Abstract
OBJECTIVE
To determine the proportion of non-concussed, neurologically normal children with failures on a vestibular and oculomotor examination for concussion performed in an acute setting.
DESIGN
This was a cross-sectional study of subjects 6–18 years old presenting to a pediatric emergency department with non-neurologic chief complaints. The examination was administered by a pediatric emergency medicine physician, and includes assessments of dysmetria, nystagmus, smooth pursuits, saccades, gaze stability, near-point of convergence, and gait/balance testing.
RESULTS
Of the 295 subjects enrolled, 24% failed at least one element of testing. 13% had >1 failed element and 5% had >2 failed elements. 29% of females and 19% of males had failed examinations. By age, 15% of subjects 6–8 years old, 32% 9–11 years, 32% 12–14 years, and 26% 15–18 years had failed examinations. Overall, 10% were unable to complete the exam due to developmental age.
CONCLUSIONS
The provider should be aware that a proportion of non-concussed children may demonstrate failure on a single element of the vestibular and oculomotor exam for. While this testing is of benefit to the acute care provider in diagnosing pediatric concussion, its utility is greatest in the context of an injury history with acute onset of concussion symptoms.
INTRODUCTION
Concussions are common injuries sustained in the pediatric population. There are nearly 1 million emergency department (ED) visits per year for mild traumatic brain injury, with the highest reported rates in children and adolescents.1 The incidence of concussion diagnosis is increasing, as reported rates have doubled over the past decade.2
Vestibular and oculomotor deficits have been recognized as a key component of the pathophysiology leading to the morbidity from concussions.3,4 Recently, studies evaluating the validity and prognostic value of vestibular and oculomotor examination have been completed.5,6 These studies, however, focus on patients presenting to specialty sports medicine clinics, likely representing a specific cohort of injured patients, though have demonstrated that a portion of this specific population exhibits abnormalities on this testing. Over the past several years, guidelines and position statements have begun recommending the use of a routine a vestibular and oculomotor assessment for the concussed pediatric patient,7 with the most recent International Consensus Statement on Concussion recommending vestibular and oculomotor testing by all providers evaluating concussion in the acute setting.4 However, there are limited data on non-concussed pediatric subjects assessed with such an examination,8 with no data describing the distribution of findings in an non-concussed population. Knowledge of these findings is imperative in order to appropriately interpret the findings on vestibular and oculomotor examination as a diagnostic tool for those presenting to the ED with concussion.
By performing a standardized version of the vestibular and oculomotor examination on a sample of non-concussed subjects with non-neurologic chief complaints presenting to a pediatric ED in a tertiary care children’s hospital, this study aimed to (1) determine the proportion of neurologically-normal, non-concussed children ages 6–18 years old with a failed examination; (2) determine the proportion of failed examinations across both sex and age groups; and (3) determine the youngest age at which the exam can be reasonably completed.
METHODS
Study Design and Patient Population
We conducted an observational, cross-sectional study of subjects age 6–18 years old presenting to a pediatric ED in a tertiary care children’s hospital with non-neurologic chief complaints, including, but not limited to, abdominal pain, chest pain, rash, mild respiratory distress (defined was respiratory distress with an emergency severity index of 3, 4, or 5).9 Subjects were screened for eligibility by the lead study investigator, and after verbal consent and assent were obtained, a brief medical record review, as well as patient and parent interview were completed to confirm eligibility. Exclusion criteria included subjects who reported any of the provocable symptoms prior to testing (headache, dizziness, or fogginess); subjects with underling neurologic disorders suggesting vestibular or visual dysfunction (including, but not limited to, migraine headaches, epilepsy, cerebral palsy, benign paroxysmal positional vertigo, bilateral or unilateral vestibular hypofunction, strabismus, diplopia); subjects who were visibly intoxicated with an illicit substance, who had a positive serum or urine drug toxicology if tested, or who had taken an opioid, benzodiazepine, or anti-epileptic medication within 24 hours; and subjects with lower extremity trauma preventing them from ambulating to complete gait testing or upper extremity trauma to prevent them from completing dysmetria testing. The study was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia.
Data Collection
295 subjects were enrolled in a convenience sample. Demographic data, including age, sex, and any excluding chronic neurologic problems were obtained from the patient’s electronic medical record and patient/parent interview. Following completion of screening, the examination was performed by the lead study investigator, a pediatric emergency medicine physician trained in performance of the exam. For 10% of subjects (29 subjects), a second clinician repeated the examination to test for inter-rater reliability 20 minutes following the initial examination. Training of the study clinicians involved demonstration and practice with a pediatric sports medicine specialist, and observed performance of the examination by the clinician on at least ten patients by the specialist. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at The Children’s Hospital of Philadelphia.10
The Vestibular and Oculomotor Examination
The vestibular and oculomotor examination performed at our institution is a modified version of the brief Vestibular/Ocular Motor Screening Assessment (VOMS) validated by the University of Pittsburgh,6 and consists of nine measures. It is standardized across providers in sports medicine, primary care, and emergency medicine at our institution and has been shown to be feasible in these settings.11 It includes assessment for dysmetria, nystagmus, smooth pursuits, fast saccades (both horizontal and vertical), gaze stability testing (both the horizontal and vertical vestibular ocular reflex), near-point of convergence testing, and gait/balance testing (as compared to the VOMS, which includes testing for visual-motion sensitivity, but not for dysmetria or gait/balance testing). The examination used in this study takes approximately 3 minutes to perform, and is conducted as described below. For those tests evaluating symptom provocation, symptoms were evaluated during testing; prior to testing the patient was prompted to inform the examiner if he or she was experiencing any of the listed symptoms. If symptoms were provoked on any exam element, the examiner allowed these symptoms to resolve before proceeding with the testing.
Dysmetria
This test is performed via finger-nose-finger, with examiner’s finger moving horizontally, for 10 repetitions. A failed exam was defined as slow reaction time, past-pointing, or an intention tremor.
Nystagmus and Smooth pursuits
This test is performed with subject following the examiner’s finger visually as it moves horizontally, progressively more rapidly, and then stopping centrally. A failed exam was defined as greater than 1 beat of nystagmus at center of visual field, eyes turning red or watering, or symptom provocation including headache, dizziness, or eye fatigue (subjective pain in the eye reported by the subject).
Fast saccades
This test is performed with the subject rapidly moving his or her eyes between a stationary object, in this case the examiner’s fingers, held at shoulder-width apart to test horizontally, and forehead-to-chest distance to test vertically, for 30 repetitions each. A failed exam was defined as eyes turning red or watering, or an inability to complete the exam due to symptom provocation including headache, dizziness, eye fatigue, or fogginess.
Gaze stability testing
This test, which assesses the vestibular ocular reflex, is performed with the patient fixing his or her gaze on the examiner’s thumb while nodding “yes,” and then shaking his or her head “no” side-to-side, for 30 repetitions each. A failed exam was defined as eyes turning red or watering, or an inability to complete the exam due to symptom provocation including headache, dizziness, eye fatigue, or fogginess.
Near-point of convergence testing
This test is performed using a standard Astron accommodative rule (Gulden Ophthalmics, Elkins Park, PA) with a single column 20/30 card.12 The ruler is placed at the center the subject’s forehead, and a measurement is taken when the patient reported the letters became double. A failed exam was defined as the letters becoming double at greater than 6 cm from the patient’s forehead.13
Gait/balance testing
This test is performed by a tandem heel-toe gait forward and backward with both eyes open and closed for 10 steps each. A failed exam was defined as a patient who raises his or her arms from the side for stability or widens gait, or has extreme truncal swaying without immediate correction.
Failure on any of the nine elements of the exam was considered a failed overall examination, and all findings for each element were weighted equally. If failure on one or more of the exam elements occurred, the lead study investigator administered a secondary questionnaire to assess for symptoms with everyday activity, including assessing for headaches that are positional or awaken the patient from sleep, headaches or dizziness that develop when reading or taking notes, and unsteadiness or balance problems with everyday activity. If the patient was symptomatic with everyday activity, the results were to be documented in the medical record and reported to the primary treating attending physician, who would determine appropriate follow-up.
Finally, for each exam element, the examiner noted if the patient was unable to complete the measure due to developmental age, defined as a patient who either was not able to initiate testing or lost attention during the testing, without reported symptoms or objective abnormalities.
Outcomes
The primary outcome of interest was the proportion of subjects with a failure on one or more of the nine elements of the vestibular and oculomotor examination. To evaluate the secondary objectives, subjects were categorized into four age groups (6–8 years old, 9–11 years old, 12–14 years old, and 15–18 years old). To determine the age at which the examination could be reliably completed, we determined the proportion of subjects developmentally unable to complete each exam element in each age group.
Power and Statistical Analysis
Based on prior studies,6,8 we estimate that as many as 20% of the neurologically normal patient population exhibits vestibular deficits on examination. To calculate this proportion with a 10% confidence interval, using an alpha of 0.05 and a power of 0.8, we estimated we would have to enroll 300 children. Demographic and baseline statistics were summarized using standard descriptive statistics. Point estimates and 95% confidence intervals were calculated for all primary and secondary endpoints using the Exact (Clopper-Pearson) method. Children who were developmentally unable to complete each exam element were excluded from the point estimate calculation. P-values were obtained using two-sample proportion testing, and testing for trends was conducted using an extension of the Wilcoxon rank-sum test. The analysis was conducted using Stata version 14.2 (StataCorp, College Station, TX).
RESULTS
A total of 295 subjects were included in the analysis. A flowchart of patient enrollment is shown in Figure 1. Information on patient demographics is presented in Table 1. Overall, 24% (n = 71) of subjects failed at least one of the nine examination elements (95% confidence interval [CI] 19% to 29%). A breakdown of the failed exam elements is shown in Table 2. The largest proportion of patients showed failures on saccade testing, with 19% (n = 52) of all patients (95% CI 14% to 24%) failing either vertical or horizontal saccades. 87% (n = 33) of those with failed horizontal saccade testing and 72% (n = 28) of those with failed vertical saccade testing only reported symptoms after 15 repetitions; 97% of those who failed either saccade testing reported symptoms after 10 repetitions. Of the 71 subjects with a failed exam, 48% (n = 34) showed an abnormality on only one of nine exam elements. More than 1 failed exam element was noted in 13% (n = 37), and 5% (n = 15) failed more than 2 exam elements. None of the subjects with failed exams reported daily symptoms on secondary questionnaire. By the most common chief complaints, 23% (95% CI 14% to 34%) of patients with upper extremity trauma, 43% (95% CI 27% to 59%) of patients with abdominal pain, 18% (95% CI 9% to 30%) of patients with respiratory distress, 23% (95% CI 12% to 39%) of patients with sore throat, and 21% (95% CI 9% to 38%) of patients with rash had a failure on at least one examination element.
Figure 1.
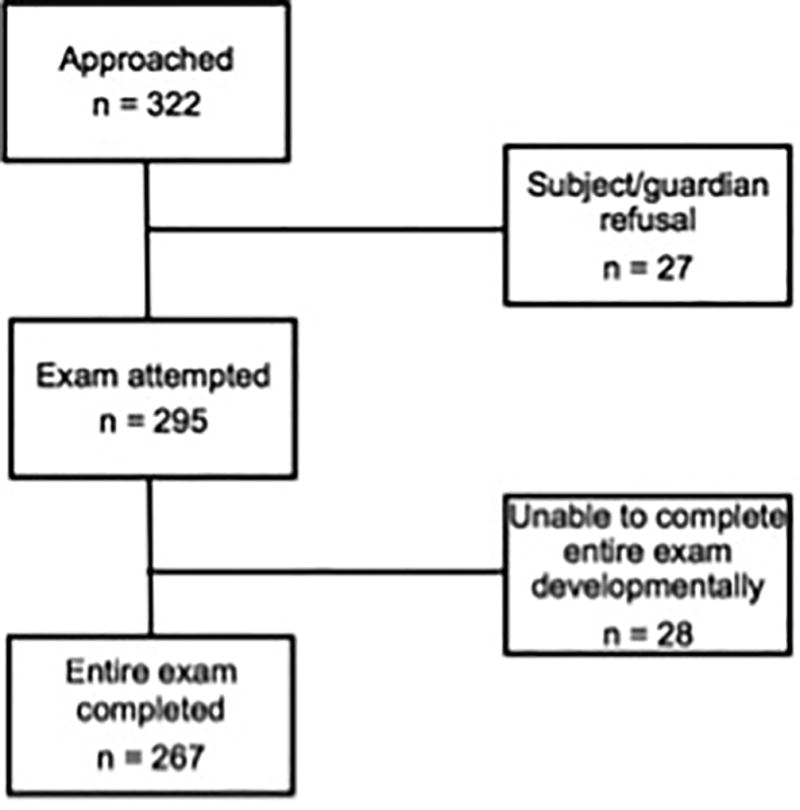
Flowchart of patient enrollment.
Table 1.
Patient demographics
| Characteristic | N (%) |
|---|---|
| All Patients | 295 |
| Male | 147 (50%) |
| Age 6–8 years | 79 (27%) |
| Age 9–11 years | 78 (26%) |
| Age 12–14 years | 83 (28%) |
| Age 15–18 years | 55 (19%) |
| Chief complaint | |
| Upper extremity trauma | 70 (24%) |
| Respiratory distress | 62 (21%) |
| Sore throat | 43 (15%) |
| Abdominal pain | 40 (14%) |
| Rash | 34 (12%) |
| Chest pain | 20 (7%) |
| Ear pain | 11 (4%) |
| Genitourinary complaint | 11 (4%) |
| Nosebleed | 4 (1%) |
Table 2.
Failures on the vestibular and oculomotor examination by exam element
| Exam Element | Abnormal % (n) | 95% CI |
|---|---|---|
| ANY | 24% (71) | (19%, 29%) |
| Dysmetria | 0% (0) | (0%, 1.2%) |
| Nysagmus | 0% (0) | (0%, 1.3%) |
| Smooth pursuits | 0.3% (1) | (0%, 1.9%) |
| Horizontal saccades | 14% (38) | (10%, 19%) |
| Vertical saccades | 14% (39) | (10%, 19%) |
| Horizontal gaze stability | 2.1% (6) | (0.8%, 4.4%) |
| Vertical gaze stability | 4.1% (12) | (2.1%, 7.0%) |
| Convergence | 2.4% (7) | (1.0%, 4.9%) |
| Tandem gait | 8.2% (24) | (5.4%, 12%) |
Females exhibited more overall failures than males (29%, n = 43 vs. 19%, n = 28; p=0.043; see Table 3), including saccades (19% [n = 26] vs. 9% [n = 12] for horizontal saccades, p=0.023; 19% [n = 25] vs. 10% [n = 14] for vertical saccades, p=0.071) and near-point of convergence (4.1% [n = 6] vs. 0.7% [ n = 1], p-=0.061). Overall the youngest age group (6–8 years old) showed the fewest overall failed exams for those able to fully complete the examination (15% [n = 12] see Table 4). Those in the youngest age group had more abnormalities on near-point of convergence (6.3% [n = 5] vs. 3.8% [n = 3], 2.5% [n = 2], and 3.6% [n = 2] in the older age groups), however this difference was not significant.
Table 3.
Failures on the vestibular and oculomotor examination by gender
| MALE | FEMALE | |||
|---|---|---|---|---|
| Exam Element | Abnormal % | 95% CI | Abnormal % | 95% CI |
| ANY | 19% | (13%, 26%) | 29% | (22%, 37%) |
| Dysmetria | 0.0% | (0%, 2.5%) | 0.0% | (0%, 2.5%) |
| Nysagmus | 0.0% | (0%, 2.6%) | 0.0% | (0%, 2.5%) |
| Smooth pursuits | 0.0% | (0%, 2.6%) | 0.7% | (0%, 3.7%) |
| Horizontal saccades | 9.1% | (4.8%, 15%) | 19% | (13%, 26%) |
| Vertical saccades | 10% | (5.8%, 17%) | 18% | (12%, 26%) |
| Horizontal gaze stability | 1.4% | (0.2%, 4.8%) | 2.8% | (0.8%, 6.9%) |
| Vertical gaze stability | 3.4% | (1.1%, 7.8%) | 4.8% | (1.9%, 9.6%) |
| Convergence | 0.7% | (0%, 3.8%) | 4.1% | (1.5%, 8.7%) |
| Tandem gait | 7.0% | (3.4%, 13%) | 9.5% | (5.3%, 15%) |
Table 4.
Failures on the vestibular and oculomotor examination by age
| Age | ||||
|---|---|---|---|---|
| Exam Element | 6–8 y | 9–11 y | 12–14 y | 15–18 y |
| ANY | 15% | 32% | 32% | 26% |
| Dysmetria | 0.0% | 0.0% | 0.0% | 0.0% |
| Nysagmus | 0.0% | 0.0% | 0.0% | 0.0% |
| Smooth pursuits | 1.4% | 0.0% | 0.0% | 0.0% |
| Horizontal saccades | 6.9% | 20% | 15% | 13% |
| Vertical saccades | 8.5% | 21% | 13% | 13% |
| Horizontal gaze stability | 2.6% | 3.8% | 1.2% | 0% |
| Vertical gaze stability | 3.9% | 6.4% | 3.6% | 1.8% |
| Convergence | 6.3% | 3.8% | 2.5% | 3.6% |
| Tandem gait | 6.7% | 9.0% | 8.4% | 9.1% |
Overall 10% of subjects (n = 28) were unable to complete at least one element of the examination (see Table 5). All subjects unable to complete part of the examination were younger than 10 years old. 24 of those subjects had difficulty completing horizontal saccades, and 23 had difficulty completing vertical saccades. The distribution of subjects unable to complete at least one examination element by age is shown in Table 6.
Table 5.
Developmentally unable to complete by examination element
| Exam Element | Unable to complete % (n) |
95% CI |
|---|---|---|
| ANY | 9.5% (28) | (6.4%, 13%) |
| Dysmetria | 0.3% (1) | (0%, 1.9%) |
| Nysagmus | 2.0% (6) | (0.7%, 4.4%) |
| Smooth pursuits | 3.1% (9) | (1.4%, 5.7%) |
| Horizontal saccades | 8.1% (24) | (5.3%, 12%) |
| Vertical saccades | 7.8% (23) | (5.0%, 12%) |
| Horizontal gaze stability | 1.0% (3) | (0.2%, 2.9%) |
| Vertical gaze stability | 0.7% (2) | (0%, 2.4%) |
| Convergence | 1.7% (5) | (0.6%, 3.9%) |
| Tandem gait | 1.4% (4) | (0.4%, 3.4%) |
Table 6.
Developmentally unable to complete examination by age
| Age group | Unable to complete % (n/total patients) |
95% CI |
|---|---|---|
| Age 6 | 47% (8/17) | (23%, 72%) |
| Age 7 | 33% (8/24) | (16%, 55%) |
| Age 8 | 2% (8/38) | (9.6%, 37%) |
| Age 9 | 13% (4/31) | (3.6%, 30%) |
| Age 6–9 | 26% (28/110) | (18%, 35%) |
Finally, each individual exam element had a percent agreement of at least 84%, with a range of 84% to 100%. Due to low prevalence of abnormalities in each individual test among the 29 subjects on whom inter-rater reliability was performed, we were unable to calculate a reliable kappa statistic for these tests.14
DISCUSSION
This study evaluated the prevalence of failures on a version of the vestibular and oculomotor examination for concussion in neurologically normal, non-concussed children and adolescents. Previous studies have shown that vestibular and oculomotor deficits are common following concussion, with 70–80% of concussed children exhibiting at least one vestibular or oculomotor deficit.5,15 Additionally, those with vestibular and oculomotor abnormalities have been shown to be at risk for prolonged recovery from concussion,5 and the examination has practical utility in assessing the everyday function of the child and adolescent student. Overall, we found that nearly one quarter of children demonstrated a failure on at least one of the nine elements of the examination. However, nearly half of these subjects only had an abnormality on only one exam element, and a substantial number of those with failed exams only had the failed test result of mild symptom provocation after 10 repetitions of either horizontal or vertical saccades.
The limited data on the performance of non-concussed children on a version of the vestibular and oculomotor examination for concussion exists only in the setting of validating the examination in comparison to concussed youth. As part of their validation study of the Vestibular/Ocular Motor Screening (VOMS) Assessment, Mucha and colleagues used a population of 78 subjects age 10–17, all of whom were athletes.6 The VOMS includes assessments of smooth pursuits, horizontal and vertical saccades, gaze stability, and near-point of convergence; it additionally includes a test for visual motion sensitivity, but unlike our version, does not include dysmetria, nystagmus, or gait/balance testing. They found 9% reported symptom provocation with gaze stability, horizontal saccades, or smooth pursuits. Yorke and colleagues evaluated the performance of 105 healthy high school students, and assessed for symptom provocation change prior to and following administration of the elements of the VOMS.8 They found a median change of 0 (range 0–5), though they do not quantify the overall percentage that reported symptom change with each exam element. They do, however, report abnormal near-point of convergence in 25% of subjects. McDevitt and colleagues evaluated a sample of 60 college-aged athletes, and found that 5.4% of controls reported symptom provocation on vestibular and oculomotor assessments. Finally, Vernau and colleagues performed near-point of convergence in 108 male hockey players age 6–18, and found 11.5% of subjects had abnormal near-point of convergence. 17 It should be noted that convergence insufficiency (a diagnosis consisting of abnormal near-point of convergence plus other accommodative defects) has been more broadly studied in the pediatric population in the optometry literature, where data has shown between 5% and 17% of children have abnormal exams;18–20 no such data, however exists for measurements of symptom provocation with saccades or vestibulo-ocular reflex testing. No children exhibited failures on our dysmetria and nystagmus testing, most likely due to the fact that these tests observed purely objective findings rather than some element of symptom provocation.
Several differences exist between the populations in these prior studies and our assessed population. The prior studies all included either only athletes or teenagers and young adults. Our study, by enrolling in the ED setting and including a larger sample and age range of children (including non-athletes), likely more accurately reflects the general population. There is a potential that high functioning athletes at baseline might have more robust oculomotor and balance systems than the average child, and symptom provocation on saccade and gaze stability testing would occur more easily in non-athletes with less exposure to repetitive motion associated with exercise. While similar to the VOMS (which was the exam performed in two of the four above studies), the examination performed in this study has several key differences, specifically including a component of balance testing, which may have increased our overall percentage of exam abnormalities (and in fact, 8% of our population had an abnormality on their tandem gait).
Additional findings from our study were the significantly higher proportion of females with failed exams as compared with males, specifically in symptom provocation during horizontal and vertical saccades. Among the various age groups, the lowest proportion of failures occurred in the lowest age range (6–8 years old) and the highest among those aged 9–11 years-old. These data reflect the findings of recent studies attempting to describe the distribution of concussion-like symptoms in non-concussed children. Iverson and colleagues, among a sample of over 31,000 non-concussed teenagers, found that a significantly higher proportion of non-concussed females reported symptoms on a 22-question post-concussion scale when compared with non-concussed males.21 Hunt and colleagues conducted a similar study among a wider age range, and found the youngest non-concussed children reported concussion-like symptoms less frequently than older teenagers.22 This may partially explain the lower proportion of young children with failed fast saccades.
This study is unique in examining this specific testing among children as young as 6 years of age. Our results show that developmentally, the exam can be reliably completed in those 10 years of age and older, and can be attempted in children as young as 6 years old, with over 50% of 6-year-olds being able to complete all exam elements. When analyzing the particular exam elements, the youngest children struggled with horizontal and vertical saccades compared to the other exam elements.
There are several limitations to this study. First, this study was conducted exclusively in the emergency department setting among subjects with non-neurologic chief complaints, a sample chosen do to the fact we believe represent the population of children who will present to acute care with concern for concussion. The same pediatric emergency medicine physician trained in the performance of the examination conducted all 295 exams. While a second pediatric emergency medicine physician repeated the examination for 10% of the sample, with high percent agreement scores (all greater than 84%), we were unable to calculate a kappa statistic due to the relatively low overall percentage of abnormalities among each exam element in the random sample.13 Finally, while the VOMS examination has been validated in comparison with concussion symptom scores, and shown high internal consistency and sensitivity,6 we acknowledge that there do exist key differences between that examination and the examination performed in this study, specifically in the area of balance testing. Future studies include assessing this examination for both internal validity and inter-rater reliability amongst a sample of concussed children.
These results have implications for the diagnosis of pediatric concussion. When using this examination to aid in the diagnosis of a child with concussion, the provider should be aware that a proportion of non-concussed children may demonstrate failures on a single exam element, specifically on the tests of horizontal and vertical saccades. The vast majority of these subjects reported symptoms after at least 10 repetitions of saccades, therefore symptom provocation with fewer repetitions is more likely to be pathologic in the setting of a head injury. The proportion of non-concussed children exhibiting a failure on more than one of the nine elements of the examination is significantly lower (13%), and it is quite rare for a non-concussed child to show greater than two abnormalities (only 5% of our population). This suggests that, while one or two failed exam elements, in the absence of other symptoms, may not be sufficient for a concussion diagnosis, the practitioner can use this examination in conjunction with a careful history and subsequent acute onset of concussion symptomatology to make the diagnosis of pediatric concussion. The examination should be used as a tool in the acute care provider’s toolkit when taking a multimodal approach to evaluating pediatric concussion.
Table 7.
Percent agreement for each exam element
| Exam Element | % Agreement |
|---|---|
| Dysmetria | 100% |
| Nysagmus | 94% |
| Smooth pursuits | 100% |
| Horizontal saccades | 87% |
| Vertical saccades | 87% |
| Horizontal gaze stability | 94% |
| Vertical gaze stability | 87% |
| Convergence | 94% |
| Tandem gait | 84% |
Acknowledgments
Funding: This study was supported by a pilot grant from the Penn Injury Science Center and the Centers for Disease Control #R49CE002474 and a training grant from the National Institutes of Health, Neurologic Clinical Epidemiology Training Grant, Grant # T32-NS-061779, both awarded to Dr. Corwin.
Abbreviations
- CI
confidence interval
- ED
emergency department
- VOMS
vestibular/ocular motor screening
- VOR
vestibular ocular reflex
Footnotes
Financial disclosure: The authors have no relevant financial relationships to disclose. No an honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Zonfrillo MR, Kim KH, Arbogast KB. Emergency Department Visits and Head Computed Tomography Utilization for Concussion Patients From 2006 to 2011. Acad Emerg Med. 2015;22:872–877. doi: 10.1111/acem.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal JA, Foraker RE, Collins CL, Comstock RD. National High School Athlete Concussion Rates From 2005–2006 to 2011–2012. Am J Sports Med. 2014;42:1710–1715. doi: 10.1177/0363546514530091. [DOI] [PubMed] [Google Scholar]
- 3.Grady MF. Concussion in the adolescent athlete. Curr Probl Pediatr Adolesc Health Care. 2010;40:154–169. doi: 10.1016/j.cppeds.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 4.McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 5.Corwin DJ, Weibe DJ, Zonfrillo MR, Grady MF, Robinson RL, Goodman AM, Master CM. Vestibular Deficits following Youth Concussion. J Pediatr. 2015;166:1221–1225. doi: 10.1016/j.jpeds.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, Marchetti G, Kontos AP. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42:2479–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matuszak JM, McVige J, McPherson J, Willer B, Leddy J. A Practical Concussion Physical Examination Toolbox: Evidence-Based Physical Examination for Concussion. Sports Health. 2016;8:260–269. doi: 10.1177/1941738116641394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yorke AM, Smith L, Babcock M, Alsalaheen B. Validity and Reliability of the Vestibular/Ocular Motor Screening and Associations With Common Concussion Screening Tools. Sports Health. 2017;9:174–180. doi: 10.1177/1941738116678411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilboy N, Tanabe P, Tavers D, Rosenau AM. Agency for Healthcare Research and Quality. Emergency Severity Index (ESI): A Triage Tool for Emergency Department Care. Version 4. 2012 http://www.ahrq.gov/
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Master CL, Grady MF. Office-based management of pediatric and adolescent concussion. Pediatr Ann. 2012;41:1–6. doi: 10.3928/00904481-20120827-08. [DOI] [PubMed] [Google Scholar]
- 12.Scheiman M, Cotter S, Kulp MT, Mitchell GL, Cooper J, Gallaway M, Hopkins KB, Bartuccio M, Chung I. Treatment of accommodative dysfunction in children: results from a randomized clinical trial. Optom Vis Sci. 2011;88:1343–1352. doi: 10.1097/OPX.0b013e31822f4d7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavrich JB. Convergence insufficiency and its current treatment. Curr Opin Ophthalmol. 2010;21:356–360. doi: 10.1097/ICU.0b013e32833cf03a. [DOI] [PubMed] [Google Scholar]
- 14.Szklo M, Nieto FJ. Epidemiology, Beyond the Basics. 3. Burlington, MA: Jones and Bartlett Publishers; 2014. Chapter 8: Quality Assurance and Control. [Google Scholar]
- 15.Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, Grady MF. Vision Diagnoses Are Common After Concussion in Adolescents. Clin Pediatr (Phila) 2016;55:260–267. doi: 10.1177/0009922815594367. [DOI] [PubMed] [Google Scholar]
- 16.McDevitt J, Appiah-Kubi KO, Tierney R, Wright WG. Vestibular and oculomotor assessments may increase accuracy of subacute concussion assessment. Int J Sports Med. 2016;37:738–747. doi: 10.1055/s-0042-100470. [DOI] [PubMed] [Google Scholar]
- 17.Vernau BT, Grady MF, Goodman A, Wiebe DJ, Basta L, Park Y, Arbogast KB, Master CL. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neurpsychol. 2015;40:7–11. doi: 10.1080/87565641.2014.971955. [DOI] [PubMed] [Google Scholar]
- 18.Scheiman M, Gallaway M, Coulter R, Restein F, Ciner E, Herzberg C, Parisi M. Prevalence of vision and ocular disease conditions in a clinical pediatric population. J Am Optom Assoc. 1996;67:193–202. [PubMed] [Google Scholar]
- 19.Hussaindeen JR, Rakshit A, Singh NK, George R, Swaminathan M, Kapur S. Prevalence of non-strabismic anomalies of binocular vision in Tamil Nadu: report 2 of BAND study. Clin Exp Optom. 2017;100:642–648. doi: 10.1111/cxo.12496. [DOI] [PubMed] [Google Scholar]
- 20.Jang JU, Park IJ. Prevalence of general binocular dysfunctions among rural schoolchildren in South Korea. T J Ophth. 2015;5:177–181. doi: 10.1016/j.tjo.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iverson GL, Silverberg ND, Mannix R, Maxwell BA, Atkins JE, Zafonte R, Berkner PD. Factors Associated With Concussion-like Symptom Reporting in High School Athletes. JAMA Pediatr. 2015;169:1132–1140. doi: 10.1001/jamapediatrics.2015.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt AW, Paniccia M, Reed N, Keightley M. Concussion-Like Symptoms in Child and Youth Athletes at Baseline: What Is “Typical”? J Athl Train. 2016;51:747–757. doi: 10.4085/1062-6050-51.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


