Abstract
The need for new therapeutic interventions to treat pharmacologically resistant focal epileptic seizures has led recently to the development of closed-loop systems for seizure control. Once a seizure is predicted/detected by the system, electrical stimulation is delivered to prevent seizure initiation or spread. So far, seizure prediction/detection has been limited to tracking non-invasive electroencephalogram (EEG) or intracranial EEG (iEEG) signals. Here, we examine seizure prediction based on local field potentials (LFPs) from a small neocortical patch recorded via a 10×10 microelectrode array implanted in a patient with focal seizures. We formulate the seizure (ictal) prediction problem in terms of discriminating between interictal and preictal neural activity. Using deep Convolutional Neural Networks (CNNs), we show that periods of preictal activity can be successfully discriminated (80% detection; no false positives) from periods of interictal activity several (2 – 18) minutes prior to seizure onset. CNN input features consisted of the spectral power of LFP channels (1-second time windows) computed in 50 frequency bands (0 – 100 Hz; 2 Hz steps). Our preliminary results show that intracortical LFPs may be a promising neural signal for seizure prediction in focal epilepsy.
I. Introduction
The prediction of epileptic seizures remains an important problem in neuroengineering. Epilepsy is one of the most common neurological disorders affecting ~ 60 million people worldwide [1]. In a substantial proportion of those, pharmacological, and even resective surgery, intervention is not a successful approach for seizure control. Alternative therapeutic interventions based on closed-loop systems for seizure prediction/detection combined with electrical stimulation have been examined (e.g. the NeuroPace clinical trial [2]). These previous studies used spatially coarser signals of neural activity (e.g. iEEGs), which consist of spatial averages over populations of millions of neurons.
Here, we present preliminary analysis of seizure prediction based on local field potentials, which consist of a much finer spatial scale with respect to iEEGs. LFPs were recorded from a high density microelectrode array implanted in the neocortex of a patient with focal epilepsy [3–5]. We have previously reported early detection of focal seizures, i.e. detection of a seizure already in its initial stages, based on these microelectrode array signals [6, 7]. In this study, we focus on actual prediction by detecting differences between interictal states, i.e. cortical states during normal periods of activity away from seizures (ictal states), and preictal states, i.e. states that occur in the period immediately preceding seizure onset.
II. Methods
A. Data Acquisition and Preprocessing
Neural data were recorded from a patient with pharmacological intractable focal epilepsy at Massachusetts General Hospital under approval by its Institutional Review Board. A 10 × 10 (4 mm × 4 mm) NeuroPort microelectrode array (Blackrock Microsystems, Utah), was implanted in the middle temporal gyrus (Figure 1). Based on subdural electrocorticograms (ECoGs), the microelectrode array was ~ 2 cm away from the putative seizure onset zone.
Figure 1.
Intracranial microelectrode array recordings of local field potentials prior and during a focal epileptic seizure. Continuous recordings were obtained from an 10×10 microelectrode array (top-middle) implanted in the temporal middle gyrus. Top-left shows the MRI-reconstruction of the patient’s left hemisphere. The black square denotes the approximate location of the microelectrode array. The bottom plot shows 8 out of 96 recorded LFP sites (marked as gray squares on the top-right image of the array). Time zero corresponds to the clinical seizure onset time (seizure 1 in Fig. 3). In this study, we defined a preictal period consisting of the 5-minute interval immediately preceding the seizure onset.
Neural data were recorded continuously for more than a week, in which the patient presented several focal seizures. In this preliminary study, we focus on a subset of these data including 5 clinical seizures. For more details on the clinical case and data recordings, see reference [4], patient P1.
Field potentials (0.3 Hz – 7.5 kHz) were originally sampled at 30 kilosamples per second. These original signals were then lowpass filtered (fourth-order Butterworth filter, cutoff at 500 Hz) and downsampled to 2 KHz. We removed 16 out of 96 recorded channels due to faulty recordings and low signal to noise ratio (SNR). Figure 1 shows example LFP recordings before and during a focal epileptic seizure.
B. Feature Extraction and Data Labeling
Features were extracted from the power spectrum of each LFP channel computed on 1-second time windows (0.5-second time overlap). We used multitaper spectral estimators [8], with a bandwidth of 4 Hz, which resulted in 3 tapers. The power spectrum in the range of 0 – 100 Hz of each time window was partitioned into 2 Hz bands, which resulted into 50 features per channel. After concatenating the features over all of the selected LFP channels, we obtained an 80×50 feature matrix for each time window. Figure 2.a shows sample feature matrices for interictal and preictal periods, respectively.
Figure 2.
(a) Feature matrix samples from interictal and preictal periods. Rows is a feature matrix are estimated separately from the 80 selected LFP channels. (b) Organization of features matrices: Red and black denote preictal and interictal samples, respectively. Ictal and post-ictal periods were not included in the training set. (c) Structure of the convolutional neural network (CNN) with three convolutional layers. The output layer (“Labels”) correspond to two activation nodes for the “preictal” and “interictal” classes.
To assess the predictability of seizures, we adopted here the following scheme based on a hypothesized distinction between interictal and preictal states or periods. A 5-minute period preceding each of the 5 seizures was defined as preictal periods. As stated above, recorded LFP time series, excluding seizures and a 20-minute postictal period, were partitioned into 1-second segments, which were then labeled as interictal or preictal. The total interictal data consisted of 8.2 hours of data, including (228, 81, 96, 44 and 43)-minute interictal sequences corresponding to seizures 1 – 5, respectively.
Figure 2.b presents a schematic diagram of preictal and interictal feature matrices, in which matrices within 5 minutes before the ictal onset are labeled as the preictal class (red), and feature matrices before that as the interictal class (black).
C. Classification via Convolutional Neural Networks
Our goal is to demonstrate that interictal and preictal samples can be classified correctly based on the spectral features defined here. A successful classification would imply that one can also successfully predict an upcoming seizure.
For classification purposes, we used deep Convolutional Neural Networks (CNNs). Multilayer CNNs have been a top-of-the-shelf machine learning tool for image and speech recognition and have also been previously used in ECoG-based seizure prediction [9]. The two nodes in the visible output layer corresponded to the two class labels, i.e. interictal and preictal, respectively. Figure 2.c shows the CNN structure with three hidden convolutional layers and two subsampling layers.
The performance of the CNN approach was assessed under a “leave-one-seizure-out” cross-validation scheme. Specifically, data corresponding to each of the five selected seizures were organized into five data sequences, each containing interictal and preictal data. A CNN was trained on four data sequences and tested on the remaining test sequence, and so on such that each sequence played the role of a test sequence once. We examined different numbers of iterations (“epochs”), ranging from 1 to 100, in the training of each CNN. The scores of the node corresponding to the “preictal” label in the topmost layer were used for prediction.
III. Results
A. Training CNNs for Seizure Prediction
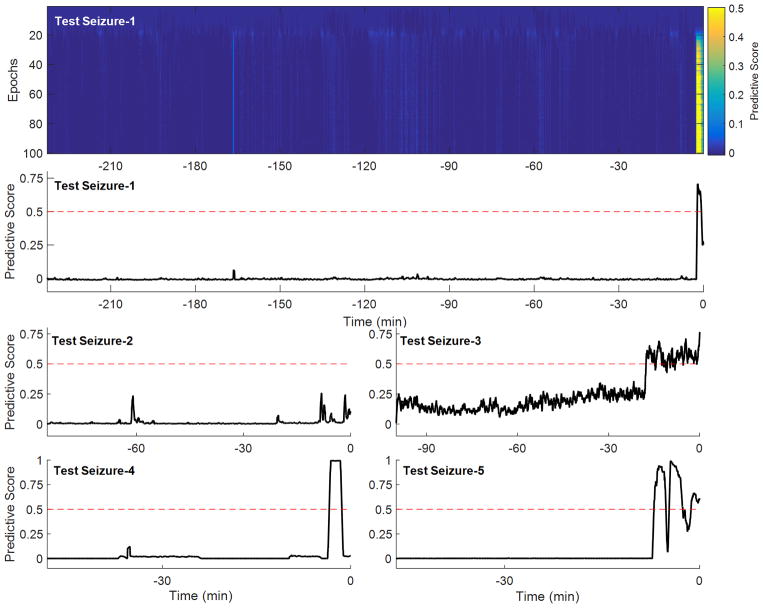
Figure 3 (first row) shows the predictive score of a CNN trained with increased number of iterations (epochs) under cross-validation. The predictive score, a number between 0 and 1, was computed for the test data sequence including interictal and preictal leading to seizure-1. It can be seen that the predictive score substantially deviates from 0 during the period immediately preceding the seizure onset (at time zero) for CNNs trained with > 20 – 30 iterations. A preictal classification made before the start of the a priori defined preictal period was considered correct if the general trend of score function extended over the preictal period. Higher number of iteration improved sensitivity and reduced false positives. Figure 3 (second row) shows the predictive score after 100 training epochs, in which the seizure is predicted at 2.5 minutes before the ictal onset. (A seizure prediction was defined as the time point where the predictive score crossed the prediction threshold, which was set here at 0.5.)
Figure 3.
First row: Predictive score of a trained CNN on interictal and preictal activity leading to seizure-1 (not introduced for training) after each epoch in the training process. Second, third and fourth rows: The predictive scores for all of the five seizures obtained after 100 epochs of CNN training.
B. Leave-one-seizure-out Analysis
Figure 3 (third and fourth rows) show the predictive scores for interictal/preictal data sequences corresponding to seizures 2 to 5, respectively. Seizures 3, 4, and 5 were predicted 18, 3.5 and 7 minutes before the ictal onset, respectively. For seizure-2, however, the predictive score did not cross the prediction threshold. Nevertheless, the predictive scores corresponding to this seizure did peak (score = 0.25) at 8 and 2 minutes before the ictal onset.
Under the chosen threshold, there were no false positives, and 4 seizures were predicted giving an 80% sensitivity, with an average 7.75 minute early prediction time.
IV. Conclusion
Our preliminary analysis in one patient with focal epilepsy shows that seizure prediction based on LFPs recorded from intracortical microelectrode arrays are a promising approach warranting further investigation in a larger datasets and number of patients, as well as detailed comparison with approaches based on other types of intracranial recordings. In this study, preictal periods were defined a priori as 5-minute periods preceding seizure onsets. The duration of preictal periods may vary from seizure to seizure and a more principled way to define these periods, especially for training data purposes, needs to be developed. In some cases, there might also be a gradual “build up” of activity from interictal to preictal periods, potentially explaining the gradual rise of the scores before their abrupt change, as for example in seizure 3. Also, a systematic approach for selection of prediction thresholds and prediction time horizons needs to be developed. In addition, we applied a multilayer CNN to very simple spectral power features. We plan to examine in the future the use of more complex CNNs and spectral features (including higher frequency bands and temporal information, e.g. spectrograms). Also, different training schemes and threshold selection, which will require a “two-stage” approach, need also to be examined in a more systematic manner. Furthermore, we hope to extend our study to include, beyond the LFP signals examined here, neuronal spiking activity in single neurons and multiunit activity recorded by these microelectrode arrays.
Acknowledgments
This research was supported by: the National Institute of Neurological Disorders and Stroke (NINDS), grants R01NS079533 (to WT) and R01NS062092 (to SSC); the U.S. Department of Veterans Affairs, Merit Review Award RX000668-01A2 (to WT); and the Pablo J. Salame ‘88 Goldman Sachs endowed Assistant Professorship of Computational Neuroscience (to WT). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank the participant in this study and nurses and physicians at MGH.
Contributor Information
Mehdi Aghagolzadeh, Department of Neuroscience, Brown University, and the Center for Neurorestoration and Neurotechnology, U.S. Department of Veterans Affairs, Providence, RI 02912.
Leigh R. Hochberg, School of Engineering and the Institute for Brain Science, Brown University, and the Center for Neurorestoration and Neurotechnology, U.S. Department of Veterans Affairs, Providence, RI 02912, and the Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114
Sydney S. Cash, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114
Wilson Truccolo, Department of Neuroscience and the Institute for Brain Science, Brown University, and the Center for Neurorestoration and Neurotechnology, U.S. Department of Veterans Affairs, Providence, RI 02912.
References
- 1.Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R, et al. Standards for epidemiologic studiesand surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 2.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, Salanova V, Cole AJ, Smith MC, Gwinn RP, Skidmore C, Van Ness PC, Bergey GK, Park YD, Miller I, Geller E, Rutecki PA, Zimmerman R, Spencer DC, Goldman A, Edwards JC, Leiphart JW, Wharen RE, Fessler J, Fountain NB, Worrell GA, Gross RE, Eisenschenk S, Duckrow RB, Hirsch LJ, Bazil C, O’Donovan CA, Sun FT, Courtney TA, Seale CG, Morrell MJ. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–41. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nature neuroscience. 2011 May 1;14(5):635–41. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truccolo W, Ahmed OJ, Harrison MT, Eskandar EN, Cosgrove GR, Madsen JR, Blum AS, Potter NS, Hochberg LR, Cash SS. Neuronal ensemble synchrony during human focal seizures. The Journal of Neuroscience. 2014 Jul 23;34(30):9927–44. doi: 10.1523/JNEUROSCI.4567-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner FB, Truccolo W, Wang J, Nurmikko AV. Spatiotemporal dynamics of optogenetically induced and spontaneous seizure transitions in primary generalized epilepsy. Journal of neurophysiology. 2015 Apr 1;113(7):2321–41. doi: 10.1152/jn.01040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YS, Hochberg LR, Eskandar EN, Cash SS, Truccolo W. Adaptive parametric spectral estimation with Kalman smoothing for online early seizure detection. Neural Engineering (NER), 2013 6th International IEEE/EMBS Conference; 2013 Nov 6; pp. 1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park YS, Hochberg LR, Eskandar EN, Cash SS, Truccolo W. Early detection of human focal seizures based on cortical multiunit activity. Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE; 2014 Aug 26; pp. 5796–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophysical journal. 1999 Feb 28;76(2):691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirowski P, Madhavan D, LeCun Y, Kuzniecky R. Classification of patterns of EEG synchronization for seizure prediction. Clinical neurophysiology. 2009 Nov 30;120(11):1927–40. doi: 10.1016/j.clinph.2009.09.002. [DOI] [PubMed] [Google Scholar]