ABSTRACT
Intestinal microbiota composition and gut-associated immune response can contribute to the toxicity of arsenic. We investigated the potential toxicity of short-term arsenic exposure on gut microbiome composition, intestinal immune status, microbial arsenic resistance gene, and arsenic metabolic profiles in adult and developmental stages of CD-1 mice. The potential toxicity of arsenite [As(III)] was determined for two life stages: (i) adult animals at 24 or 48 h after single gavage (0.05 mg/kg body weight [b.w.] [low dose], 0.1 mg/kg b.w. [medium dose], and 0.2 mg/kg b.w. [high dose]) and repeated exposure at 1 mg/liter for 8 days and (ii) postnatal day 10 (PND10) and PND21 after single gavage (0.05 mg/kg b.w.). Dose- and time-dependent responses in bacterial recovery/microbial composition were observed in adults after a single gavage. Repeated exposure caused a transient decrease in the recovery of intestinal bacteria, a shift in the bacterial population with abundance of arsenic resistance genes, and evidence for host metabolism of arsenite into less-reactive trivalent methylated species. Arsenic exposure in adult animals induced high levels of CC chemokines and of proinflammatory and anti-inflammatory cytokine secretion in intestine. Arsenic exposure at PND21 resulted in the development of distinct bacterial populations. Results of this study highlight significant changes in the intestinal microbiome and gut-associated immune status during a single or repeated exposure to arsenic in juvenile and adult animals. The data warrant investigation of the long-term effects of oral arsenic exposure on the microbiome and of immune system development and responses.
KEYWORDS: arsenic, arsenic resistance genes, arsenic speciation, developmental toxicity, immune response, intestinal microbiome
IMPORTANCE
Transformation of organic arsenic to toxic inorganic arsenic (iAs) is likely carried out by intestinal bacteria, and iAs may alter the viability of certain microbial populations. This study addressed the impact of arsenic exposure on intestinal microbiota diversity and host gut-associated immune mediators during early development or adulthood using scenarios of acute or repeated doses. During acute arsenic exposure, animals developed defense functions characterized by higher abundances of bacteria that are involved in arsenic resistance or detoxification mechanisms. Arsenite had a negative effect on the abundance of bacterial species that are involved in the conversion of protein to butyrate, which is an alternative energy source in the intestine. The intestinal mucosal immune cytokine profile reflected a mechanism of protection from arsenic toxicity.
INTRODUCTION
The health risks associated with environmental arsenic exposure have been an area of interest for a long time. Due to the presence of this element in soil and water, the interaction of arsenic with the environmental microbial population had gained much attention (1–3). The U.S. Environmental Protection Agency (EPA) has set regulatory standards for the arsenic level in drinking water at 0.01 mg/liter or 10 ppb (4). In 2016, FDA proposed an action level of 100 ppb for iAs (inorganic arsenic) in infant rice products (5). Larger amounts of arsenic are known to be associated with gastrointestinal disturbance and other adverse effects (6–8). These adverse health hazards could include cancer or other health disorders (5, 7, 9–12). Specifically, exposure to arsenic by ingestion is known to be associated with liver, lung, kidney, and bladder cancer (see reference 13). The lifetime risk of dying from cancer of the liver, lung, kidney, or bladder from drinking 1 liter/day of water was predicted to be as high as 13 per 1,000 persons for a level of arsenic in drinking water of 50 ppb (13). These data need to be reevaluated with the current regulatory standards (50 ppb versus 10 ppb).
Transformation of organic arsenic to more-toxic iAs is likely carried out by the intestinal gut bacteria and some of the host enzymes involved in methylation (14, 15). Arsenic toxicity is related to its metabolism from iAs into organic forms, particularly the trivalent species (monomethylarsonic acid [MMA] and dimethylarsinic acid [DMA]) (16, 17). The metabolism of arsenic is very complicated; however, in general, iAs is more toxic than organic As (MMA and DMA), and the toxicity of arsenite [AsIII] is higher than that of AsV, but there is interconversion of AsIII and AsV. Intestinal microbiota play a key role in the speciation of the arsenic that enters the gastrointestinal tract (GIT) via consumption of contaminated water and food (16, 18). Bacterial genera, including Bacteroides, Clostridium, Alistipes, and Bilophila, carry As resistance genes, with an ability to methylate As (19, 20). Bacteroides vulgatus possesses an arsenic resistance operon consisting of eight continuous genes, with functions as an As(III)-responsive transcriptional repressor (arsR) and in detoxification of inorganic arsenic (arsDABC), whereas the functions of the other three genes (orf1, orf2, and orf3) in arsenic resistance are unknown (21).
Introduction of a prebiotic (oligofructose) and a probiotic (Lactobacillus rhamnosus GR-1) was shown to be protective for arsenic-induced alterations in the gastrointestinal microbiome (22). Lactobacillus rhamnosus GR-1 was shown to decrease the bioaccumulation of arsenic in pregnant women and children (23). Arsenic can also cause adverse effects in progeny (24, 25). Newborns (both laboratory animals and human) are more prone to develop metabolic diseases, cardiovascular irregularities, and behavioral, cognitive, and motor disabilities if the mother is exposed (chronically) to arsenic during gestation (26–35).
A change in the microbiome composition of C57BL/6 mice was observed after exposure to 10 ppm arsenic in drinking water for 4 weeks (36). Mice exposed for 2, 5, or 10 weeks to 0, 10, or 250 ppb arsenite (AsIII) showed changes in the colonic microbial population, metabolic phenotype, and levels of metabolites in the tissue and serum (37). Another study showed that 100 ppb arsenic exposure for 13 weeks changed the gut microbial composition and altered important microbial functional pathways (carbohydrate metabolism [especially pyruvate fermentation], short-chain fatty acid synthesis, and starch utilization) that could influence host metabolism (38). Moreover, child gut microbiota exhibit high levels of AsIII, the more toxic form of arsenic, which could result in increased health risk (39).
The immune response to arsenic exposure shows a spectrum ranging from immune-suppressive to immune-stimulatory effects depending on the type of experimental model, dose, concentration, the duration of exposure of arsenic (40), and on the levels of trivalent arsenic species (41). In a population-based longitudinal study, arsenic exposure was shown to be associated with oxidative stress, inflammation, and immune disruption in human placenta and cord blood (42). The results of those studies clearly depict multifaceted interactions of arsenic with the microbiome and immune system that could result in a compromised health status. However, further studies are needed for assessment of the risk represented by arsenic during single or repeated exposures in adults as well as during developmental phases.
The experimental approach in the current investigation addressed the following questions. (i) Does a single or repeated exposure to arsenic have any effect on the commensal microbiota, arsenic resistance, and on the gut mucosa-associated immune status? (ii) Are the responses to repeated arsenic exposure transient and correlated with arsenic speciation in the GIT? (iii) Does exposure of neonates to arsenic lead to any changes in the gut bacterial population?
RESULTS
Rationale for study design.
This study aimed to assess changes in the murine intestinal microbial population, relative expression levels of arsenic resistance genes in gut bacteria, and the intestinal mucosal secretion of chemokines and cytokines during exposure to arsenite under different conditions in two different life stages as described in Fig. 1. Typical arsenic levels in highly contaminated drinking water could be in the milligram-per-liter range, thus reaching a total daily intake of ~50 µg/kg body weight (b.w.) (43). For this reason, we selected three different doses for the single-gavage exposure effect (50 µg/kg b.w., 100 µg/kg b.w., and 200 µg/kg b.w.). Note that the exposure in the present study was performed only once (single dose [SD]) or for 8 days (repeated dose [RD]). During the repeated dosing, the sodium arsenite content (1 mg/liter or 1 ppm as arsenic) in drinking water predicted the total daily intake in the range of 100 to 200 µg/kg b.w. According to our recently published study (44), this arsenite concentration reflected the finding that individual drinking events throughout the day would produce acute intakes similar to a 50 µg/kg b.w. dose. This concentration is also similar to the upper limits of arsenic contamination in drinking water in heavily contaminated communities across the world (43).
FIG 1 .
Arsenic exposure strategy during different life stages of CD1 mice. The figure depicts the experimental strategies regarding exposure to arsenic during two life stages (adult and postnatal) of animals. The rectangles represent phases of the life cycle as indicated, and each rectangle presents a description of the detailed experimental plan.
The first phase of the study investigated the differential effects of arsenite in the adult female with single-gavage exposure (0.05 mg/kg b.w. [low dose; LD], 0.1 mg/kg b.w. [medium dose; MD], and 0.2 mg/kg b.w. [high dose; HD]) or a repeated-dose (RD) 8-day exposure (1 mg/liter, ~200 µg/kg b.w./day) via drinking water. The second phase of the study investigated the effects of a single exposure of arsenite on microbial populations in neonates. Moreover, the samples were collected 24 and 48 h postexposure, a strategy that allowed the bacterial population in the intestine to stabilize after a single gavage of arsenic.
16S rRNA sequencing analysis at the bacterial phylum level.
The predominant bacterial phyla in all the samples were Firmicutes and Bacteroidetes as assessed by 16S RNA sequencing (Fig. 2A). The percent abundance range for Firmicutes and Bacteroidetes for various experimental groups is provided as Table S1 in the supplemental material. There was a decrease of about 50% in the abundance of bacteria that belong to Bacteroidetes in PND21 animals after 48 h. Bacteria that belong to the phyla Actinobacteria, Proteobacteria, “Candidatus Saccharibacteria,” Verrucomicrobia, Deferribacteres, and Spirochaetes were also present in the control and treatment groups and were classified as “other phyla” (Fig. 2B). There was a predominance of bacteria that belong to the phylum “Candidatus Saccharibacteria” in control neonatal mice. Bacteria that belong to the phylum Deferribacteres were significantly higher in abundance and contributed to more than 70% of the “other phyla” category in the PND21 mice 48 h after exposure to arsenite (Fig. 2B).
FIG 2 .
Effect of single or repeated arsenic exposure on the abundance of bacteria representative of specific phylum. Data represent the relative abundances of bacterial populations representing the phyla Firmicutes and Bacteroidetes (A) or other phyla (A and B) in the fecal samples of mice included in the study. The y axis in panel a shows a 100% stack column to emphasize the ratio of Firmicutes and Bacteroidetes phyla in the total bacterial population measured. The stack column values represent results from an average of two to six experimental animals.
Percent abundance range for Firmicutes and Bacteroidetes in various experimental groups. Download TABLE S1, PDF file, 0.1 MB (118.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
16S rRNA sequencing analysis at the bacterial genus level.
To assess the separation between different experimental groups and to observe the similarities within an experimental group at the genus level, we used partial least-squares discriminant analysis (PLS-DA) (Fig. 3A). Some distinct features that could be observed from these data were as follows: (i) bacterial populations in adult controls were quite distinct from those in the PND21 control at the genus level; (ii) a single arsenite dose had an effect distinct from that seen with a repeated dose; (iii) during the single-dose exposure, the bacterial populations within a treatment group were more similar to each other at 48 h of exposure than at 24 h postexposure; (iv) repeated dosing with arsenite resulted in increased abundance of bacteria that belong to distinct genera compared with exposure to arsenite using single gavage; and (v) the levels of bacterial diversity did not differ much between the individuals on PND21 within an experimental group.
FIG 3 .
Effect of arsenic exposure on the abundance of bacteria representative of specific genera. PLS-DA was conducted to classify the metagenomics data at the genus level (A). Here the x abscissa presents the bacterial genera, and the y ordinate variables represent results of comparisons of observations of different treatment groups. The group designations were as follows: low dose (LD), 0.05 mg/kg b.w.; medium dose (MD), 0.1 mg/kg b.w.; high dose (HD), 0.2 mg/kg b.w. Animals were sacrificed at 24 or 48 h postgavage and are designated LD24, LD48, MD24, MD48, HD24, or HD48. Another group of adult females were given continuous exposure (dosed water; 1 mg/liter) for 8 days (repeated dose [RD]), and some animals from this group were provided only water during the last 24 h (RDCE). Postnatal day 21 (PND21) female mice were exposed to single doses of arsenic (oral gavage, 0.05 mg/kg b.w.), and the microbiome was analyzed at 24 h and 48 h postexposure (PND21 24h and PND21 48h, respectively). The controls were an adult control (C) and a PND21 control (PNDC). (B) A heat map was generated to show the alpha, beta, and gamma diversity in the animals across all the experimental groups. The data for the top 50 genera were determined after applying ANOVA. Here the distance was measured by applying Pearson correlation and the Ward hierarchical clustering algorithm. The heat map provides an inbuilt visualization of the OTU abundance. Each colored cell in each column corresponds to an individual OTU abundance within specific samples (alpha diversity), and the rows correspond to individual samples within each of the experimental groups/samples (beta diversity).
To provide a clear view of the predominance of the bacteria representing specific genera, we focused on the top 50 genera after applying analysis of variance (ANOVA) (Fig. 3B) and representing the data as a heat map. Despite sample-to-sample variation, bacterial genera in control adults showed a predominance of Clavibacter, Pirellula, and Intestinimonas, whereas the PND21 control showed a predominance of Neisseria, Lactococcus, Escherichia, Staphylococcus, and Peptoclostridium. There was a lower abundance of bacteria of the genus Intestinimonas in animals exposed to arsenite; this decrease was most prominent in the RD group.
Experimental groups exposed to a single dose or repeated doses of arsenite showed the presence of bacteria belonging to the Bilophila genus; however, these were almost absent in the control adults as well as in PND21 animals. The genera that were exclusively predominant in PND21 after 48 h of arsenic exposure were Butyricicoccus, Phyllobacterium, and Parasporobacterium (Fig. 3B).
16S rRNA sequence analysis at the bacterial species level.
The adult experimental animals gavaged with single doses of arsenite showed a clear separation with respect to the presence of bacterial species compared to control animals (Fig. 4A). There was a clear difference in the abundances of species in PND21 animals. The alpha and beta diversity data are provided here as the measure of intensity in the heat map for clear visualization. The heat map data indicate the number of species (i.e., the species richness) in the treatment groups (individual columns in one class, top row). Comparisons of all the experimental groups (with classes as depicted in heat map) reveal the change in species diversity between the experimental groups and thus the beta diversity. Beta diversity was higher at the species level (Fig. 4B). The bacterial species Dorea formicigenerans was predominant in the adult animals. Responses to the arsenite exposure (single gavage) differed significantly among the mice within the same treatment groups. Clostridium sulfatireducens bacteria were consistently detected in most of the single-gavage LD animals after 24 h. However, the abundance of these bacterial species was only transient, as they were absent when the sampling was conducted at 48 h. Alistipes massiliensis and Lactobacillus johnsonii emerged following repeated exposure to arsenite.
FIG 4 .
Effect of arsenic exposure on the abundance of bacterial species. (A) PLS-DA was conducted to classify the metagenomics data at the species level. The group designation are as follows: low dose (LD), 0.05 mg/kg b.w.; medium dose (MD), 0.1 mg/kg b.w.; high dose (HD), 0.2 mg/kg b.w. Animals were sacrificed at 24 or 48 h postgavage and are designated LD24, LD48, MD24, MD48, HD24, or HD48. Another group of adult females were given continuous arsenic exposure (dosed water; 1 mg/liter) for 8 days (repeated dose [RD]), and some animals from this group were provided only water for the last 24 h (RDCE). Postnatal day 21 (PND21) female mice were exposed to a single dose of arsenic (oral gavage, 0.05 mg/kg b.w.), and their microbiome was analyzed 24 h and 48 h postexposure (PND21 24h or PND21 48h, respectively). The controls were an adult control (C) and a PND21 control (PNDC). A heat map was generated to show the alpha, beta, and gamma diversity in the animals across all the experimental groups. Panel B shows the data for the top 50 genera determined after applying ANOVA. The red arrows show the presence of distinctive bacterial species in a particular experimental group.
Arsenite exposure during development (PND21) resulted in the appearance of a very distinct population of bacterial species. This difference was more noticeable in the samples collected 48 h after exposure by a single gavage than in those collected from 24 h after exposure. Some predominant species in this group were Eubacterium plexicaudatum, Lachnoclostridium, and Mucispirillum schaedleri, along with several species of the genera Roseburia and Parasporobacterium.
Arsenic speciation in blood and ileal tissue.
The three gavage dosing levels used in acute treatments produced fluxes of “free” pentavalent and "bound" trivalent arsenic metabolites that peaked after ~1 h and were largely absent by 24 h (44). For example, the maximal plasma concentrations of “free” pentavalent DMA (DMAV) produced by the low, medium, and high arsenite doses were ~200, 400, and 900 nM at 1 h. Peak erythrocyte levels of bound DMAIII were 300, 400, and 800 nM at 1 to 2 h. In ileal tissue, the 0.05 mg/kg b.w. dose produced 870, 465, and 501 pmol/g for free AsIII, DMAV, and MMAV and 400, 1,814, and 222 pmol/g for bound DMAIII, MMAIII, and AsIII, respectively (not shown). In contrast, the repeated dosing in drinking water (1 mg/liter arsenite) produced ileal levels of free DMAV and bound DMAIII, MMAIII, and AsIII in the range of approximately 100 pmol/g; these metabolites were eliminated by 24 h upon cessation of arsenic dosing (Fig. 5).
FIG 5 .
Arsenic speciation in the intestinal tract and blood. Pentavalent and trivalent arsenic species were quantified by using LC-ICP/MS in the ileal tissue (GI tract) as well as in plasma and erythrocytes from nonpregnant female CD-1 mice after repeated dosing in drinking water (1 mg/liter) for 8 days (top panel) and after transfer to control drinking water for 24 h (bottom panel).
Gut-associated immune status.
The levels of CXC chemokine (KC) were not noticeably affected during the arsenite exposure. Only single-dose-gavaged animals (LD 24 h) showed a significant increase in the level of KC (Fig. 6A).
FIG 6 .
Effect of arsenic exposure on the gut-associated profile of cytokines and chemokines. Gut-associated mucosal chemokine (A to C) and cytokine (D and E) levels were evaluated in the tissue lysate using Bio-Plex mouse cytokine multiplex kits. The experimental groups for this comparison were as follows: adult control (C), i.e., mice treated with dosed water; 1 mg/liter (RD), i.e., repeatedly dosed mice kept on water for 24 h (RDCE); low dose 24 h (LD24); and low dose 48 h (LD48).
Among CC chemokines, secretion of eotaxin was higher in all the experimental groups than in the control; however, statistically significant differences were observed only in the cessation group and in adult LD24 (adult female exposed to a single dose of arsenite and analyzed after 24 h) (Fig. 6A to C).
All the experimental groups showed higher levels of monocyte chemoattractant protein 1 (MCP-1). In contrast, macrophage inflammatory protein 1α (MIP-1α) and MIP-1β did not show significant changes in secretion levels compared to control animals. The production of tumor necrosis factor alpha (TNF-α) was higher in all treatment groups. Interleukin-12 (IL-12) is a heterodimeric cytokine and is encoded by two separate genes, IL-12A(p35) and IL-12β(p40). The active heterodimer IL-12(p70) and a homodimer, IL-12(p40), are formed following protein synthesis. In the present study, most of the experimental groups showed significantly higher secretion of IL-12(p70) (Fig. 6D). Furthermore, the production of IL-13 and IL-1β was stimulated in most of the experimental groups (Fig. 6D). The acute single gavage of arsenite caused increased IL-1α production (Fig. 6E) in adult. A significantly higher level of gamma interferon (IFN-γ) was present only in the animals exposed repeatedly to arsenite; the cessation of arsenic exposure for 24 h brought the levels of IFN-γ back to those seen with the control animals (Fig. 6E). The levels of another proinflammatory cytokine, IL-17, were higher in most of the experimental groups. Levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-10 were also increased in all groups.
DISCUSSION
Our study data indicate that the activity of the microbiome-mediated adaptation mechanism against deleterious effects of arsenic probably starts at the neonatal stage (PND21). The difference in the patterns corresponding to the recovery of bacteria at 24 and 48 h in both adults and neonatal mice may have been a consequence of the growth of the bacteria that survived the single-dose exposure of arsenic and proliferated over the next 48 h. (Please see Text S1, Fig. S1, and Fig. S2 in the supplemental material.) There have been reports of arsenic thiolation occurring during in vitro digestion of iAs in bioreactors simulating gastric conditions with human intestinal microbiota and in in vivo animal models (18, 44). In fact, the abundance of bacteria with putative thioreductase (orf1/2) was higher during LD arsenic exposure in our study, though large variability was observed in individual animals (see Table S2 in the supplemental material; see also Fig. S3). The transient appearance of Clostridium sulfatireducens, a sulfate-reducing bacterium (SRB), in LD single-gavaged animals could indicate the involvement of these bacteria in thiolation of arsenic (45). An increased abundance of bacteria that belong to the phylum Actinobacteria in HD-gavaged animals at 24 and 48 h postexposure as well as in the group of repeatedly dosed animals and in the animals in the cessation group could represent a detoxification mechanism (46, 47).
Supplemental methods and supplemental results. Download TEXT S1, DOC file, 0.04 MB (39.5KB, doc) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Effect of single versus repeated exposure to arsenic in adult mice on the intestinal bacterial recovery. Animals were exposed to single gavage using a low dose (LD; 0.05 mg/kg b.w.), medium dose (MD; 0.1 mg/kg b.w.), or high dose (HD; 0.2 mg/kg b.w.). These animals were sacrificed at 24 or 48 h postgavage (Single Dose 24 hr or Single Dose 48 hr). Another group of adult females were given continuous exposure (dosed water; 1 mg/liter) for 8 days (Repeated Dose), and samples were collected either on day 8 or after transfer to regular water for 24 h (cessation). Fecal samples were collected, and the suspension was made under anaerobic conditions. The fecal suspension was plated for the enumeration of total CFU for aerobic bacteria (a), anaerobic bacteria (b), or for three specific bacterial genera (c). Bacterial culture for each animal sample was done in two dilutions, and each dilution was done in duplicate. All groups were compared with the control animals that were not exposed to arsenic. Each column presents mean data, and the error bars represent the standard errors of the means (SEM) of results from 2 to 5 animals in each group. Download FIG S1, PDF file, 0.1 MB (114.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Effect of single exposure to arsenic in neonatal mice on the intestinal bacteria recovery. Neonatal (postnatal day 10 [PND10] and PND21) animals (both male and female) were exposed to a single dose of arsenic (oral gavage; 0.05 mg/kg b.w.), and the microbiome was analyzed 24 h and 48 h postexposure. The fecal suspension was plated for the enumeration of total CFU for aerobic and anaerobic bacteria (a) or bacterial culture on selective media (b and c). All groups were compared with the age-matched controls. The bars represent means and SEM of results from 2 animals in each group. Bacterial culture for each animal sample was done in two dilutions and each dilution in duplicate. Thus, each data point represents the value determined for four plates. Download FIG S2, PDF file, 0.1 MB (112.1KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Arsenic resistance gene abundance. Fold change in the presence of the arsenic resistance gene was analyzed by real-time PCR for the arsenic resistance genes. Data were normalized to the 16S gene. The group designations are as follows: low dose (LD), 0.05 mg/kg b.w.; medium dose (MD), 0.1 mg/kg b.w.; high dose (HD), 0.2 mg/kg b.w. Animals were sacrificed at 24 or 48 h postgavage and are designated LD24 (n = 8), LD48 (n = 8), MD24 (n = 2), MD48 (n = 2), HD24 (n = 2), or HD48 (n = 2). Download FIG S3, PDF file, 0.05 MB (52.5KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
List of arsenic resistance gene primers. Download TABLE S2, DOCX file, 0.01 MB (14KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Repeated exposure to arsenite in adult mice led to an abundance of bacteria that belong to genera Alistipes, Bilophila, and Lactobacillus johnsonii. Members of genus Bilophila are known to be involved in intra-abdominal infections and other infections that lead to inflammatory bowel disease (IBD) (48). Furthermore, B. wadsworthia is an immunogenic sulfite-reducing opportunistic pathobiont and is involved in modifying conjugation states of bile acids (49, 50). The abundance of B. wadsworthia promoted a Th1-mediated immune response and the development of colitis in IL-10−/− mice (49). Arsenic is considered one of the causative agents for IBD and ulcerative colitis (51, 52). Furthermore, several bacterial species, including Alistipes and Bilophila bacteria, had positive correlations with the formation of iAsIII, iAsV, and total arsenic (TAs), which is indicative of higher arsenic resistance (19). Likewise, L. johnsonii has been shown to carry an arsenic resistance cassette (53). Bacterial species that belong to the phylum Deferribacteres are known to harbor arsenic resistance genes (54); thus, in our study it is tempting to speculate that the observed changes in the abundance of bacterial population may represent one of the mechanisms adapted by neonatal animals for surviving the toxic effects of the arsenite. In particular, the arsenic-inducible transcriptional unit (arsD) conferred resistance to trivalent arsenic species, with MMAIII being the most effective inducer, followed by AsIII (21). Ileal tissue showed efficient conversion of arsenite into pentavalent and trivalent methylated metabolites. Methylation of arsenite (either due to bacterium-induced conversion or due to host factors) is generally viewed as a detoxification reaction because DMAV is the predominant species excreted in urine and methylation appears to protect mice from acute toxicity of arsenite (55). However, trivalent DMA and MMA and arsenite itself are reactive species whose low-molecular-weight thiol complexes (e.g., glutathione) represent mobile forms that may be capable of transferring bound arsenic to critical thiol throughout the host tissues or the associated microbiota (44). Further studies are required to understand if the single exposure can increase the risk of disease in the animals; it would be very pertinent to assess such effects with a challenge study or to assess the immune status of single-dose-arsenic-exposed animals at a later time.
Remarkably, arsenite and its metabolic products exert a proinflammatory response in vitro when the dose is approximately 5 µM (56). By comparison, the arsenic species (peak concentrations) whose levels were measured in ileal tissue following gavage dosing at 50 µg/kg b.w. (not shown) included bound arsenic species (400, 1,814, and 222 pmol/g for AsIII, MMAIII, and DMAIII, respectively) and free arsenic species (870, 465, and 501 pmol/g for AsIII, MMAV, and DMAV, respectively); the levels were similar to the effective in vitro concentrations (56). It should be noted that these peak levels were transitory, since the “steady-state” levels achieved after repeated arsenite dosing through the drinking water (~200 µg/kg b.w./day) produced concentrations in ileal tissue that were up to 10-fold lower.
The abundance of bacteria that belong to the phylum Deferribacteres or genera Butyricicoccus, Phyllobacterium, and Parasporobacterium could play an adaptive role against arsenic assault. Members of the phylum Deferribacteres are known to harbor arsenic resistance genes (54). Members of the genus Butyricicoccus are tolerant to changing intestinal conditions, produce butyrate, and have probiotic potential (57, 58). In addition, Butyricicoccus is known to inhibit growth of potentially harmful intestinal pathogens and protect the intestine from the formation of necrotic enteritis in broilers (59). Members of the genus Phyllobacterium are known to have heavy metal resistance genes. Bacteria that belong to the genus Parasporobacterium were shown to convert aromatic residues (gallate) into acetate and butyrate (60). Eubacterium plexicaudatum (61) and Roseburia (62) are able to produce butyric acid from fermented carbohydrates, whereas Parasporobacterium produces dimethyl sulfide and methanethiol from methoxy-containing aromatic compounds and sulfide (60). In the current study, all these species were predominant in PND21 animals after 48 h of exposure to arsenic. The exact role of these species during the arsenite exposure needs to be explored in further detail. Some bacterial species that belong to Lactobacillus and Lactococcus (lactis) were predominant in the PND21 control group. L. lactis subsp. lactis bacteria are among the early colonizers in the human infant gut (63).
Lower abundance of members of the Intestinimonas genus in most of the arsenite-exposed animals may result in low butyrate production. Butyrate, a metabolic product of dietary fiber digestion via intestine-microbe interaction, is a major energy source in the colon that is crucial to maintaining intestinal health (64, 65). Genus Intestinimonas bacteria produce butyrate from protein and Amadori products. Instead of a sole focus on butyrate generation from fibers/carbohydrate, protein fermentation by Intestinimonas could also contribute to the production of energy and intestinal homeostasis.
Mucispirillum schaedleri, a bacterial species that colonizes the mucus layer of the GIT, was predominant in samples from the animals exposed to a single dose of arsenic and in samples collected after 48 h in the PND21 arsenite-exposed group. M. schaedleri is a commensal pathobiont. The higher abundance of these bacteria at 48 h than at 24 h could be due to its tolerance of arsenic toxicity. By 48 h, the bacteria that survived the detrimental effects of arsenic proliferated more and thus resulted in higher abundance. However, the bacterial population is more diverse in adult animals and could result in the different abundances of populations of bacterial species or bacterial species that harbor an arsenic resistance gene to mitigate the arsenic toxicity. M. schaedleri was shown to reduce nitrate and to help in scavenging oxygen and reactive oxygen species in vivo (66). M. schaedleri harbors a type VI secretion system and putative effector proteins and can modify gene expression in mucosal tissue during inflammation (67). The close proximity of M. schaedleri to intestinal mucosal tissue and expansion during arsenic perturbations may produce intimate interactions with its host and possibly play a role in inflammation. In the present study, microbiome analysis was conducted in the fecal samples. Studies are ongoing to assess the adherent microbiota in the gut mucosa in the same animals. Another possibility that could not be ruled out is that exposure to arsenic might cause the intestinal mucosa to undergo a perturbation that ends in depleting the mucosal surfaces so much that these populations end up in the feces. All the experimental groups examined in the present study showed higher levels of MCP-1, a chemokine whose levels have been previously shown to be elevated during exposure of vascular smooth muscle cells to arsenic (68). However, neutrophil granulocytes may not be involved in the arsenic-induced toxicity in the GIT, as the secretion of CXC chemokine remained unchanged.
Lipopolysaccharide is known to increase the levels of IL-12 (69, 70). Enhanced production of murine IL-12 is proposed to be mediated by Lactococcus lactis (71), a bacterial species that was also significantly abundant in the arsenite-exposed PND21 group. In fact, trivalent arsenic species have been shown to induce changes in mRNA expression levels and in secretion levels of proinflammatory cytokines in intestinal epithelial cells. To address whether this was the case during in vivo exposure, we quantified the arsenic speciation in the animals that were provided arsenic in the drinking water continuously for 8 days and in those in the cessation group. Our data support the idea of extensive metabolism of arsenic within the GIT to MMA and DMA in both the trivalent (reactive) and pentavalent states (72). This further supports the notion that metabolism of arsenite within the gut could produce diffusible species that could interact with the microbiota and host in ways that affect production of chemokine/cytokines.
Overall, this study addressed some of the knowledge gaps concerning the effects of arsenic exposure during early development as well as in the scenario where someone is exposed to arsenic only acutely, or chronically for only a short period, with an emphasis on its impact on microbiota diversity and host gut-associated immune mediators. During acute arsenic exposure, the first response observed in the animals was a defense mechanism characterized by either an increase in the bacterial population containing arsenic resistance genes or a higher abundance of bacteria that are involved in the arsenic detoxification mechanism. Bacterial species that harbor arsenic efflux systems are presumed to have evolved to purge the toxic effects of this deleterious chemical. These efflux systems include members of the multidrug resistance protein family, bacterial exchangers, and ATP-driven efflux pump (73). Interestingly, arsenite showed a negative effect on the presence/abundance of bacterial populations that are known to convert protein to butyrate, an alternative energy source. However, to compensate for that, there was an emergence of bacterial species in the arsenite-exposed animals (during repeated exposure, as well as during the developmental stages) that are proposed to be alternate producers of butyrate. This clearly corresponds to and supports the Baas Becking and Beijerinck microbe distribution hypothesis: “Everything is everywhere, but the environment selects” (74).
Furthermore, we show that the intestinal mucosal immune response pattern reflects a mechanism of protection from arsenic toxicity (Fig. 7). Altered levels of biologically relevant CC chemokines in most of the arsenite-exposed groups indicate an inflammatory response to arsenic toxicity. There was abundance of bacterial species during repeated exposure to arsenic that could contribute to dysbiosis and lead to the production of heat shock proteins (HSP). This may trigger/induce an autoimmune reaction due to the molecular analogy between HSP from bacterial origin and human HSP (40, 75, 76). Thus, it is essential to investigate the in utero as well as long-term effects of arsenic exposure in relation to gastrointestinal toxicity (specifically for the neoplastic transformation of epithelial cells or for susceptibility to infection) and overall homeostasis. Transplacental movements of xenobiotics are proposed to negatively interact with fetal immune stem cell maturation. Detailed studies are in progress to dissect the immune responses of developmental exposure and to assess if these are in fact sex dependent and if there is any correlation with the microbiome changes that occur during the early developmental stages. Studies are also in progress to assess if gestational exposure to arsenic has any effect on the dam and on the intestinal immune development of the fetus. Of particular interest with respect to this study is the developmental exposure that could potentially affect the immune system development and responses to diseases during adulthood.
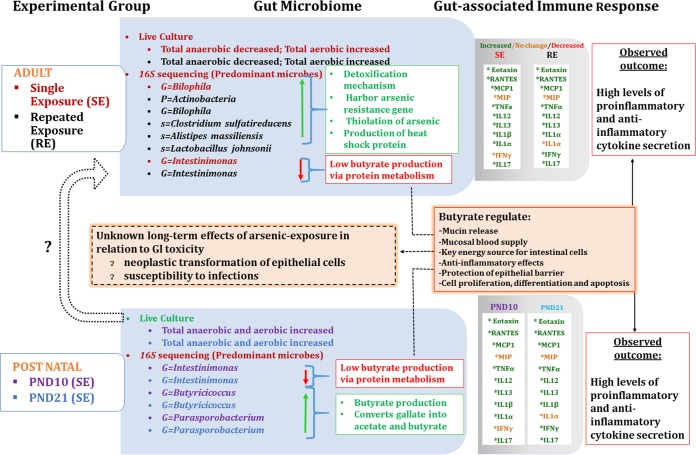
FIG 7 .
Summary of the effects of arsenic exposure on the gut microbiome and gut-associated immune response. This schematic diagram depicts the outcome of the exposure to arsenic at the developmental stages of CD-1 mice. The leftmost box (shaded blue) shows the adult exposure or postnatal exposure to arsenic in the form of either single exposure (SE) or repeated exposure (RE). Experimental groups are identified and matched with the individually colored fonts for the gut microbiome (sky-blue box). The outcome of the microbial population shift is summarized in the white box. The impact of the exposure to arsenic on the gut-associated immune response is shown as increased secretion (green), no change in secretion (orange), or decreased secretion (red) of cytokines within the shaded gray box. The overall effects of changes in the levels of cytokines/chemokines are indicated in the rightmost white box.
In conclusion, our report reveals that the interactions among xenobiotics, gut microbiome, and the gut-associated immune response and the impact on the host represent a complex and dynamic process. The results warrant further investigation into arsenic developmental immunotoxicity to examine how the presence of the distinct microbial population during the early developmental stages leads to colonization of the bacterial population at the adult stage and the critical windows of dysbiosis, immunotoxicity, and differential susceptibility based on gender.
MATERIALS AND METHODS
Animal exposure strategy.
All studies were approved by the Animal Care and Use Committee at the National Center for Toxicological Research, Jefferson, AR (44). The strategy for the life stage animal groups and arsenic exposure is provided in Fig. 1.
In brief, this study consisted of exposure of mice to sodium arsenite during the developmental stages of the mice (adult nonpregnant and postnatal). Female CD-1 mice (7 to 8 weeks of age) (designated the "first group" below) and pregnant CD-1 mice (unspecified age; designated the “second group” below) were procured from Charles River, Inc. (Wilmington, MA). The CD-1 strain of mouse was chosen for this study due to its susceptibility to the carcinogenic effects of sodium arsenite under conditions of exposure to 50 to 5,000 ppb in drinking water (77). For at least 10 days, mice were provided a common low-arsenic basal diet (5K96; Test Diets, Purina Mills, Richmond, IN) to reduce the background level of arsenic. Animal for individual experiments were housed in groups. In the first group, adult females were exposed to a single gavage consisting of three concentrations of sodium arsenite (low dose [LD], 0.05 mg/kg b.w.; medium dose [MD], 0.1 mg/kg b.w.; high dose [HD], 0.2 mg/kg b.w.; expressed as arsenic equivalents). These animals were compared to the animals that were not exposed to arsenic. Animals were sacrificed at 24 or 48 h after gavage. Mice in another group of nonpregnant adult females (n = 6) were given continuous exposure (dosed water; 1 mg/liter) for 8 days (repeated dose [RD]). Preparation of the dosed water was described in detail earlier (72). Based on measurements of drinking water consumption and body weight data for pregnant and nonpregnant mice, the ingested daily dose was ~200 mg/kg b.w. (range, 168 to 291; data not shown). In the RD group, the animals in one set were provided control water for an additional 24 h (ninth day) and then sacrificed (referred to here as the “repeated-dose-cessation group” [RDCE group] [n = 3]). All of the repeatedly dosed animals were compared to the animals that were not exposed to arsenic and were sacrificed along with single-gavaged dosed animals. The second group of animals consisted of those with postnatal exposure. In this group, both the male and female pups (postnatal day 10 [PND10] and PND21) were exposed to a single oral dose of arsenite (by gavage; 0.05 mg/kg b.w.) and the microbiome was analyzed at 24 h and 48 h postexposure and compared with the the microbiome of age-matched and sex-matched control animals.
Collection of biological samples.
Animals were fasted overnight and euthanized by exposure to carbon dioxide. Verification of euthanasia by exsanguination was done prior to initiation of necropsy. Blood was collected by cardiac puncture and was immediately processed into plasma and erythrocyte fractions and stored at −60°C until analysis for arsenic speciation.
The intestine was exposed and flushed with normal saline solution, and 3-cm sections of terminal ileum were collected in individual cryo-tubes for RNA and protein extraction. These cryo-tubes were immediately flash frozen for later use.
Fecal sample collection for culture-based bacteriology and DNA extraction.
Feces was collected from the colon of euthanized animals and immediately transferred to the anaerobic chamber for analysis of live bacteria (for details, please see Text S1 in the supplemental material).
Two or three pellets of feces were collected from the colon in a cryovial and immediately stored at 80°C. Fecal DNA was extracted by a bead beating and organic extraction method as described earlier (78, 79). The DNA pellet was suspended in DNase-and-RNase-free water, quantified using a Nanodrop ND-1000 spectrophotometer (NanoDrop, Wilmington, DE) and double-stranded DNA (dsDNA) quantification by Qubit (Thermo, Fisher Scientific, Waltham, MA), and used as the template for 16S rRNA sequencing for microbial population and real-time PCR analysis of expression of arsenic resistance genes.
16S rRNA gene sequencing of bacterial population.
Fecal DNA was used to perform community bacterial population analysis. The 16S rRNA gene V4 variable region was amplified using PCR primers 515/806. Pooled and purified PCR products were used to prepare an Illumina DNA library. Sequences were joined and depleted of barcodes, and then sequences of <150 bp and sequences with ambiguous base calls were removed. Sequences were denoised, operational taxonomic units (OTUs) were generated, and chimeras were removed. OTUs were defined by clustering at 3% divergence (97% similarity). Final OTUs were taxonomically classified using BLASTN against a curated database derived from RDPII and NCBI.
For statistical analysis of microbiome data, the effect of arsenic exposure on the bacteria representative of a specific phylum was calculated as the percent abundance (79). For genus-level and species-level analysis, orthogonal partial least-squares discriminant analysis (PLS-DA) was used to observe the separation between different experimental groups and to observe the similarities within an experimental group (80–82). This analysis requires n = 3; but there were only n = 2 samples available in some experimental groups (e.g., PND). This lacuna was overcome by obtaining an additional value by averaging two values, with the results depicted as a heat map for an individual sample.
Hierarchical clustering was performed with the hclust function in the stat package (83, 84). The distance was measured by applying Pearson correlation and the Ward hierarchical clustering algorithm. The outcome is presented as heat maps, which provide an inbuilt visualization of the levels of OTU abundance. Comparison of the data corresponding to each animal (in the same color box) reveals the alpha diversity. Comparison of the data corresponding to all the experimental groups reveals the change in species diversity between the experimental groups and thus the beta diversity.
Arsenic speciation in blood and ileal tissue.
Pentavalent and trivalent arsenic species (DMAV, MMAV, AsIII, and AsV) were quantified in plasma, erythrocytes, and ileal tissue homogenate in nonpregnant female CD-1 mice using ion exchange liquid chromatographic separation (LC separation or speciation), and detection/quantification using inductively coupled plasma mass spectrometry (ICP/MS) was performed as reported earlier (44).
Protein extraction from the ileal tissue.
Ileal tissue protein extraction was performed using a gentleMACS dissociator (Miltenyi Biotec, Inc., Auburn, CA). The lysate was centrifuged at 4°C for 10 min at 750 rpm, and the clear homogenate was transferred to 2-ml Eppendorf tubes and centrifuged at 4°C for 15 min at 12,000 rpm. The clear supernatant was transferred into new 2-ml Eppendorf tubes, and these were stored at −80°C until cytokine profiling. The protein concentration was measured spectrophotometerically using Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA).
Multiplex cytokine assay.
Gut-associated mucosal chemokine and cytokine levels were evaluated in the tissue lysate using a Bioplex mouse cytokine 23-plex panel and Th17 plex (Bio-Rad, Hercules, CA) following the manufacturer’s instructions and as previously described (85) using the multiplex Bioplex assay.
Statistical analysis for cytokine data.
The Bioplex data were exported to Bioplex Manager, and comparisons of different treatment groups were performed using the Mann-Whitney test. All statistical analysis was two sided, and a P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
This study was funded by the National Toxicology Program under an Interagency Agreement between FDA and NIEHS (FDA IAG # 224-17-0502 and NIH IAG # AES12013). We gratefully acknowledge Paul Howard, Office of Scientific Coordination, NCTR, Jefferson, AR, and Suresh Pillai, Texas A&M University, College Station, TX, for helpful discussions.
The findings/opinions presented here represent our views. They do not reflect the views of the U.S. Food and Drug Administration.
Footnotes
Citation Gokulan K, Arnold MG, Jensen J, Vanlandingham M, Twaddle NC, Doerge DR, Cerniglia CE, Khare S. 2018. Exposure to arsenite in CD-1 mice during juvenile and adult stages: effects on intestinal microbiota and gut-associated immune status. mBio 9:e01418-18. https://doi.org/10.1128/mBio.01418-18.
REFERENCES
- 1.Ahmann D, Roberts AL, Krumholz LR, Morel FM. 1994. Microbe grows by reducing arsenic. Nature 371:750. doi: 10.1038/371750a0. [DOI] [PubMed] [Google Scholar]
- 2.Shariatpanahi M, Anderson AC, Abdelghani AA, Englande AJ, Hughes J, Wilkinson RF. 1981. Biotransformation of the pesticide sodium arsenate. J Environ Sci Health B 16:35–47. doi: 10.1080/03601238109372237. [DOI] [PubMed] [Google Scholar]
- 3.Zhu YG, Yoshinaga M, Zhao FJ, Rosen BP. 2014. Earth abides arsenic biotransformations. Annu Rev Earth Planet Sci 42:443–467. doi: 10.1146/annurev-earth-060313-054942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US EPA 2002. Implementation guidance for the arsenic rule. Drinking water regulations for arsenic and clarifications to compliance and new source contaminants monitoring. EPA-816-K-02-018 US EPA, Washington, DC. [Google Scholar]
- 5.Nachman KE, Ginsberg GL, Miller MD, Murray CJ, Nigra AE, Pendergrast CB. 2017. Mitigating dietary arsenic exposure: current status in the United States and recommendations for an improved path forward. Sci Total Environ 581–582:221–236. doi: 10.1016/j.scitotenv.2016.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong CW, Stroube RB, Rubio T, Siudyla EA, Miller GB Jr.. 1984. Outbreak of fatal arsenic poisoning caused by contaminated drinking water. Arch Environ Health 39:276–279. doi: 10.1080/00039896.1984.10545849. [DOI] [PubMed] [Google Scholar]
- 7.Arslan B, Djamgoz MBA, Akün E. 2017. Arsenic: a review on exposure pathways, accumulation, mobility and transmission into the human food chain. Rev Environ Contam Toxicol 243:27–51. doi: 10.1007/398_2016_18. [DOI] [PubMed] [Google Scholar]
- 8.Ishinishi N, Kodama Y, Nobutomo K, Inamasu T, Kunitake E, Suenaga Y. 1977. Outbreak of chronic arsenic poisoning among retired workers from an arsenic mine in Japan. Environ Health Perspect 19:121–125. doi: 10.1289/ehp.7719121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan F, Momtaz S, Niaz K, Hassan FI, Abdollahi M. 2017. Epigenetic mechanisms underlying the toxic effects associated with arsenic exposure and the development of diabetes. Food Chem Toxicol 107:406–417. doi: 10.1016/j.fct.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Wang SL, Chiou JM, Chen CJ, Tseng CH, Chou WL, Wang CC, Wu TN, Chang LW. 2003. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect 111:155–159. doi: 10.1289/ehp.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal A, Zhang H, Karmaus WJJ, Everson TM, Marsit CJ, Karagas MR, Tsai SF, Wen HJ, Wang SL. 2017. Genome-wide DNA methylation at birth in relation to in utero arsenic exposure and the associated health in later life. Environ Health 16:50. doi: 10.1186/s12940-017-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. 1992. Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall LL, George SE, Kohan MJ, Styblo M, Thomas DJ. 1997. In vitro methylation of inorganic arsenic in mouse intestinal cecum. Toxicol Appl Pharmacol 147:101–109. doi: 10.1006/taap.1997.8269. [DOI] [PubMed] [Google Scholar]
- 15.Martinez VD, Vucic EA, Adonis M, Gil L, Lam WL. 2011. Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol Biol Int 2011:718974. doi: 10.4061/2011/718974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akter KF, Owens G, Davey DE, Naidu R. 2005. Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol 184:97–149. doi: 10.1007/0-387-27565-7_3. [DOI] [PubMed] [Google Scholar]
- 17.Benramdane L, Bressolle F, Vallon JJ. 1999. Arsenic speciation in humans and food products: a review. J Chromatogr Sci 37:330–344. doi: 10.1093/chromsci/37.9.330. [DOI] [PubMed] [Google Scholar]
- 18.Van de Wiele T, Gallawa CM, Kubachka KM, Creed JT, Basta N, Dayton EA, Whitacre S, Du Laing G, Bradham K. 2010. Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ Health Perspect 118:1004–1009. doi: 10.1289/ehp.0901794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Wu B, Zhang XX, Liu S, Yu J, Cheng S, Ren HQ, Ye L. 2016. Arsenic metabolism and toxicity influenced by ferric iron in simulated gastrointestinal tract and the roles of gut microbiota. Environ Sci Technol 50:7189–7197. doi: 10.1021/acs.est.6b01533. [DOI] [PubMed] [Google Scholar]
- 20.Yin N, Zhang Z, Cai X, Du H, Sun G, Cui Y. 2015. In vitro method to assess soil arsenic metabolism by human gut microbiota: arsenic speciation and distribution. Environ Sci Technol 49:10675–10681. doi: 10.1021/acs.est.5b03046. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Mandal G, Rosen BP. 2016. Expression of arsenic resistance genes in the obligate anaerobe Bacteroides vulgatus ATCC 8482, a gut microbiome bacterium. Anaerobe 39:117–123. doi: 10.1016/j.anaerobe.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massey VL, Stocke KS, Schmidt RH, Tan M, Ajami N, Neal RE, Petrosino JF, Barve S, Arteel GE. 2015. Oligofructose protects against arsenic-induced liver injury in a model of environment/obesity interaction. Toxicol Appl Pharmacol 284:304–314. doi: 10.1016/j.taap.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinder M, Bisanz JE, Burton JP, Reid G. 2015. Probiotic lactobacilli: a potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Benef Microbes 6:841–847. doi: 10.3920/BM2015.0022. [DOI] [PubMed] [Google Scholar]
- 24.Kozul-Horvath CD, Zandbergen F, Jackson BP, Enelow RI, Hamilton JW. 2012. Effects of low-dose drinking water arsenic on mouse fetal and postnatal growth and development. PLoS One 7:e38249. doi: 10.1371/journal.pone.0038249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE, Yao HH. 2016. Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ Health Perspect 124:336–343. doi: 10.1289/ehp.1509703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed S, Ahsan KB, Kippler M, Mily A, Wagatsuma Y, Hoque AM, Ngom PT, El Arifeen S, Raqib R, Vahter M. 2012. In utero arsenic exposure is associated with impaired thymic function in newborns possibly via oxidative stress and apoptosis. Toxicol Sci 129:305–314. doi: 10.1093/toxsci/kfs202. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger AS, Zota AR, Amarasiriwardena CJ, Hopkins MR, Schwartz J, Hu H, Wright RO. 2009. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect 117:1059–1064. doi: 10.1289/ehp0800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, Marsit CJ, Karagas MR, Robbins DJ. 2013. Association between in utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environ Health 12:58. doi: 10.1186/1476-069X-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, Hertz-Picciotto I. 2003. Arsenic exposure from drinking water and birth weight. Epidemiology 14:593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- 30.Kippler M, Wagatsuma Y, Rahman A, Nermell B, Persson LÅ, Raqib R, Vahter M. 2012. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: a longitudinal study in rural Bangladesh. Reprod Toxicol 34:504–511. doi: 10.1016/j.reprotox.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey KA, Larcombe AN, Sly PD, Zosky GR. 2013. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacol Toxicol 14:13. doi: 10.1186/2050-6511-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha KK, Engström A, Hamadani JD, Tofail F, Rasmussen KM, Vahter M. 2012. Pre- and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural Bangladesh. Environ Health Perspect 120:1208–1214. doi: 10.1289/ehp.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. 2010. Maternal exposure to low-level heavy metals during pregnancy and birth size. J Environ Sci Health A Tox Hazard Subst Environ Eng 45:1468–1474. doi: 10.1080/10934529.2010.500942. [DOI] [PubMed] [Google Scholar]
- 34.States JC, Singh AV, Knudsen TB, Rouchka EC, Ngalame NO, Arteel GE, Piao Y, Ko MS. 2012. Prenatal arsenic exposure alters gene expression in the adult liver to a proinflammatory state contributing to accelerated atherosclerosis. PLoS One 7:e38713. doi: 10.1371/journal.pone.0038713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferm VH, Hanlon DP. 1985. Constant rate exposure of pregnant hamsters to arsenate during early gestation. Environ Res 37:425–432. doi: 10.1016/0013-9351(85)90124-0. [DOI] [PubMed] [Google Scholar]
- 36.Lu K, Abo RP, Schlieper KA, Graffam ME, Levine S, Wishnok JS, Swenberg JA, Tannenbaum SR, Fox JG. 2014. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect 122:284–291. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dheer R, Patterson J, Dudash M, Stachler EN, Bibby KJ, Stolz DB, Shiva S, Wang Z, Hazen SL, Barchowsky A, Stolz JF. 2015. Arsenic induces structural and compositional colonic microbiome change and promotes host nitrogen and amino acid metabolism. Toxicol Appl Pharmacol 289:397–408. doi: 10.1016/j.taap.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chi L, Bian X, Gao B, Tu P, Ru H, Lu K. 2017. The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci 160:193–204. doi: 10.1093/toxsci/kfx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin N, Du H, Wang P, Cai X, Chen P, Sun G, Cui Y. 2017. Interindividual variability of soil arsenic metabolism by human gut microbiota using SHIME model. Chemosphere 184:460–466. doi: 10.1016/j.chemosphere.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Ferrario D, Gribaldo L, Hartung T. 2016. Arsenic exposure and immunotoxicity: a review including the possible influence of age and sex. Curr Environ Health Rep 3:1–12. doi: 10.1007/s40572-016-0082-3. [DOI] [PubMed] [Google Scholar]
- 41.Calatayud M, Gimeno-Alcañiz JV, Vélez D, Devesa V. 2014. Trivalent arsenic species induce changes in expression and levels of proinflammatory cytokines in intestinal epithelial cells. Toxicol Lett 224:40–46. doi: 10.1016/j.toxlet.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekström EC, Vahter M, Raqib R. 2011. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect 119:258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.EPA 2010. Toxicological review of inorganic arsenic. https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=219111.
- 44.Twaddle NC, Vanlandingham M, Churchwell MI, Doerge DR. 2018. Metabolism and disposition of arsenic species from controlled oral dosing with sodium arsenite in adult female CD-1 mice. I. Pilot study to determine dosing, analytical measurements, and sampling strategies. Food Chem Toxicol 111:482–493. doi: 10.1016/j.fct.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 45.D C Rubin SS, Alava P, Zekker I, Du Laing G, Van de Wiele T. 2014. Arsenic thiolation and the role of sulfate-reducing bacteria from the human intestinal tract. Environ Health Perspect 122:817–822. doi: 10.1289/ehp.1307759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. 2015. Role of the normal gut microbiota. World J Gastroenterol 21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuentes MS, Briceño GE, Saez JM, Benimeli CS, Diez MC, Amoroso MJ. 2013. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. Biomed Res Int 2013:392573. doi: 10.1155/2013/392573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devkota S, Chang EB. 2015. Interactions between diet, bile acid metabolism, gut microbiota, and inflammatory bowel diseases. Dig Dis 33:351–356. doi: 10.1159/000371687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang I, Eibach D, Kops F, Brenneke B, Woltemate S, Schulze J, Bleich A, Gruber AD, Muthupalani S, Fox JG, Josenhans C, Suerbaum S. 2013. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to Helicobacter hepaticus-induced colitis. PLoS One 8:e70783. doi: 10.1371/journal.pone.0070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller PD, Benowitz NL. 1989. Toxicologic causes of acute abdominal disorders. Emerg Med Clin North Am 7:667–682. [PubMed] [Google Scholar]
- 52.Wang X, Mandal AK, Saito H, Pulliam JF, Lee EY, Ke ZJ, Lu J, Ding S, Li L, Shelton BJ, Tucker T, Evers BM, Zhang Z, Shi X. 2012. Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/beta-catenin signaling pathway. Toxicol Appl Pharmacol 262:11–21. doi: 10.1016/j.taap.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger B, Pridmore RD, Barretto C, Delmas-Julien F, Schreiber K, Arigoni F, Brüssow H. 2007. Similarity and differences in the Lactobacillus acidophilus group identified by polyphasic analysis and comparative genomics. J Bacteriol 189:1311–1321. doi: 10.1128/JB.01393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mumford AC, Yee N, Young LY. 2013. Precipitation of alacranite (As8S9) by a novel As(V)-respiring anaerobe strain MPA-C3. Environ Microbiol 15:2748–2760. doi: 10.1111/1462-2920.12136. [DOI] [PubMed] [Google Scholar]
- 55.Currier JM, Douillet C, Drobná Z, Stýblo M. 2016. Oxidation state specific analysis of arsenic species in tissues of wild-type and arsenic (+3 oxidation state) methyltransferase-knockout mice. J Environ Sci 49:104–112. doi: 10.1016/j.jes.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trouba KJ, Germolec DR. 2004. Micromolar concentrations of sodium arsenite induce cyclooxygenase-2 expression and stimulate p42/44 mitogen-activated protein kinase phosphorylation in normal human epidermal keratinocytes. Toxicol Sci 79:248–257. doi: 10.1093/toxsci/kfh132. [DOI] [PubMed] [Google Scholar]
- 57.Geirnaert A, Steyaert A, Eeckhaut V, Debruyne B, Arends JB, Van Immerseel F, Boon N, Van de Wiele T. 2014. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe 30:70–74. doi: 10.1016/j.anaerobe.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Steppe M, Van Nieuwerburgh F, Vercauteren G, Boyen F, Eeckhaut V, Deforce D, Haesebrouck F, Ducatelle R, Van Immerseel F. 2014. Safety assessment of the butyrate-producing Butyricicoccus pullicaecorum strain 25-3(T), a potential probiotic for patients with inflammatory bowel disease, based on oral toxicity tests and whole genome sequencing. Food Chem Toxicol 72:129–137. doi: 10.1016/j.fct.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 59.Eeckhaut V, Wang J, Van Parys A, Haesebrouck F, Joossens M, Falony G, Raes J, Ducatelle R, Van Immerseel F. 2016. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front Microbiol 7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lomans BP, Leijdekkers P, Wesselink JJ, Bakkes P, Pol A, van der Drift C, den Camp HJ. 2001. Obligate sulfide-dependent degradation of methoxylated aromatic compounds and formation of methanethiol and dimethyl sulfide by a freshwater sediment isolate, Parasporobacterium paucivorans gen. nov., sp. nov. Appl Environ Microbiol 67:4017–4023. doi: 10.1128/AEM.67.9.4017-4023.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkins TD, Fulghum RS, Wilkins JH. 1974. Eubacterium plexicaudatum sp. nov., an anaerobic bacterium with a subpolar tuft of flagella, isolated from a mouse cecum. Int J Syst Evol Microbiol 24:408–411. [Google Scholar]
- 62.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. 2014. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 63.Park HK, Shim SS, Kim SY, Park JH, Park SE, Kim HJ, Kang BC, Kim CM. 2005. Molecular analysis of colonized bacteria in a human newborn infant gut. J Microbiol 43:345–353. [PubMed] [Google Scholar]
- 64.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 66.Robertson BR, O’Rourke JL, Neilan BA, Vandamme P, On SL, Fox JG, Lee A. 2005. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int J Syst Evol Microbiol 55:1199–1204. doi: 10.1099/ijs.0.63472-0. [DOI] [PubMed] [Google Scholar]
- 67.Loy A, Pfann C, Steinberger M, Hanson B, Herp S, Brugiroux S, Gomes Neto JC, Boekschoten MV, Schwab C, Urich T, Ramer-Tait AE, Rattei T, Stecher B, Berry D. 2017. Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems 2:e00171-16. doi: 10.1128/mSystems.00171-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee PC, Ho IC, Lee TC. 2005. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol Sci 85:541–550. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- 69.Moreno SE, Alves-Filho JC, Alfaya TM, da Silva JS, Ferreira SH, Liew FY. 2006. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J Immunol 177:3218–3224. doi: 10.4049/jimmunol.177.5.3218. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi M, Ogasawara K, Takeda K, Hashimoto W, Sakihara H, Kumagai K, Anzai R, Satoh M, Seki S. 1996. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol 156:2436–2442. [PubMed] [Google Scholar]
- 71.Fernandez A, Horn N, Wegmann U, Nicoletti C, Gasson MJ, Narbad A. 2009. Enhanced secretion of biologically active murine interleukin-12 by Lactococcus lactis. Appl Environ Microbiol 75:869–871. doi: 10.1128/AEM.01728-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Twaddle NC, Vanlandingham M, Beland FA, Doerge DR. 2018. Metabolism and disposition of arsenic species after repeated oral dosing with sodium arsenite in drinking water. II. Measurements in pregnant and fetal CD-1 mice. Food Chem Toxicol 115:178–184. doi: 10.1016/j.fct.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Yang HC, Fu HL, Lin YF, Rosen BP. 2012. Pathways of arsenic uptake and efflux. Curr Top Membr 69:325–358. doi: 10.1016/B978-0-12-394390-3.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Wit R, Bouvier T. 2006. ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ Microbiol 8:755–758. doi: 10.1111/j.1462-2920.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 75.Munera-Picazo S, Ramírez-Gandolfo A, Burló F, Carbonell-Barrachina AA. 2014. Inorganic and total arsenic contents in rice-based foods for children with celiac disease. J Food Sci 79:T122–T128. doi: 10.1111/1750-3841.12310. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Y, Wen G, Qiao Z, Xu H, Sun Q, Huang H, Shan S, Mu Z, Zhang J. 2013. Effects of tetra-arsenic tetra-sulfide on BXSB lupus-prone mice: a pilot study. Lupus 22:469–476. doi: 10.1177/0961203313478302. [DOI] [PubMed] [Google Scholar]
- 77.Waalkes MP, Qu W, Tokar EJ, Kissling GE, Dixon D. 2014. Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses. Arch Toxicol 88:1619–1629. doi: 10.1007/s00204-014-1305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khare S, Ficht TA, Santos RL, Romano J, Ficht AR, Zhang S, Grant IR, Libal M, Hunter D, Adams LG. 2004. Rapid and sensitive detection of Mycobacterium avium subsp. paratuberculosis in bovine milk and feces by a combination of immunomagnetic bead separation-conventional PCR and real-time PCR. J Clin Microbiol 42:1075–1081. doi: 10.1128/JCM.42.3.1075-1081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams K, Milner J, Boudreau MD, Gokulan K, Cerniglia CE, Khare S. 2015. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 9:279–289. doi: 10.3109/17435390.2014.921346. [DOI] [PubMed] [Google Scholar]
- 80.Al-Momani H, Perry A, Stewart CJ, Jones R, Krishnan A, Robertson AG, Bourke S, Doe S, Cummings SP, Anderson A, Forrest T, Griffin SM, Brodlie M, Pearson J, Ward C. 2016. Microbiological profiles of sputum and gastric juice aspirates in cystic fibrosis patients. Sci Rep 6:26985. doi: 10.1038/srep26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rabbi MF, Munyaka PM, Eissa N, Metz-Boutigue MH, Khafipour E, Ghia JE. 2016. Human catestatin alters gut microbiota composition in mice. Front Microbiol 7:2151. doi: 10.3389/fmicb.2016.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang M, Zhang M, Zhang C, Du H, Wei G, Pang X, Zhou H, Liu B, Zhao L. 2009. Pattern extraction of structural responses of gut microbiota to rotavirus infection via multivariate statistical analysis of clone library data. FEMS Microbiol Ecol 70:21–29. doi: 10.1111/j.1574-6941.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- 83.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. 2017. MicrobiomeAnalyst: a Web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia J, Wishart DS. 2016. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics 55:14.10.1–14.10.91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 85.Gokulan K, Khare S, Williams K, Foley SL. 2016. Transmissible plasmid containing Salmonella enterica Heidelberg isolates modulate cytokine production during early stage of interaction with intestinal epithelial cells. DNA Cell Biol 35:443–453. doi: 10.1089/dna.2015.3142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent abundance range for Firmicutes and Bacteroidetes in various experimental groups. Download TABLE S1, PDF file, 0.1 MB (118.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Supplemental methods and supplemental results. Download TEXT S1, DOC file, 0.04 MB (39.5KB, doc) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Effect of single versus repeated exposure to arsenic in adult mice on the intestinal bacterial recovery. Animals were exposed to single gavage using a low dose (LD; 0.05 mg/kg b.w.), medium dose (MD; 0.1 mg/kg b.w.), or high dose (HD; 0.2 mg/kg b.w.). These animals were sacrificed at 24 or 48 h postgavage (Single Dose 24 hr or Single Dose 48 hr). Another group of adult females were given continuous exposure (dosed water; 1 mg/liter) for 8 days (Repeated Dose), and samples were collected either on day 8 or after transfer to regular water for 24 h (cessation). Fecal samples were collected, and the suspension was made under anaerobic conditions. The fecal suspension was plated for the enumeration of total CFU for aerobic bacteria (a), anaerobic bacteria (b), or for three specific bacterial genera (c). Bacterial culture for each animal sample was done in two dilutions, and each dilution was done in duplicate. All groups were compared with the control animals that were not exposed to arsenic. Each column presents mean data, and the error bars represent the standard errors of the means (SEM) of results from 2 to 5 animals in each group. Download FIG S1, PDF file, 0.1 MB (114.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Effect of single exposure to arsenic in neonatal mice on the intestinal bacteria recovery. Neonatal (postnatal day 10 [PND10] and PND21) animals (both male and female) were exposed to a single dose of arsenic (oral gavage; 0.05 mg/kg b.w.), and the microbiome was analyzed 24 h and 48 h postexposure. The fecal suspension was plated for the enumeration of total CFU for aerobic and anaerobic bacteria (a) or bacterial culture on selective media (b and c). All groups were compared with the age-matched controls. The bars represent means and SEM of results from 2 animals in each group. Bacterial culture for each animal sample was done in two dilutions and each dilution in duplicate. Thus, each data point represents the value determined for four plates. Download FIG S2, PDF file, 0.1 MB (112.1KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Arsenic resistance gene abundance. Fold change in the presence of the arsenic resistance gene was analyzed by real-time PCR for the arsenic resistance genes. Data were normalized to the 16S gene. The group designations are as follows: low dose (LD), 0.05 mg/kg b.w.; medium dose (MD), 0.1 mg/kg b.w.; high dose (HD), 0.2 mg/kg b.w. Animals were sacrificed at 24 or 48 h postgavage and are designated LD24 (n = 8), LD48 (n = 8), MD24 (n = 2), MD48 (n = 2), HD24 (n = 2), or HD48 (n = 2). Download FIG S3, PDF file, 0.05 MB (52.5KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
List of arsenic resistance gene primers. Download TABLE S2, DOCX file, 0.01 MB (14KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.