Abstract
Objective:
Major Depressive Disorder (MDD) is associated with increased risk of mortality and aging-related diseases. Here, it was examined whether MDD is associated with higher epigenetic aging (EA) in blood as measured by DNA methylation (DNAm) patterns, whether clinical characteristics of MDD further impact these patterns, and whether findings replicate in brain tissue.
Method:
DNAmAge was estimated using all methylation sites in blood of 811 depressed patients and 319 controls from the Netherlands Study of Depression and Anxiety. The residuals of the DNAmAge estimates regressed on chronological age were calculated to indicate epigenetic aging (EA). MDD diagnosis and clinical characteristics were assessed with questionnaires and psychiatric interviews. Analyses were adjusted for sociodemographics, lifestyle, and health status. Post-mortem brain samples of 74 depressed patients and 64 controls were used for replication. Pathway enrichment analysis was conducted using ConsensusPathDB to gain insight into the biological processes underlying EA in blood and brain.
Results:
Higher EA was observed in MDD patients compared to controls (P=0.008; Cohen’s d=0.18), with a dose-effect with increasing symptom severity in the overall sample (P=0.001). Within MDD patients, EA was positively associated with childhood trauma scores (P=0.02). The case-control difference was replicated in an independent dataset of post-mortem brain samples (P=0.03). Top significantly enriched Gene Ontology terms included neuronal processes.
Conclusions:
As compared to controls, MDD patients show higher epigenetic aging in blood and brain tissue, suggesting that they are biologically older than their corresponding chronological age. This effect was even more profound in the presence of childhood trauma.
INTRODUCTION
A growing body of literature suggests that Major Depressive Disorder (MDD) is associated with increased risk of mortality and aging-related phenotypes and diseases, including cardiovascular disease, diabetes, obesity(1) cancer(2), cognitive impairment(3), and frailty(4). Given the associated negative impact on the patient’s quality of life and health care costs(5), it is of interest to investigate if MDD patients are prone to accelerated aging.
Current literature provides evidence for advanced biological aging in MDD as indicated by shorter telomere length(6;7) and advanced brain aging(8). Recently, alternative markers of biological age derived from DNA methylation have been developed (also known as “epigenetic clocks”). Chronological age can be accurately predicted from methylation data and yields estimates of DNA methylation age (DNAmAge)(9;10). Further, DNAmAge can be studied as either “decelerated” or “accelerated” by regressing it on chronological age to get a measure of epigenetic aging (EA). Thus, this measure is a promising candidate for reliably investigating accelerated or premature aging in MDD.
Previous studies have shown EA in Down syndrome(11), HIV-positive patients(12), and obesity(13). In addition, EA has been associated with poorer physical and cognitive fitness(14), increased smoking and alcohol use(15), cancer(16), Alzheimer disease(17), cardiovascular disease(16), and increased risk of mortality(18). A few studies have investigated DNAmAge in relation to schizophrenia(19;20), life stress(21), and post-traumatic stress disorder(22;23), with mixed findings. However, studies examining epigenetic aging in relation to MDD are currently lacking.
In this study, we examined whether MDD is associated with higher EA in blood, using a large clinically well-phenotyped sample to further explore associations with clinical characteristics. Moreover, we aimed to replicate findings in post-mortem brain tissue. To examine these aims, we used a sequencing based approach(24;25) that yields almost complete coverage of the CpG methylome which allowed us to obtain the most accurate DNAmAge estimates for our sample and to better explore the biological processes underlying epigenetic aging.
METHODS
The Netherlands Study of Depression and Anxiety
Participants were from the Netherlands Study of Depression and Anxiety (NESDA), an ongoing longitudinal, multi-center, cohort study designed to investigate the long-term course and consequences of depression and anxiety disorders(26). Its 2981 participants (18–65 years) include patients with a current or lifetime diagnosis of depression and/or anxiety disorder and controls (without any lifetime depressive disorder and/or anxiety disorder). Participants were recruited from the general population, general practices, and mental health organizations in order to reflect various settings and the entire range of psychopathology. Presence of MDD was ascertained with the DSM-IV based Composite International Diagnostic Interview (CIDI version 2.1(27)) assessed by trained research staff. Exclusion criteria were: a) clinically overt primary diagnosis of other psychiatric conditions, e.g. psychotic, obsessive compulsive, bipolar, or severe substance use disorder, and b) not being fluent in Dutch. The study was approved by the ethical committee of all participating centers, and participants provided written informed consent.
A total sample of N=1130 participants were selected that were divided into control (no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology (IDS)<14), n=319) and current MDD (within the past 6 months, IDS≥14, n=811) groups, leaving out those that did not meet criteria for either of the two groups. The sample selection was further based on good quality GWAS genotype information available from a previous investigation(28).
Assessed phenotypes
Sex, education (in years), and body mass index (BMI) data were collected during interviews. Alcohol was calculated as mean number of drinks/week. Smoking behavior was represented by cotinine levels, an adequate marker for calculating recent tobacco exposure(29). Physical activity was assessed using the International Physical Activity Questionnaire and indicated by Metabolic Equivalent Total-minutes per week. Health status was assessed as number of chronic diseases for which participants received medical treatment.
In all subjects, depression severity was measured with the 30-item IDS self-report version(30). Childhood trauma was assessed using the NEMESIS childhood trauma interview with personal history questions including a structured inventory of trauma exposure during childhood. Finally, frequent use of antidepressants was assessed through container inspection and categorized using World Health Organization Anatomical Therapeutic Chemical classifications: tricyclic antidepressants, selective serotonin reuptake inhibitors, and other antidepressants.
In those with MDD, depression duration was measured by the Life Chart interview, utilizing a calendar method to assess the percentage of time in which symptoms were present during the past four years(31). Also, current comorbid anxiety (Panic disorder, generalized anxiety disorder, agoraphobia, social phobia) and alcohol disorder, as well as age of onset of depression were assessed with the CIDI. A more detailed description of all phenotypes can be found in the supplement.
DNA methylation measurements
To assay the methylation status of the approximately 28 million common CpG sites in the human genome, we used an optimized protocol for MBD-seq(25). With this approach, genomic DNA is fragmented and the methylated fragments are then bound to the MBD2 protein that has high affinity for methylated DNA. The non-methylated fraction is washed away and only the methylation-enriched fraction is sequenced (for more detail, see supplement). This optimized protocol assesses about 94% of the CpGs in the methylome(25). The sequenced reads were aligned to the reference genome (build hg19/GRCh37) with Bowtie2(32) using local and gapped alignment. Aligned reads were further processed using the RaMWAS Bioconductor package(33) to perform quality control and calculate methylation scores for each CpG.
DNAmAge estimation
While existing algorithms(9;34) have gone through demonstrations of utility and reliability, estimating DNAmAge with those prediction models will be suboptimal for the current study. These algorithms were derived using methylation data from a different platform in study populations with different characteristics (e.g., age distribution). Commonly used methods for assaying DNA methylation depend on the Illumina arrays, platforms that generate variables representing percentage methylated (ranging from 0 to 1). The current study used MBD-seq, generating methylation data that is semi quantitative (scores may range from 0–20) (24). As the weights assigned to individual CpGs when making age predictions directly depend on the platform and study population, they will not optimally capture the effects of CpGs on age in the current study. Therefore, we “re-calibrated” the DNAmAge estimate in a way that is optimal for the current study. It is important to stress that we aimed to obtain the best possible DNAmAge estimates for MBD-seq data in our sample. We have not developed a new clock to be generalized to data from platforms other than MBD-seq. Furthermore, as we already collected MBD-seq data, we did not attempt to reduce the predictor set to the smallest number of CpGs as pruning sites may reduce the precision of the DNAmAge estimates.
Our approach for estimating DNAmAge is similar to the one taken by Horvath(34). Specifically, we used elastic-nets, a variable selection method that is particularly useful when the number of predictors is much larger than the number of observations(35). Parameter alpha was set to zero (i.e. ridge regression, retaining all sites in the model) where chronological age was used as outcome and methylation sites as predictors. To estimate predictive power and obtain DNAmAge estimates for each subject, k fold cross validation was used with k = 10. Thus, the sample was randomly partitioned into 10 equal sized subsamples. Of the 10 subsamples, 9 were used as training data and the remaining subsample as validation data. This ensures that in samples with the same properties and platform, our results would “replicate” and provide unbiased estimates of DNAmAge. In the RaMWAS implementation, a cycle of MWAS, marker selection, and estimation via ridge regression was repeated in each training dataset with the resulting model applied to the test data to obtain unbiased estimates of DNAmAge for each of the k= 10 iterations.
Validation of the use of MBD-seq data to estimate DNAmAge
Several analyses were performed to validate the model. First, the model used to estimate DNAmAge contained 80,000 CpGs (Supplementary Table S1). 10-fold cross-validation showed that chronological age could be predicted very well with a correlation of 0.95 (P<0.001, Figure 1). Second, when analyzing assessed phenotypes in NESDA with DNAmAge, we confirmed some similar determinants of DNAmAge found in prior studies validating our outcome measure (supplementary Table S3). Male sex(36), and higher BMI(13;37;38) were associated with higher EA. Third, to validate calculation of DNAmAge, we used both ridge regression (current study), as well as the lasso method (used by Horvath). The additional elastic-net model with parameter alpha set to 0.5, resulted in a quite comparable correlation of 0.93 between chronological age and predicted DNAmAge in our dataset (vs. 0.95 with ridge regression and alpha=0), indicating that parameter set point did not largely impact our outcome measure. Finally, to ensure no systematic bias was introduced by training the model on both cases and controls, we also trained the prediction model in controls only. This resulted in a slightly lower correlation between DNAmAge estimates and chronological age (r=0.93) since the controls represented only a third of our total sample (i.e., lower statistical power). However, the correlation between the DNAmAge estimates obtained in the full sample and those obtained using controls-only was high (r=0.98, P<0.001), indicating that psychiatric status did not impact the estimation of DNAmAge.
FIGURE 1. DNA methylation age (DNAmAge) prediction using Methyl-CpG binding domain protein-enriched genome sequencing (MBD-seq) in NESDA.
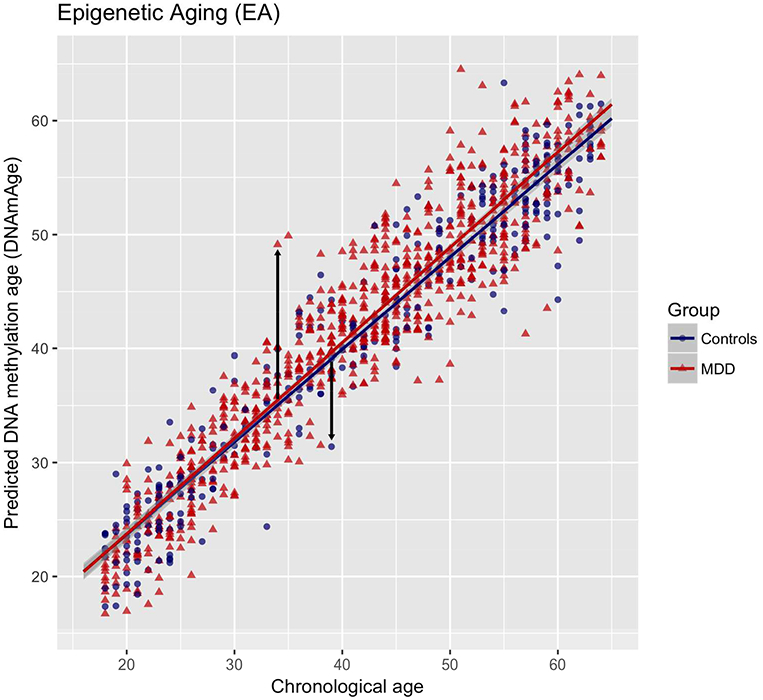
The plot shows the prediction of DNAmAge using MBD-seq across groups in blood. The lines indicate regression lines (controls: r=0.94, major depressive disorder (MDD): r=0.96, both P’s<0.001). Each circle or triangle represents an individual subject (N=1130): red triangles represent patients with MDD (n=811) and blue circles represent controls (n=319). The arrows indicate the outcome variable Epigenetic Aging (EA), representing higher epigenetic aging if the individual’s estimated DNAmAge exceeds chronological age (upward arrow), whereas negative EA indicates lower epigenetic aging (downward arrow).
Post-mortem brain samples
We pooled data of five brain sample collections from four different brain banks (Victorian Brain Bank Network, Australia; Harvard Brain Bank; the Netherlands Brain Bank; Stanley Medical Institute), including a total of 141 brain samples from BA10 and BA25 brain regions. Presence of MDD (n=74) was determined by at least one psychiatrist by using information obtained from a family member who is well acquainted with the deceased. Controls (n=67) had no history of psychiatric disorders. Post-mortem interval (hours) and pH were recorded in brain collections, with the exception of the Harvard Brain Bank.
To further test the reliability and validity of our methods, we used the same approach to predict DNAmAge in these samples with MBD-seq methylation data generated from the SOLiD 5500 W platform (Life Technologies). The model used to predict DNAmAge contained 100,000 CpGs (supplementary Table S2), obtaining a correlation of 0.69 between predicted DNAmAge and chronological age. The lower correlation with age is likely because the methylation data was generated with an older platform with lower quality (e.g. lower alignment of reads) from a more heterogeneous dataset. More details about the samples and methods can found in the supplement.
Statistical analyses for discovery
To investigate case-control differences in EA, we conducted linear regression models with EA as the outcome and all covariates as predictors. To correct for the relative abundance of cell types that may be differentially associated with MDD an additional model included cell-type proportions as covariates(39). Other linear regression models were used to examine the relationship between EA and IDS-score across groups and clinical characteristics within MDD patients. All analyses were corrected for all sociodemographic, lifestyle and health covariates, using two-tailed tests considering P<0.05 significant.
Statistical analyses for replication
Within post-mortem brain samples, we constructed a linear mixed model in R using the nlme package to account for the heterogeneity of EA across brain collections. Thus, brain collection was entered as random effect and sex as fixed effect. The P-value was derived by a likelihood ratio test, hypothesis-driven one-sided tested, and considered significant at P<0.05.
Bioinformatics analyses
To perform enrichment tests of top MWAS findings in brain and blood, we used the shiftR R-package with 1 million permutations for each test and used three thresholds (0.5, 1% and 5%) to define “top findings”. To account for this “multiple testing”, shiftR uses the same thresholds in the permutations where the test statistic distribution under the null hypothesis is generated from most significant (combination of) thresholds. A more in-depth description is provided in the supplement. To gain insight into the overlapping biological pathways affecting EA in blood and brain, we used ConsensusPathDB(40) to test whether genes harboring EA-associated CpGs were enriched for level-5 Gene Ontology GO terms. Methylation sites with P<1×10−5 were selected and had to be within gene boundaries. At least four genes had to be present in the GO term to be considered. Finally, we also evaluated the overlap between chronological age-associated and EA-associated CpG sites to examine whether similar biological processes were involved in chronological and biological aging.
RESULTS
Higher epigenetic aging and MDD in NESDA
The mean age of the NESDA sample was 41.5 years (s.d. = 13.0 years, range 18–64 years) with 64.5% of females (Table 1). The groups did not differ in age (P=0.67), but the MDD group was more often female (P=0.02) and less educated (P<0.001). As anticipated, MDD patients reported higher levels of depression severity and use of antidepressants (all P’s<0.001). Childhood trauma scores were also higher in the depressed group (P<0.001).
TABLE 1.
Participant characteristics of the Netherlands Study of Depression and Anxiety
| Characteristic | Controls (N=319) | MDD (N=811) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Sociodemographic | ||||
| Age (years) | 41.6 | 14.63 | 41.5 | 12.26 |
| Education (years) | 13.1 | 3.15 | 11.5 | 3.20 |
| N | % | N | % | |
| Female Sex | 188 | 58.9 | 541 | 66.7 |
| Mean | SD | Mean | SD | |
| Lifestyle and health | ||||
| Body Mass Index | 25.2 | 4.50 | 25.9 | 5.31 |
| Cotinine levels (ng/ml) | 70.9 | 200.8 | 103.3 | 183.9 |
| Alcohol intake (mean number of drinks/week) | 7.10 | 7.11 | 6.32 | 9.12 |
| Physical activity (1000 MET-minutes/week) | 3.91 | 2.86 | 3.49 | 3.17 |
| Number of chronic diseases | 0.46 | 0.74 | 0.69 | 0.92 |
| Mean | SD | Mean | SD | |
| Clinical characteristics | ||||
| Severity (Inventory of Depressive Symptoms score) | 5.02 | 3.54 | 33.8 | 10.9 |
| Childhood trauma index score | 0.30 | 0.69 | 1.23 | 1.24 |
| Age of onset (years) | NA | NA | 27.0 | 12.5 |
| Symptom duration (% time in the past 4 years) | NA | NA | 0.39 | 0.30 |
| N | % | N | % | |
| Comorbid anxiety disorder | NA | NA | 538 | 66.4 |
| Comorbid alcohol disorder | NA | NA | 270 | 33.3 |
| Antidepressant use | ||||
| Tricyclic antidepressants | 0 | 0.0 | 38 | 4.7 |
| Selective serotonin reuptake inhibitor | 1 | 0.3 | 243 | 30.0 |
| Other antidepressants | 0 | 0.0 | 90 | 11.2 |
| Mean | SD | Mean | SD | |
| Epigenetic Aging | ||||
| EA | −0.45 | 3.37 | 0.18 | 3.65 |
Abbreviations: MDD, major depressive disorder; MET-minutes, metabolic equivalent of number of calories spent per minute; NA, not applicable; Epigenetic Aging (EA), unstandardized residuals of DNAmAge regressed on chronological age.
EA showed (by design) a mean of zero (s.d.=3.58 years), ranging from −13.26 to 15.00. MDD patients had significantly higher EA compared to controls (b=0.64, t=2.65, P=0.008) (effect size, Cohen’s d=0.18) indicating patients were estimated to be 0.64 years (or 7.68 months) older than controls after full adjustment for covariates (Table 2). Additional analyses correcting for cell type proportions did not change results and produced a Cohen’s d of 0.14 (supplement). Consistent with a dose-response effect, a fully-adjusted linear regression showed that greater EA was significantly associated with higher IDS-score in the overall sample β=0.10, P=0.001). As expected from the high correlation between the DNAmAge estimates generated by both models (r=0.98), above mentioned results remained unchanged when performing the same analyses with the DNAmAge estimates from the controls-only model (supplement).
TABLE 2.
Estimated marginal means of epigenetic aging by major depressive disorder status and association with depression severity in the overall sample in basic-and fully adjusted analyses
| Model | Controls (N=319) | MDD (N=811) | MDD versus Controls | IDS-score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | 95% CI | Mean | SE | 95% CI | p | Cohen’s d | β | p | |
| Basic adjusteda | −0.51 | 0.20 | −0.90, −0.11 | 0.20 | 0.13 | −0.05, 0.45 | 0.004 | 0.20 | 0.12 | <0.001 |
| Fully adjustedb | −0.46 | 0.20 | −0.86, −0.06 | 0.18 | 0.13 | −0.07, 0.43 | 0.008 | 0.18 | 0.10 | 0.001 |
Abbreviations: SE. standard error; CI, confidence intervals; MDD, major depressive disorder; IDS, Inventory of Depressive Symptoms.
Adjusted for sex and education.
Adjusted for sex, education, body mass index, cotinine levels, alcohol use, physical activity, and number of chronic diseases.
Exploratory analyses of epigenetic aging and clinical characteristics
Within MDD cases, we found EA to be positively associated with childhood trauma scores β=0.09, P=0.02, see Table 3). The association between EA and IDS-score in the overall sample did not remain significant when analyzed only within MDD patients β=0.05, P=0.21), likely due to reduced variation in symptom severity. No further significant associations with clinical characteristics were found.
TABLE 3.
Associations between epigenetic aging and clinical characteristics in major depressive disorder patients (N=811)
| Variable | β | pa | |
|---|---|---|---|
| Severity (IDS score) | 0.05 | 0.21 | |
| Duration | −0.02 | 0.58 | |
| Age of Onset | 0.03 | 0.42 | |
| Comorbid anxiety disorder | −0.02 | 0.53 | |
| Comorbid alcohol dependence disorder | 0.05 | 0.21 | |
| Childhood trauma index score | 0.09 | 0.02 | |
| Antidepressant Use | Tricyclic antidepressants | 0.02 | 0.67 |
| Selective serotonin reuptake inhibitors | −0.04 | 0.31 | |
| Other antidepressants | −0.04 | 0.29 |
Abbreviations: Epigenetic aging, the residuals of DNA methylation age regressed on chronological age; IDS, Inventory of Depressive Symptoms.
Analyses are adjusted for sex, education, body mass index, cotinine levels, alcohol use, physical activity, and number of chronic diseases.
Further analyses revealed that MDD patients with childhood trauma showed the highest EA compared to controls without childhood trauma (P=0.001, Cohen’s d=0.29), highlighting that this MDD and childhood trauma subgroup is associated with the highest EA (Supplementary Figure S1). Important to note, more severe symptomatology of chronic MDD was correlated with childhood trauma (r=0.39, P<0.001), making it difficult to discern which of these two factors drives increased EA. Linear regression indeed showed that both childhood trauma (β=0.08, P=0.01) and depression severity scores (β=0.07, P=0.03) were significant predictors of EA when analyzed in the same model.
Replication in post-mortem brain samples
The mean age of the post-mortem brain samples was 55.2 years (s.d.=19.3, age range 20–100), with 45.4% of females. Groups were matched on age and sex. Mean post-mortem interval was 35.1 hours (s.d.=21.1) and mean pH was 6.51 (s.d.=0.25) across samples. EA was uncorrelated to pH or Post-mortem interval (both P’s>0.05). Table 4 shows an overview of the descriptive characteristics by brain collection. Only the control (n=67) and MDD (n=74) samples from the same brain collection were included in analyses (see supplement for more detail).
TABLE 4.
Descriptive characteristics of the post-mortem brain samples
| Characteristic | Controls (N=67) | MDD (N=74) | ||
|---|---|---|---|---|
| Brain collection 1* | N=30 | N=30 | ||
| Mean | SD | Mean | SD | |
| Age (years) | 51.63 | 12.94 | 51.93 | 18.61 |
| N | % | N | % | |
| Female sex | 17 | 56.7 | 18 | 60.0 |
| Post-mortem interval (hours) | 47.04 | 14.63 | 41.74 | 15.74 |
| pH | 6.32 | 0.21 | 6.50 | 0.28 |
| Brain collection 2 | N=4 | N=3 | ||
| Mean | SD | Mean | SD | |
| Age (years) | 77.00 | 12.25 | 83.00 | 13.00 |
| N | % | N | % | |
| Female sex | 1 | 25 | 1 | 33.3 |
| PMI (hours) | NA | NA | NA | NA |
| pH | NA | NA | NA | NA |
| Brain collection 3 | N=9 | N=9 | ||
| Mean | SD | Mean | SD | |
| Age (years) | 85.67 | 8.57 | 86.44 | 8.37 |
| N | % | N | % | |
| Female sex | 6 | 66.7 | 2 | 66.7 |
| Post-mortem interval (hours) | 5.28 | 0.78 | 6.59 | 1.93 |
| pH | 6.56 | 0.15 | 6.49 | 0.23 |
| Brain collection 4 | N=11 | N=22 | ||
| Mean | SD | Mean | SD | |
| Age (years) | 48.00 | 11.95 | 42.32 | 11.15 |
| N | % | N | % | |
| Female sex | 4 | 36.4 | 10 | 45.5 |
| Post-mortem interval (hours) | 26.72 | 9.79 | 30.14 | 12.84 |
| pH | 6.64 | 0.19 | 6.65 | 0.13 |
| Brain collection 5 | N=13 | N=10 | ||
| Mean | SD | Mean | SD | |
| Age (years) | 51.15 | 8.35 | 48.80 | 8.35 |
| N | % | N | % | |
| Female sex | 0 | 0 | 1 | 10 |
| Post-mortem interval (hours) | 24.00 | 15.00 | 49.00 | 49.50 |
| pH | 6.69 | 0.18 | 6.64 | 0.23 |
Note: N is number of samples left after quality control.
Abbreviations: MDD, major depressive disorder; NA, not applicable.
Brain collection 1 contains tissue dissected from BA25, all other collections contain tissue from BA10.
Our replication findings in independent brain samples supported our findings in NESDA and again showed that the EA was higher in MDD cases than in controls (b=1.11, χ2=3.41, P=0.03). The beta indicates that the MDD group was estimated to be on average 1.11 years older than controls. The phenotype information available from the post-mortem samples was limited, and therefore we were unable to attempt any replication of the exploratory clinical associations observed for e.g., childhood trauma in NESDA.
Enrichment testing and gene ontology analyses
When evaluating the overlap between both epigenetic aging indicators, we found that after correcting for multiple testing the top 1% findings from the EA MWAS in blood were significantly enriched for CpGs in the top 0.5% of the EA MWAS from brain (odds ratio=1.19, P<0.001). To examine possible processes underlying epigenetic aging in both tissues, we performed pathway analyses on the 1084 overlapping CpGs associated with EA, leading to 330 genes (90.7%) that were present in at least one GO category. Subsequently, this resulted in 53 significantly enriched GO terms (Supplementary Table S4). Top GO terms included neurogenesis (P-value=9.79×10−9), neuron differentiation (P-value=5.34×10−8) and regulation of neuron death (P-value=4.67×10−5), indicating that several MDD-relevant pathways were enriched in the cross-tissue EA indicators.
DISCUSSION
To the best of our knowledge, this is the first time higher epigenetic aging in MDD patients compared to controls is shown. Exploratory analyses suggested even more pronounced epigenetic aging in MDD patients with more childhood trauma. The case-control difference in blood was replicated in post-mortem brain tissue. Finally, analyses showed significantly enriched neuronal pathways associated with the overlap between EA-associated CpGs from blood and brain tissue.
Replication of our main finding in post-mortem brain tissue bolstered confidence in the observed higher epigenetic aging in MDD. Moreover, the significantly enriched overlap suggests that at least some processes affecting epigenetic aging are at play in both blood and brain. There is some evidence that blood and brain show concordance in methylation(41) and epigenetic aging(34). However, considering the interactions between stress, central and peripheral immune processes, and neurobiology(42), it is plausible and likely EA in MDD is also dictated by many systemic processes. Nonetheless, more work is needed to confirm higher epigenetic aging findings and better characterize advanced aging associated genes and their implications in MDD.
DNAmAge is just one of the several available markers of biological aging(36). The current study confirms advanced or premature biological aging in MDD with a novel platform and is consistent with literature regarding telomere length as biological marker of aging in MDD(43)(7). Also in line with other studies(44;45), post-hoc analyses between telomere length and EA showed non-significant relationships, suggesting that both measures likely independently track different aspects of biological aging. Similarly, other post-hoc analyses showed that telomere length did not alter this study’s findings when accounted for, providing further evidence that EA captures significant aging signal different from telomere length (supplementary results).
We found that childhood trauma was positively associated with higher epigenetic aging in MDD patients. It seems conceivable that MDD and accumulated stress throughout the lifetime due to childhood trauma may alter the epigenetic landscape and influence genomic regulation and function(46). However, this study did not further identify additional relationships between higher epigenetic aging and more cumulative clinical characteristics such as earlier onset age or longer duration of MDD. Rather, our findings suggest that higher epigenetic aging in MDD may be largely driven by severity of disease.
Alternatively, childhood trauma may produce long-lasting epigenetic “scars” that impact MDD and advanced or premature aging processes later in life. Individuals with childhood trauma and depressive disorders have earlier onset age, higher symptom severity, more comorbidities, increased suicidality, and poorer treatment response than patients without childhood trauma(47;48). As Teicher & Samson (2013) suggest, presence of childhood trauma is associated with a clinically and neurobiologically distinct subtype of depression.
Strikingly, three out of ten top GO categories enriched across tissues included neuronal pathways. Epigenetic mechanisms are critical in early brain development, adult neurogenesis, and late-stage brain maturation(49). Being processes that all seem markedly aberrant in MDD(50), the implicated pathways suggest EA in MDD directly contributes to disease symptomology. Additionally, the degree of overlap between the top 1% findings of EA from blood/brain and top 0.5% findings of chronological age in NESDA (odds ratio: 85.31, P<0.001/odds ratio: 1.64, P<0.001) was highly significant, suggesting that biological aging is overlapping with the same epigenetic processes underlying chronological aging.
Although effect sizes such as those observed in the current study are common in MDD research (e.g. oxidative stress, brain-derived neurotrophic factor, and cortisol yield effect sizes ranging from 0.15–0.31(51–53)), it is possible that this is an underestimate. The reason is that DNAmAge is estimated from the residuals of the regression of methylation data on chronological age. This residual variance comprises two components: i) true unique variance associated with DNAmAge and ii) measurement errors. However, because the residual variance is very small (the correlation between chronological age and methylation data was 0.95), even small measurement errors can have a large negative effect on the reliability of the DNAmAge that is defined as: reliability = Var(DNAmAge)/[Var(DNAmAge)+VAR(errors of measurement)]. As a less than perfect reliability will attenuate the correlation of DNAmAge and MDD status, the real effect size may have been underestimated.
Strengths of this study are the replication in post-mortem brain tissue and inclusion of a large clinically well-characterized and representative sample including many potential confounders that did not explain our findings. Given the full methylome coverage, we were also able to examine which biological pathways seemingly underlie epigenetic aging. However, the findings of our study should also be considered against some limitations. A direct comparison of our MBD-seq-based epigenetic clock and existing Illumina array-based clocks was not possible and beyond the current scope. However, a side-by-side comparison is an interesting endeavor for a future methodological paper. Furthermore, with the current cross-sectional data we were not able to disentangle whether greater EA in MDD truly reflects aging acceleration over time or if subjects have increased EA from birth or before adulthood that continues to be stable thereafter(54). Future studies with longitudinal designs are needed to distinguish the two possibilities.
In conclusion, our findings show that DNAmAge from both blood and brain of MDD patients is higher than their corresponding chronological age, which may contribute to their increased risk for mortality and aging-related diseases. Further, higher childhood trauma scores correlated with higher epigenetic aging in MDD patients. Taken together, our findings suggest that higher methylation aging in MDD is present in both blood and brain, and that higher epigenetic aging largely overlaps with the same underpinnings associated with chronological aging. Further research is needed to investigate the causal relationships between age-associated alterations in DNA methylation and MDD.
Supplementary Material
Overview of the 80,000 CpG sites used to estimate DNA methylation age in blood samples of patients with major depressive disorder (MDD) and controls in The Netherlands Study of Depression and Anxiety.
Overview of the 100,000 CpG sites used to estimate DNA methylation age in post-mortem brain samples from patients with major depressive disorder (MDD) and controls.
The relationship between all selected covariates and Epigenetic Aging (EA).
ConsensusPathDB-human enrichment analysis results for the overlap between epigenetic aging-associated CpG sites of blood and brain tissue.
Epigenetic aging (EA) in major depressive disorder (MDD) and childhood trauma (CT).
Disclosures and acknowledgements
The NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Zon-MW, grant number 10000–1002) and participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe). This work was supported by grant R01MH099110 from the National Institute of Mental Health and the EMGO+ Travel Grant. Tissues were received from four brain banks: the Victorian Brain Bank, supported by The Florey Institute of Neuroscience and Mental Health, The Alfred and Victorian Forensic Institute of Medicine and funded by Australia’s National Health & Medical Research Council and Parkinson’s Victoria; the Stanley Medical Research Institute; The Netherlands Brain Bank, Netherlands Institute of Neuroscience, Amsterdam; the Harvard Brain Tissue Resource Center.
Footnotes
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest. BP has received research funding –non-related to the current study – from Jansen Research and Boehringer Ingelheim.
REFERENCES
- 1.Penninx BWJH: Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms [Internet]. Neurosci. Biobehav. Rev 2016; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0149763415303559 [DOI] [PubMed] [Google Scholar]
- 2.Spiegel D, Giese-Davis J: Depression and cancer: Mechanisms and disease progression. Biol. Psychiatry 2003; 54:269–282 [DOI] [PubMed] [Google Scholar]
- 3.McDermott LM, Ebmeier KP: A meta-analysis of depression severity and cognitive function. [Internet]. J. Affect. Disord 2009; 119:1–8[cited 2013 Mar 14] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19428120 [DOI] [PubMed] [Google Scholar]
- 4.Buigues C, Padilla-Sanchez C, Garrido JF, Navarro-Martinez R, Ruiz-Ros V, Cauli O: The relationship between depression and frailty syndrome: a systematic review. Aging Ment Heal 2015; 19:762–772 [DOI] [PubMed] [Google Scholar]
- 5.Ben-Avraham D, Muzumdar RH, Atzmon G: Epigenetic genom-wide association methylation in aging and longevity. Ep 2012; 4:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schutte NS, Malouff JM: The association between depression and leukocyte telomere length: A meta-analysis. Depress. Anxiety 2015; 32:229–238 [DOI] [PubMed] [Google Scholar]
- 7.Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR: Depression and telomere length: A meta-analysis. J. Affect. Disord 2016; 191:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, Falkai P, Riecher-Rössler A, Möller HJ, Reiser M, Pantelis C, Meisenzahl E: Accelerated brain aging in schizophrenia and beyond: A neuroanatomical marker of psychiatric disorders. Schizophr. Bull 2014; 40:1140–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K: Genomewide Methylation Profiles Reveal Quantitative Views of Human Aging Rates [Internet]. Mol. Cell 2013; 49:359–367 Available from: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath S: DNA methylation age of human tissues and cell types. [Internet]. Genome biol 2013; 14:R115 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4015143&tool=pmcentrez&rendertype=abstract%5Cnhttp://genomebiology.com/2013/14/10/R115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath S, Garagnani P, Bacalini MG, Pirazzini C, Salvioli S, Gentilini D, Di Blasio AM, Giuliani C, Tung S, Vinters HV, Franceschi C: Accelerated epigenetic aging in Down syndrome. Aging Cell 2015; 14:491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine AJ, Quach A, Moore DJ, Achim CL, Soontornniyomkij V, Masliah E, Singer EJ, Gelman B, Nemanim N, Horvath S: Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders [Internet]. J. Neurovirol 2015; 366–375 Available from: http://link.springer.com/10.1007/s13365-015-0406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schönfels W, Ahrens M, Heits N, Bell JT, Tsai P-C, Spector TD, Deloukas P, Siebert R, Sipos B, Becker T, Röcken C, Schafmayer C, Hampe J: Obesity accelerates epigenetic aging of human liver [Internet]. Proc. Natl. Acad. Sci 2014; 201412759 Available from: http://www.pnas.org/content/early/2014/10/10/1412759111%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/25313081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Gibson J, Redmond P, Cox SR, Pattie A, Corley J, Taylor A, Murphy L, Starr JM, Horvath S, Visscher PM, Wray NR, Deary IJ: The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol 2015; 44:1388–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beach SRH, Dogan MV., Lei MK, Cutrona CE, Gerrard M, Gibbons FX, Simons RL, Brody GH, Philibert RA: Methylomic Aging as a Window onto the Influence of Lifestyle: Tobacco and Alcohol Use Alter the Rate of Biological Aging. J. Am. Geriatr. Soc 2015; 63:2519–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perna L, Zhang Y, Mons U, Holleczek B, Saum K-U, Brenner H: Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort [Internet]. Clin. Epigenetics 2016; 8:64 Available from: http://clinicalepigeneticsjournal.biomedcentral.com/artides/10.1186/s13148-016-0228-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine ME, Lu AT, Bennett DA, Horvath S: Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging (Albany. NY). 2015; 7:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen BH, Marioni RE, Colicino E, Peters MJ, Ward CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Bressler J, Fornage M, Studenski S, Vandiver AR, Tanaka T, Kiel DP, Liang L, Vokonas P, Lunetta KL, Murabito JM, Bandinelli S, Dena G, Melzer D, Nalls M, Pilling LC, Price TR, Andrew B, Gieger C, Holle R, Kretschmer A, Kronenberg F, Visscher PM, Shah S, Wray NR, Mcrae AF, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Levy D, Baccarelli A, Van Meurs J, Bell JT: DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany. NY). 2016; 8:1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voisey J, Lawford BR, Morris CP, Wockner LF, Noble EP, Young RM, Mehta D: Epigenetic analysis confirms no accelerated brain aging in schizophrenia [Internet]. npj Schizophr. 2017; 3:26 Available from: http://www.nature.com/articles/s41537-017-0026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA: DNA methylation age is not accelerated in brain or blood of subjects with schizophrenia [Internet]. Schizophr. Res 2017; Available from: 10.1016/j.schres.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A, Lange JF, Bruckl T, Ising M, Wray NR, Erhardt A, Binder EB, Mehta D: Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling [Internet]. Genome Biol 2015;16:266 Available from: http://genomebiology.biomedcentral.com/artides/10.1186/s13059-015-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf EJ, Logue MW, Hayes JP, Sadeh N, Schichman SA, Stone A, Salat DH, Milberg W, McGlinchey R, Miller MW: Accelerated DNA methylation age: Associations with PTSD and neural integrity. Psychoneuroendocrinology 2016; 63:155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boks MP, van Mierlo HC, Rutten BPF, Radstake TRDJ, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers CH, Broen JCA, Vermetten E: Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology 2015; 51:506–512 [DOI] [PubMed] [Google Scholar]
- 24.Aberg KA, Mcclay JL, Nerella S, Xie LY, Clark SL, Hudson AD, Bukszár J, Adkins D, Schizophrenia Consortium S, Hultman CM, Sullivan PF, Magnusson PIKE, van den Oord EJCG: MBD-seq as a cost-effective approach for methylome-wide assocciation studies: demonstration in 1500 case-control samples. Epigenomics 2012; 4:605–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan RF, Shabalin AA, Xie LY, Adkins DE, Zhao M, Turecki G, Clark SL, Aberg KA, Van Den Oord EJCG: Enrichment methods provide a feasible approach to comprehensive and adequately powered investigations of the brain methylome. Nucleic Acids Res. 2017; 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penninx BWJH, Beekman ATF, Smit JH, Zitman FG, Nolen WA, Spinhoven P, Cuijpers PIM, Jong PJDE, Marwijk HWJVAN, Assendelft WJJ, Meer KVANDER: The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. 2008; 17:121–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittchen HU: Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): A critical review. J. Psychiatr. Res 1994; 28:57–84 [DOI] [PubMed] [Google Scholar]
- 28.Boomsma DI, Willemsen G, Sullivan PF, Heutink P, Meijer P, Sondervan D, Kluft C, Smit G, Nolen W a, Zitman FG, Smit JH, Hoogendijk WJ, van Dyck R, de Geus EJC, Penninx BWJH: Genome-wide association of major depression: description of samples for the GAIN Major Depressive Disorder Study: NTR and NESDA biobank projects. Eur. J. Hum. Genet 2008; 16:335–342 [DOI] [PubMed] [Google Scholar]
- 29.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J: Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol 2009; 169:236–248 [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH: The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol. Med 1996; 26:477–486 [DOI] [PubMed] [Google Scholar]
- 31.Lyketsos CG, Nestadt G, Cwi J, Heithoff K, et al. : The Life Chart Interview: A standardized method to describe the course of psychopathology. Int. J. Methods Psychiatr. Res 1994; 4:143–155 [Google Scholar]
- 32.Langmead B, Salzberg SL Fast gapped-read alignment with Bowtie 2. Nat Methods 2013; 9:357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shabalin AA, Clark S, Hattab MW, Aberg KA, van den Oord EJCG: RaMWAS: Fast Methylome-Wide Association Study Pipeline for Enrichment Platforms. Bioinformatics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horvath S: DNA methylation age of human tissues and cell types. [Internet]. Genome Biol 2013; 14:R115 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4015143&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman J, Hastie T, Tibshirani R: Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw 2010; 33 [PMC free article] [PubMed] [Google Scholar]
- 36.Jylhava J, Pedersen NL, Hagg S: Biological Age Predictors [Internet]. EBioMedicine 2017; 21:29–36 Available from: 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, Ritz B, Bandinelli S, Neuhouser ML, Beasley JM, Snetselaar L, Wallace RB, Tsao PS, Absher D, Assimes TL, Stewart JD, Li Y, Hou L, Baccarelli AA, Whitsel EA, Horvath S: Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany. NY). 2017; 9:419–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weidner CI, Lin Q, Koch CM, Eisele L, Beier F, Ziegler P, Bauerschlag DO, Jöckel K- H, Erbel R, Mühleisen TW, Zenke M, Brümmendorf TH, Wagner W: Aging of blood can be tracked by DNA methylation changes at just three CpG sites. [Internet]. Genome Biol. 2014; 15:R24 Available from: http://genomebiology.com/2014/15/2/R24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattab MW, Shabalin AA, Clark SL, Zhao M, Kumar G, Chan RF, Xie LY, Jansen R, Han LKM, Magnusson PKE, van Grootheest G, Hultman CM, Penninx BWJH, Aberg KA, van den Oord EJCG: Correcting for cell-type effects in DNA methylation studies: reference-based method outperforms latent variable approaches in empirical studies [Internet]. Genome Biol. 2017; 18 Available from: http://genomebiology.biomedcentral.com/articles/10.1186/s13059-017-1149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamburov A, Stelzl U, Lehrach H, Herwig R: The ConsensusPathDB interaction database: 2013 Update. Nucleic Acids Res. 2013; 41:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tylee DS, Kawaguchi DM, Glatt SJ: On the outside, looking in: A review and evaluation of the comparability of blood and brain “-omes.” Am. J. Med. Genet. Part B Neuropsychiatr. Genet 2013; 162:595–603 [DOI] [PubMed] [Google Scholar]
- 42.Wohleb ES, Franklin T, Iwata M, Duman RS: Integrating neuroimmune systems in the neurobiology of depression [Internet]. Nat. Rev. Neurosci 2016; 17:497–511 Available from: http://www.nature.com/doifinder/10.1038/nrn.2016.69 [DOI] [PubMed] [Google Scholar]
- 43.Henje Blom E, Han LKM, Connolly CG, Ho TC, Lin J, LeWinn KZ, Simmons AN, Sacchet MD, Mobayed N, Luna ME, Paulus M, Epel ES, Blackburn EH, Wolkowitz OM, Yang TT: Peripheral telomere length and hippocampal volume in adolescents with major depressive disorder. [Internet]. Transl. Psychiatry 2015; 5:e676 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26556285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breitling LP, Saum K-U, Perna L, Schöttker B, Holleczek B, Brenner H: Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. [Internet]. Clin. Epigenetics 2016; 8:21 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4768341&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marioni RE, Harris SE, Shah S, Mcrae AF, Von Zglinicki T, Martin-ruiz C, Wray NR, Visscher PM, Deary IJ: The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int. J. Epidemiol 2016; 1–927433568 [Google Scholar]
- 46.Gassen NC, Chrousos GP, Binder EB, Zannas AS: Life stress, glucocorticoid signaling, and the aging epigenome: implications for aging-related diseases [Internet]. Neurosci. Biobehav. Rev 2016; Available from: 10.1016/j.neubiorev.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 47.Nanni V, Uher R, Danese A: Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 2012; 169:141–151 [DOI] [PubMed] [Google Scholar]
- 48.Teicher MH, Samson JA: Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 2013; 170:1114–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matt S, Roth ED, Roth TL: Chapter 5 Role of Epigenetics in the Brain [Internet]. Elsevier Inc.; 2014. Available from: 10.1016/B978-0-12-417114-5.00005-X [DOI] [Google Scholar]
- 50.Balu DT, Lucki I: Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. [Internet]. Neurosci. Biobehav. Rev 2009; 33:232–52[cited 2014 May 31] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2671071&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BWJH: Is depression associated with increased oxidative stress? A systematic review and meta-analysis [Internet].Psychoneuroendocrinology 2015; 51:164–175 Available from: 10.1016/j.psyneuen.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 52.Vreeburg S a., Hoogendijk WJG, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Pennix BWJ: Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity. Arch. Gen. Psychiatry 2009; 66:617–626 [DOI] [PubMed] [Google Scholar]
- 53.Molendijk ML, Bus BAA, Spinhoven P, Penninx BWJH, Kenis G, Prickaerts J, Voshaar RO, Elzinga BM: Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment [Internet]. Mol. Psychiatry 2011; 16:1088–1095 Available from: http://www.nature.com/doifinder/10.1038/mp.2010.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kananen L, Marttila S, Nevalainen T, Kummola L, Junttila I, Mononen N, Kähönen M, Raitakari OT, Hervonen A, Jylhä M, Lehtimäki T, Hurme M, Jylhävä J: The trajectory of the blood DNA methylome ageing rate is largely set before adulthood: evidence from two longitudinal studies. [Internet]. Age (Dordr). 2016; 38:65 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27300324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of the 80,000 CpG sites used to estimate DNA methylation age in blood samples of patients with major depressive disorder (MDD) and controls in The Netherlands Study of Depression and Anxiety.
Overview of the 100,000 CpG sites used to estimate DNA methylation age in post-mortem brain samples from patients with major depressive disorder (MDD) and controls.
The relationship between all selected covariates and Epigenetic Aging (EA).
ConsensusPathDB-human enrichment analysis results for the overlap between epigenetic aging-associated CpG sites of blood and brain tissue.
Epigenetic aging (EA) in major depressive disorder (MDD) and childhood trauma (CT).


