Abstract
Background
ATX-101 (deoxycholic acid injection) is the only injectable drug approved for submental fat (SMF) reduction. In the phase 3 REFINE trials, adults with moderate or severe SMF who were dissatisfied with the appearance of their face/chin were eligible to receive up to 6 treatment sessions with ATX-101 (2 mg/cm2) or placebo. Primary and secondary endpoints, evaluated at 12 weeks after last treatment, significantly favored ATX-101 supporting its efficacy for reducing SMF and the psychological impact of SMF, and increasing satisfaction with the appearance of the face/chin.
Objectives
To evaluate the efficacy and safety of ATX-101 by treatment session.
Methods
This post hoc analysis used pooled data from the REFINE trials to evaluate efficacy endpoints and adverse events following each treatment session to further characterize the ATX-101 treatment response and safety profile.
Results
In both treatment groups, mean injection volume declined over subsequent treatment sessions, though more markedly in the ATX-101 group. The majority of ATX-101–treated patients achieved a ≥1-grade improvement in SMF within 2 to 4 treatment sessions based on either clinician or patient assessment. Furthermore, 19.1% of ATX-101–treated patients (vs 3.9% of placebo-treated patients) received fewer than 6 treatment sessions owing to patient satisfaction with treatment or lack of sufficient SMF for further treatment. In both treatment groups, the incidence/severity of common injection-site adverse events declined over subsequent treatment sessions.
Conclusions
Although up to 6 treatment sessions were permitted in the REFINE trials, most ATX-101–treated patients achieved an improvement in SMF within 2 to 4 treatment sessions.
Level of Evidence: 3 
Submental fullness can present as an unappealing submental profile and have a negative impact on the well-being of an individual.1 Until recently, patients who desired to improve their submental contour had to rely on surgical procedures and/or targeted liposuction. However, ATX-101 (deoxycholic acid injection; Kybella [United States] and Belkyra [Canada, Australia, and Europe]; Kythera Biopharmaceuticals, Inc., Parsippany, NJ [an affiliate of Allergan]),2,3 offers patients a nonsurgical treatment option for reduction of submental fat (SMF).
ATX-101 was approved for improving the appearance of moderate to severe convexity or fullness associated with SMF based, in part, on data from the phase 3 REFINE trials.4,5 REFINE-1 (NCT01542034) and REFINE-2 (NCT01546142) were identical randomized controlled trials conducted to evaluate the efficacy and safety of ATX-101 treatment. In the REFINE trials, patients were eligible to receive up to 6 treatment sessions with study drug, and efficacy assessments were conducted at 12 weeks after last treatment. Both REFINE trials met their a priori primary and secondary endpoints, demonstrating ATX-101 as an effective treatment for reducing SMF and the psychological impact of SMF, and increasing satisfaction with the appearance of the face/chin.4,5
ATX-101 has been shown to cause adipocytolysis when injected subcutaneously into fat.6 Histological evidence indicated that ATX-101 results in a local tissue response including macrophage infiltration, which clears cellular debris and liberated lipids from the treatment area, and fibroblast recruitment, which may be responsible for collagen production.6 ATX-101 is a locally acting drug that requires multiple injections at each treatment session as well as multiple treatment sessions to achieve a satisfactory reduction in SMF. The ATX-101 treatment paradigm allows physicians to tailor treatment for each patient based on their SMF presentation, response to treatment, and treatment goals.
In the REFINE trials, the total volume of study drug injected at each treatment session was at the discretion of the clinician (up to a maximum of 10 mL administered via 0.2-mL injections).4,5 Subjects were eligible to receive up to 6 treatment sessions. Reasons for receiving fewer than 6 treatments included patient satisfaction with treatment, lack of sufficient SMF for further treatment (which was the single largest reason for receiving fewer than 6 treatment sessions in the REFINE trials), administrative reasons, or safety/tolerability concerns.4,5 The current post hoc analysis was conducted to evaluate the efficacy and safety of ATX-101 following each treatment session using pooled data from the REFINE trials to further characterize the ATX-101 treatment response and safety profile.
METHODS
Study Design
The REFINE-1 trial was conducted between February 29, 2012 and August 22, 2013, while the REFINE-2 trial was conducted between March 15, 2012 and August 20, 2013. The Institutional Review Board/Research Ethics Board at each site (Appendix A) reviewed and approved the protocol and informed consent form for each trial prior to site initiation under the requirements of the International Conference on Harmonisation Guideline for Good Clinical Practice. Full details on the study design for the REFINE trials, which were conducted at 70 sites in the United States and Canada, have been previously reported. An overview of the study design is presented schematically in Supplementary Figure 1.4,5 Briefly, adults with a moderate (grade 2) or severe/large amount of SMF (grade 3) (as graded by both the clinician using the validated Clinician-Reported SMF Rating Scale [CR-SMFRS] and the patient using the validated Patient-Reported SMF Rating Scale [PR-SMFRS]; details of all scales reported in Table 1) who were dissatisfied with the appearance of their face/chin (based on rating of 0 [extremely dissatisfied], 1 [dissatisfied], or 2 [slightly dissatisfied] on the Subject Self-Rating Scale [SSRS]) were randomized 1:1 to either ATX-101 (area-adjusted dose, 2 mg/cm2) or placebo for up to 6 treatment sessions (every 28 ± 5 days). Randomization was implemented using an automated Interactive Web-based Randomization System (eCaseLink; DSG Inc., Malvern, PA). The randomization schedules were predetermined using a block size that was not disclosed to the sites. Subjects were randomized based on a within- site randomization scheme in the order in which they completed baseline evaluations. For sites selected to conduct magnetic resonance imaging (MRI), confirmation of a qualified baseline MRI was required before randomization.
Table 1.
Scales Used in the REFINE Trials
| CR-SMFRSa | PR-SMFRSa | PR-SMF line drawings | PR-SMFISa | SSRS | SMSLG | |
|---|---|---|---|---|---|---|
| Evaluates | SMF severity | SMF severity | SMF severity | Psychological impact of SMF | Satisfaction with the appearance of the face/chin | Skin laxity |
| Evaluated by | Clinician | Patient | Patient | Patient | Patient | Clinician |
| Description | 10 line drawings that included 2 examples of each CR-SMFRS grade provided to the patient in a random order Patients selected the drawing that best represented their submental profile |
Self-perception of 6 emotional/visual characteristics (how happy, bothered, self-conscious, or embarrassed are you with the appearance of your chin fat? How much older or overweight do you look because of your chin fat?) Scores for the 6 items combined to generate TSS |
A responder was a patient whose response was slightly satisfied, satisfied, or extremely satisfied | Integration of 3 features: skin wrinkling, adherence to underlying neck structures (bone and muscle), and redundancy (horizontal and vertical folds) Each grade defines the maximal allowed limit for skin wrinkling, adherence to underlying structures, and redundancy |
||
| Scale | 0 = absent 1 = mild 2 = moderate 3 = severe 4 = extreme |
0 = no chin fat 1 = slight amount 2 = moderate amount 3 = large amount 4 = very large amount |
0 (lowest score) to 4 (highest score) Lower score is better |
0 (no impact) to 10 (greatest negative impact) Lower scores indicate improvement or reduced negative impact |
0 = extremely dissatisfied 1 = dissatisfied 2 = slightly dissatisfied 3 = neither satisfied nor dissatisfied 4 = slightly satisfied 5 = satisfied 6 = extremely satisfied |
1 = none 2 = mild 3 = moderate 4 = severe The feature with the highest grade determines the score |
CR-SMFRS, Clinician-Reported Submental Fat Rating Scale; PR-SMFIS, Patient-Reported Submental Fat Impact Scale; PR-SMFRS, Patient-Reported Submental Fat Rating Scale; SMF, submental fat; SMSLG, Submental Skin Laxity Grade; SSRS, Subject Self-Rating Scale; TSS, total scale score.
aValidated scale.
Subjects were excluded who had a body mass index >40 kg/m2, had excessive submental skin laxity based on clinician judgment, had recently received aesthetic treatment(s) in the neck/chin area (radiofrequency, lasers, chemical peels, or dermal fillers within 12 months; botulinum toxin within 6 months), or had previously received treatment(s) to reduce SMF (liposuction, surgery, or treatment with lipolytic agents at any time). Male patients were required to be clean shaven throughout the trials to allow for assessment of their submental area. At preselected sites, MRI was performed as an objective assessment of reduction in submental volume following ATX-101 treatment. At these sites, patients who had any condition that would render them unsuitable for an MRI also were excluded.
For patient comfort, ice/cold packs or topical/local anesthetics could be applied to the treatment area at the discretion of the clinician. An injection grid was applied to the treatment area to assist with injection spacing. Study drug was administered via 0.2-mL subcutaneous injections at 1.0-cm intervals into the preplatysmal SMF using a 30-gauge, 0.5-inch needle. Subjects could receive up to 50 injections (10 mL [100 mg]) of study drug at each treatment session; the number of injections administered was at the discretion of the clinician based on SMF presentation.
The coprimary endpoints in the REFINE trials were percentage of patients who achieved a ≥1-grade improvement in SMF from baseline based on both clinician (CR-SMFRS) and patient (PR-SMFRS) assessment (composite CR-1/PR-1 response), and percentage of patients who achieved a ≥2-grade improvement in SMF from baseline (composite CR-2/PR-2 response). Secondary endpoints included the percentage of patients who achieved a ≥10% reduction from baseline in submental volume based on MRI (defined a priori as an MRI responder) and mean change from baseline in the psychological impact of SMF using the validated Patient-Reported SMF Impact Scale (PR-SMFIS) total scale score. All endpoints were evaluated at 12 weeks after last treatment regardless of the number of treatment sessions received by a patient.
In the REFINE trials, all patient-reported outcome (PRO) assessments were completed independently by the patient (in writing) prior to any clinician assessments. PRO assessments were provided in English only and patients were required to complete the assessments at the study site during scheduled visits. For visits in which multiple PRO assessments were conducted, assessments were performed in the following order: PR-SMFRS, PR-SMFIS, SSRS, and Patient-Reported SMF (PR-SMF) line drawings.
Safety was assessed throughout the trials via spontaneous reports of adverse events (AEs) and findings from clinical laboratory tests, vital sign assessments, and physical examinations. Skin laxity, evaluated by the clinician using the Submental Skin Laxity Grade scale, was included as an additional safety measure. Standardized photography was performed at baseline, and at 12 and 24 weeks after last treatment for documentation purposes. MRIs were conducted at baseline and at 12 weeks after last treatment following procedures previously reported.4,5
Statistical Analysis
Data from the 2 REFINE trials were pooled, and coprimary and secondary endpoints and AEs were analyzed in the pooled population. The pooling of these data was appropriate because the REFINE trials were conducted concurrently in the same geographic region, had identical protocols, and there were no meaningful differences in the results between the trials. Details regarding the methodology for the statistical analyses conducted in the REFINE trials have been reported.4,5 Briefly, formal hypothesis tests were conducted for the coprimary endpoints (α = 0.05) and for the 2 secondary endpoints (using a Bonferroni-Holm adjustment for 2 tests) with multiple imputation of missing data. A Cochran-Mantel-Haenszel analysis stratified by site was performed for responder endpoints. Continuous variables were analyzed using an analysis of covariance model, with treatment as fixed effect and baseline value as covariate.
In the REFINE trials, MRIs were performed at centers identified before the start of the trial. The first approximately 200 patients randomized in each trial at the preselected centers underwent MRI. The power for the secondary hypothesis testing in the REFINE trials was to be >99% based on the observed MRI responder rates in a previous ATX-101 trial with n = 90 per treatment group; response was defined as ≥10% reduction in submental volume. Allowing for a 10% dropout rate, approximately 100 patients per treatment group were to be enrolled in the MRI cohort in each of the REFINE trials.
To characterize the timing of the ATX-101 treatment response, a post hoc analysis of efficacy measures assessed at each treatment visit was performed. The percentage of patients who achieved a ≥1-grade improvement in SMF based on either clinician assessment (CR-1 response) or patient assessment (PR-1 response) following each treatment session, and mean change from baseline in patient assessment of SMF based on standardized line drawings of submental convexity (PR-SMF line drawings) following each treatment session were determined. In addition, the median number of treatment sessions to achieve a CR-1, PR-1, or composite CR-1/PR-1 response was determined. CR-2, PR-2, and composite CR-2/PR-2 response rates, the more stringent regulatory criteria required for registration in the United States, were not included in this analysis, as the ≥1-grade response has been shown to be clinically meaningful, with high rates of patient satisfaction.5 The incidence and severity of AEs were evaluated at each treatment session. All statistical analyses were performed using the SAS System (Cary, NC) version 9.2 or higher.
RESULTS
Demographic and Baseline Characteristics
Of the 1022 patients randomized in the REFINE trials (REFINE-1 [n = 506] and REFINE-2 [n = 516]), 1022 patients were included in the efficacy population (ATX-101 [n = 514] and placebo [n = 508]) and 1019 patients were included in the safety population for the current analysis (ATX-101 [n = 515] and placebo [n = 504]). Table 2 summarizes the demographic and baseline characteristics of the efficacy population. Overall, most patients were female (84.7% [866/1022]) and white (87.0% [889/1022]), with a mean age of 48.7 years (range, 19.0-65.0 years) and a mean body mass index of 29.3 kg/m2 (range, 18.2-45.6 kg/m2). At baseline, there was an even distribution of patients with moderate and severe SMF based on clinician assessment (via the CR-SMFRS) in each treatment group. By patient assessment (via the PR-SMFRS), however, there was a greater proportion of patients with a moderate vs large amount of SMF at baseline in both treatment groups.
Table 2.
Demographic and Baseline Characteristics
| Placebo (n = 508) | ATX-101 (n = 514) | |
|---|---|---|
| Age, mean (SD), years | 48.5 (9.2) | 48.8 (9.3) |
| Sex, female, % | 85.0 | 84.4 |
| Race, % | ||
| White | 88.4 | 85.6 |
| Black | 6.7 | 9.3 |
| Asian | 2.0 | 2.1 |
| BMI, mean (SD), kg/m2 | 29.3 (4.3) | 29.2 (4.6) |
| CR-SMFRS grade, % | ||
| Moderate (2)/severe (3) | 51.4/48.2 | 49.8/50.0 |
| PR-SMFRS grade, % | ||
| Moderate amount (2)/large amount (3) | 62.2/37.4 | 63.4/36.4 |
| Fitzpatrick skin type, % | ||
| I-III/IV-VI | 71.5/28.5 | 68.1/31.9 |
| SMSLG grade, % | ||
| None (1)–mild (2) | 82.9 | 83.7 |
| Moderate (3)–severe (4) | 16.7 | 16.1 |
BMI, body mass index; CR-SMFRS, Clinician-Reported Submental Fat Rating Scale; PR-SMFRS, Patient-Reported Submental Fat Rating Scale; SD, standard deviation; SMSLG, Submental Skin Laxity Grade.
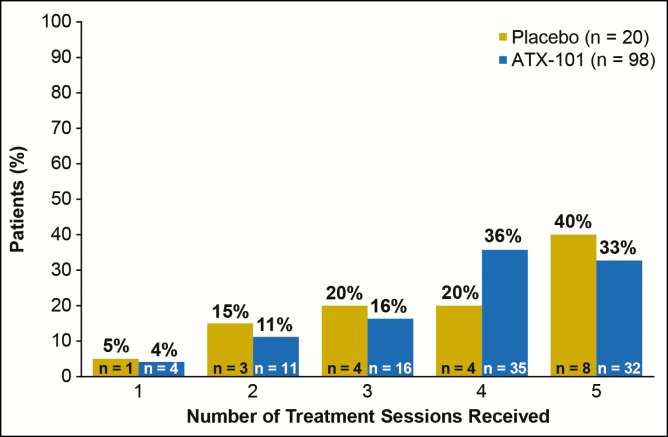
Supplementary Figure 2 shows the patient disposition in the pooled REFINE population. In total, 41.1% of ATX-101–treated patients and 18.9% of placebo-treated patients received fewer than 6 treatment sessions. These percentages include 98 patients (19.1%) in the ATX-101 group and 20 patients (3.9%) in the placebo group who received fewer than 6 treatment sessions owing to patient satisfaction with treatment or lack of sufficient SMF remaining for further treatment. Figure 1 shows the number of treatment sessions received among patients who received fewer than 6 treatment sessions owing to patient satisfaction or lack of sufficient SMF. Of the ATX-101–treated patients who received fewer than 6 treatment sessions owing to patient satisfaction or lack of sufficient SMF, 67.3% (66/98) of patients received 4 or fewer treatment sessions.
Figure 1.
Number of treatment sessions received among patients who underwent fewer than 6 treatment sessions owing to patient satisfaction with treatment or lack of sufficient submental fat.
Treatment Characteristics
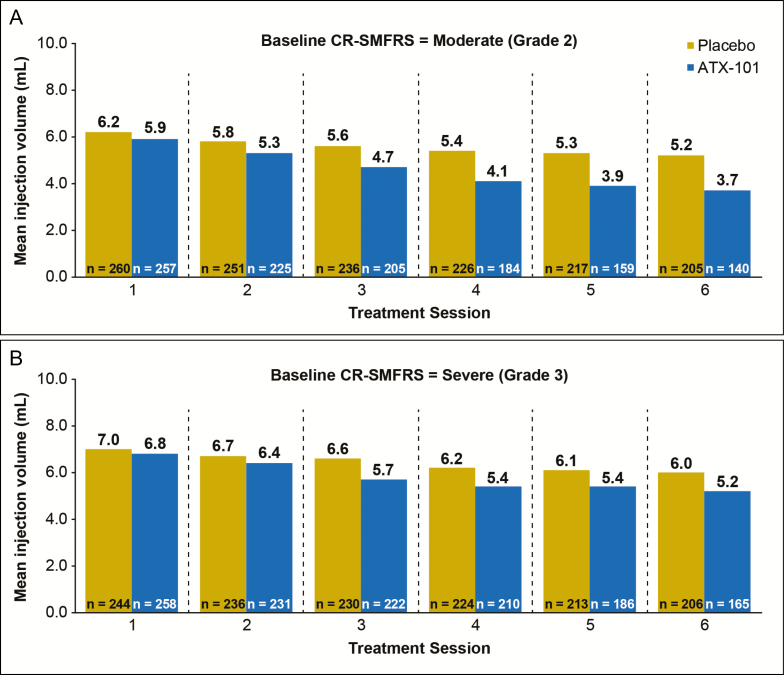
The total volume of study drug injected across all treatment sessions (mean ± standard deviation) was 25.3 ± 14.0 mL in the ATX-101 group and 32.8 ± 14.1 mL in the placebo group. Mean injection volume per treatment session was 5.4 ± 2.1 mL in the ATX-101 group and 6.0 ± 2.1 mL in the placebo group. Figures 2A and 2B show the mean injection volume at each treatment session for patients with moderate and severe baseline SMF, respectively, based on clinician assessment (via the CR-SMFRS). Regardless of baseline SMF severity, mean injection volume declined over subsequent treatment sessions in both groups with the decline being more marked in the ATX-101 group.
Figure 2.
Mean injection volume of study drug administered at each treatment session for patients with (A) moderate (grade 2) submental fat (SMF) and (B) severe (grade 3) SMF at baseline based on clinician assessment via the validated Clinician-Reported SMF Rating Scale (CR-SMFRS).
At each treatment session, patients were eligible to receive up to 50 injections of study drug. In both groups, the median number of injections administered per treatment session (27 in the ATX-101 group and 31 in the placebo group) was considerably lower than the maximum allowed per protocol. In total, ATX-101–treated patients received a mean of 252.9 mg (25.3 mL) of study drug relative to the 600 mg maximum allowed over the 6 treatment sessions.
Primary and Secondary Endpoints
The coprimary endpoints in the REFINE trials, composite CR-1/PR-1 and composite CR-2/PR-2 responses, were achieved by 68.2% of ATX-101–treated patients vs 20.5% of placebo-treated patients (P < 0.001) and 16.0% vs 1.5%, respectively (P < 0.001). Figure 3 shows the percentage of patients in each treatment group who achieved either a CR-1 or a PR-1 response at 12 weeks after last treatment.
Figure 3.

Percentage of patients achieving a ≥1-grade improvement in submental fat (SMF) from baseline based on clinician assessment via the validated Clinician-Reported SMF Rating Scale (CR-SMFRS [CR-1 response]), patient assessment via the validated Patient-Reported SMF Rating Scale (PR-SMFRS [PR-1 response]), and both clinician and patient assessment (via both the CR-SMFRS and PR-SMFRS [composite CR-1/PR-1 response]) at 12 weeks after last treatment. P < 0.001 for all comparisons between ATX-101 and placebo treatment.
MRIs were acquired for 449/1022 patients in the REFINE trials and an MRI response was achieved by 43.3% of ATX-101–treated patients vs 5.3% of placebo-treated patients (P < 0.001). The overall reduction in submental volume as measured by MRI was significantly greater following ATX-101 vs placebo treatment (P < 0.001).
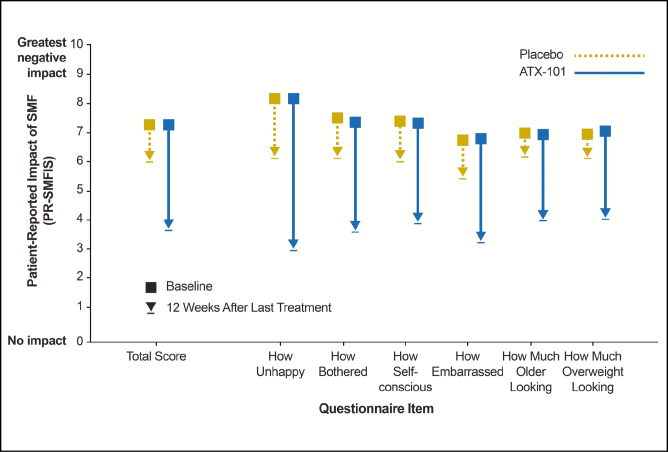
At baseline, psychological impact of SMF was suggested in both treatment groups based on the PR-SMFIS total scale score (mean ± standard deviation, 7.3 ± 1.7 in both groups [10 = greatest negative impact]). Following treatment, the psychological impact of SMF was reduced by 3.7 in the ATX-101 group vs 1.3 in the placebo group (P < 0.001). Improvements in the 6 individual components of the PR-SMFIS following ATX-101 treatment are shown in Figure 4.
Figure 4.
Reduction from baseline in the psychological impact of submental fat (SMF) at 12 weeks after last treatment using the validated Patient-Reported SMF Impact Scale (PR-SMFIS). Lower scores indicate improvement or reduced negative impact. P < 0.001 for all comparisons between ATX-101 and placebo treatment.
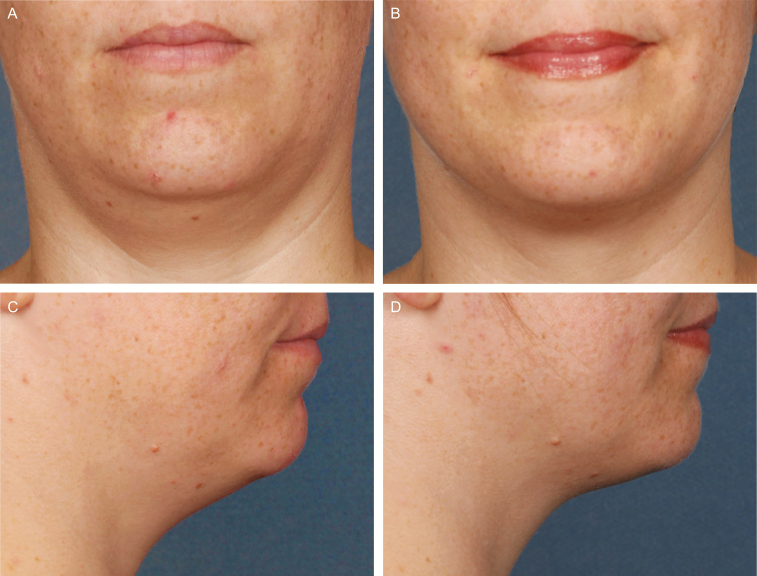
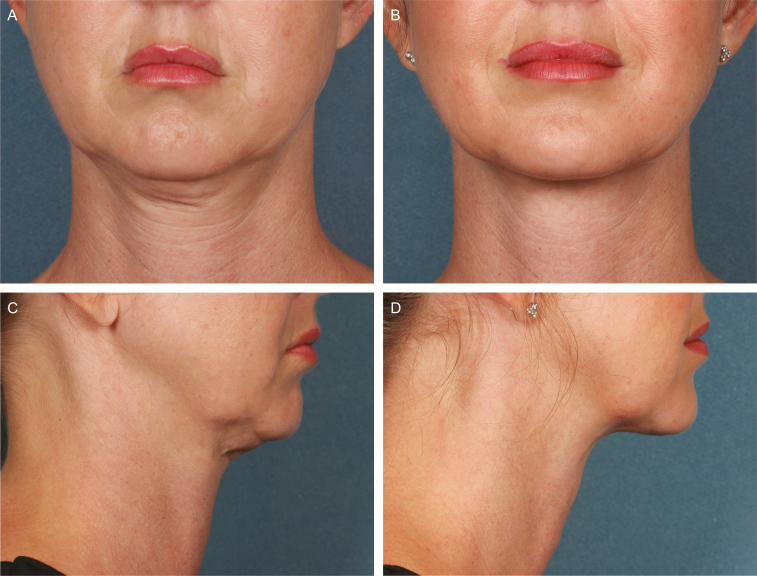
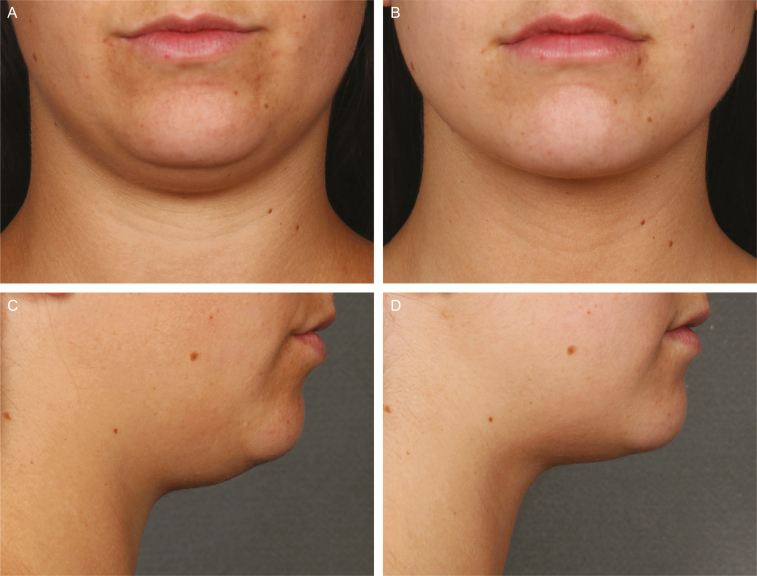
Figures 5-7 show representative before/after photographs of ATX-101–treated responders.
Figure 5.
Representative photographs and efficacy results at baseline (A [front facing] and C [right facing]) and 12 weeks after last treatment with ATX-101 (B [front facing] and D [right facing]). 36-year-old female who received 2 ATX-101 treatment sessions (10.6 mL total volume) and achieved a 1-grade improvement in submental fat based on both clinician and patient assessment. At baseline, CR-SMFRS was moderate (2); PR-SMFRS was moderate (2); SSRS was slightly dissatisfied (2); PR-SMFIS TSS was 8.8/10 (greatest negative impact); SMSLG was none (1); and BMI was 24.9 kg/m2. At 12 weeks after last treatment, CR-SMFRS was absent (0); PR-SMFRS was slight (1); SSRS was satisfied (5); PR-SMFIS TSS was 2.5; SMSLG was none (1); and BMI was 25.8 kg/m2. BMI, body mass index; CR-SMFRS, Clinician-Reported SMF Rating Scale; PR-SMFIS, Patient-Reported SMF Impact Scale; PR-SMFRS, Patient-Reported SMF Rating Scale; SMSLG, Submental Skin Laxity Grade; SSRS, Subject Self-Rating Scale; TSS, total scale score.
Figure 6.
Representative photographs and efficacy results at baseline (A [front facing] and C [right facing]) and 12 weeks after last treatment with ATX-101 (B [front facing] and D [right facing]). 39-year-old female who received 3 ATX-101 treatment sessions (total volume: 10.8 mL) and achieved a 1-grade improvement in submental fat based on both clinician and patient assessment. At baseline, CR-SMFRS was moderate (2); PR-SMFRS was moderate (2); SSRS was extremely dissatisfied (0); PR-SMFIS TSS was 8.0/10 (greatest negative impact); SMSLG was none (1); and BMI was 19.7 kg/m2. At 12 weeks after last treatment, CR-SMFRS was absent (0); PR-SMFRS was slight (1); SSRS was satisfied (5); PR-SMFIS TSS was 2.2; SMSLG was none (1); and BMI was 20.4 kg/m2. BMI, body mass index; CR-SMFRS, Clinician-Reported SMF Rating Scale; PR-SMFIS, Patient-Reported SMF Impact Scale; PR-SMFRS, Patient-Reported SMF Rating Scale; SMSLG, Submental Skin Laxity Grade; SSRS, Subject Self-Rating Scale; TSS, total scale score.
Figure 7.
Representative photographs and efficacy results at baseline (A [front facing] and C [right facing]) and 12 weeks after last treatment with ATX-101 (B [front facing] and D [right facing]). 22-year-old female who received 4 ATX-101 treatment sessions (total volume: 23.2 mL) and achieved a 2-grade improvement in submental fat based on both clinician and patient assessment. At baseline, CR-SMFRS was moderate (2); PR-SMFRS was moderate (2); SSRS was extremely dissatisfied (0); PR-SMFIS TSS was 7.0/10 (greatest negative impact); SMSLG was none (1); and BMI was 27.0 kg/m2. At 12 weeks after last treatment, CR-SMFRS was absent (0); PR-SMFRS was none (0); SSRS was extremely satisfied (6); PR-SMFIS TSS was 0.8; SMSLG was none (1); and BMI was 25.2 kg/m2. BMI, body mass index; CR-SMFRS, Clinician-Reported SMF Rating Scale; PR-SMFIS, Patient-Reported SMF Impact Scale; PR-SMFRS, Patient-Reported SMF Rating Scale; SMSLG, Submental Skin Laxity Grade; SSRS, Subject Self-Rating Scale; TSS, total scale score.
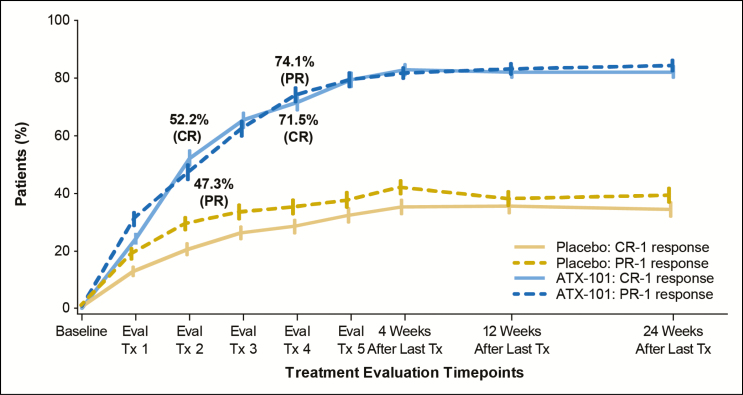
Efficacy by Treatment Session
Among the composite CR-1/PR-1 responders in the ATX-101 group, the median number of treatment sessions to achieve the response was 3. The median number of treatment sessions to achieve either a CR-1 or a PR-1 response with ATX-101 treatment was 2. Figure 8 shows the CR-1 and PR-1 response rate in each treatment group over time. The majority of ATX-101–treated patients achieved a CR-1 response within 2 to 4 treatment sessions (52.2% [after second treatment session] and 71.5% [after fourth treatment session]). Similarly, many patients treated with ATX-101 achieved a PR-1 response within 2 to 4 treatment sessions (47.3% [after second treatment session] and 74.1% [after fourth treatment session]).
Figure 8.
Most ATX-101–treated patients achieved a ≥1-grade improvement in submental fat (SMF) based on either clinician assessment via the validated Clinician-Reported SMF Rating Scale (CR-SMFRS [CR-1 response]) or patient assessment via the validated Patient-Reported SMF Rating Scale (PR-SMFRS [PR-1 response]) within 2 to 4 treatment sessions. Tx, treatment.
In the ATX-101 group, the mean PR-SMF line drawing assessments decreased from baseline by a progressively greater amount at each treatment session and then were maintained through 12 weeks after last treatment. In contrast, the mean change from baseline in the PR-SMF line drawing assessments was relatively constant over the 6 treatment sessions and through 12 weeks after last treatment in the placebo group. The change from baseline in the PR-SMF line drawing was significantly different between the ATX-101 and placebo group at each treatment session with the exception of the week 4 time point (ie, assessment following first treatment session; Supplementary Figure 3; P < 0.001).
Safety
Overall, 97.3% of ATX-101–treated patients and 89.7% of placebo-treated patients experienced an AE. Most AEs were mild (80.9% of AEs in the ATX-101 group and 88.9% in the placebo group) or moderate (17.5% and 10.4%, respectively). Serious AEs were reported by 2.5% of ATX-101–treated patients and 4.4% of placebo-treated patients; none were considered to be related to study drug. In total, 7.4% of patients in the ATX-101 group and 1.2% of patients in the placebo group discontinued treatment owing to an AE, while 1.6% and 1.0%, respectively, discontinued the REFINE trials owing to an AE.
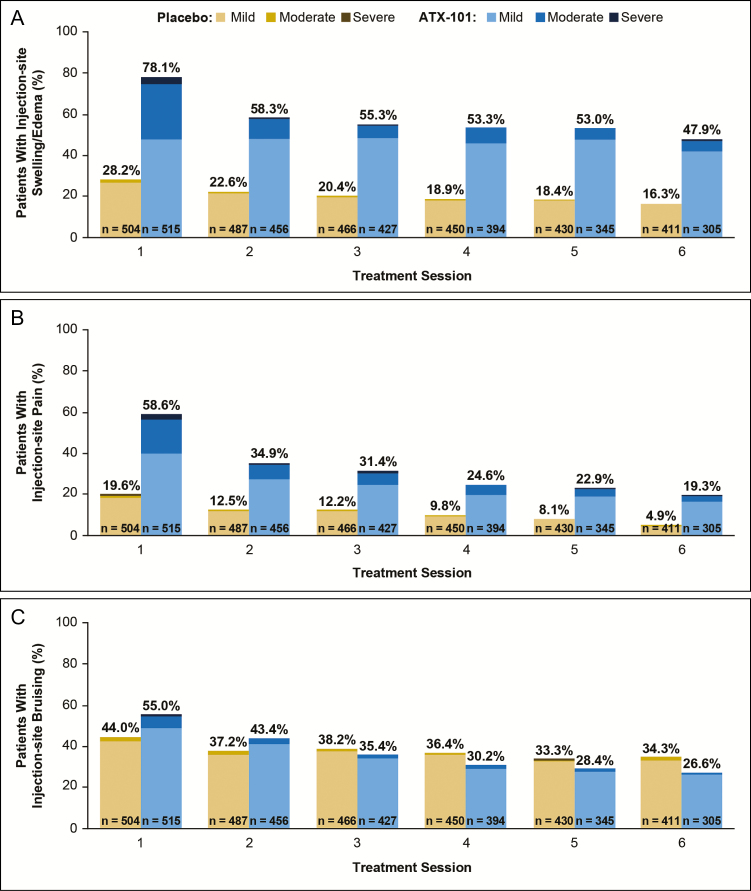
In both groups, the overall incidence of AEs was highest at the first treatment session (94.4% of ATX-101–treated patients and 69.8% of placebo-treated patients) and decreased over subsequent treatment sessions to reach its lowest value at treatment session 6 (46.4% and 53.6%, respectively). Although the incidence of AEs declined over time in both groups, the reduction was greater in the ATX-101 group. Table 3 summarizes the incidence and median duration of the common injection-site AEs reported in the REFINE trials. In both the ATX-101 and placebo group, the incidence of edema/swelling, pain, and hematoma (bruising) declined after the first treatment session and continued to decline over subsequent treatment sessions (Figure 9A-C, respectively). In addition, the severity of these common injection-site AEs declined after the first treatment session.
Table 3.
Incidence and Median Duration of Common Injection-site Adverse Events
| Placebo (n = 504) | ATX-101 (n = 515) | |||
|---|---|---|---|---|
| Patients, % | Median, days | Patients, % | Median, days | |
| Hematoma (bruising) | 70.0 | 9 | 71.5 | 9 |
| Pain | 31.3 | 3 | 69.5 | 7 |
| Anesthesia (numbness) | 5.8 | 2 | 66.2 | 43 |
| Edema | 29.2 | 4 | 60.4 | 10 |
| Swelling | 15.7 | 4 | 33.2 | 11 |
| Erythema | 17.9 | 2 | 26.6 | 3 |
| Induration | 2.6 | 6 | 23.3 | 28 |
| Paresthesia | 3.8 | 2 | 13.8 | 10 |
| Nodule | 2.6 | 5 | 13.4 | 23 |
| Pruritus | 6.0 | 3 | 12.4 | 7 |
Figure 9.
The incidence and severity of (A) edema/swelling, (B) pain, and (C) hematoma/bruising declined after the first treatment session in both treatment groups.
AEs of special interest included marginal mandibular nerve (MMN) paresis, dysphagia, skin ulceration, and injection-site alopecia. MMN paresis, which often presented as an asymmetric smile, occurred in 4.3% of ATX-101–treated patients vs 0.4% of placebo-treated patients, corresponding to a relative session frequency of 0.009 and <0.001, respectively. All events of MMN paresis resolved (after a median duration of 42 days [ATX-101 group] or 85 days [placebo group]). Dysphagia, often associated with the volume of injection and/or postinjection swelling, occurred in 1.9% of ATX-101–treated patients (median duration, 3 days) vs 0.2% of placebo-treated patients (1 day). Skin ulceration, likely resulting from inappropriate injection technique, occurred in 0.2% of ATX-101–treated patients vs 0% of placebo-treated patients. The single event of skin ulceration was mild and resolved within 23 days. Injection-site alopecia was reported in 0.4% of ATX-101–treated patients (median duration, 151 days) vs 0.6% of placebo-treated patients (272 days); all events were mild or moderate. Submental skin laxity was unchanged or improved from baseline in 93.4% of ATX-101–treated patients and 91.1% of placebo-treated patients at 12 weeks after last treatment.
DISCUSSION
In the REFINE trials, ATX-101 treatment resulted in statistically significant and clinically meaningful reductions in SMF compared with placebo at the primary time point (12 weeks after last treatment) based on both clinician-reported and PROs as well as objective evaluation via MRI. It is worth noting that the volume of study drug and number of treatment sessions was not prespecified in the REFINE trials; rather, patients could receive up to 10 mL of study drug per treatment session and up to 6 treatment sessions, as needed, to achieve an improvement in SMF. Based on baseline SMF severity, individual treatment response, and treatment expectations, some patients required fewer than the 6 treatment sessions. In fact, the majority of ATX-101–treated patients achieved a ≥1-grade improvement in SMF based on either clinician or patient assessment within 2 (52.2% or 47.3%, respectively) to 4 treatment sessions (71.5% or 74.1%). It is worth noting that achievement of a CR-1 and/or PR-1 response, though shown to be clinically meaningful to the patient, may not necessarily equate to achievement of the overall aesthetic treatment goal for the patient. This may explain why some patients continued ATX-101 treatment in the REFINE trials despite achieving a 1-grade improvement in their SMF.
ATX-101 is a locally acting drug that destroys adipocytes within the treatment area leading to a gradual reduction in SMF over time. As such, it is important to assess the submental area at each treatment session to ensure the presence of sufficient SMF to warrant additional ATX-101 treatment. The observed decrease in the number of patients receiving treatment, the fewer number of injections, and the lower injection volume at each subsequent treatment session support a reduction in SMF volume over time. Furthermore, nearly 20% of the ATX-101–treated patients in the REFINE trials received fewer than 6 treatment sessions owing to patient satisfaction with treatment or lack of sufficient SMF. Overall, these results underscore the importance of initially tailoring ATX-101 treatment for each patient based on their amount of SMF and treatment goal as well as the importance of reevaluating their SMF prior to each treatment session.
Improvements in SMF were assessed at each treatment visit in the REFINE trials via multiple PROs, including the PR-SMFRS and the PR-SMF line drawings. Both endpoints demonstrated the efficacy of ATX-101 treatment for reducing SMF and improving the submental profile. Importantly, the results of these PROs demonstrate that patients perceived an improvement in their SMF over the full course of treatment consistent with the mechanism of action of ATX-101.
The safety profile for ATX-101 was consistent with the injection procedure, the mechanism of action of ATX-101, and expected local tissue response. Most AEs were mild or moderate and decreased in both incidence and severity over successive treatment sessions. It is possible that the incidence and severity of common injection-site AEs decreased at subsequent treatment sessions owing to the presence of less SMF over time as well as lower ATX-101 injection volumes over time. As such, the extent of adipocytolysis and resulting inflammatory response would likely be reduced. Injection-site AEs, while common, typically resolved within 14 days without medication or procedures. Despite some AEs lasting for longer durations, these events did not typically prevent patients from receiving subsequent treatments or lead to study discontinuation. In fact, AEs rarely led to either treatment discontinuation or discontinuation from the REFINE trials. Patient comfort measures (cold packs/ice, injectable/topical anesthetics) were used at the discretion of the clinician in these trials. In clinical practice, patient comfort measures can be utilized to help minimize the common injection-site AEs, such as swelling, pain, and bruising.
Appropriate selection of patients may help reduce the AEs associated with ATX-101 treatment. For example, screening out patients whose submental fullness may be caused by factors other than excess SMF; patients who have excessive skin laxity, prominent platysmal bands, or scar tissue (as these conditions may lead to poor aesthetic outcomes); patients who have undergone prior surgical/aesthetic procedures in the submental area; or patients with a history of dysphagia or facial nerve injury (as these conditions may increase risk for AEs). Proper injection technique (injecting ATX-101 midway into the preplatysmal SMF to avoid the dermis, salivary glands/ducts, lymph nodes, and muscles; not injecting above a line drawn 1.0 to 1.5 cm below the inferior border of the mandible from the gonion to the mentum) is also important to help reduce the risk of AEs, including risk of MMN injury.
At 12 weeks after last treatment, submental skin laxity was unchanged or improved relative to baseline in the majority of ATX-101–treated and placebo-treated patients. Furthermore, the proportion of ATX-101–treated patients who had unchanged or improved skin laxity was similar to the proportion of placebo-treated patients who had unchanged or improved skin laxity, supporting that skin laxity did not worsen following ATX-101 treatment despite significant reductions in SMF and submental volume (confirmed by MRI). It is possible that the local inflammatory response elicited in response to ATX-101 treatment induces neocollagenesis, which may contribute to concomitant skin retraction within the treatment area as SMF is removed.
The analysis of efficacy by treatment session was not prespecified in the REFINE protocols for all endpoints and thus, there are some limitations. Several endpoints were only evaluated following completion of treatment (at 12 weeks after last treatment) including MRI assessment of submental volume reduction and various PROs assessing the psychological impact of SMF, satisfaction with the appearance of the face/chin, and satisfaction with treatment. As such, the current analysis focused on efficacy data collected following each treatment session. The Condition of Submental Fullness and Treatment Outcomes Registry (CONTOUR) has collected data regarding the management of submental fullness in clinical practice as well as data on the real-world use of ATX-101.7 For example, investigators in the REFINE trials were encouraged to achieve a 2-grade improvement in SMF and patients did not pay for study drug; however, in clinical practice, the desire to reduce patient costs and/or achievement of individual patient goals may lead to administration of fewer ATX-101 treatment sessions. Overall, data from CONTOUR may provide insight regarding how to achieve the best aesthetic outcome with ATX-101 treatment, either alone or in combination with other available therapies for facial rejuvenation.
CONCLUSIONS
ATX-101 is approved for reduction of SMF in adults with up to 6 treatment sessions allowed; however, the number of treatment sessions needed to achieve a satisfactory improvement in the submental profile is dependent on the amount of SMF for each patient, how each patient responds to treatment, and their individual treatment goals. The well-characterized efficacy and safety profile of ATX-101 in combination with its nonsurgical nature may make this treatment option for submental contouring appealing to a wide patient population.
Supplementary Material
This article contains supplementary material located online at www.aestheticsurgeryjournal.com.
Disclosures
Drs Dayan, Schlessinger, Beer, Donofrio, Jones, Humphrey, and Carruthers were investigators for Kythera Biopharmaceuticals, Inc. (an affiliate of Allergan). Drs Lizzul, Gross, Beddingfield, and Ms Somogyi are former employees of Kythera Biopharmaceuticals, Inc.
Funding
The REFINE trials were sponsored by Kythera Biopharmaceuticals, Inc. (an affiliate of Allergan). Writing and editorial assistance was provided to the authors by Karen Stauffer, PhD, CMPP, of Evidence Scientific Solutions, Philadelphia, PA and funded by Allergan plc, Dublin, Ireland. Neither honoraria nor payments were made for authorship.
Acknowledgments
We thank the investigators from the REFINE-1 (Leslie Baumann, Karen Beasley, Kenneth Beer, Brian Biesman, Michael Bukhalo, Cheryl Burgess, Valerie D. Callender, Jean Carruthers, Lisa Donofrio, Sabrina Fabi, Peter Geldner, Michael H. Gold, Melinda Gooderham, Lawrence J. Green, Kenneth Gross, Derek H. Jones, John H. Joseph, Carmen M. Kavali, Steven Kempers, Leon Kircik, Jane Lim, Catherine Maari, Anna Magee, Gary D. Monheit, Girish Munavalli, Mark G. Rubin, Daniel Schachter, Joel Schlessinger, Joel S. Shavin, Nowell Solish, Linda Stein-Gold, Heidi Waldorf, Gary Waterman, William P. Werschler, Darryl Scott Wong, Christopher Zachary) and REFINE-2 trials (Jeffrey M. Adelglass, Lorne Albrecht, Sarah Arron, Diane Baker, Stephan Baker, Lawrence Bass, Vince Bertucci, Ashish C. Bhatia, Fredric S. Brandt, Joel Cohen, Steven Davis, Steven H. Dayan, Jeanine Downie, Zoe Draelos, Robert Fixler, Peter Fodor, Holly Hake-Harris, William Hanke, Shannon Humphrey, Michael Jarratt, Jonathan Kantor, Bruce Katz, Rebecca Kazin, Corey Maas, Ronald Moy, Rhoda Narins, Gary Petrus, Sheetal Sapra, Ava Shamban, Daniel Stewart, Jonathan Sykes, Derek Woolner, Douglas Woseth, Leroy Young, Brian Zelickson).
REFERENCES
- 1. Humphrey S, Dayan SH, Shridharani SM, Baumann L, Gallagher CJ. Personal and social impacts of submental fat in the US population [abstract]. Presented at the Fall Clinical Dermatology Conference; October 20–23, 2016; Las Vegas, NV, USA. [Google Scholar]
- 2. Allergan Inc. Belkyra (deoxycholic acid injection) [product monograph] 2016; http://allergan-web-cdn-prod.azureedge.net/allergancanadaspecialty/allergancanadaspecialty/media/actavis-canada-specialty/en/products/pms/belkyra-pm-2016-03-28_e.pdf. Accessed February 16, 2017.
- 3. Kythera Biopharmaceuticals, Inc. Kybella (deoxycholic acid) injection [prescribing information] https://www.allergan.com/assets/pdf/kybella_pi. Accessed April 7, 2017.
- 4. Humphrey S, Sykes J, Kantor J et al. . ATX-101 for reduction of submental fat: A phase III randomized controlled trial. J Am Acad Dermatol. 2016;75(4):788-797. [DOI] [PubMed] [Google Scholar]
- 5. Jones DH, Carruthers J, Joseph JH et al. . REFINE-1, a multi center, randomized, double-blind, placebo-controlled, phase 3 trial with ATX-101, an injectable drug for submental fat reduction. Dermatol Surg. 2016;42(1):38-49. [DOI] [PubMed] [Google Scholar]
- 6. Walker P, Lee D, Toth BA. A histological analysis of the effects of single doses of ATX-101 on subcutaneous fat: results from a phase 1 open-label safety study of ATX-101 [abstract]. Presented at the Annual Meeting of the American Society for Dermatologic Surgery; October 3–6, 2013; Chicago, IL, USA. [Google Scholar]
- 7. Beer K, Callender VD, Shridharani SM et al. . Real world use of ATX-101 treatment for reduction of submental fat: final data from CONTOUR [abstract]. Presented at the Annual Meeting of the American Society for Dermatologic Surgery; October 5–8, 2017; Chicago, IL, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.