Abstract
To assess the potential therapeutic effects of adipose tissue-derived mesenchymal stem cells (ASCs) for the treatment of type 2 diabetes (T2D), we compared the phenotype and functionality of ASCs isolated from high-fat diet and streptozotocin (STZ)-induced T2D and the leptin receptor-deficient (db/db) mice with cells from healthy C57BL/6 mice. ASCs from T2D or db/db mice showed similar expression patterns of cellular markers and abilities to differentiate into adipocytes, osteoblasts, and chondrocytes. However, the rate of proliferation was reduced. ASCs from db/db mice secreted less hepatocyte growth factor (HGF). T2D mice receiving a single intravenous injection of T2D or db/db ASCs showed increased insulin sensitivity, reduced inflammation and fat content in adipose tissue and the liver and increased pancreatic β cell mass through 5 weeks post-infusion. Our data show that, although ASCs from T2D or db/db mice had inferior proliferative capacity compared to cells from healthy controls, improved insulin sensitivity and less β cell death was seen in T2D mice receiving mesenchymal stem cell (MSC) therapy. This study offers evidence that ASCs from diabetic donors have the potential to be used for cell therapy in the treatment of insulin resistance and T2D.
Keywords: adipose stem cells, type 2 diabetes, insulin resistance, β cell mass, cell therapy, type 2 diabetes mouse model, db/db mice
Graphical Abstract
Wang and colleagues show that adipose tissue-derived mesenchymal stem cells (ASCs) harvested from two type 2 diabetes (T2D) mouse models had slightly inferior proliferative capacity compared to cells from healthy control mice. Infusion of ASCs improved insulin sensitivity and preserved pancreatic β cell mass in T2D mice. This study offers evidence that ASCs from diabetic donors have the potential to be used for cell therapy in the treatment of diabetes.
Introduction
Type 2 diabetes (T2D) has become pandemic and a threat to global public health.1 T2D is characterized by impaired insulin sensitivity in insulin-targeting tissues2 and pancreatic β cell dysfunction and death.3, 4 The liver, adipose tissue, and skeletal muscles are the three major insulin-targeting tissues responsible for insulin metabolism and maintenance of blood glucose homeostasis.5 Inflammation adversely affects insulin sensitivity and worsens diabetic complications, including retinopathy, neuropathy, and vasculopathy.6, 7 Proinflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β), produced by macrophages and other innate immune cells, have negative impacts on insulin sensitivity.8, 9, 10, 11 β cell dysfunction and death is another major characteristics of T2D and often happens in patients within several years of diabetes onset.12 T2D patients have to rely on insulin sensitizers or exogenous insulin injection to maintain blood glucose within a normal range. However, these treatments are burdensome and cannot completely block the development of diabetes complications. Therefore, better therapies that can improve insulin sensitivity and promote survival and function of pancreatic β cells would bring benefit to T2D patients.
Mesenchymal stem cells (MSCs) are a population of self-renewable adult stem cells that can be harvested from multiple tissues, including bone marrow, adipose tissue, and umbilical cord.13, 14 Most early studies have used MSCs derived from bone marrow or umbilical cord for cell therapy. In animal studies, injection of bone marrow-derived MSCs improved insulin sensitivity in rodent models of T2D.15 In human clinical trials, injection of allogeneic MSCs harvested from the umbilical cord or bone marrow into T2D patients improved the function of pancreatic β cells, reduced the incidence of diabetic complications, and led to insulin independence in some patients.16, 17, 18
Compared to MSCs from bone marrow or umbilical cord, MSCs harvested from adipose tissue (ASCs) have become popular for therapy based on the ease of acquisition, high proliferative capacity, low immunogenicity, and high levels of multipotency. The therapeutic effects of ASCs have also been studied in animal models of diabetes.15, 19, 20, 21, 22 For example, ASCs exert insulin-sensitizing effects via the restoration of insulin-stimulated glucose uptake.4 Infusion of ASCs ameliorates hyperglycemia in T2D rats.15 Injection of human ASCs improved glucose tolerance, preserved β cell mass, and increased β cell proliferation in STZ-treated NOD-SCID mice,22 and it improved glucose tolerance and metabolic balance in high-fat diet (HFD)-induced mouse models of insulin resistance.23, 24, 25
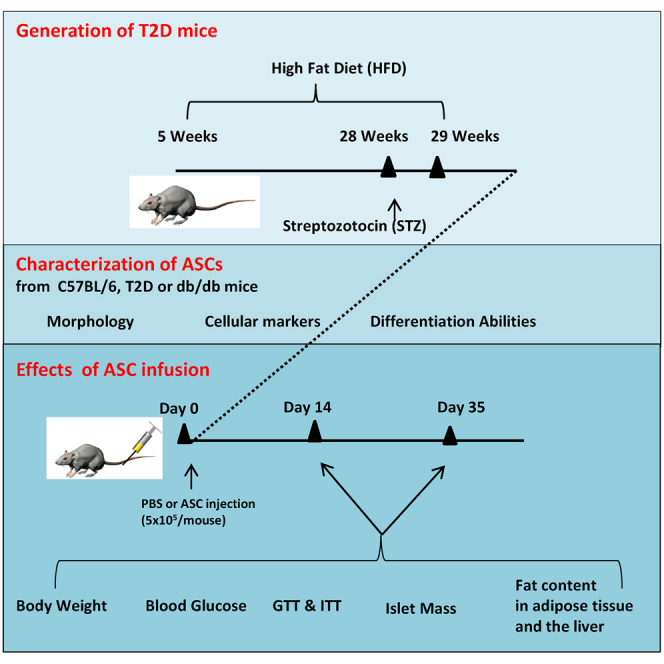
Since autologous cell therapy is preferred because of lack of immune rejection after infusion, we assessed the potential of using ASCs harvested from the HFD and streptozotocin (STZ)-treated T2D mice and the leptin receptor-deficient (db/db) mice for the treatment of insulin resistance and reduced β cell mass. The phenotypes and functionality of these cells were measured in vitro in the cell culture system and in vivo after injection into T2D recipients. A systemic comparison of the therapeutic effects of diabetic ASCs in the treatment of insulin resistance and β cell death could facilitate our understanding of the mechanisms and potential clinical application of autologous ASCs for the treatment of T2D.
Results
Generation of T2D Mice
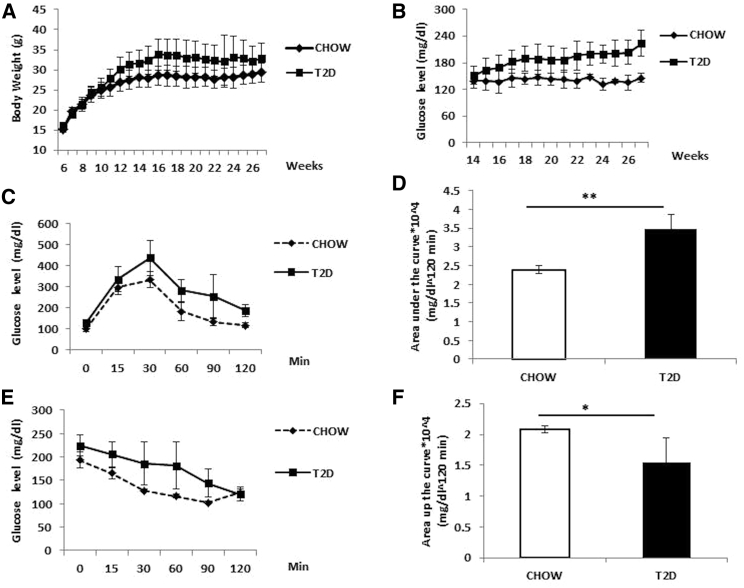
We generated T2D mouse models by HFD feeding and multiple low-dose STZ injections. At 24 weeks of HFD and STZ injections, the body weights of mice increased to 32.97 ± 2.1 g compared to 28.9 ± 1.2 g in chow-fed mice (Figure 1A). The blood glucose levels of mice were also significantly higher than the chow-fed mice (Figure 1B). T2D mice showed significant impaired glucose tolerance, as indicated by the intraperitoneal glucose tolerance test (IPGTT) (Figure 1C) and area under the curve during the IPGTT (Figure 1D). Reduced insulin sensitivity was also observed in those mice as measured by the insulin tolerance test (ITT) (Figure 1E) and area above the curve during the ITT (Figure 1F). We selected mice with insulin resistance and blood glucose levels >200 mg/dL (defined as T2D mice) for ASC collection, and we also used them as T2D recipients for all the cell therapy experiments.
Figure 1.
Generation of T2D Mouse Model by HFD Feeding and STZ Injection
(A) Changes in body weights in C57BL/6 mice fed with normal chow (chow, n = 15) or high-fat diet with STZ (T2D, n = 39). (B) Random (non-fasting) blood glucose levels in C57BL/6 mice fed with normal chow or high-fat diet and STZ injection. (C and D) Blood glucose levels (C) and area under the curve (D) in chow and T2D mice during the IPGTT. (E and F) Blood glucose levels (E) and area above the curve (F) during the ITT in chow and T2D mice before cell infusion (5–10 mice were included in each group). *p < 0.05 and **p < 0.01, ANOVA test. Error bars represent SD.
Characterization of ASCs Harvested from C57BL/6, T2D, and db/db Mice
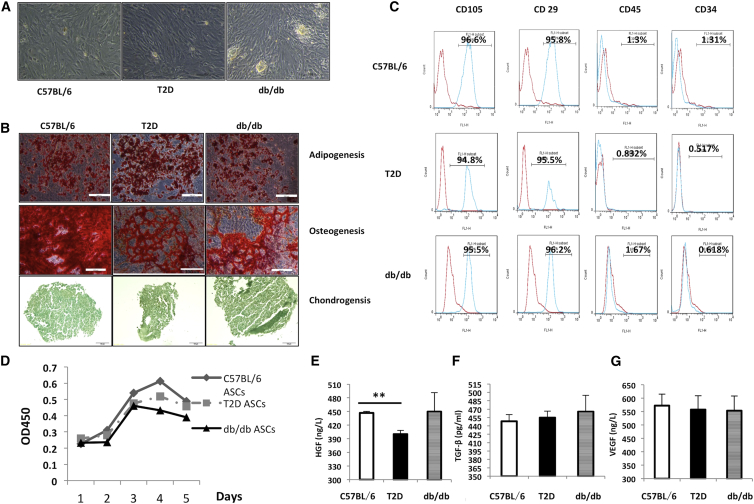
We first compared the impact of diabetes status on the phenotypes of ASCs isolated from different mouse models by measuring their morphology, expression of cellular markers, and differentiation abilities. ASCs from normal C57BL/6, T2D, or db/db mice were fibroblast-like, attached to cell culture plates (Figure 2A). They could differentiate into adipocytes, chondrocytes, and osteocytes under standard cell induction conditions (Figure 2B). All three types of cells were positive for CD105 and CD29 and negative for CD45 (<5%) and CD34 (<5%) (Figure 2C). T2D and db/db ASCs grew slower than C57BL/6 ASCs, particularly at the first and second passages (Figure 2D). We further measured secretion of hepatocyte growth factor (HGF), transforming growth factor β (TGF-β), and vascular endothelial growth factor (VEGF) in ASCs. C57BL/6 and db/db mice secreted more HGF than T2D ASCs (Figure 2E). All three cell lines secreted comparable amounts of TGF-β and VEGF (Figures 2F and 2G). Therefore, it seems that ASCs from db/db mice and T2D mice retained their major phenotypes and differentiation abilities, except that they grew slower and T2D ASCs secreted less HGF.
Figure 2.
Characterization of ASCs Harvested from Different Donors
(A) Representative micrographs of ASCs from C57BL/6, T2D, and db/db mice observed under light microscopy. (B) Representative micrographs of ASC-derived adipocytes identified by oil red O staining, osteocytes by alizarin red staining, and chondrocytes by toluidine blue staining. Scale bars, 100 μm. (C) Expression of CD105, CD29, CD45, and CD34 in ASCs analyzed by flow cytometry. Red lines represent cells stained with corresponding isotype control antibodies, and blue lines represent cells stained with individual antibodies. (D) Growth curves of C57BL/6, T2D, and db/db ASCs. (E–G) Amounts of HGF (E), TGF-β (F), and VEGF (G) secreted by different ASCs. Cells at passage three were used for the experiments. Data are from at least three individual experiments. **p < 0.01, ANOVA test. Error bars represent SD.
Effects of ASC Infusion on Body Weight and Blood Glucose Levels of T2D Mice
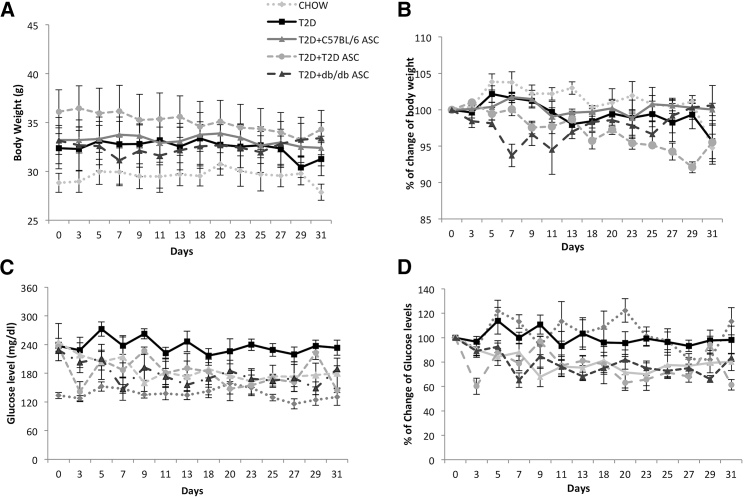
We injected cells into T2D mice and followed them for 5 weeks. There was no significant difference in body weight between T2D and any ASC-treated mice (Figures 3A and 3B). Mice receiving C57BL/6, T2D, or db/db ASCs all showed reductions in blood glucose levels compared to T2D controls (Figures 3C and 3D). These data demonstrated that ASCs from db/db or T2D mice still had the capability to reduce blood glucose of the T2D mice. In addition, we didn’t observe any significant difference in food intake among T2D mice receiving PBS or various ASCs throughout the study (data not shown).
Figure 3.
The Effects of ASC Infusion on Mouse Body Weights and Blood Glucose Levels
(A and B) Changes in body weights (A) and percentages of body weight changes (B) after ASC injection in chow-fed mice receiving PBS; T2D mice receiving PBS; and T2D mice receiving C57BL/6, T2D, or db/db ASCs. (C and D) Non-fasting blood glucose levels (C) and percentages of changes of blood glucoses (D) in chow-fed mice and T2D mice receiving C57BL/6, T2D, or db/db ASCs. Each group contains 6–8 mice. Error bars represent SD.
Effects of ASC Infusion on Glucose Tolerance and Insulin Sensitivity of T2D Mice
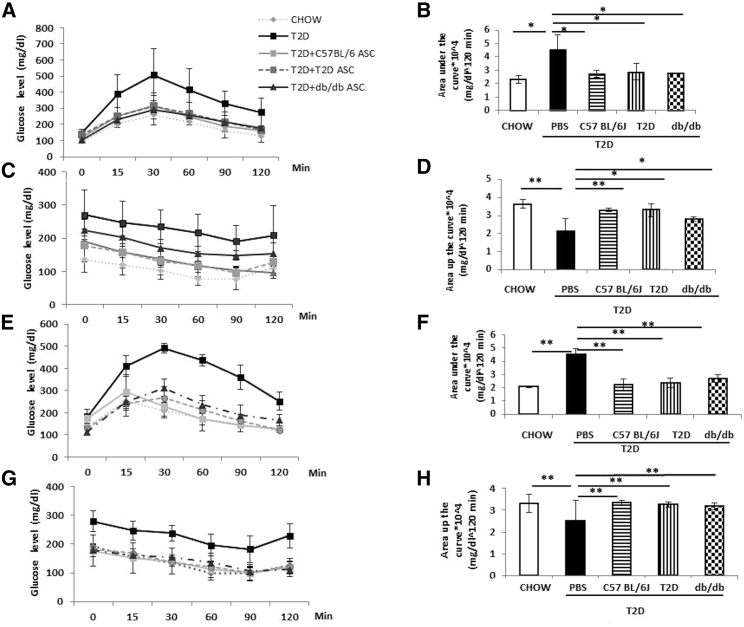
T2D mice showed impaired blood glucose disposal and reduced insulin sensitivity. We assessed the effect of ASC infusion on glucose tolerance and insulin sensitivity. At 2 weeks after cell infusion, T2D mice receiving C57BL/6, T2D, or db/db ASCs showed faster glucose clearance during the IPGTT compared to T2D controls (Figures 4A and 4B). Mice receiving C57BL/6 or T2D ASCs also showed increased insulin sensitivity with dramatic reductions in blood glucose levels during the ITT (Figures 4C and 4D). Mice receiving db/db ASCs also showed increased insulin sensitivity, but the effect was not as dramatic as those receiving C57BL/6 and T2D ASCs at 2 weeks after cell infusion (n = 4–5 per group).
Figure 4.
Metabolic Phenotypes of Mice at 2 and 5 Weeks after Treatment
(A and B) Blood glucose levels (A) and areas under the curve (B) during the IPGTT in chow-fed mice and T2D mice receiving PBS or C57BL/6, T2D, or db/db ASCs at 2 weeks after treatment. (C and D) Blood glucose levels (C) and areas under the curve (D) in mice at 2 weeks after cell infusion. (E and F) Blood glucose levels (E) and areas under the curve (F) during the IPGTT in chow-fed mice or T2D mice receiving C57BL/6, T2D, or db/db ASCs at 5 weeks after ASC or PBS infusion. (G and H) Blood glucose levels (G) and areas above the curve (H) during the ITT at 5 weeks after cell infusion. n = 4–6 per group; *p < 0.05 and **p < 0.01, Student’s t test. Error bars represent SD.
To determine the duration of the protective effect of ASC therapy, we performed IPGTT and ITT again at 5 weeks after cell infusion. Mice receiving all three kinds of ASCs showed significantly faster glucose disposal (Figures 4E and 4F) and increased insulin sensitivity (Figures 4G and 4H). These data suggest that a single injection of T2D or db/db ASCs improved glucose clearance and insulin sensitivity to a similar extent as the normal C57BL/6 ASCs in the T2D mice for at least 5 weeks post-cell infusion.
Effect of ASC Infusion on Pancreatic β Cell Mass
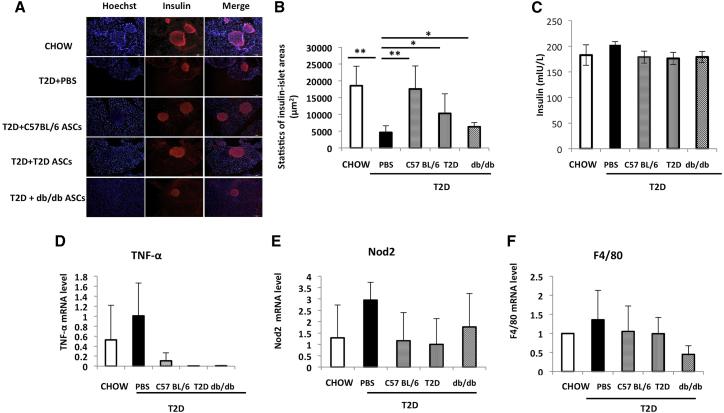
To assess the effect of ASC treatment on pancreatic β cells, we measured pancreatic β cell mass in mice receiving C57BL/6, T2D, or db/db ASCs at 5 weeks after treatment. Compared to mice fed a chow diet, T2D control mice showed dramatically reduced β cell mass (Figures 5A and 5B). Infusion of C57BL/6 ASCs ameliorated the destruction to pancreatic islets and restored β cell mass. Treatment with T2D or db/db ASCs both increased pancreatic β cell mass, but to a much lesser extent (Figures 5A and 5B). We measured plasma insulin levels in those mice. There were no significant differences in plasma insulin levels in mice from different groups (n = 6 in each group; Figure 5C). Next, we measured the expression of TNF-α, nucleotide-binding oligomerization domain-containing protein 2 (Nod2), and the macrophage marker F4/80 in pancreases harvested from different groups. T2D control pancreases had increased mRNA expression of TNF-α and F4/80 compared with pancreases from chow mice. ASC-treated pancreases showed reduced mRNA expression of TNF-α compared with T2D control pancreases. Expressions of NOD2 and F4/80 were also reduced in mice receiving ASCs, but the differences were not significant compared to T2D pancreases (Figures 5D–5F). These data indicated that the restoration of pancreatic β cell mass by ASC infusion is associated with less inflammation in the pancreas.
Figure 5.
ASC Infusion Increases Pancreatic β Cell Mass
(A) Representative immunofluorescence staining of pancreases harvested from chow-fed mice and T2D mice receiving PBS or C57BL/6, T2D, or db/db ASCs. Red represents insulin+ cells. Blue stains for nuclei. Scale bars, 100 μm. (B) Statistics of insulin+ area in pancreases from each treatment group. (C) Plasma insulin levels in chow-fed mice and T2D mice receiving PBS or C57BL/6, T2D, or db/db ASCs (n = 6 in each group). (D–F) Relative mRNA expressions of TNF-α (D), NOD2 (E), and F4/80 (F) in pancreases of chow-fed mice or T2D mice receiving PBS or C57BL/6, T2D, or db/db ASCs at 5 weeks after cell infusion, measured by RT-PCR analysis. At least three mice were included in each group. *p < 0.05 and **p < 0.01, ANOVA test. Error bars represent SD.
Effects of ASC Infusion on Fat Content in Adipose Tissue and the Liver
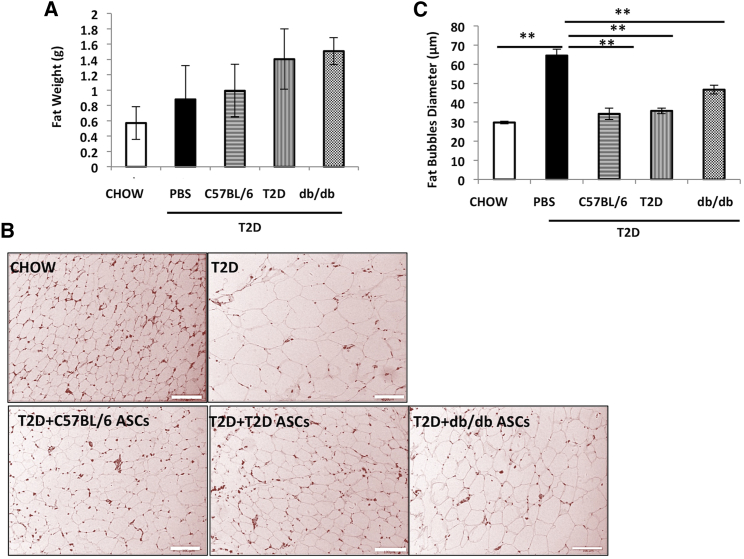
To determine the effects of ASCs on fat content in the adipose tissue, epididymal fat weights and fat bubble diameters were measured at the end of the experiments. There was no significant difference in epididymal fat weights between T2D controls and T2D mice receiving ASCs (Figure 6A). However, the average diameter of adipocytes in epididymal fat was significantly reduced in mice receiving C57BL/6, T2D, or db/db ASCs (Figures 6B 6C), suggesting that ASC infusion reduced the sizes of adipocytes caused by the HFD feeding.
Figure 6.
ASC Infusion Reduces Adiposity in Adipose Tissue
(A) Epididymal fat weights of chow-fed mice and T2D mice receiving PBS or C57BL/6, T2D, or db/db ASCs. (B) Representative micrographs of H&E staining of adipose tissue sections from mice from each treatment group. Scale bar, 100 μm. (C) Mean fat bubble diameters (n = 200) of epididymal adipocytes of mice from each group. Tissues from at least 3 mice in each group were analyzed. p < 0.05 and **p < 0.01, Student’s t test. Error bars represent SD.
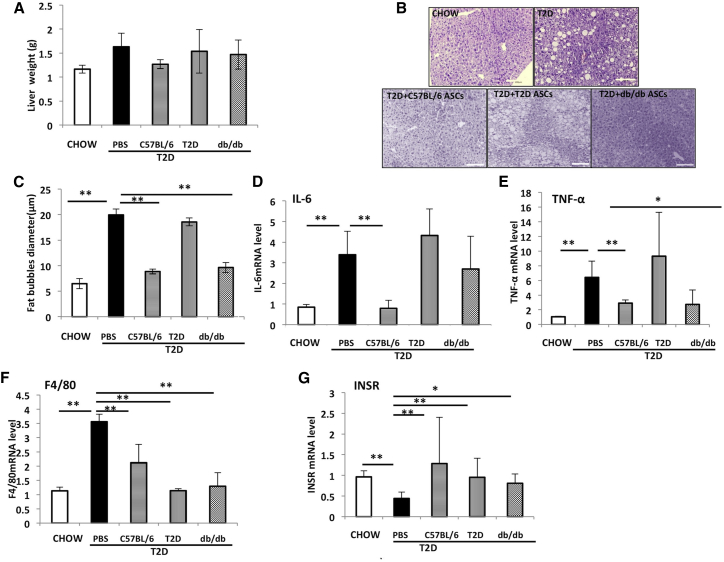
We next measured the impact of ASC infusion on liver steatosis. T2D mice showed increased liver weights and fat accumulation as demonstrated by vacuole area in the liver (Figures 7A–7C). Injection of C57BL/6 ASCs reduced liver weight and steatosis. Both T2D and db/db ASCs had little effect on liver weights (Figure 7A). Liver steatosis was improved in mice receiving db/db ASCs, but not T2D ASCs (Figure 7B). Inflammation in the liver can cause liver steatosis and reduces insulin sensitivity. As previously reported,25 HFD plus STZ upregulates proinflammatory genes, including IL-6 and TNF-α, and macrophage infiltration (as indicated by the F4/80 expression (Figures 7D–7F), and it causes a reduction in the expression of insulin receptor (InsR) gene (Figure 7G) that might have contributed to the insulin resistance in the liver. Livers from mice receiving db/db or C57BL/6 ASCs showed reduced IL-6, TNF-α, and F4/80 and increased InsR expression (Figures 7D–7G). The impact of T2D ASC infusion was not as dramatic as that of C57BL/6 or db/db ASCs. These data suggested that ASCs reduced adiposity and restored insulin sensitivity via the suppression of inflammation and restored expression of InsR in the liver. T2D ASCs showed less effect than the C57BL/6 or db/db ASCs.
Figure 7.
ASC Infusion Reduces Liver Steatosis and Inflammation and Increases Insulin Receptor Expression in Liver
(A) Liver weights in chow-fed mice and T2D mice receiving PBS or C57BL/6, T2D, or db/db ASCs at 5 weeks after cell infusion. (B) Representative micrographs of H&E staining of liver tissue sections. Scale bar, 100 μm. (C) Mean vacuole diameters in livers. (D–G) Relative mRNA expressions of IL-6 (D), TNF-α (E), F4/80 (F), and InsR (G) in livers. Samples from 5–6 individual mice were analyzed. *p < 0.05 and **p < 0.01, one-way ANOVA. Error bars represent SD.
Discussion
Emerging data from animal studies and clinical trials have demonstrated that infusion of ASCs reduces blood glucose levels in diabetic rodents and humans.19, 26, 27 We have shown that a single intravenous infusion of ASCs harvested from C57BL/6 mice reduced obesity and improved insulin resistance and glucose homeostasis in HFD-induced obese mice.25 In this study, we further compared the phenotypes and therapeutic effects of ASCs harvested from HFD feeding and STZ-induced T2D mice and the db/db mice in the treatment of insulin sensitivity, obesity, and β cell death in the T2D model. We found that ASCs from T2D or db/db mice had similar phenotypes compared to normal ASCs from C57BL/6 mice, although T2D ASCs secreted less HGF. A single intravenous infusion of T2D or db/db ASCs reduced blood glucose levels and improved insulin sensitivity in the diabetic mice. This was associated with reduced inflammation, increased pancreatic β cell mass, and reduced fat deposition in adipose tissue and the liver in treated mice. These findings provide evidence that, although ASCs harvested from diabetic donors were slightly inferior to ASCs from normal mice, they still possess significant protective effects and might be used for the treatment of diabetes, particularly since the effects on glucose tolerance and insulin sensitivity were not different at 5 weeks after ASC administration among any of the models.
Insulin resistance and β cell destruction are key features of T2D. We generated a T2D mouse model, characterized by insulin resistance and impairment of β cell survival and function, that mimics the natural progression of T2D in human by HFD feeding combined with multiple low-dose STZ injections. We selected mice with non-fasting blood glucose levels >200 mg/dL as T2D ASC donors, and we compared their phenotypes and functionalities in vitro with ASCs harvested from the db/db mice who had more severe insulin resistance and hyperglycemia. Cells harvested from normal C57BL/6 mice were used as controls. The T2D mice were also used as recipients to evaluate the therapeutic effects of various ASCs in vivo. Compared to ASCs harvested from controls, ASCs from T2D or db/db mice proliferated much slower. The reduced proliferation rate of diabetic ASCs might be caused by cell senescence seen in diabetic conditions.28 The proliferation was recovered after cell culture for two to three passages, suggesting that the impact of diabetes on ASCs might be determined by the specific donor microenvironment29 and their therapeutic ability can recover when removed from the detrimental microenvironment.
In contrast to other studies showing that ASCs from T2D patients failed to differentiate into functional adipocytes and myocytes,30, 31 we were able to differentiate T2D ASCs and db/db ASCs into adipocytes, osteocytes, and chondrocytes, using standard protocols. The discrepancy may be due to differences in donor species, disease severity, and cell passages when differentiation was initiated. We always culture cells for at least 2–3 passages before differentiation induction to allow cells to recover from stress. Although there was no difference in cell differentiation among three types of ASCs, T2D ASCs showed reduced secretion of HGF, a critical growth factor that is important for the function of ASCs. HGF plays a major role in liver repair and regeneration during injury,32 and it improves alcohol-related fatty liver disease.33 Overexpression of HGF dramatically ameliorates HFD-induced fatty liver via activation of the triglyceride transfer protein and apolipoprotein B.34 Therefore, it is reasonable to assume that T2D ASCs, which secreted the least amount of HGF, showed reduced resolution of hepatic steatosis. Further studies are needed to confirm the role of HGF in mediating the ASC effects in the liver. We do not know if diabetes impacts other ASC growth factors since we only measured HGF, VEGF, and TGF-β. Nevertheless, these data suggest that insulin resistance and hyperglycemia selectively reduced proliferating rates and HGF secretion of ASCs from diabetic mice.
ASCs from T2D or db/db mice were effective in restoring insulin sensitivity and reducing blood glucose levels. At a minimum, infused ASCs targeted multiple organs, including the pancreas, adipose tissue, and the liver. Proper function of islets requires sufficient pancreatic β cell mass and integrity.35 In this study, we found that, although the blood glucose levels of T2D mice were only >200 mg/dL, there was already a dramatic reduction in pancreatic β cell mass. T2D mice receiving T2D or db/db ASCs both had restored β cell mass with reduced inflammatory cytokine expression. In addition, the T2D mice with or without cell treatment showed similar levels of plasma insulin levels compared to chow diet mice, which might have been caused by hyperinsulinemia due to decreased insulin clearance in those mice, as observed by other studies.36, 37
Our data are not sufficient to define if the reduction of inflammation is necessary for the increase seen in pancreatic β cell mass. One benefit of cell-based therapy is that multiple simultaneous effects can sometimes be seen. Studies have shown that MSCs alleviated insulin resistance via promoting the conversion of macrophages from classically activated M1 macrophages (pro-inflammatory) into alternatively activated M2 macrophages (anti-inflammatory), which may help explain the alleviated insulin resistance in mice receiving MSCs.38, 39 In this study, ASCs suppressed HFD-induced inflammation in insulin-targeting tissues, although the T2D ASC benefits were reduced. This is in agreement with another study showing that umbilical cord-derived MSCs diminished insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in T2D rats.40
Whether infused MSCs can migrate to injured tissues after intravenous (i.v.) infusion is still controversial. It is generally believed that a majority of the infused cells would be trapped in the lung and the liver immediately after cell infusion. We have shown in our previous study that ASCs could migrate to an injured pancreas, suppress pro-inflammatory cytokine expression, and contribute to the survival of pancreatic islets.25 Other groups also showed that MSCs migrated to the pancreases and transdifferentitated into islet cells in the diabetic rat.41 Nevertheless, there is sufficient evidence to support that MSCs could deliver a profound clinical effect, without homing to target organs in significant numbers, without transdifferentiation, and despite the cell’s disappearance within short periods of time.42
Multiple studies reported that diabetes impairs MSCs. For example, diabetes induces oxidative stress and causes dysfunction in MSC differentiation.30 MSCs from db/db mice showed impaired capacity to augment postischemic neovascularization in the db/db mice. A significant decrease in the abundance of circulating MSC-like cells was correlated with the complications in individuals with T2D.43 ASCs from T2D patients showed senescence and apoptosis44 and had impaired function.45 Treating Wharton jelly-derived MSCs with diabetic patient-derived serum decreased the viability of MSCs, and it reduced their secretion of VEGF, reduced antioxidant capacity,46 reduced angiogeneic and restorative effects, and impaired cell migration.47 Diabetes also alters autophagy signaling48 and the secretome composition of MSCs.28 Human umbilical cord MSCs from obese and diabetic mothers showed impaired differentiation and immunomodulatory potential, and they did not appear to be suitable for cell therapy.49 ASCs from diabetic patients also showed increased expression of early growth response factor-1 (EGR-1) and its target genes PTEN and GGPS1, and they had impaired wound healing after being injected into the ischemic flap mouse model.50, 51 Diabetic ASCs also showed reduced fibrinolytic activity.52
Our experiments are different in focus. Although subject to the limitations of short-term mouse models, we have been able to prove the functionality of ASCs from 2 diabetic models in improving pancreatic inflammation, β cell mass, glucose tolerance, and insulin sensitivity in the HFD-induced obese mice given small doses of STZ to achieve baseline glucose levels >200 mg/dL. These data would suggest that cellular therapies with ASCs should be attempted in T2D. The studies destined for humans should focus on the duration of T2D, the baseline β cell mass, and the degree of insulin resistance as modifiers of response. Optimal handling of diabetic ASCs to restore proliferative activity should be explored.
In summary, ASCs from diabetic mice had reduced proliferation and HGF secretion compared to those harvested from normal mice. However, they still possess significant insulin-sensitizing and pancreas-protective effects, and they could be used in the treatment of insulin resistance and T2D.
Materials and Methods
Animals
Male C57BL/6 mice (4 weeks old) and db/db mice at 8 weeks of age were purchased from Nanjing Biomedical Research Institute at the Nanjing University (Nanjing, China). Mice were fed a regular chow diet ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee at Qingdao Agricultural University.
Generation of T2D Mice
Mice were allowed to adapt to the new environment for 1 week. At 5 weeks of age, C57BL/6 mice were fed with either an HFD (60% of calories from fat) or a standard chow diet (10% of calories from fat) for a total of 24 weeks. At 23 weeks of HFD feeding, mice were injected with 40 mg/kg STZ (Sigma-Aldrich, St. Louis, MO, USA) daily for 3 consecutive days to generate the T2D mouse model.53, 54 Body weights and blood glucose levels of mice were measured twice per week.
ASC Isolation and Culture
ASCs were isolated from the epididymal fat of C57BL/6 mice fed with normal chow, db/db mice, or T2D mice (at 7 days after the last STZ injection), according to a previously reported protocol.25 Tissues were digested with collagenase type 1 (Sigma-Aldrich, St. Louis, MO, USA) in PBS by incubation in a shaker at 37°C for 15–30 min. The digestion was terminated by the addition of 10% fetal bovine serum (FBS). The mixture was centrifuged at 1,200 rpm for 5 min. Cells were resuspended in complete medium made of DMEM/F12 medium supplemented with 10% FBS and 1% penicillin and streptomycin. Cells were cultured in 37°C at 5% CO2 atmosphere. Floating cells were removed at 24 hr, and cell culture medium was replaced with fresh complete medium every 2–3 days. Cells were passaged at 90% confluence by digestion with 0.25% trypsin-EDTA. ASCs at passage 3 were used in all the experiments.
ASC Characterization
Expressions of cellular markers, including CD105, CD29, CD45, and CD34, were measured by flow cytometry analysis as described.55 ASCs were induced to differentiate into adipocytes, osteocytes, and chondrocytes using cell differentiation kits according to the manufacturer’s recommendation (Mo Bi Tec., Lorzestrasse, Germany).56 The presence of adipocytes was determined by oil red O staining, osteoblasts by alizarin red staining, and chondrocytes by Alkaline Blue staining as described.25 VEGF, TGF-β, and HGF secreted by ASCs were measured by respective ELISAs. In a separate set of experiments, 1,000 cells from each ASC line were seeded in 12-well plates, cell numbers from three individual wells were counted daily for 5 days, and cell growth curves were plotted.
ASC Infusion
ASCs from C57BL/6, db/db, or T2D mice at passage 3 were infused intravenously (5 × 105/mouse, in 0.2 mL PBS) via the tail vein into the T2D recipients generated by HFD and STZ injections. C57BL/6 mice fed a chow diet and T2D mice receiving 0.2 mL PBS were used as controls.
Monitoring of Mouse Behavior, Body Weights, and Blood Glucose Levels
Mice activities (e.g., food intake, drinking, licking, and other behaviors) were monitored daily. Non-fasting blood glucose levels were measured daily using the Freestyle Lite blood glucometer (Abbott). IPGTT (2 g/kg glucose) and ITT (0.5 unit/kg insulin) were performed before and at 2 and 5 weeks after cell infusion, as previously described.25 Blood glucose area under the curve during an IPGTT and area above the curve below the basal line during the ITT were calculated using the Trapezoidal method.57
Tissue Collection
At 5 weeks after cell infusion, bloods were collected from all mice. Mice were then anesthetized and adipose tissue and liver were dissected and weighed. Half of the tissue was cut into small pieces and snap-frozen in liquid nitrogen for gene expression analysis. The other half was fixed in 4% formalin for histological analysis. Plasma insulin levels were measured using the Insulin Kit (APCOL).
RT-PCR Analysis
Total RNA was extracted from liver and pancreas tissues using the RNeasy Kit (QIAGEN, Venlo, the Netherlands). RNA was converted into cDNA by reverse transcription. Expressions of InsR, interleukin-6 (IL-6), F4/80, and TNF-α were quantified by RT-PCR analysis, as described previously.58 Primers were purchased from Life Technologies (Invitrogen, Shanghai, China). Beta-actin expression was quantified in each sample and used as an endogenous control, and relative gene expression was calculated.
H&E Staining
H&E staining was performed as described in a previous study.59 In brief, liver or adipose tissues were fixed in formalin and embedded in paraffin blocks. Sections of 5 μm were collected and stained with H&E (Sigma-Aldrich). Slides were observed using an Olympus BX51 microscope (Olympus, Tokyo, Japan), and images were captured using an Olympus DP72 digital camera. Diameters of individual fat cells in adipose tissue or fat bubbles in the liver (n = 200 in each group) were calculated using the Olympus CellSens software.
Measurement of Pancreatic β Cell Mass
The whole pancreas fixed in paraffin was continuously sectioned every 100 μm with 5-μm thickness tissue sections. About 20–25 sections were collected from each pancreas and stained with guinea pig anti-insulin antibody. Texas red-conjugated goat anti-guinea pig antibody was used to detect insulin expression. After staining, four areas of the same size were randomly selected from each tissue section. Insulin+ area in each section was calculated and used to calculate total β cell mass per mouse. An average β cell mass from at least 3 individual animals was used to compare β cell mass among groups.
Statistical Analysis
Data were analyzed by one-way ANOVA and unpaired Student’s t tests with Bonferroni correction; p values of less than 0.05 were considered statistically significant. Unless otherwise stated, data were expressed as mean ± SD.
Author Contributions
M.W. performed and analyzed all experiments and was involved in drafting the manuscript. L.S. contributed to the scientific design, data analysis, and manuscript drafting. C.S. revised the manuscript critically. H.W. and X.D. designed the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province, China (ZR2014CM011 and ZR2017MC030) and the High-level Talent Scientific Research Foundation of Qingdao Agricultural University (6631117017). Dr. Wang was supported by NIH grant DK105183.
Contributor Information
Xiao Dong, Email: 1163155358@qq.com.
Hongjun Wang, Email: wangho@musc.edu.
References
- 1.Herman W.H., Zimmet P. Type 2 diabetes: an epidemic requiring global attention and urgent action. Diabetes Care. 2012;35:943–944. doi: 10.2337/dc12-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda-Massari J.R., Gonzalez M.J., Fernando A.S., Cidre C., Paz I.M., Charvel J., Martínez V., Duconge J., Aponte A., Ricart C.M. Metabolic Correction as a tool to improve diabetes type 2 management. Bol. Asoc. Med. P. R. 2015;107:54–59. [PubMed] [Google Scholar]
- 3.Kahn S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 4.Shree N., Bhonde R.R. Conditioned Media From Adipose Tissue Derived Mesenchymal Stem Cells Reverse Insulin Resistance in Cellular Models. J. Cell. Biochem. 2017;118:2037–2043. doi: 10.1002/jcb.25777. [DOI] [PubMed] [Google Scholar]
- 5.Boura-Halfon S., Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 6.Vikram A., Tripathi D.N., Kumar A., Singh S. Oxidative stress and inflammation in diabetic complications. Int. J. Endocrinol. 2014;2014:679754. doi: 10.1155/2014/679754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada J., Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. (Lond.) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 8.Yan M., Mehta J.L., Zhang W., Hu C. LOX-1, oxidative stress and inflammation: a novel mechanism for diabetic cardiovascular complications. Cardiovasc. Drugs Ther. 2011;25:451–459. doi: 10.1007/s10557-011-6342-4. [DOI] [PubMed] [Google Scholar]
- 9.Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab. J. 2013;37:165–172. doi: 10.4093/dmj.2013.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastard J.P., Maachi M., Lagathu C., Kim M.J., Caron M., Vidal H., Capeau J., Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 11.Tack C.J., Stienstra R., Joosten L.A., Netea M.G. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunol. Rev. 2012;249:239–252. doi: 10.1111/j.1600-065X.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 12.Prentki M., Nolan C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshmipathy U., Verfaillie C. Stem cell plasticity. Blood Rev. 2005;19:29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Si Y., Zhao Y., Hao H., Liu J., Guo Y., Mu Y., Shen J., Cheng Y., Fu X., Han W. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61:1616–1625. doi: 10.2337/db11-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J., Wang Y., Gong H., Yu C., Guo C., Wang F., Yan S., Xu H. Long term effect and safety of Wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes. Exp. Ther. Med. 2016;12:1857–1866. doi: 10.3892/etm.2016.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan L.X., Guan H., Li H.B., Ren C.A., Liu L., Chu J.J., Dai L.J. Therapeutic efficacy of umbilical cord-derived mesenchymal stem cells in patients with type 2 diabetes. Exp. Ther. Med. 2015;9:1623–1630. doi: 10.3892/etm.2015.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong D., Zhuang X., Wang D., Qu H., Jiang Y., Li X., Wu W., Xiao J., Liu X., Liu J. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin. Lab. 2014;60:1969–1976. doi: 10.7754/clin.lab.2014.140305. [DOI] [PubMed] [Google Scholar]
- 19.Ezquer F.E., Ezquer M.E., Parrau D.B., Carpio D., Yañez A.J., Conget P.A. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol. Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Si Y.L., Zhao Y.L., Hao H.J., Fu X.B., Han W.D. MSCs: Biological characteristics, clinical applications and their outstanding concerns. Ageing Res. Rev. 2011;10:93–103. doi: 10.1016/j.arr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Song H., Song B.W., Cha M.J., Choi I.G., Hwang K.C. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin. Biol. Ther. 2010;10:309–319. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- 22.Kono T.M., Sims E.K., Moss D.R., Yamamoto W., Ahn G., Diamond J., Tong X., Day K.H., Territo P.R., Hanenberg H. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells. 2014;32:1831–1842. doi: 10.1002/stem.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao H., Liu J., Shen J., Zhao Y., Liu H., Hou Q., Tong C., Ti D., Dong L., Cheng Y. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats. Biochem. Biophys. Res. Commun. 2013;436:418–423. doi: 10.1016/j.bbrc.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 24.Ji A.T., Chang Y.C., Fu Y.J., Lee O.K., Ho J.H. Niche-dependent regulations of metabolic balance in high-fat diet-induced diabetic mice by mesenchymal stromal cells. Diabetes. 2015;64:926–936. doi: 10.2337/db14-1042. [DOI] [PubMed] [Google Scholar]
- 25.Cao M., Pan Q., Dong H., Yuan X., Li Y., Sun Z., Dong X., Wang H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res. Ther. 2015;6:208. doi: 10.1186/s13287-015-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali H., Al-Yatama M.K., Abu-Farha M., Behbehani K., Al Madhoun A. Multi-lineage differentiation of human umbilical cord Wharton’s Jelly Mesenchymal Stromal Cells mediates changes in the expression profile of stemness markers. PLoS ONE. 2015;10:e0122465. doi: 10.1371/journal.pone.0122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdi R., Fiorina P., Adra C.N., Atkinson M., Sayegh M.H. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Lu H., Chen Z., Wang Y., Lin J., Xu S., Zhang C., Wang B., Yuan Z., Feng X. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol. Med. Rep. 2017;16:1685–1690. doi: 10.3892/mmr.2017.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribot J., Caliaperoumal G., Paquet J., Boisson-Vidal C., Petite H., Anagnostou F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J. Cell. Mol. Med. 2017;21:349–363. doi: 10.1111/jcmm.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbagallo I., Li Volti G., Galvano F., Tettamanti G., Pluchinotta F.R., Bergante S., Vanella L. Diabetic human adipose tissue-derived mesenchymal stem cells fail to differentiate in functional adipocytes. Exp. Biol. Med. (Maywood) 2017;242:1079–1085. doi: 10.1177/1535370216681552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin P., Zhang X., Wu Y., Li L., Yin Q., Zheng L., Zhang H., Sun C. Streptozotocin-induced diabetic rat-derived bone marrow mesenchymal stem cells have impaired abilities in proliferation, paracrine, antiapoptosis, and myogenic differentiation. Transplant. Proc. 2010;42:2745–2752. doi: 10.1016/j.transproceed.2010.05.145. [DOI] [PubMed] [Google Scholar]
- 32.Lalani N., Poulsom R., Stamp G., Fogt F., Thomas P., Nanji A.A. Expression of hepatocyte growth factor and its receptor c-met, correlates with severity of pathological injury in experimental alcoholic liver disease. Int. J. Mol. Med. 2005;15:811–817. [PubMed] [Google Scholar]
- 33.Tahara M., Matsumoto K., Nukiwa T., Nakamura T. Hepatocyte growth factor leads to recovery from alcohol-induced fatty liver in rats. J. Clin. Invest. 1999;103:313–320. doi: 10.1172/JCI4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosone T., Takagi H., Horiguchi N., Ariyama Y., Otsuka T., Sohara N., Kakizaki S., Sato K., Mori M. HGF ameliorates a high-fat diet-induced fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G204–G210. doi: 10.1152/ajpgi.00021.2007. [DOI] [PubMed] [Google Scholar]
- 35.Cerf M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne) 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winzell M.S., Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 37.Mosser R.E., Maulis M.F., Moullé V.S., Dunn J.C., Carboneau B.A., Arasi K., Pappan K., Poitout V., Gannon M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am. J. Physiol. Endocrinol. Metab. 2015;308:E573–E582. doi: 10.1152/ajpendo.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odegaard J.I., Chawla A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Z., Hao H., Tong C., Cheng Y., Liu J., Pang Y., Si Y., Guo Y., Zang L., Mu Y., Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34:627–639. doi: 10.1002/stem.2238. [DOI] [PubMed] [Google Scholar]
- 40.Sun X., Hao H., Han Q., Song X., Liu J., Dong L., Han W., Mu Y. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res. Ther. 2017;8:241. doi: 10.1186/s13287-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhansali S., Kumar V., Saikia U.N., Medhi B., Jha V., Bhansali A., Dutta P. Effect of mesenchymal stem cells transplantation on glycaemic profile & their localization in streptozotocin induced diabetic Wistar rats. Indian J. Med. Res. 2015;142:63–71. doi: 10.4103/0971-5916.162116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtz A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells. 2008;1:1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu M., He X., Wang X.H., Qiu W., Xing W., Guo W., An T.C., Ao L.Q., Hu X.T., Li Z. Complement C5a induces mesenchymal stem cell apoptosis during the progression of chronic diabetic complications. Diabetologia. 2017;60:1822–1833. doi: 10.1007/s00125-017-4316-1. [DOI] [PubMed] [Google Scholar]
- 44.Cramer C., Freisinger E., Jones R.K., Slakey D.P., Dupin C.L., Newsome E.R., Alt E.U., Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19:1875–1884. doi: 10.1089/scd.2010.0009. [DOI] [PubMed] [Google Scholar]
- 45.Januszyk M., Sorkin M., Glotzbach J.P., Vial I.N., Maan Z.N., Rennert R.C., Duscher D., Thangarajah H., Longaker M.T., Butte A.J., Gurtner G.C. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes. 2014;63:3047–3056. doi: 10.2337/db13-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali F., Aziz F., Wajid N. Effect of type 2 diabetic serum on the behavior of Wharton’s jelly-derived mesenchymal stem cells in vitro. Chronic Dis. Transl. Med. 2017;3:105–111. doi: 10.1016/j.cdtm.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rezaie J., Mehranjani M.S., Rahbarghazi R., Shariatzadeh M.A. Angiogenic and Restorative Abilities of Human Mesenchymal Stem Cells Were Reduced Following Treatment With Serum From Diabetes Mellitus Type 2 Patients. J. Cell. Biochem. 2018;119:524–535. doi: 10.1002/jcb.26211. [DOI] [PubMed] [Google Scholar]
- 48.Rezabakhsh A., Cheraghi O., Nourazarian A., Hassanpour M., Kazemi M., Ghaderi S., Faraji E., Rahbarghazi R., Avci Ç.B., Bagca B.G., Garjani A. Type 2 Diabetes Inhibited Human Mesenchymal Stem Cells Angiogenic Response by Over-Activity of the Autophagic Pathway. J. Cell. Biochem. 2017;118:1518–1530. doi: 10.1002/jcb.25814. [DOI] [PubMed] [Google Scholar]
- 49.Montanucci P., Pescara T., Pennoni I., Alunno A., Bistoni O., Torlone E., Luca G., Gerli R., Basta G., Calafiore R. Functional Profiles of Human Umbilical Cord-Derived Adult Mesenchymal Stem Cells in Obese/Diabetic Versus Healthy Women. Curr. Diabetes Rev. 2016 Published online June 28, 2016. PMID 27363411. [PubMed] [Google Scholar]
- 50.Trinh N.T., Yamashita T., Ohneda K., Kimura K., Salazar G.T., Sato F., Ohneda O. Increased Expression of EGR-1 in Diabetic Human Adipose Tissue-Derived Mesenchymal Stem Cells Reduces Their Wound Healing Capacity. Stem Cells Dev. 2016;25:760–773. doi: 10.1089/scd.2015.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dzhoyashvili N.A., Efimenko A.Y., Kochegura T.N., Kalinina N.I., Koptelova N.V., Sukhareva O.Y., Shestakova M.V., Akchurin R.S., Tkachuk V.A., Parfyonova Y.V. Disturbed angiogenic activity of adipose-derived stromal cells obtained from patients with coronary artery disease and diabetes mellitus type 2. J. Transl. Med. 2014;12:337. doi: 10.1186/s12967-014-0337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acosta L., Hmadcha A., Escacena N., Pérez-Camacho I., de la Cuesta A., Ruiz-Salmeron R., Gauthier B.R., Soria B. Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes. 2013;62:4266–4269. doi: 10.2337/db13-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung J.Y., Lim Y., Moon M.S., Kim J.Y., Kwon O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr. Metab. (Lond.) 2011;8:18. doi: 10.1186/1743-7075-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie M., Hao H.J., Cheng Y., Xie Z.Y., Yin Y.Q., Zhang Q., Gao J.Q., Liu H.Y., Mu Y.M., Han W.D. Adipose-derived mesenchymal stem cells ameliorate hyperglycemia through regulating hepatic glucose metabolism in type 2 diabetic rats. Biochem. Biophys. Res. Commun. 2017;483:435–441. doi: 10.1016/j.bbrc.2016.12.125. [DOI] [PubMed] [Google Scholar]
- 55.Hu J., Fu Z., Chen Y., Tang N., Wang L., Wang F., Sun R., Yan S. Effects of autologous adipose-derived stem cell infusion on type 2 diabetic rats. Endocr. J. 2015;62:339–352. doi: 10.1507/endocrj.EJ14-0584. [DOI] [PubMed] [Google Scholar]
- 56.Ho J.H., Ma W.H., Tseng T.C., Chen Y.F., Chen M.H., Lee O.K. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng. Part A. 2011;17:255–266. doi: 10.1089/ten.TEA.2010.0106. [DOI] [PubMed] [Google Scholar]
- 57.Tai M.M. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 58.Wang H., Lee S.S., Gao W., Czismadia E., McDaid J., Ollinger R., Soares M.P., Yamashita K., Bach F.H. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005;54:1400–1406. doi: 10.2337/diabetes.54.5.1400. [DOI] [PubMed] [Google Scholar]
- 59.Dong H., Huang H., Yun X., Kim D.S., Yue Y., Wu H., Sutter A., Chavin K.D., Otterbein L.E., Adams D.B. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology. 2014;155:818–828. doi: 10.1210/en.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]