Summary
Maxillofacial osteosynthetic surgeries require stable fixation for uneventful boney healing and optimal remodeling. Although conventional titanium plates and screws for osteofixation are considered the gold standard for rigid fixation in maxillofacial surgeries, bioresorbable implants of plates and screw systems are commonly used for various maxillofacial osteosynthetic surgeries such as orthognathic surgery, maxillofacial fractures, and reconstructive surgery. Titanium plates are limited by their palpability, mutagenic effects, and interference with imaging, which may lead to the need for subsequent removal; the use of a biologically resorbable osteofixation system could potentially address these limitations. However, several problems remain including fundamental issues involving decreased mechanical strength and stability, slow biodegradation, complex procedures, and the available bioresorbable implant materials. Major advances in bioresorbable plate systems have been made with the use of bioactive/resorbable osteoconductive materials and an accelerator of bioresorption, such as polyglycolic acid. This report presents an overview of currently available resorbable implant materials and their applications, with a focus on recent innovative advances and new developments in this field.
Keywords: Bioresorbable plate, Bioactive, Resorbable plate, Maxillofacial osteosysnthesis, Bone regeneration, Fixation, Osteoconductivity
1. Introduction
Essential prerequisites for the stable fixation and sound healing of maxillofacial boney segments in maxillofacial osteosynthetic surgeries, such as maxillofacial fractures and bimaxillary osteotomies in orthognathic surgery, include sufficient vascularization, reduction or repositioning of bone segments, immobilization with stable fixation, uneventful boney healing and optimal remodeling [1], [2], [3], [4], [5]. Recent developments for standard treatment in maxillofacial surgical implant biomaterials have led to the achievement of stable fixation using a titanium plate system [1], [3], [4], [5]. This contributes to patients’ masticatory functional load immediately after such surgeries. The mechanical properties of titanium including its strength, ease of handling, lack of dimensional changes, minimal scatter on computed tomography (CT) scanning, and compatibility with radiography and magnetic resonance imaging have prompted its widespread adoption as the general standard [1], [6], [7].
However, as the need for fixation is only temporary and as metallic materials cause stress shielding of the underlying bone, these plates are often removed after the maxillofacial boney healing [1], [2], [3]. In 5–40% of cases, the titanium plates and screws are removed in a second operation once the bone has healed as titanium is associated with potential effects on facial growth, thermal sensitivity, plate migration, and interference with diagnostic imaging [3], [4], [8], [9]. Other adverse effects of retained metallic devices include osteopenia of cortical bone induced by stress and corrosion [7], [8], [9]. Moreover, titanium particles have been found in scar tissue covering these plates, as well as in locoregional lymph nodes, and imperfect contact can occur between the metal plate and bone surface [6], [7], [8], [9]. Most recently, it was reported that even titanium miniplates are a risk factor for the development of bisphosphonate-related osteonecrosis of the jaw [9], [10]. Therefore, titanium osteofixation implant materials should be removed [5], [6], [7], [8], [9], and resorbable bone fixation implant materials for plate and screw devices have been developed.
Bioresorbable and biodegradable osteosynthetic fixation implants have been considered an effective fixation system that offers several advantages over titanium fixation, including the absence of corrosion and of accumulation of metal in tissues, and of the need to remove the implants after osseous healing; radiolucency; decreased pain; and reduced stress-shielding as the implants bear a smaller load initially and gradually transfer the load as they degrade [1], [2], [3], [7], [9]. The first study on the use of biodegradable implants was published in 1966 by Kulkarni et al. [11], who studied the biocompatibility of poly-l-lactic acid (PLLA) in animals. The use of PLLA plates and screws to fix mandibular fractures in dogs was studied, and the material was non-toxic and gradually degraded [11]. Another study presented the results of PLLA sutures in mandibular fractures with no serious tissue reactions such as severe inflammatory and immunological responses [12].
Bioresorbable implant materials allow newly formed tissue to grow into any surface irregularities. Thus, a resorbable osteofixation implant material is free of toxic and mutagenic effects. Nonetheless, there are some problems related to the use of these materials, such as an inflammatory response, rapid loss of initial implant strength, higher refracture rates, inadequate stiffness of the implants, and weakness compared to metallic implants [13], [14], [15]. Biodegradable osteofixation implants are characterized by biomaterials that disintegrate after implantation with no sign of elimination from the body. The biodegradation process depends on contact with body fluids, temperature, motion, molecular weight, the crystal form and geometry of the material, and the tissue that is implanted [13], [14], [15]. The ideal biodegradable osteofixation implant material provides appropriate strength while degrading in a predictable fashion throughout the healing process without causing adverse reactions [1], [6], [7], [8], [9].
On the other hand, the limitations of biodegradable osteofixation implants are associated mainly with their mechanical properties [13], [14], [16]; they are weaker than conventional such titanium metal implants, leading to low confidence levels regarding the stability of reduced fractures [13], [16]. Biocompatibility may be another limitation of these materials as they can provoke adverse tissue responses that have characteristics of an inflammatory, bacterial foreign-body reaction [16], [17].
However, bioresorbable implant materials for oral and maxillofacial osteosynthetic applications are currently becoming more common. These materials are safe, effective, and sufficiently flexible for use at many maxillofacial boney surgical sites [13], [14], [15], [16], [17]. Numerous clinical studies and several review articles have documented the feasibility of bioresorbable materials and these osteofixation plate and screw systems, showing comparable results between the use of resorbable and titanium plates and screws in various maxillofacial surgeries. Furthermore, novel products and feasible modified systems have been introduced for clinical applications [16], [18], [19].
This report presents an overview of currently available resorbable materials in Japan and their applications, with a focus on innovative advances, modifications and developments in the field of oral and maxillofacial surgery.
2. Variety of bioresorbable osteosynthesis materials
Common constituents include polyhydroxyl acids: polymers and copolymers of PLLA, poly-d-lactic acid (PDLA), polyglycolic acid (PGA), and polydioxanone sulfate so far [13], [17]. Developed bioresorbable osteosynthesis materials comprise a composite of PLLA as a base, an osteoconductive material such as hydroxyapatite, and an accelerator of bioresorption such as polyglycolic acid [7], [18]. Table 1 summarizes the commercially available resorbable osteosynthesis implant materials that have been approved by the Japanese national government for use in oral and maxillofacial surgery within the coverage of national medical insurance.
Table 1.
Commercially available resorbable and bioresorbable plate systems for maxillofacial osteosynthesis in Japan.
| Plate system | Plate/screw comformation | Plate thickness | Screw diameter | Indication for osteosynthesis | Manufacturer | Biodegradation period |
|---|---|---|---|---|---|---|
| GrandFix® | PLLA (100%) | 1.0 mm/1.5 mm | 2.2 mm | Mid-face/mandible | GUNZE, Kyoto, Japan | More than 3 years |
| GrandFix, Flat-type® | PLLA (100%) | 0.95 mm | 2.2 mm | Mid-face | GUNZE, Kyoto, Japan | More than 3 years |
| SonicWeld Rx® | PDLLA (100%) | 0.8 mm/1.0 mm (0.3 mm/0.6 mm) | 1.6 mm/2.1 mm | Mid-face | KLS Martin GmbH & Co, Tuttlingen, Germany | 12–30 months |
| LactoSorb® | PLLA (82%) + PGA (18%) | 0.9 mm/1.4 mm (0.5 mm) | 1.5 mm/2.0 mm | Mid-face | Biomet Inc, Jacksonville, FL, USA | 12–18 months |
| RapidSorb® | PLLA (85%) + PGA (15%) | 0.8 mm/1.2 mm (0.5 mm) | 1.5 mm/2.0 mm | Mid-face | DePuy Synthes CMF, West Chester, PA, USA | 12 months |
| FIXORB-MX® | PLLA (100%) | 1.5 mm | 2.0 mm | Mid-face/mandible | TEIJIN Medical Corp., Osaka, Japan | More than 3 years |
| SuperFIXORB-MX® (OsteotransMX®) | Plate: PLLA (60 wt%) + u-HA (40 wt%) Screw: PLLA (70 wt%) + u-HA (30 wt%) |
1.0 mm/1.4 mm (0.3 mm/0.5 mm) | 2.0 mm | Mid-face/mandible | TEIJIN Medical Corp., Osaka, Japan | 5.5 years |
PLLA, poly-l-lactic acid; PDLLA, poly-d-l-lactic acid; PGA, polyglycolic acid; u-HA, unsintered hydroxyapatite.
The use of biodegradable materials to stabilize the maxillofacial skeleton was first reported for facial fracture surgery in 1971 [20]. Since then, resorbable polymeric plates and screws have been used in pediatric patients with maxillofacial fractures in trauma patients because permanent osteofixation may hinder facial growth [21], [22]. These bioresorbable polymers are mainly high-molecular-weight aliphatic polyesters with repeating units of α-hydroxy acid (HOCHR-COOH) derivatives manufactured by ring-opening polymerization [21], [22], [23]. The resorption of these polymers begins with depolymerization through the hydrolysis of their ester bonds and subsequent metabolism, probably by macrophages, in the citric acid cycle into water and carbon dioxide [17], [21]. In the first era, the strength of resorbable plates and screws was poor, but the strength of the devices was increased by using self-reinforcement technology [17], [21], [22], [23]. Encouraging results were reported for the treatment of mandibular fractures and orthognathic surgery [22], [23], [24]. Recently, the concept has shifted from simply “resorbable” to actual “bioresorbable,” which represents biodegradation plus the stimulation of bioactivity, such as an innovative osteoconductivity [7], [18].
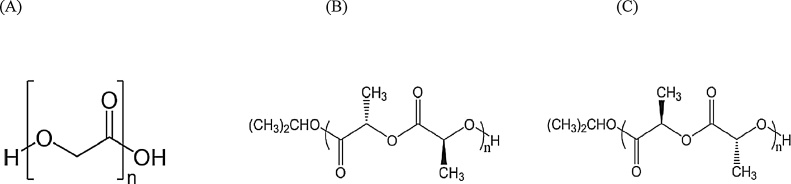
The ideal biodegradable material should not only support boney fragments during healing but also resorb fully once the healing process is completed. The resulting metabolites should not cause any local or systemic problems. Additionally, the amount of material required must be small, and the material itself must be flexible enough to be applied at various maxillofacial bone sites [16], [17], [18], [19]. These aforementioned three resorbable materials, PGA, PLLA, and PDLA, have been commonly well introduced for the products [13], [17] (Fig. 1).
Figure 1.
Structural formulas of polyglycolic acid (A), poly-l-lactic acid (B), and poly-d-lactic acid (C) (adapted and modified from Ref. [13]).
2.1. Polyglycolic acid (PGA)
The first bioresorbable polymer to be used clinically was poly(glycolic acid) (PGA), a highly crystalline and high-molecular-weight molecule with limited clinical use for osteosynthesis because of its susceptibility to rapid degradation [2], [8]. Approximately 4–7 weeks after implantation (a duration that is insufficient to allow for complete bone healing), PGA loses its mechanical strength in vivo [8], [25]. Additionally, adverse effects of PGA have been observed during its clinical use; these are due to difficulties in clearing the accumulated acid-degradation products. These negative effects have resulted in minimal use of pure PGA in maxillofacial osteosynthesis [8], [25].
2.2. Poly(lactic acid) (PLA): PLLA and PDLA
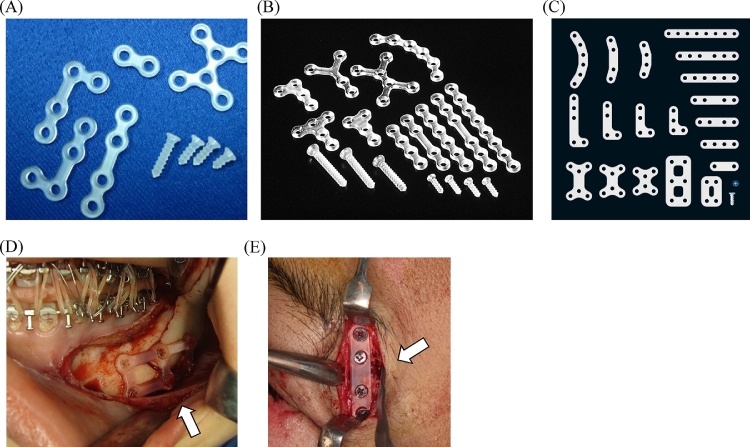
Poly(lactic acid) (PLA) is another high-molecular-weight bioresorbable polymer; its optically active carbon in lactic acid generates two stereoisomeric forms; namely, poly-l-lactide (PLLA) and poly-d-lactide (PDLA) [17]. Since the early 1990s, PLLA has been used as a maxillofacial osteosynthesis material as “the first generation” bioresorbable osteosynthetic material [2], [8], [26] (Fig. 2). Due to its crystallinity and hydrophobicity, PLLA is resistant to hydrolysis; thus, bioresorption with complete loss of strength in vitro does not occur within the first 2 years of implantation [8], [26]. In a clinical setting, the total resorption time of PLLA appears to be over 3.5 years [27], [28]. Two PLLA devices, FixsorbMX® (TEIJIN Medical Corp., Osaka, Japan) and GrandFix® (GUNZE, Kyoto, Japan), have been used in maxillofacial bone surgery [13], [16]. Reported problems include insufficient intensity of materials, foreign-body reactions, and a late-degradation tissue response [16], [27], [28]. On the other hand, PDLA has lower crystallinity and is less resistant to hydrolysis. Because of its slower degradation rate, PDLA is highly biocompatible and applicable for whole facial osteosysnthetic surgeries, including mid-face and mandible, although crystalline particles resistant to degradation may elicit an inflammatory response [13], [17], [27].
Figure 2.
Maxillofacial osteosynthesis systems using first-generation bioresorbable materials. (A) The GRAND FIX® system. (B) The FIXORB-MX® system. (C) The GRAND FIX® flat-type system. (D) Three-dimensional bioresorbable plate osteosynthesis of setback mandibular bilateral saggital split ramus osteotomy (BSSRO) using the GRAND FIX® system in orthognathic surgery. (E) Thin/flat-type bioresorbable plate osteosynthesis (GRAND FIX®, flat type) for the internal fixation of the left frontozygomatic suture in a complex zygomatico-maxillary fracture.
Despite the advances in the polymers used for maxillofacial osteosynthesis systems, there has been no significant improvement in their strength compared with that of titanium plate systems; sufficient thickness is still required to maintain strength, and complications such as palpability are related to the thickness of bioresorbable plate systems [16]. Although the PLLA bioresorbable plate system eventually degrades, it takes several years for complete absorption to occur. Therefore, the influence of thickness on osteosynthesis plates is important. In this respect, the shape of GrandFix-Flat type® (Gunze, Kyoto, Japan) was modified products from its original system to develop a novel system that was made commercially available as a thin, flat, bioresorbable plate system; this is only ideal use for mid-facial osteosynthesis due to the durability for strength (to date, limited approval has been obtained in Japan). The plate is stiff, has mechanical strength similar to that of conservative PLLA plates owing to its width, and is well-suited to reducing the burden of facial palpability, especially at easily facial palpated areas, such as the periorbital rim and frontozygomatic sutures, as reported previously [16].
2.3. Copolymers of PGA, PLLA, and PDLA
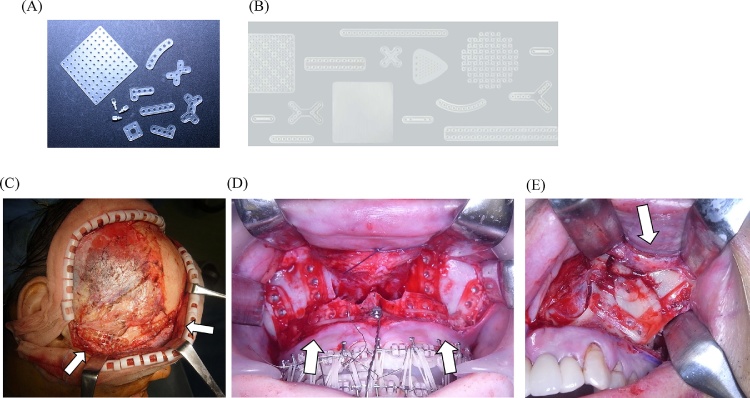
Copolymers of PGA, PLLA, and PDLA were preferred over pure PGA and PLLA as “the second generation” as rapidly bioresorbable osteosynthetic materials [8], [13] (Fig. 3). Their properties can be controlled by varying the ratio of glycolide to lactide for different compositions. The rates of hydration and hydrolysis can increase when crystalline PGA is co-polymerized with PLLA [13]. Therefore, through the copolymerization of different derivatives of α-hydroxy acids, a variety of different mechanical qualities and degradation rates can be achieved. The degradation time of the co-polymer depends on the ratio of monomers used during synthesis [8]. In general, a higher glycolide content leads to a faster rate of degradation. For example, the degradation time is 5 months for an 85:15 PDLA:PGA co-polymer. However, an exception to this rule is the 50:50 ratio of PGA:PLLA, which shows the fastest degradation time [13], [29].
Figure 3.
Maxillofacial osteosynthesis systems using second-generation rapidly bioresorbable materials. (A) The LactoSorb® system. (B) The RapidSorb® system. (C) Three-dimensional bioresorbable plate osteosynthesis of Le Fort II/III mid-face fractures using the LactoSorb® system. (D) Double-L-shape bioresorbable plate osteosynthesis (LactoSorb®) for the internal fixation of Le Fort I advancement in orthognathic surgery. (E) Bioresorbable plate osteosynthesis (RapidSorb®) for the internal fixation of a complex left zygomatico-maxillary fracture.
Copolymers of l-,d-lactides, such as SR-P(L/DL)LA 70/30, a copolymer composed of 70% PLLA and 30% PDLA, lose all strength in vitro after 48 weeks of implantation. Copolymers of l-lactide and glycolide (PLGA) have been used extensively owing to the wide range of physiochemical properties of the components. Lactosorb® (Biomet Inc., Jacksonville, Florida, USA) is a copolymer of PLLA (82%) and PGA (18%) [19], [29], [30], [31]. RapidSorb ® (DePuy Synthes CMF, West Chester, PA, USA) is composed of the same polymers in almost the same ratio of 85:15; both these products are only ideal for mid-face and maxillary osteosynthesis (to date, limited approval has been obtained in Japan) [32]. The copolymer is structured to provide adequate strength for 6–8 weeks and for a complete resorption time of 12–18 months, those of which products are really feasible in a clinical application for midfacial osteosynthesis as secure and rapid bioresorbable materials, and such plate systems [8], [9], [19], [29]. The recent retrospective clinical study by Sukegawa et al. [30] elucidated and stressed the feasibility of PLLA/PGA copolymer plate systems in maxillofacial osteosynthesis with relatively small minor postoperative complication rates. It is metabolized via the citric acid cycle and is eventually excreted by the lungs as carbon dioxide and water [31], [32], [33]. Because of their amorphous structure, copolymers do not release any crystalline particles, and the rate of degradation is slow enough to facilitate high biocompatibility [8], [13].
2.4. u-HA/PLLA bioactive/resorbable material
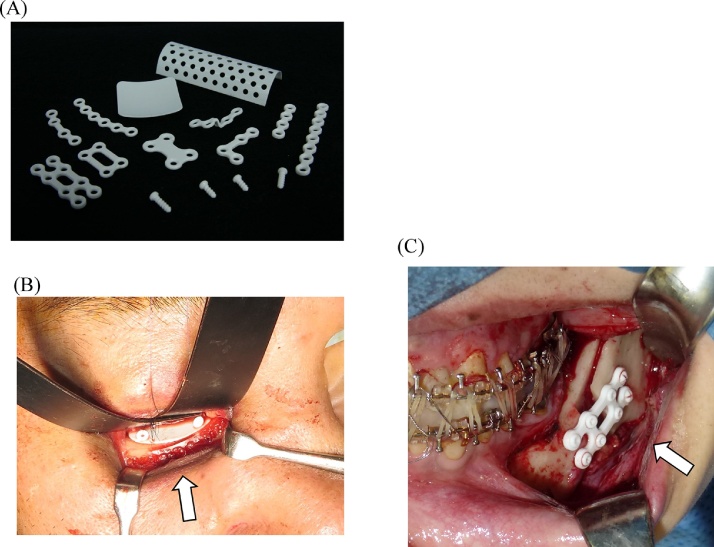
Recently, hydroxyapatite has been incorporated into PLLA because of its documented osteoconductive capacity [34], [35]. SuperFIXORB-MX® (TEIJIN Medical Corp., Osaka, Japan) (also known as OSTEOTRANS MX® overseas) plates are made of a composite material of fine particles of unsintered hydroxyapatite (u-HA) and carbonate ions combined with PLLA [7], [18]. As “the third generation” bioactive/bioresorbable osteosynthetic material, forged composites of unsintered hydroxyapatite/poly-l-lactide (u-HA/PLLA) are processed by machining or milling treatments into various miniscrews and miniplates, containing 30% and 40% weight fractions of u-HA (raw hydroxyapatite, neither calcined nor sintered material) particles in composites, respectively (hereinafter referred to as u-HA 30% miniscrew and u-HA 40% miniplate) [7], [18], [34], [35], [36] (Fig. 4). Because they are osteoconductive and biodegradable, the u-HA/PLLA nano-composites can be used for complete replacement by bony tissue [34], [35], [36], [37].
Figure 4.
Maxillofacial osteosynthesis systems using third-generation bioactive/bioresorbable materials. (A) The SuperFIXORB-MX® (OsteotransMS®) system. (B) Bioresorbable sheet and tack fixation for right orbital reconstruction in a case with naso-orbito-ethmoidal (midfacial) fractures using the SuperFIXORB-MX® (OsteotransMS®) system, with use of the RapidSorb® system for infraorbital rim fixation. (C) Three-dimensional bioresorbable plate osteosynthesis of advancement mandibular BSSRO using the SuperFIXORB-MX® (OsteotransMS®) system in orthognathic surgery.
Furthermore, these devices maintain a bending strength equal to that of human cortical bone for 25 weeks in vivo [34], [35], [36], [37]. Once PLLA has been implanted, its hydrolysis by body fluids and biodegradation begins. The molecular weight of PLLA decreases and the u-HA fraction increases for about 2 years [34], [35]. The PLLA matrix is completely absent from the composites after 4 years, and the majority of u-HA particles have been replaced by bone after 5.5 years [35], [36]. Although this long-term resorption can cause additional complications, such as palpable discomfort when using a resorbable plate in an area of very thin facial skin areas (e.g., the periorbital region), it is possible that discomfort may be exacerbated over time because of a further increase in volume due to fibrous tissue covering the plate [35], [36]. Compared with early bioresorbable polymers, u-HA/PLLA osteoconductive composites provide more stable boney segment retention during maxillofacial surgery and are approved for clinical use with whole facial osteosynthetic surgeries of the mid-face and mandible [35], [36], [37].
The u-HA/PLLA composite material has higher mechanical strength, including bending strength, bending modulus, shear strength, and impact strength, than PLLA devices. The recent in vitro model study has clearly demonstrated the efficacy of such higher stable mechanical properties of the u-HA/PLLA plates system using a 3-dimensional model of bilateral sagittal split ramus osteotomy of the mandible upon biomechanical loading evaluation [38]. u-HA/PLLA plates can bond directly to bone and possess osteoconductivity. Recent clinical reports have elucidated this innovative bioactive osteoconductive direct bone healing effects of this plate system based on scanning capacitance microscopy analysis on an incompletely exposed plate after its removals in maxillofacial regions [18], [36], [37], [38], [39], [40], [41] (Fig. 5). Deposits of bone-like regenerative tissue were evident on the bone-contacting surface of the removed plates and screws [7], [39]. This suggested that the plates directly bonded to bone and supported the osteoconductivity of the u-HA/PLLA plate system to accelerate boney healing in maxillofacial boney segments. The early osteoconductive bioactivity is advantageous for early functional improvements in maxillofacial osteosynthesis, such as in maxillofacial fractures and osteotomies (Fig. 6). Thus, because of its bioactive, osteoconductive, and biodegradable properties, this u-HA/PLLA composite material has significantly innovative clinical advantages and may have broad indications in maxillofacial surgery as a next-generation material [34], [35], [36], [37], [38], [39], [40], [41].
Figure 5.
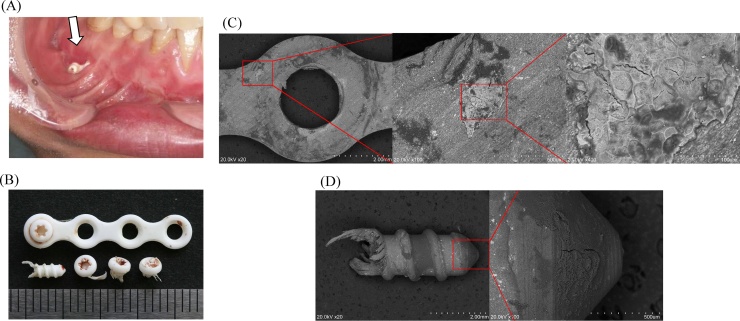
(A) Plate exposure (SuperFIXORB-MX® system) occurred in a patient with a mandibular fracture 4 months after surgery. We removed the exposed plate and screws at the outpatient clinic (B), and then performed a close examination. The molecular weight and crystallinity of the removed plate were 64 kDa and 54.1%, and those of the screws were 60 kDa and 54.4%, respectively, which signified the occurrence of the natural process of planned bioresorption in vivo. Fracture healing was uneventful (adapted and modified from Ref. [7]) (C) Scanning electron microscopic (SEM) imaging (S-3400N; Hitachi, Ltd., Tokyo, Japan) of the removed plate and tissue attached to the bone–plate interface at the site of complete fracture healing. Marked bony regeneration is visible around the plate (red circle) and remodeled trabecular bone formation is visible toward the center. The plate was observed to bond directly to the fractured bone surface, with no interposition of unmineralized tissue [7]. The red square indicates the area of the magnified view. SEM magnifications, from left to right: ×20, ×100, and ×400. (D) SEM imaging of the screw. Bone regeneration was also confirmed around the screw surface. The red square indicates the area of the magnified view. SEM magnifications, from left to right: ×20 and ×100 (adapted and modified from Ref. [7]). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Figure 6.
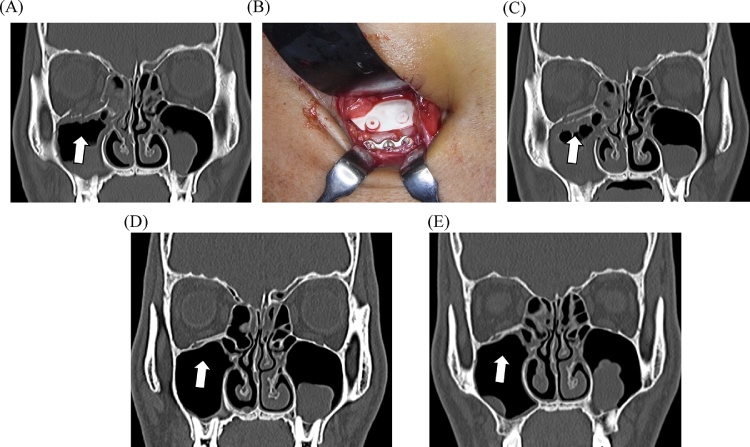
A patient with a naso-orbito-ethmoidal fracture with defects on the orbital floor and medial wall. (A) Preoperative computed tomographic (CT) image. (B) Intraoperative view showing orbital reconstruction using bioresorbable sheet and tack fixation [SuperFIXORB-MX® (OsteotransMS®) system]. (C) CT image obtained immediately after surgery showing the feasible orbital reconstruction. (D) CT image obtained 6 months after surgery showing the optimal mechanical potential and bioactive, osteoconductive/bioresorbable characteristics. (E) CT image obtained 12 months after surgery showing complete bony healing with feasible functioning of the bioactive osteoconductive potential in the orbit.
3. Clinical significance
The main applications of bioresorbable osteosynthetic implants are to stabilize fractures, osteotomies, and bone grafts in oral and maxillofacial regions of facial bones [2], [6], [7], [8], [9], [41], [42]. The mid-facial skeleton is an acceptable location for the use of bioresorbable osteosynthetic implants given the relatively easy access to fractures in this region and the low biomechanical stress to which they are exposed. Furthermore, based on the recent aforementioned innovative advances, improvements and developments, this bioresorbable osteosynthetic system can also be well applied to the mandible.
3.1. Clinical applications in orthognathic surgery
In orthognathic surgery, bioresorbable implants offer a clinical advantage over titanium plates by eliminating the possible need for a second operation for their removal [12], [13], [24], [42], [43]. Le Fort I osteotomies are stabilized with four L-shaped bioabsorbable plates and are secured bilaterally in the pyriform aperture and zygomatic buttress [2], [8], [13]. Reliable results have also been reported using a biodegradable mesh [8], [13]. Segmental Le Fort I osteotomy is stabilized with the above standard bioresorbable fixation [41]. Fedorowicz et al. [45] evaluated the effectiveness of bioresorbable fixation in orthognathic surgery by a systemic review study. Adverse effects were observed in some plate exposures, mainly in the posterior maxillary region [30], [45]. Infection was associated with loosened screws and wound dehiscence. A recent meta-analysis discussed two trials comparing the complications of bioresorbable and conventional titanium fixation in Le Fort I orthognathic surgery as well as three other trials comparing the complications of resorbable and titanium fixation in the bimaxillary operation category Le Fort I plus bilateral saggital split ramus osteotomy (BSSRO) [9]. They concluded that there was no significant difference between these two groups in terms of complication rates [9].
Regarding mandibular osteotomy, the bioresorbable plate application in BSSRO has been well-described [42], [43]. Standard methods for the osteosynthesis of mandibular BSSRO are the triangular placement of bicortical screws or the use of two PLLA and HA/PLLA mini-plates and monocortical screws [9], [13]. When two plates are applied, one is located above the inferior alveolar canal and the other is located below the canal. Regardless of the fixation method, mandibular setback is the more unstable movement compared with mandibular advancement [13], [44], [45], [46], [47], [48]. One mini-resorbable plate for fixation of mandibular BSSRO with mandibular setback can lead to segment mobility during the early postoperative period [46], [47]. A 0.7-mm-thick u-HA/PLLA mesh can also be applied after mandibular BSSRO, especially when major segmental movements have been performed [46], [47]. Osteosynthesis using u-HA/PLLA devices in orthognathic surgery is reliable because of their rigidity as well as their osteoconductivity and bone-bonding capacity [38], [39], [40], [44]. Furthermore, a recent meta-analysis study showed no significant difference between the bioresorbable and titanium plate fixation groups in BSSRO [9]. Additionally, a subgroup analysis was performed for complications, including infection, temporomandibular disorders (TMDs), paresthesia, palpability, dehiscence, material-related complications, exposure, and relapse [9]. These results showed the same rate of complications in the bioresorbable and titanium groups. Bioresorbable fixation systems tended to have a similar favorable safety profile compared to titanium fixation during bimaxillary operations, BSSRO, and Le Fort I operations, in which the resorbable fixation was superior to titanium fixation with regard to palpability, and no plate removal was required [2], [9], [13], [48], [49].
3.2. Clinical application in maxillofacial trauma surgery
The application of bioresorbable osteosynthesis in maxillofacial trauma surgery has been well-documented [7], [16], [17], [18], [19]. Previous studies have shown that mid-facial fractures fixation stability can be achieved with satisfactory results when uhydroxyapatite/poly-(l-lactic) acid (u-HA/PLLA) and poly-l-lactic acid (PLLA) plates are used (similar to titanium plates) [7], [8], [9], [16], [17], [18], [19], [49]. In maxillofacial fracture surgery, the feasibility of applying biodegradable plates and screws for zygomatic fracture fixation was first shown by Bos et al. in 1987 [50]. This technique was soon extended to other craniomaxillofacial fracture surgical procedures. Additionally, application has been extended to craniomaxillofacial trauma regions, from the mandible to the mid-face, especially in three-dimensional orbital trauma reconstruction [15], [16], [17], [18], [19], [51]. The resorbable system is applicable for rigid internal fixation under specific conditions where muscular and stress forces are not a determining factor in fragment displacement, including mid-face and whole mandibular fractures [14], [15], [16], [17], [18], [19], [51]. In paediatric patients, the effective use of resorbable polyglycolic and poly-l-lactic acid plating systems was well reported by Stanton et al. [52], when combined with a brief postoperative period of intermaxillary fixation for mandibular fractures surgical treatment. However, the stability of fixation, the length of time required for their degradation, and the possibility of complications (e.g., foreign-body reactions) have not yet been fully explored. Park et al. [53] suggested that resorbable plate and screw systems should be selected carefully depending on the fracture site and whether there is an accompanying infection. It is important to select the optimal method for the patient’s situation. The use of biodegradable plates should be recommended for minimally loaded maxillofacial fracture situations [2], [16], [37], [39]. Additionally, the degradation process of these devices, such as PLLA into carbon dioxide and water, may take up to 3 years [26], [27], [28]. Therefore, the use of resorbable plates and screws may be applicable in less complicated fractures both in the mid-face and mandible while considering the loading of mastication and masticatory muscles at surgical sites [9], [53], [54].
Recent meta-analysis studies discussed pooled clinical evidence from five trials of the maxillofacial fracture fixation operation, and the combined results of the five clinical trials involving both mid-face and mandible fractures showed that the bioresorbable group had a significantly lower rate of complications compared with the titanium group [8], [9], [55], [56], [57], [58]. Subgroup analysis was further performed for complications, including infection, paresthesia, foreign-body reactions, palpability, dehiscence, malocclusion, material-related complications, exposure, and mobility [9], [55]. Palpability was more common in patients fixed with titanium than in patients who received a resorbable fixation. Additionally, the resorbable group did not show a significant increase in infection, paranesthesia, foreign-body reactions, dehiscence, malocclusion, material-related complications, exposure, or mobility, but metal ions were present near the site, suggesting that metal gradually leached out through the action of body fluids [8], [9], [36], [40], [55], [56], [57], [58]. The bioresorbable fixation systems tend to have a favorable safety profile, similar to titanium fixation. Furthermore, next-generation uHA/PLLA bioactive/resorbable plates will be suitable for use as internal bone fixation devices for maxillofacial fractures not only because of their durable mechanical properties but also their bioactivities, including osteoconductive potential [34], [35], [36], [37], [38], [39], [40].
Therefore, a bioresorbable fixation system may be applicable for maxillofacial fracture fixation surgery, although further large-scale randomized, prospective trials of the bioresorbable fixation systems used in maxillofacial surgery are required to support the safety of this approach [9], [42], [59].
4. Conclusions and perspectives
There have been significant advances in bioresorbable osteosynthesis plate systems for oral and maxillofacial surgery. These have been developed, improved and modified for optimal clinical feasibility for both patients and oral and maxillofacial surgeons and offer important advantages over traditional titanium metal plate systems. This review study presents an overview of, and update on, the currently available bioresorbable osteosynthesis materials and osteofixation systems.
Although the use of bioresorbable osteosynthetic materials is still associated with several complications that need to be resolved, compared with conventional titanium fixation with the need for a second operation and associated interference with boney healing, optimal remodeling and radiological evaluation, the currently available bioresorbable osteosynthesis systems for maxillofacial surgery are feasible. Furthermore, based on recent clinical and biological reports, bioabsorbable osteosynthesis systems are reliable for osteofixation in various maxillofacial surgeries, including fragment fixation in orthognathic procedures and maxillofacial trauma surgery. The use of bioresorbable devices leads to predictable postoperative stable fixation for uneventful boney healing and optimal remodeling, as well as skeletal stability similar to that provided by conventional titanium devices for specific limited applications.
The updated third-generation bioresorbable material of uHA/PLLA may be applicable for maxillofacial surgery as it has optimal mechanical potential and bioactive, osteoconductive/bioresorbable characteristics. This material retains its strength long enough to support bone healing and then gradually and harmlessly disintegrates in the patient’s body. The material properties and degradation characteristics of these implants can be engineered.
Further future development of these materials as maxillofacial osteosynthetic plate systems should focus on the reduction of foreign-body reactions and the enhancement of biocompatibility, mechanical strength and bioactivities, or bioresorption rate/speed controllability, and even for such customizability. Overall, further such improvements are required in the field of oral and maxillofacial surgery and maxillofacial boney osteosynthesis.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank our colleagues at the Departments of Oral and Maxillofacial Surgery, Shimane University Faculty of Medicine and at the Division of Oral and Maxillofacial Surgery, Kagawa Prefectural Central Hospotal. This work was in part supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#17K11803 to Dr. T.K.).
References
- 1.Buijs G.J., van Bakelen N.B., Jansma J., de Visscher J.G., Hoppenreijs T.J., Bergsma J.E. A randomized clinical trial of biodegradable and titanium fixation systems in maxillofacial surgery. J Dent Res. 2012;91:299–304. doi: 10.1177/0022034511434353. [DOI] [PubMed] [Google Scholar]
- 2.van Bakelen N.B., Buijs G.J., Jansma J., de Visscher J.G., Hoppenreijs T.J., Bergsma J.E. Decision-making considerations in application of biodegradable fixation systems in maxillofacial surgery—a retrospective cohort study. J Craniomaxillofac Surg. 2014;42:417–422. doi: 10.1016/j.jcms.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Haers P.E., Suuronen R., Lindqvist C., Sailer H. Biodegradable polylactide plates and screws in orthognathic surgery: technical note. J Craniomaxillofac Surg. 1998;26:87–91. doi: 10.1016/s1010-5182(98)80045-0. [DOI] [PubMed] [Google Scholar]
- 4.Ray M.S., Matthew I.R., Frame J.W. Metallic fragments on the surface of miniplates and screws before insertion. Br J Oral Maxillofac Surg. 1999;37:14–18. doi: 10.1054/bjom.1998.0264. [DOI] [PubMed] [Google Scholar]
- 5.Erkmen E., Şimşek B., Yücel E., Kurt A. Three-dimensional finite element analysis used to compare methods of fixation after sagittal split ramus osteotomy: setback surgery-posterior loading. Br J Oral Maxillofac Surg. 2005;43:97–104. doi: 10.1016/j.bjoms.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Al-Moraissi E.A., Ellis E., Jr. Biodegradable and titanium osteosynthesis provide similar stability for orthognathic surgery. J Oral Maxillofac Surg. 2015;73:1795–1808. doi: 10.1016/j.joms.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Sukegawa S., Kanno T., Katase N., Shibata A., Takahashi Y., Furuki Y. Clinical evaluation of an unsintered hydroxyapatite/poly-l-lactide osteoconductive composite device for the internal fixation of maxillofacial fractures. J Craniofac Surg. 2016;27:1391–1397. doi: 10.1097/SCS.0000000000002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumann P., Lindhorst D., Wagner M.E.H., Schramm A., Gellrich L.C., Rücker M. Perspectives on resorbable osteosynthesis materials in craniomaxillofacial surgery. Pathobiology. 2013;80:211–217. doi: 10.1159/000348328. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Xu M., Jin X., Xu J., Lu J., Zhang C. Complications of absorbable fixation in maxillofacial surgery: a meta-analysis. PLoS One. 2013;8:e67449. doi: 10.1371/journal.pone.0067449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siniscalchi E.N., Catalfamo L., Allegra A., Musolino C., De Ponte F.S. Titanium miniplates: a new risk factor for the development of the bisphosphonate-related osteonecrosis of the jaw. J Craniofac Surg. 2013;24:e1–2. doi: 10.1097/SCS.0b013e31826d07b9. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni R.K., Pani K.C., Neuman C., Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93:839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni R.K., Moore E.G., Hegyeli A.F., Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- 13.Park Y.W. Bioabsorbable osteofixation for orthognathic surgery. Maxillofac Plast Reconst Surg. 2015;37:1–9. doi: 10.1186/s40902-015-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bali Rishi K., Parveen Sharma, Shalu Jindal, Shivani Gaba. To evaluate the efficacy of biodegradable plating system for fixation of maxillofacial fractures: a prospective study. Natl J Maxillofac Surg. 2013;4:167–172. doi: 10.4103/0975-5950.127645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yolcu U., Alan H., Malkoc S., Bozkurt S.B., Hakki S.S. Cytotoxicity evaluation of bioresorbable fixation screws on human gingival fibroblasts and mouse osteoblasts by real-time cell analysis. J Oral Maxillofac Surg. 2015;73:e1–10. doi: 10.1016/j.joms.2015.03.067. [DOI] [PubMed] [Google Scholar]
- 16.Sukegawa S., Kanno T., Nagano D., Shibata A., Sukegawa-Takahashi Y., Furuki Y. The clinical feasibility of newly developed thin flat-type bioresorbable osteosynthesis devices for the internal fixation of zygomatic fractures: is there a difference in healing between bioresorbable materials compare with titanium osteosynthesis? J Craniofac Surg. 2016;27:2124–2129. doi: 10.1097/SCS.0000000000003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pina S., Ferreira J.M.F. Bioresorbable plates and screws for clinical applications: a review. J Healthc Eng. 2012;3:243–260. [Google Scholar]
- 18.Kanno T., Karino M., Yoshino A., Koike T., Ide T., Tatsumi H. Feasibility of single folded unsintered hydroxyapatite particles/poly-l-lactide composite sheet in combined orbital floor and medial wall fracture reconstruction. J Hard Tissue Biol. 2017;26:237–244. [Google Scholar]
- 19.Koike T., Kanno T., Sekine J. A case of nasoorbitoethmoid fracture following airbag deployment. J Oral Maxillofac Surg Pathol Med. 2015;27:522–524. [Google Scholar]
- 20.Cutright D.E., Hunsuck E.E., Beasley J.D. Fracture reduction using a biodegradable material, polylactic acid. J Oral Surg. 1971;29:393–397. [PubMed] [Google Scholar]
- 21.Suuronen R., Kallela I., Lindqvist C. Bioabsorbable plates and screws: current state of the art in facial fracture repair. J Craniomaxillofac Trauma. 2000;6:19–27. [PubMed] [Google Scholar]
- 22.Ylikontiola L., Sundqvuist K., Sàndor G.K., Törmälä P., Ashammakhi N. Self-reinforced bioresorbable poly-l/dl-lactide [SR-P(L/DL)LA] 70/30 miniplates and miniscrews are reliable for fixation of anterior mandibular fractures: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:312–317. doi: 10.1016/j.tripleo.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed-Hashem I.K., Mitchell D.A. Resorbable implants (plates and screws) in orthognathic surgery. J Orthod. 2000;27:198–199. doi: 10.1093/ortho/27.2.198. [DOI] [PubMed] [Google Scholar]
- 24.Turvey T.A., Bell R.B., Tejera T.J., Proffit W.R. The use of self-reinforced biodegradable bone plates and screws in orthognathic surgery. J Oral Maxillofac Surg. 2002;60:59–65. doi: 10.1053/joms.2002.28274. [DOI] [PubMed] [Google Scholar]
- 25.Vasenius J., Vainionpaa S., Vihtonen K., Makela A., Rokkanen P., Mero M. Comparison of in vitro hydrolysis, subcutaneous and intramedullary implantation to evaluate the strength retention of absorbable osteosynthesis implants. Biomaterials. 1990;11:501–504. doi: 10.1016/0142-9612(90)90065-x. [DOI] [PubMed] [Google Scholar]
- 26.Pihlajamaki H., Bostman O., Hirvensalo E., Tormala P., Rokkanen P. Absorbable pins of self-reinforced poly-l-lactic acid for fixation of fractures and osteotomies. J Bone Jt Surg Br. 1992;74:853–857. doi: 10.1302/0301-620X.74B6.1447246. [DOI] [PubMed] [Google Scholar]
- 27.Bergsma E.J., de Bruijn W.C., Rozema F.R., Bos R.R., Boering G. Late degradation tissue response to poly(l-lactide) bone plates and screws. Biomaterials. 1995;16:25–31. doi: 10.1016/0142-9612(95)91092-d. [DOI] [PubMed] [Google Scholar]
- 28.Bergsma E.J., Rozema F.R., Bos R.R., de Bruijn W.C. Foreign body reaction to resorbable poly(l-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J Oral Maxillofac Surg. 1993;51:666–670. doi: 10.1016/s0278-2391(10)80267-8. [DOI] [PubMed] [Google Scholar]
- 29.Surronen R., Haers P.E., Lindqvist C., Sailer H.F. Update on bioresorbable plates in maxillofacial surgery. Facial Plast Surg. 1999;15:61–72. doi: 10.1055/s-2008-1064301. [DOI] [PubMed] [Google Scholar]
- 30.Sukegawa S., Kanno T., Matsumoto K., Sukegawa-Takahashi Y., Masui M., Furuki Y. Complications of a poly-l-lactic acid and polyglycolic acid osteosynthesis device for internal fixation in maxillofacial surgery. Odontology. 2018 doi: 10.1007/s10266-018-0345-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Wiltfang J., Merten H.A., Schultze-Mosgau S., Schrell U., Wenzel D., Kessler P. Biodegradable miniplates (LactoSorb): long-term results in infant minipigs and clinical results. J Craniofac Surg. 2000;11:239–243. doi: 10.1097/00001665-200011030-00006. [DOI] [PubMed] [Google Scholar]
- 32.Edwards R.C., Kiely K.D., Eppley B.L. The fate of resorbable poly-l-lactic/polyglycolic acid (LactoSorb) bone fixation devices in orthognathic surgery. J Oral Maxillofac Surg. 2001;59:19–25. doi: 10.1053/joms.2001.19267. [DOI] [PubMed] [Google Scholar]
- 33.Young S.M., Sundar G., Lim T.C., Lang S.S., Thomas G., Amrith S. Use of bioresorbable implants for orbital fracture reconstruction. Br J Ophthalmol. 2017;101:1080–1085. doi: 10.1136/bjophthalmol-2016-309330. [DOI] [PubMed] [Google Scholar]
- 34.Shikinami Y., Hata K., Okuno M. Ultra-high-strength resorbable implants made from bioactive ceramic particles/polylactide composites. Bioceramics. 1996;9:391–394. [Google Scholar]
- 35.Shikinami Y., Matsusue Y., Nakamura T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly-l-lactide (F-u-HA/PLLA) Biomaterials. 2005;26:5542–5551. doi: 10.1016/j.biomaterials.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Sukegawa S., Kanno T., Kawai H., Shibata A., Sukegawa-Takahashi Y., Nagatsuka H. Long-term bioresorption of bone fixation devices made from composites of unsintered hydroxyapatite particles and poly-l-lactide. J Hard Tissue Biol. 2015;24:219–224. [Google Scholar]
- 37.Kanno T., Tatsumi H., Karino M., Koike T., Ide T., Sekine J. The applicability of an unsintered hydroxyapatite particles/poly-l-lactide composite sheet with tack fixation for orbital fracture reconstruction. J Hard Tissue Biol. 2016;25:329–334. [Google Scholar]
- 38.Sukegawa S., Kanno T., Manabe Y., Matsumoto K., Sukegawa-Takahashi Y., Masui M. Biomechanical loading evaluation of unsintered hydroxyapatite/poly-l-lactide plate system in bilateral sagittal split ramus osteotomy. Materials (Basel) 2017;10:E764. doi: 10.3390/ma10070764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sukegawa S., Kanno T., Koyama Y., Matsumoto K., Sukegawa-Takahashi Y., Nagatsuka H. Precision of post-traumatic orbital reconstruction using unsintered hydroxyapatite particles/poly-l-lactide composite bioactive/resorbable mesh plate with and without navigation: a retrospective study. J Hard Tissue Biol. 2017;26:274–280. [Google Scholar]
- 40.Landes C., Ballon A., Ghanaati S., Tran A., Sader R. Treatment of malar and midfacial fractures with osteoconductive forged unsintered hydroxyapatite and poly-l-lactide composite internal fixation devices. J Oral Maxillofac Surg. 2014;72:1328–1338. doi: 10.1016/j.joms.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 41.van Bakelen N.B., Buijs G.J., Jansma J., de Visscher J.G., Hoppenreijs T.J., Bergsma J.E. Comparison of biodegradable and titanium fixation systems in maxillofacial surgery: a two-year multi-center randomized controlled trial. J Dent Res. 2013;92:1100–1105. doi: 10.1177/0022034513508953. [DOI] [PubMed] [Google Scholar]
- 42.Gareb B., van Bakelen N.B., Buijs G.J., Jansma J., de Visscher J.G.A.M., Hoppenreijs T.J.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: a multicenter randomized controlled trial. PLoS One. 2017;11:e0177152. doi: 10.1371/journal.pone.0177152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Bakelen N.B., Boermans B.D., Buijs G.J., Jansma J., Pruim G.J., Hoppenreijs T.J. Comparison of the long-term skeletal stability between a biodegradable and a titanium fixation system following BSSO advancement—a cohort study based on a multicenter randomised controlled trial. Br J Oral Maxillofac Surg. 2014;52:721–728. doi: 10.1016/j.bjoms.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Landes C.A., Ballon A., Tran A., Ghanaati S., Sader R. Segmental stability in orthognathic surgery: hydroxyapatite/poly-l-lactide osteoconductive composite versus titanium miniplate osteosyntheses. J Craniomaxillofac Surg. 2014;42:930–942. doi: 10.1016/j.jcms.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Fedorowicz Z., Nasser M., Newton J.T., Oliver R.J. Resorbable versus titanium plates for orthognathic surgery. Cochrane Database Syst Rev. 2007:CD006204. doi: 10.1002/14651858.CD006204.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Ueki K., Marukawa K., Shimada M., Nakagawa K., Alam S., Yamamoto E. Maxillary stability following Le Fort I osteotomy in combination with sagittal split ramus osteotomy and intraoral vertical ramus osteotomy: a comparative study between titanium miniplate and poly-l-lactic acid plate. J Oral Maxillofac Surg. 2006;64:74–80. doi: 10.1016/j.joms.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Ko E.W., Huang C.S., Lo L.J., Chen Y.R. Alteration of masticatory electromyographic activity and stability of orthognathic surgery in patients with skeletal class III malocclusion. J Oral Maxillofac Surg. 2013;71:1249–1260. doi: 10.1016/j.joms.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Paeng J.Y., Hong J., Kim C.S., Kim M.J. Comparative study of skeletal stability between bicortical resorbable and titanium screw fixation after sagittal split ramus osteotomy for mandibular prognathism. J Craniomaxillofac Surg. 2012;40:660–664. doi: 10.1016/j.jcms.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Sukegawa S., Kanno T., Shibata A., Sukegawa-Takahashi Y., Furuki Y. Use of templates and self-tapping metal screws for temporary fixation of a resorbable plate system. Ann Maxillofac Surg. 2015;5:231–233. doi: 10.4103/2231-0746.175763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bos R.R., Boering G., Rozema F.R., Leenslag J.W. Resorbable poly(l-lactide) plates and screws for the fixation of zygomatic fractures. J Oral Maxillofac Surg. 1987;45:751–753. doi: 10.1016/0278-2391(87)90194-7. [DOI] [PubMed] [Google Scholar]
- 51.Singh M., Singh R.K., Passi D., Aggarwal M., Kaur G. Management of pediatric mandibular fractures using bioresorbable plating system—efficacy, stability, and clinical outcomes: our experiences and literature review. J Oral Biol Craniofac Res. 2016;6:101–106. doi: 10.1016/j.jobcr.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanton D.C., Liu F., Yu J.W., Mistretta M.C. Use of bioresorbable plating systems in paediatric mandible fractures. J Craniomaxillofac Surg. 2014;42:1305–1309. doi: 10.1016/j.jcms.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Park C.H., Kim H.S., Lee J.H., Hong S.M., Ko Y.G., Lee O.J. Resorbable skeletal fixation systems for treating maxillofacial bone fractures. Arch Otolaryngol Head Neck Surg. 2011;137:125–129. doi: 10.1001/archoto.2010.241. [DOI] [PubMed] [Google Scholar]
- 54.Buijs Gerrit J., van der Houwen Eduard B., Stegenga Boudewijn, Bos Rudulf R.M., Verkerke Gijsbertus J. Mechanical strength and stiffness of biodegradable and titanium osteofixation systems. J Oral Maxillofac Surg. 2007;65:2148–2158. doi: 10.1016/j.joms.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Dorri M., Nasser M., Oliver R. Resorbable versus titanium plates for facial fractures. Cochrane Database Syst Rev. 2009:CD007158. doi: 10.1002/14651858.CD007158.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Lee H.B., Oh J.S., Kim S.G., Kim H.K., Moon S.Y., Kim Y.K. Comparison of titanium and biodegradable miniplates for fixation of mandibular fractures. J Oral Maxillofac Surg. 2010;68:2065–2069. doi: 10.1016/j.joms.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt K., Roychoudhury A., Bhutia O., Trikha A., Seith A., Pandey R.M. Equivalence randomized controlled trial of bioresorbable versus titanium miniplates in treatment of mandibular fracture: a pilot study. J Oral Maxillofac Surg. 2010;68:1842–1848. doi: 10.1016/j.joms.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Meningaud J.P., Poupon J., Bertrand J.C., Chenevier C., Galliot-Guilley M., Guilbert F. Dynamic study about metal release from titanium miniplates in maxillofacial surgery. Int J Oral Maxillofac Surg. 2001;30:185–188. doi: 10.1054/ijom.2000.0039. [DOI] [PubMed] [Google Scholar]
- 59.van Bakelen N.B., Vermeulen K.M., Buijs G.J., Jansma J., de Visscher J.G., Hoppenreijs T.J. Cost-effectiveness of a biodegradable compared to a titanium fixation system in maxillofacial surgery: a multicenter randomized controlled trial. PLoS One. 2015;20:e0130330. doi: 10.1371/journal.pone.0130330. [DOI] [PMC free article] [PubMed] [Google Scholar]