from the other wolf spider treatments (Fig. 1B and SI Appendix, Table S4).
Similar models to those described above did not detect any significant differences in belowground fungal or bacterial biomass under the warming or wolf spider density treatments (all P values >0.07; Table 1; mean biomass by treatment is shown in SI Appendix, Table S2). Fungal biomass was higher in plots with higher soil moisture (Table 1).
Effects of Wolf Spider Density and Warming on Decomposition, Litter N, and Available Soil Nutrients.
We used litter bags to measure the effects of wolf spiders and warming on litter decomposition and litter N loss using locally collected leaves of the dominant plant, Eriophorum vaginatum. To isolate where wolf spiders might have the largest indirect effects, one litter bag was placed on the soil surface and another was buried in the litter layer just below the moss surface (ca. 5–10 cm) in each plot. Litter bags were collected 14 mo after the experimental start date, at which point remaining litter was weighed and analyzed for total N content. We also measured treatment effects on available soil N, phosphorus (P), and potassium (K) using ion exchange membranes that were incubated over 6 wk during the second summer of the experiment. We fitted linear mixed effects models to estimate interactive treatment effects of wolf spider density and warming on litter decomposition (percent litter mass remaining from initial amount); litter percent N (in relation to initial litter N content); and total available soil N [total N, ammonium (NH4+), and nitrate (NO3−)], P, and K. Litter moisture content was included as a covariate in the litter-related models, while average soil moisture was used instead in the soil nutrient models; experimental block was included as a random effect in all models.
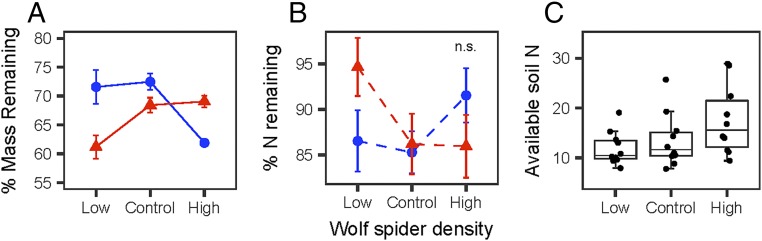
Within the litter layer, we found that there were significant interactive treatment effects of wolf spider density and warming on decomposition (Fig. 2A, Table 2, and SI Appendix, Table S2). Under ambient temperatures and at high spider densities, we observed faster litter decomposition rates. However, experimentally warmed plots with high spider densities experienced less litter decomposition than those with low spider densities. Higher litter moisture content was also associated with faster decomposition in the litter layer. When models were split using only ambient temperature or warmed plots to investigate pairwise effects of the wolf spider density treatments, we found that the rate of belowground decomposition was significantly affected by wolf spider densities under both temperature treatments (SI Appendix, Table S4). Specifically, under ambient temperature, decomposition was significantly faster in plots with high densities of wolf spiders than in those with control densities of wolf spiders (P = 0.0103; Fig. 2A and SI Appendix, Table S4). Under warming, decomposition was significantly slower under control and high wolf spider densities than under low wolf spider densities (control/low P = 0.0041, high/low P = 0.0034; Fig. 2A and SI Appendix, Table S4). There were no significant treatment effects on N loss or gain from the belowground litter bags (Fig. 2B and Table 2).
Fig. 2.
Effects of altered wolf spider densities and experimental warming on decomposition in the litter layer (A; percent litter mass remaining from the initial amount), percent N remaining in litter (B; in relation to initial N content of litter), and total available soil N (C; milligrams of NH4+ per 10 cm−2) over the peak 6-wk summer season. Blue circles are from ambient temperature plots, and red triangles are from the experimentally warmed plots. Points are mean treatment effects, and error bars are SEs. Solid lines in A represent a significant interaction between the treatments, and dotted lines in B indicate a nonsignificant interaction. Full model results are shown in Table 2 and SI Appendix, Tables S3 and S5.
Table 2.
Effects of altered wolf spider densities and warming on belowground litter decomposition and litter N
| Response | Fixed effects terms | Estimate | SE | df | t | P |
| Mass remaining, % | Intercept | 98.03 | 5.74 | 19 | 17.07 | 0.000 |
| Litter moisture content, % | −0.36 | 0.08 | 19 | −4.84 | 0.0001 | |
| Control SD | 3.58 | 2.54 | 19 | 1.41 | 0.175 | |
| High SD | −6.34 | 2.57 | 19 | −2.47 | 0.0232 | |
| Warming | −8.14 | 2.52 | 19 | −3.23 | 0.0044 | |
| Control SD × warming | 4.82 | 3.51 | 19 | 1.37 | 0.186 | |
| High SD × warming | 15.01 | 3.54 | 19 | 4.24 | 0.0004 | |
| N remaining, % | Intercept | 111.81 | 13.95 | 19 | 8.02 | 0.000 |
| Litter moisture content, % | −0.35 | 0.18 | 19 | −1.90 | 0.073 | |
| Control SD | 1.30 | 5.98 | 19 | 0.22 | 0.830 | |
| High SD | 8.20 | 6.06 | 19 | 1.35 | 0.192 | |
| Warming | 10.27 | 5.935 | 19 | 1.73 | 0.0997 | |
| Control SD × warming | −8.66 | 8.28 | 19 | −1.05 | 0.309 | |
| High SD × warming | −16.14 | 8.34 | 19 | −1.94 | 0.068 |
Mixed effects model results of belowground ecosystem function response variables, as predicted by wolf spider density (SD; reference category: low SD), warming (reference category: ambient temperature), and average litter moisture content. The decomposition rate is expressed as percent litter mass remaining in proportion to initial mass. Litter N is percent N remaining in litter in relation to initial N content of litter. Litter bags were buried below the tundra litter layer and underwent 14 mo of experimental treatment. Model results with control SD as the reference category for the wolf spider treatment are shown in SI Appendix, Table S5.
We did not observe any treatment effects on litter decomposition or litter N content at the soil surface. Similarly, variation in surface decomposition and litter N was not related to litter moisture content (SI Appendix, Table S1). A possible explanation for these results is that decomposition at the soil surface is driven by variations in biotic and abiotic conditions (45) that were not captured through our measurements in the soil and litter layers.
However, our results did indicate that increasing wolf spider densities resulted in higher availability of soil N (Fig. 2C and SI Appendix, Tables S2 and S3). Specifically, plots with high wolf spider densities showed a trend toward having higher levels of total N than those with low wolf spider densities (SI Appendix, Table S3a). This pattern was driven by significantly higher levels of soil NH4+ in plots with high wolf spider densities than in those with low wolf spider densities (Fig. 2C and SI Appendix, Table S3a); there was no difference in soil NH4+ between plots with control and low or control and high wolf spider densities (all Pcritical values >0.0167; SI Appendix, Table S3). Neither total N nor NH4+ was related to the warming treatment. Moreover, we did not find any effects of wolf spider density or warming on soil available NO3−, P, or K (SI Appendix, Tables S2 and S3). Soil moisture was positively and significantly associated with availability of total N and NH4+ (SI Appendix, Table S3) and marginally negatively correlated with soil P (P = 0.0129; SI Appendix, Table S3a).
Effect Sizes of Treatments on Belowground Community and Ecosystem Function.
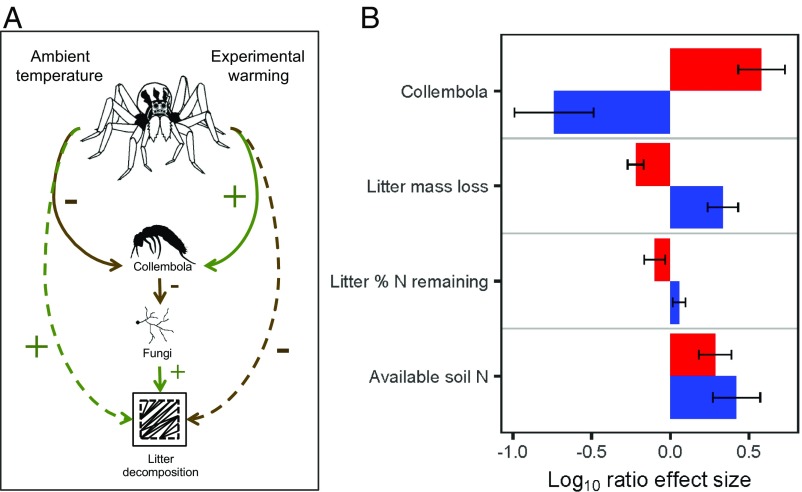
We compared the mean effect sizes of high wolf spider densities relative to low wolf spider densities for several key response variables (Collembola, belowground litter decomposition, percent litter N remaining, and soil available NH4+). Effect sizes were estimated under ambient temperatures and warming by calculating the log10 (Xa/Xb) effect magnitudes for each experimental block, where Xa and Xb are the values for a given response variable when spider densities are high or low, respectively (46, 47). We found that having high densities of wolf spiders resulted in mean log10 effect magnitudes of −0.739 to 0.422 under ambient temperatures and −0.219 to 0.581 under experimental warming relative to those plots with experimentally reduced wolf spider densities (Fig. 3B). Warming caused opposite responses to high wolf spider densities for most response variables. The exception was soil NH4+, which increased under high wolf spider densities, irrespective of the warming treatment.
Fig. 3.
(A) Conceptualization of the hypothesized pathways by which warming alters the cascading effects of wolf spiders on decomposition via fungivorous Collembola. Solid lines denote direct effects of the wolf spiders, and dashed lines indicate indirect effects of the wolf spiders. Positive trophic effects are shown in green, and negative effects are shown in brown. (B) Mean (±SE) effect sizes of high wolf spider densities on Collembola, decomposition (belowground litter mass loss), percent N remaining in litter, and available soil N (total milligrams of NH4+ per 10 cm−2) under ambient temperatures (shown in blue) and experimental warming (shown in red) across the five experimental blocks. The log10 ratios are comparisons between plots with low vs. high densities of wolf spiders; positive log ratios10 indicate a positive effect of high wolf spider densities on the response variable.
Discussion
A central goal of ecological and environmental research is to understand how climate change is affecting biological communities and ecosystem functioning. Wolf spiders inhabit nearly every terrestrial habitat and are among the globally dominant groups of spiders in terms of local abundance and diversity (48, 49). The cascading effects of these predators on detrital food webs and decomposition may be a common phenomenon across many ecosystems (e.g., refs. 26, 28, 50), suggesting that responses by wolf spiders to environmental disturbances such as climate change could have widespread ecosystem-level effects. We have shown here that in the Arctic, an ecosystem that is rapidly warming and where changes in biogeochemical cycling have disproportionate effects on global processes (51), wolf spiders indirectly alleviate the effects of warming on decomposition rates. Specifically, we found that experimentally increasing wolf spider densities tended to reduce densities of belowground Collembola, with associated increases in litter decomposition and soil available N (NH4+). However, the direction of these effects was, in general, reversed under experimental warming (a conceptual model is shown in Fig. 3A). Increasing wolf spider densities did not have measurable effects on the densities of any of the other common members of the soil community (i.e., oribatid mites, predatory mites, soil-dwelling intermediate predators) under ambient temperature or warming. Therefore, we interpret our findings as warming mediating the direct, and potentially indirect, effects of wolf spiders on Collembola, which subsequently influences decomposition. Higher densities of wolf spiders were also associated with higher soil N availability irrespective of the warming treatment, suggesting that these predators have an indirect influence on primary production as well. Given that wolf spiders comprise a large portion of animal biomass across much of the Arctic (52) (SI Appendix), these impacts could scale across the region.
As has been previously documented in other ecosystems (5, 53), aboveground tundra predators can have important indirect effects on the functioning of the belowground community. The findings of our experiment suggest that under ambient conditions, moist tundra exhibits a classic trophic cascade, where high densities of a top predator (wolf spiders) tend to suppress intermediate consumers (Collembola; Fig. 1A), which presumably releases the fungal community from predation, and indirectly results in faster litter decomposition (Fig. 2A). While the effects of increasing wolf spider densities on Collembola densities were not strong, they nevertheless corresponded to important changes in decomposition. Collembola can influence decomposition and nutrient cycling via their consumption of both detritus and fungal decomposers (e.g., ref. 44). The pattern of faster decomposition that we observed under modest declines in Collembola densities suggests that in this system, Collembola primarily affect decomposition indirectly via consumption of the microbial community. Otherwise, the trend toward lower Collembola densities should have corresponded to slower, not faster, decomposition rates. Similarly, the slower decomposition rates observed under warming and high wolf spider densities may be explained by the slightly higher densities of Collembola exerting stronger predation pressure on fungal decomposers under that treatment. The effects of actively hunting spiders can vary depending on moisture availability in temperate (29, 54) and tropical systems (55). This study was conducted in moist acidic tundra, which is the dominant ecosystem type in the Alaskan Arctic (56) and is also common in many subarctic areas (57); the extent to which our findings may be generalized to drier tundra habitats warrants further study.
Notably, despite previous work suggesting that predators are more likely to regulate processes in belowground food webs dominated by bacteria and bacteria-consuming organisms (39, 58), our findings suggest that trophic cascades also occur in systems where the fungal energy channel (i.e., fungi, fungal feeders) is responsible for the majority of nutrient cycling. The changes to the function of the belowground community (i.e., decomposition, soil N) that were brought about by higher wolf spider densities in our experiment are similar in magnitude to other documented trophic cascades by invertebrate predators on plant biomass (e.g., ref. 47) (Fig. 3B). As such, these results suggest that trophic cascades initiated by generalist predators that derive substantial energy from belowground systems can be equal to those in aboveground systems (59).
Our results suggest a change in both the strength and the nature of wolf spider effects under warming. We found that reduced numbers of predators could still suppress prey populations (as observed in the low wolf spider density treatment), possibly due to higher predator activity rates under warming (42). However, the unexpected positive trend in Collembola densities (and concurrent decline in decomposition) as wolf spider densities increased suggests that in some cases, climate change may not simply alter the strength of top-down effects but could, instead, induce different types of trophic cascades altogether (29, 55, 60, 61) (Fig. 3). At least two possible scenarios may explain the increasing, albeit weak, trend in Collembola with increasing wolf spider densities under warming. First, although we did not observe changes in the Collembola community at the order level, warming could have caused turnover in Collembola species composition (62) or ecologically relevant traits (63), which would likely have consequences for the functional roles of these animals (64). In plots with high wolf spider densities, intensified predation on preferred Collembola species may have released less palatable species (65) from competition and enabled them to reach higher abundances under warming. Alternatively, under elevated wolf spider densities, warming-associated increases in spider activity could result in more frequent antagonistic interactions with other predators (66). Strong predator–predator interactions or even intraguild predation, which is fairly common in this system (22), can relax the collective effect of predator communities, resulting in increased prey populations and weaker trophic cascades (67, 68). Under warming, we observed that numbers of intermediate predators declined as wolf spider densities increased (Fig. 1B). The reduction in intermediate predators corresponds to a progressive increase in Collembola, suggesting that under warming, wolf spiders may have preferentially preyed upon intermediate predators, effectively releasing Collembola from predation by both groups.
Our results lead us to believe that the differential effects of wolf spiders on decomposition under warming are mediated by trophic interactions with Collembola (Fig. 1A) and possibly also related to interactions with other predators (Fig. 1B). As species-level data for the Collembola and potential changes in wolf spider diets are not available for our experimental plots, it is possible that the apparent shift in community-level effects of wolf spiders under warming was driven by an alternative mechanism. However, among the six organismal groups that we measured within this food web (including all major soil microarthropod groups), Collembola and intermediate predators were the only ones for which there were interactive treatment effects. Importantly, the trends in altered Collembola densities with increasing wolf spider densities were negatively related to the changes we observed in decomposition rates in both ambient and warmed plots. Overall, our results highlight the importance of accounting for changes in species interactions between and within trophic guilds (i.e., predator–prey interactions and predator–predator interactions) for predicting ecosystem responses to climate change.
Wolf spiders also influenced N cycling in this N-limited ecosystem, suggesting an important link between predators and plant nutrient availability in the tundra. We observed higher availability of total soil N, driven by increasing levels of NH4+, with increasing wolf spider densities (Fig. 2C). Higher availability of soil N may reflect increased N excretion by stressed prey when exposed to high predator densities (69) or microbial predation by microarthropods, which can result in remobilization of excess nutrients (ref. 70 and references therein). It is unclear whether the mechanism for higher soil N under high spider densities was the same or different under ambient temperatures and warming. Moreover, it remains to be seen whether such an increase in soil N would provide more of a benefit to the microbes or plants in N-limited tundra ecosystems, but previous work in our study region found that increased nutrient availability resulted in a net ecosystem loss of C despite increases in plant productivity (71).
Tundra decomposition rates and C losses are likely to increase under warming (e.g., ref. 72), yet we show that predators may indirectly dampen some of these impacts. While populations of many northern species are projected to decline under climate change (73), wolf spiders may actually benefit from warmer Arctic temperatures (34). Given that close to half of the world’s terrestrial C is stored in the Arctic permafrost (7, 51), slight changes in regional C dynamics, even those driven by predators as small as the wolf spider, could have disproportionate effects on the global C cycle (72). The results of this study therefore highlight the importance of considering trophic interactions in efforts to understand ecosystem responses to global change and suggest that those studies that do not consider species interactions (particularly top-down effects of predators) may potentially miscalculate the projected effects of warming on decomposition and nutrient cycling.
Materials and Methods
Study System and Experimental Design.
Our study took place from early June 2011 through late July 2012 near Toolik Field Station (68°38′N and 149°43′W, elevation = 760 m), a well-studied area of moist acidic tundra (74) on the North Slope of Alaska. The mean annual temperature at Toolik is −10 °C, with the majority of positive temperatures occurring during the summer months (average July temperature is 14 °C), and the annual precipitation is 200–400 mm (75). We explored the effects of wolf spider density and warming on community composition, litter decomposition, litter N loss, and available soil nutrients through a fully factorial field experiment. Mesocosms were circular, 1.5 m in diameter, and enclosed with aluminum flashing that was buried 20 cm belowground and stood 20 cm above the soil surface to contain the belowground community and wolf spiders, who are not skilled climbers. Plots were distributed across five blocks and randomly assigned to one of the six spider density/warming treatments. For the warming treatment, we placed ITEX OTCs over half of the plots during the summer seasons only. The ITEX method typically increases mean air temperature within the OTCs by 1–2 °C (43).
Wolf spider density treatments included the following: (i) low, (ii) control, and (iii) high density. At the beginning of each summer, we removed all possible wolf spiders from low-density plots and added spiders to high-density plots such that they would have approximately twice the early season average density of control plots, which was 1.1 wolf spiders per square meter. We continued to check and remove individuals from the removal plots throughout each summer. Visual inspection and live pitfall trapping at the end of the summer seasons verified that we successfully manipulated wolf spider densities during both summers of the experiment. In 2011, the mean and SE values of wolf spider densities within each plot over a series of three 24-h live pitfall trapping bouts (July 20–22, 2011) were 0.8 ± 0.22 wolf spiders in high-density plots, 0.167 ± 0.075 wolf spiders in control plots, and 0.20 ± 0.10 wolf spiders in low-density plots. ANOVA and post hoc Tukey tests for these 2011 data showed that the average number of spiders was significantly higher in high-density plots than in control (P = 0.0145) and removal (P = 0.021) plots but that catches did not differ between control and removal plots (P = 0.986). At the end of the second summer, we visually surveyed each plot to get a more complete count of the total number of wolf spiders per plot (as opposed to pitfalls, which catch a smaller subset of spiders present) and found that wolf spider densities differed significantly according to their preassigned treatments (ANOVA: F2,27 = 21.85, P = < 0.0001); low-density (removal) plots had an average of 0.3 ± 0.213 wolf spiders, control plots had 1.8 ± 0.20 wolf spiders, and high-density plots had 3.3 ± 0.47 wolf spiders each.
Sampling of Community Composition.
We sampled the belowground community by taking soil samples of the upper soil organic layer (average sample volume = 176 cm3) in each plot on two midseason dates (June 20 and July 17, 2012). Microarthropods were removed through heat extraction using Berlese–Tullgren funnels (BioQuip Products) into 90% ethanol over a period of 5 d. Groups that were present in at least 70% of our soil samples included Collembola, which were identified to order (Entomobrymorpha, Poduromorpha, and Symphypleona); oribatid mites; and predatory mites. There were also some intermediate predators in the soil samples (centipedes; unidentifiable juvenile spiders; and other small spiders from the Dysderidae, Thomisidae, Linyphiidae, and Gnaphosidae families). Although none of these predator types were present in abundance, at least one intermediate predator was caught in 73% of the soil samples. These animals are likely predators to the detritivore groups and could potentially be prey to wolf spiders; due to their low numbers in the soil samples, we did not differentiate among different soil-dwelling intermediate predators but, instead, used a measure of the total density of these predators. In all subsequent analyses of soil-dwelling microarthropods, we used the average densities from the June and July samplings.
To measure treatment effects on other intermediate predators that could affect detritivore densities, particularly Collembola, and/or be prey to wolf spiders (e.g., surface-active crab spiders, linyphiid spiders, juvenile spiders, centipedes), we used live pitfall traps over several dates during the second summer season (June 13–15, June 29, July 9, July 11, and July 20–23). Live traps were checked daily; surface-active intermediate predators and other bycatch were immediately returned to their respective experimental plots. Counts were corrected by the number of live pitfall traps within each plot and expressed as the average number of surface-active intermediate predators caught per plot over the summer.
Fungal biomass and bacterial biomass were estimated using epiflourescent microscopy techniques (76) from 5-g soil samples that were collected from the upper organic layer at the conclusion of the experiment in late July. Fungal samples were stained with Calcofluor fluorescent brightener (77) and read between 334-nm and 365-nm wavelengths. Bacterial samples were stained with 5-(4,6 dichlorotriazin-2-yl) aminofluorescein and read at a 490-nm wavelength. We estimated active fungal biomass as 10% of the total fungal biomass (78).
Measures of Decomposition and Nutrient Availability.
We measured decomposition rates and litter N loss in the experimental plots using litter bags that were filled with standing dead leaves of the dominant plant, E. vaginatum, that were collected from an area adjacent to our experimental plots in June 2010. Litter was dried at 40° C for 48 h, mixed, and subsampled for litter bag preparation. Litter bags were 8 × 8 cm with a 3-mm mesh size on the top and bottom to allow access by most arthropods, and each bag contained 1.5 g of litter. Subsamples of the initial litter mixture were ground and processed for total N content using a CE Elantech Flash EA 1112 Elemental Analyzer (CE Elantech, Inc.) at Duke University.
We deployed a pair of litter bags in each plot during mid-June 2011 by placing one litter bag on the soil surface and burying the other in the lower litter layer under the moss surface (ca. 5–10 cm). We collected both litter bags in August 2012 (14 mo) after the experimental start date and manually removed accumulated soil, ingrown moss and roots, and microarthropods from the decomposed leaves before drying them at 40° C for 72 h. Litter bag contents were weighed to determine proportional mass loss from the initial litter, and subsamples were ground and analyzed for percentage of N as described above.
We used ion exchange membranes (Plant Root Simulator probes; Western Ag Innovations, Inc.) to measure treatment effects on available soil N, P, and K. Three cation-exchange and three anion-exchange resin membranes were inserted at equal spacing within each plot in June 2012 and incubated 3 wk before collection. The initial probes were replaced with a second set of probes that were similarly incubated for an additional 3 wk. Probes were analyzed by Western Ag Innovations, Inc. for NO3−; NH4+; and total N, P, and K. Nutrient supply rates are expressed as the total over these two periods.
Moisture Availability.
Experimental warming can reduce soil moisture, which can affect litter decomposition (e.g., refs. 79, 80), microarthropod abundances (e.g., ref. 62), and microarthropod effects on decomposition (81). To account for this, we took measures of soil moisture at three locations within each plot at the beginning, middle, and end of the 2012 summer season using a HydroSense portable soil moisture probe (Campbell Scientific). Those data indicated that the warming treatments had no effect on average soil moisture content (mixed effects model: df = 24, t = 0.683, P = 0.501; mean ± SE values of soil moisture in ambient plots were 34.31 ± 6.57 and 37.37 ± 4.81 in warmed plots). Furthermore, we did not observe effects of the warming treatment on percent moisture content within litter bags, although the moisture content of surface litter was significantly lower than that of litter from bags that were buried in the litter layer (mixed effects model of litter moisture content as the response variable, with warming treatment and litter bag location as fixed effects and experimental block as a random effect; intercept = 78.75 ± 4.54, df = 53, t = 17.36; warming = 1.46 ± 4.78, df = 53, t = 0.306, P = 0.761; surface = −50.99 ± 4.78, df = 53, t = −10.68, P < 0.0001); the results of a test of potential interactive effects between the warming treatment and litter bag location were also nonsignificant.
Analyses.
We used linear mixed effects models estimated through the lmer function of the nlme package (82) in R (83) to investigate the influence of our treatments and their potential interactive effects (spider density × warming) on the relevant response variables. Spider density was treated as a categorical variable (low density, control, high density), and treatment block was included as a random effect. Average percent moisture from the nine measures of soil moisture was also included as a continuous independent predictor. We fitted separate models for the average densities of each of the soil arthropod groups (Collembola, oribatid mites, predatory mites, and soil-dwelling intermediate predators) from the June and July samplings, as well as the average total abundance of surface-active intermediate predators from the dry pitfall traps and biomasses of fungi and bacteria from the upper organic horizon. Soil animal densities (per cubic centimeter) and abundances of surface-active intermediate predators (per plot) were log-transformed following the technique of Osborne (84) to conform to the assumptions of linear models. In the Collembola model, Collembola order (i.e., Entomobrymorpha, Poduromorpha, Symphypleona) was included as a fixed effect and study plot nested within experimental block was included as a random effect. Bacterial biomass in one plot was 399 standard deviations (SDs) above the mean biomass of other plots within the same treatment, and was thus excluded from analyses. Similarly, fungal biomass estimates from three plots were between 12 and 24 SDs above the other biomass measures in those treatments, and were therefore also excluded from analyses.
We individually assessed the interactive effects of the spider density and warming treatments on decomposition, litter N content, and available soil nutrients (N, P, and K). Litter moisture content was included as a covariate in the litter-related models, while average soil moisture was included in the soil nutrient models. Decomposition rate was expressed as cumulative mass loss over the 14 mo of the experiment, and the percent gain or loss in litter N was expressed as the percent difference from the initial N content.
Post hoc tests (e.g., Tukey) are not available for linear mixed effects models with interactions; therefore, we investigated the direction of significant interaction effects by running separate models for data under ambient temperature and warming treatments. From these models, we evaluated the significance of pairwise differences in wolf spider density using Bonferroni correction (Pcritical = 0.05/3 = 0.01).
Finally, we estimated the mean (±SE) effect sizes of wolf spiders on several key response variables (Collembola, belowground litter decomposition, percent litter N remaining, and soil available NH4+) under ambient conditions and warming across all experimental blocks. To do this, we calculated the log10 (Xa/Xb) effect magnitude for each response variable within a given experimental block, where Xa and Xb are the values for a given response variable when spider densities are high or low, respectively (46, 47).
All data used in the analyses described above are archived by the LTER Network Data Portal (85).
Supplementary Material
Acknowledgments
We thank undergraduate assistants Kiki Contreras and Samantha Walker, PolarTREC (Teachers and Researchers Exploring and Collaborating) teacher Nick LaFave, Greg Selby, Rod Simpson, Jennie McLaren, Jason Stuckey, and the staff of the Toolik Field Station for assistance in the field and Ashley Asmus, Laura Gough, and two anonymous reviewers for helpful feedback on earlier drafts of this manuscript. Funding for this research was provided from the US National Science Foundation Grant 1106401 (to A.M.K.); the National Geographic Committee for Research and Exploration; Duke University; Conservation, Research and Education Opportunities International; Alaska Geographic; the Kappa Delta Foundation; the Lewis and Clark Fund; Western Ag Innovations; and the Arctic Institute of North America. A.T.C. was funded by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, Terrestrial Ecosystem Sciences Program under Award DE-SC0010562.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808754115/-/DCSupplemental.
References
- 1.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol Lett. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 2.Walther G-R. Community and ecosystem responses to recent climate change. Philos Trans R Soc Lond B Biol Sci. 2010;365:2019–2024. doi: 10.1098/rstb.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz OJ. Terrestrial food webs and vulnerability of the structure and functioning of ecosystems to climate. In: Pielke RA, editor. Climate Vulnerability. Academic; Oxford: 2013. pp. 213–222. [Google Scholar]
- 4.Chapin FS, 3rd, Matson PA, Vitousek P. Principles of Terrestrial Ecosystem Ecology. 2nd Ed Springer; New York: 2012. [Google Scholar]
- 5.Bardgett RD, Wardle DA. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change. Oxford Univ Press; Oxford: 2010. [Google Scholar]
- 6.IPCC . In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, et al., editors. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 7.Tarnocai C, et al. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem Cycles. 2009;23:GB2023. [Google Scholar]
- 8.Zimov SA, Schuur EAG, Chapin FS., 3rd Climate change. Permafrost and the global carbon budget. Science. 2006;312:1612–1613. doi: 10.1126/science.1128908. [DOI] [PubMed] [Google Scholar]
- 9.McGuire AD, Chapin FS, Walsh JE, Wirth C. Integrated regional changes in Arctic climate feedbacks: Implications for the global climate system. Annu Rev Environ Resour. 2006;31:61–91. [Google Scholar]
- 10.Wookey PA, et al. Ecosystem feedbacks and cascade processes: Understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Global Change Biol. 2009;15:1153–1172. [Google Scholar]
- 11.Sjögersten S, van der Wal R, Woodin S. Impacts of grazing and climate warming on C pools and decomposition rates in Arctic environments. Ecosystems (N Y) 2012;15:349–362. [Google Scholar]
- 12.Cahoon SMP, Sullivan PF, Post E, Welker JM. Large herbivores limit CO2 uptake and suppress carbon cycle responses to warming in West Greenland. Global Change Biol. 2012;18:469–479. [Google Scholar]
- 13.Little CJ, Cutting H, Alatalo J, Cooper EJ. Short-term herbivory has long-term consequences in warmed and ambient high Arctic tundra. Environ Res Lett. 2017;12:025001. [Google Scholar]
- 14.Tveit AT, Urich T, Frenzel P, Svenning MM. Metabolic and trophic interactions modulate methane production by Arctic peat microbiota in response to warming. Proc Natl Acad Sci USA. 2015;112:E2507–E2516. doi: 10.1073/pnas.1420797112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briones MJI, Ostle NJ, McNamara NP, Poskitt J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol Biochem. 2009;41:315–322. [Google Scholar]
- 16.David J-F, Gillon D. Combined effects of elevated temperatures and reduced leaf litter quality on the life-history parameters of a saprophagous macroarthropod. Global Change Biol. 2009;15:156–165. [Google Scholar]
- 17.Coulson SJ, et al. Effects of experimental temperature elevation on high-arctic soil microarthropod populations. Polar Biol. 1996;16:147–153. [Google Scholar]
- 18.Ruess L, Michelsen A, Schmidt IK, Jonasson S. Simulated climate change affecting microorganisms, nematode density and biodiversity in subarctic soils. Plant Soil. 1999;212:63–73. [Google Scholar]
- 19.Singh BK, Bardgett RD, Smith P, Reay DS. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat Rev Microbiol. 2010;8:779–790. doi: 10.1038/nrmicro2439. [DOI] [PubMed] [Google Scholar]
- 20.Sistla SA, et al. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature. 2013;497:615–618. doi: 10.1038/nature12129. [DOI] [PubMed] [Google Scholar]
- 21.Scheu S. Plants and generalist predators as links between the below-ground and above-ground system. Basic Appl Ecol. 2001;2:3–13. [Google Scholar]
- 22.Koltz AM, Asmus A, Gough L, Pressler Y, Moore JC. The detritus-based microbial-invertebrate food web contributes disproportionately to carbon and nitrogen cycling in the Arctic. Polar Biol. September 20, 2017 doi: 10.1007/s00300-017-2201-5. [DOI] [Google Scholar]
- 23.Rich ME, Gough L, Boelman NT. Arctic arthropod assemblages in habitats of differing shrub dominance. Ecography. 2013;36:994–1003. [Google Scholar]
- 24.Snyder WE, Wise DH. Contrasting trophic cascades generated by a community of generalist predators. Ecology. 2001;82:1571–1583. [Google Scholar]
- 25.Sitvarin MI, Rypstra AL. Fear of predation alters soil carbon dioxide flux and nitrogen content. Biol Lett. 2014;10:20140366. doi: 10.1098/rsbl.2014.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maran AM, Pelini SL. Predator contributions to belowground responses to warming. Ecosphere. 2016;7:e01457. [Google Scholar]
- 27.Lawrence KL, Wise DH. Spider predation on forest-floor Collembola and evidence for indirect effects on decomposition. Pedobiologia (Jena) 2000;44:33–39. [Google Scholar]
- 28.Lawrence KL, Wise DH. Unexpected indirect effect of spiders on the rate of litter disappearance in a deciduous forest. Pedobiologia (Jena) 2004;48:149–157. [Google Scholar]
- 29.Lensing JR, Wise DH. Predicted climate change alters the indirect effect of predators on an ecosystem process. Proc Natl Acad Sci USA. 2006;103:15502–15505. doi: 10.1073/pnas.0607064103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirta HK, Weingartner E, Hambäck PA, Roslin T. Extensive niche overlap among the dominant arthropod predators of the high Arctic. Basic Appl Ecol. 2015;16:86–92. [Google Scholar]
- 31.Wise DH. Wandering spiders limit densities of a major microbi-detritivore in the forest-floor food web. Pedobiologia (Jena) 2004;48:181–188. [Google Scholar]
- 32.Oelbermann K, Langel R, Scheu S. Utilization of prey from the decomposer system by generalist predators of grassland. Oecologia. 2008;155:605–617. doi: 10.1007/s00442-007-0927-4. [DOI] [PubMed] [Google Scholar]
- 33.Visakorpi K, Wirta HK, Ek M, Schmidt NM, Roslin T. No detectable trophic cascade in a high-Arctic arthropod food web. Basic Appl Ecol. 2015;16:652–660. [Google Scholar]
- 34.Høye TT, Hammel JU, Fuchs T, Toft S. Climate change and sexual size dimorphism in an Arctic spider. Biol Lett. 2009;5:542–544. doi: 10.1098/rsbl.2009.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall SD, Gittleman JL. Clutch size in spiders: Is more better? Funct Ecol. 1994;8:118–124. [Google Scholar]
- 36.Shaver GR, et al. Alaska’s Changing Arctic: Ecological Consequences for Tundra, Streams and Lakes. Oxford Univ Press; New York: 2014. Terrestrial ecosystems at toolik lake, Alaska; pp. 90–142. [Google Scholar]
- 37.Laakso J, Setälä H. Population- and ecosystem-level effects of predation on microbial-feeding nematodes. Oecologia. 1999;120:279–286. doi: 10.1007/s004420050859. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz OJ. Predators have large effects on ecosystem properties by changing plant diversity, not plant biomass. Ecology. 2006;87:1432–1437. doi: 10.1890/0012-9658(2006)87[1432:phleoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Mikola J, Setälä H. Productivity and trophic-level biomasses in a microbial-based soil food web. Oikos. 1998;82:158–168. doi: 10.1007/s004420050673. [DOI] [PubMed] [Google Scholar]
- 40.Martikainen E, Huhta V. Interactions between nematodes and predatory mites in raw humus soil: A microcosm experiment. Revue d’Écologie et de Biologie du Sol. 1990;27:13–20. [Google Scholar]
- 41.Ford MJ. Metabolic costs of the predation strategy of the spider Pardosa amentata (Clerck) (Lycosidae) Oecologia. 1977;28:333–340. doi: 10.1007/BF00345988. [DOI] [PubMed] [Google Scholar]
- 42.Kruse PD, Toft S, Sunderland KD. Temperature and prey capture: Opposite relationships in two predator taxa. Ecol Entomol. 2008;33:305–312. [Google Scholar]
- 43.Marion GM, et al. 1993. Open-top devices for manipulating field temperatures in tundra ecosystems. Proceedings of the Fourth International Symposium on Thermal Engineering and Science for Cold Regions, eds Lunardini VJ, Bowen SL, CRREL Special Report 93–22 (US Army Corps of Engineers, Hanover, NH), pp 205–210.
- 44.Moore JC, Walter DE, Hunt HW. Arthropod regulation of micro- and mesobiota in below-ground detrital food webs. Annu Rev Entomol. 1988;33:419–435. [Google Scholar]
- 45.Bradford MA, et al. A test of the hierarchical model of litter decomposition. Nat Ecol Evol. 2017;1:1836–1845. doi: 10.1038/s41559-017-0367-4. [DOI] [PubMed] [Google Scholar]
- 46.Beschta RL, Ripple WJ. Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol Conserv. 2009;142:2401–2414. [Google Scholar]
- 47.Borer ET, et al. What determintes the strength of a trophic cascade? Ecology. 2005;86:528–537. [Google Scholar]
- 48.World Spider Catalog 2018 World Spider Catalog, Version 18.5 (Natural History Museum Bern, Switzerland). Available at https://wsc.nmbe.ch/. Accessed May 10, 2018.
- 49.Murphy NP, et al. Phylogenetic reconstruction of the wolf spiders (Araneae: Lycosidae) using sequences from the 12S rRNA, 28S rRNA, and NADH1 genes: Implications for classification, biogeography, and the evolution of web building behavior. Mol Phylogenet Evol. 2006;38:583–602. doi: 10.1016/j.ympev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Wise D, Snyder W, Tuntibunpakul P, Halaj J. Spiders in decomposition food webs of agroecosystems: Theory and evidence. J Arachnol. 1999;27:363–370. [Google Scholar]
- 51.McGuire AD, et al. Sensitivity of the carbon cycle in the Arctic to climate change. Ecol Monogr. 2009;79:523–555. [Google Scholar]
- 52.Dondale CD, Redner JH. The Insects and Arachnids of Canada. Part 17. Biosystematics Research Centre; Ottawa: 1990. The wolf spiders, nurseryweb spiders, and lynx spiders of Canada and Alaska. Araneae: Lycosidae, Pisauridae, and Oxyopidae. [Google Scholar]
- 53.Mancinelli G, Mulder C. Detrital dynamics and cascading effects on supporting ecosystem services. In: Woodward G, Bohan DA, editors. Advances in Ecological Research. Vol 53. Academic; New York: 2015. pp. 97–160. [Google Scholar]
- 54.McCluney KE, Sabo JL. Water availability directly determines per capita consumption at two trophic levels. Ecology. 2009;90:1463–1469. doi: 10.1890/08-1626.1. [DOI] [PubMed] [Google Scholar]
- 55.Liu S, et al. Spider foraging strategy affects trophic cascades under natural and drought conditions. Sci Rep. 2015;5:12396. doi: 10.1038/srep12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein HE, et al. The nature of spatial transitions in the Arctic. J Biogeogr. 2004;31:1917–1933. [Google Scholar]
- 57.Walker DA, et al. The circumpolar Arctic vegetation map. J Veg Sci. 2005;16:267–282. [Google Scholar]
- 58.Wardle DA. Communities and Ecosystems: Linking the Aboveground and Belowground Components. Princeton Univ Press; Princeton: 2002. [Google Scholar]
- 59.Halaj J, Wise DH. Terrestrial trophic cascades: How much do they trickle? Am Nat. 2001;157:262–281. doi: 10.1086/319190. [DOI] [PubMed] [Google Scholar]
- 60.Marino NAC, et al. Rainfall and hydrological stability alter the impact of top predators on food web structure and function. Global Change Biol. 2017;23:673–685. doi: 10.1111/gcb.13399. [DOI] [PubMed] [Google Scholar]
- 61.Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315:640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 62.Hodkinson ID, et al. Global change and Arctic ecosystems: Conclusions and predictions from experiments with terrestrial invertebrates on Spitsbergen. Arct Alp Res. 1998;30:306–313. [Google Scholar]
- 63.Makkonen M, et al. Traits explain the responses of a sub-arctic Collembola community to climate manipulation. Soil Biol Biochem. 2011;43:377–384. [Google Scholar]
- 64.Filser J. The role of Collembola in carbon and nitrogen cycling in soil: Proceedings of the Xth International Colloquium on Apterygota, České Budějovice 2000: Apterygota at the beginning of the third millennium. Pedobiologia (Jena) 2002;46:234–245. [Google Scholar]
- 65.Toft S. Prey choice and spider fitness. J Arachnol. 1999;27:301–307. [Google Scholar]
- 66.Lang B, Rall BC, Brose U. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J Anim Ecol. 2012;81:516–523. doi: 10.1111/j.1365-2656.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- 67.Sitvarin MI, Rypstra AL. The importance of intraguild predation in predicting emergent multiple predator effects. Ecology. 2014;95:2936–2945. [Google Scholar]
- 68.Finke DL, Denno RF. Intra-guild predation relaxes natural enemy impacts on herbivore populations. Ecol Entomol. 2003;28:67–73. [Google Scholar]
- 69.Hawlena D, Strickland MS, Bradford MA, Schmitz OJ. Fear of predation slows plant-litter decomposition. Science. 2012;336:1434–1438. doi: 10.1126/science.1220097. [DOI] [PubMed] [Google Scholar]
- 70.Moore JC, McCann K, Setälä H, De Ruiter PC. Top-down is bottom-up: Does predation in the rhizosphere regulate aboveground dynamics? Ecology. 2003;84:846–857. [Google Scholar]
- 71.Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature. 2004;431:440–443. doi: 10.1038/nature02887. [DOI] [PubMed] [Google Scholar]
- 72.Schuur EAG, et al. Vulnerability of permafrost carbon to climate change: Implications for the global carbon cycle. Bioscience. 2008;58:701–714. [Google Scholar]
- 73.Callaghan TV, et al. Effects on the structure of Arctic ecosystems in the short- and long-term perspectives. Ambio. 2004;33:436–447. doi: 10.1579/0044-7447-33.7.436. [DOI] [PubMed] [Google Scholar]
- 74.Bliss LC, Matveyeva NN. Circumpolar arctic vegetation. In: Chapin FS 3rd, et al., editors. Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective. Academic; San Diego: 1992. pp. 59–89. [Google Scholar]
- 75.van Wijk MT, Williams M, Shaver GR. Tight coupling between leaf area index and foliage N content in arctic plant communities. Oecologia. 2005;142:421–427. doi: 10.1007/s00442-004-1733-x. [DOI] [PubMed] [Google Scholar]
- 76.Bloem J. Fluorescent staining of microbes for total direct counts. In: Akkermans ADL, Van Elsas JD, De Bruijn FJ, editors. Molecular Microbial Ecology Manual. Springer Dordrecht; The Netherlands: 1995. pp. 367–378. [Google Scholar]
- 77.Frey SD, Elliott ET, Paustian K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem. 1999;31:573–585. [Google Scholar]
- 78.Ingham ER, Klein DA. Soil fungi: Relationships between hyphal activity and staining with fluorescein diacetate. Soil Biol Biochem. 1984;16:273–278. [Google Scholar]
- 79.Makkonen M, et al. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett. 2012;15:1033–1041. doi: 10.1111/j.1461-0248.2012.01826.x. [DOI] [PubMed] [Google Scholar]
- 80.Aerts R. The freezer defrosting: Global warming and litter decomposition rates in cold biomes. J Ecol. 2006;94:713–724. [Google Scholar]
- 81.Wall DH, et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Global Change Biol. 2008;14:2661–2677. [Google Scholar]
- 82.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team 2014 nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-131. Available at https://CRAN.R-project.org/package=nlme. Accessed June 26, 2018.
- 83.R Core Team 2017 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at https://www.R-project.org/. Accessed June 26, 2018.
- 84.Osborne J. Notes on the use of data transformations. Pract Assess Res Eval. 2002;9:42–50. [Google Scholar]
- 85.Koltz A. 2018 Effects of experimentally altered wolf spider densities and warming on soil microarthropods, litter decomposition, litter N, and soil nutrients near Toolik Field Station, AK in summer 2012. Environmental Data Initiative. Available at https://portal.lternet.edu/nis/mapbrowse?scope=knb-lter-arc&identifier=20049. Accessed June 26, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.