Summary
Odontoblasts located in the outermost layer of dental pulp form a natural barrier between mineralized tissues, dentin, and soft tissues, dental pulp, of the vital tooth, and they first recognize caries-related pathogens and sense external irritations. Therefore, odontoblasts possess a specialized innate immune system to fight oral pathogens invading into dentin. Generally, the rapid initial sensing of microbial pathogens, especially pathogen-associated molecular patterns (PAMPs) shared by microorganisms, are mediated by pattern recognition receptors (PRRs), such as Toll-like receptor and the nucleotide-binding oligomerization domain (NOD). The innate immune responses in odontoblasts initiated by sensing oral pathogens provide host protective events, such as inflammatory reactions, to produce a variety of pro-inflammatory mediators, including chemokines and cytokines. These attract various inflammatory cells and cause antibacterial reactions, such as the production of defensins, to kill microorganisms in the proximal region of the odontoblast layer. This review focuses on innate immunity, especially cellular and molecular mechanisms regarding the sensing of PAMPs from oral pathogens by PRRs, in odontoblasts and provides information for future studies for the development of novel therapeutic strategies, including diagnosis and treatment, to prevent exceeding dental pulp inflammation and preserve the dental pulp tissues.
Keywords: Odontoblast, Pattern recognition receptor, Pathogen-associated molecular pattern, Inflammation, Pro-inflammatory mediator, Damage-associated molecular pattern
1. Introduction
Odontoblasts have a long cell process extended to the dentinal tubule and their cell bodies are located on the surface of the dental pulp. Odontoblasts also form a layer along the interface between the dental pulp tissue and dentin, and they function as a natural barrier between mineralized tissues, dentin, and soft tissues, dental pulp, of the vital tooth [1]. Therefore, odontoblasts are the primary biologically active cells that maintain the dentin and protect the living pulp tissue by the deposition of reactionary dentin in response to mild stimulation with bacterial products at an early stage of dental caries and are involved in innate and adaptive immunity of the dental pulp to combat invading bacteria. The dental pulp under this odontoblast layer is a vascularized tissue with a dense capillary plexus. When bacteria and their products invade deeply into dentinal tubules, odontoblasts are the first pulpal cells encountered by these dentin-invading microorganisms and sense pathogen-associated molecular patterns (PAMPs) shared by microorganisms through specialized pattern recognition receptors (PRRs) at the dentin-pulp interface. This triggers host-protective events, such as inflammatory reactions, to produce a variety of pro-inflammatory mediators, including chemokines and cytokines. These attract various inflammatory cells and antibacterial reactions, such as the production of defensins, to kill microorganisms in proximity to the odontoblasts by initiating innate immune responses. When low-grade inflammation occurs, odontoblasts act to promote regenerative mechanisms through angiogenesis and dentinogenesis pathways and to increase pulp defense capability, finally leading to reactionary dentin formation. However, when intense and/or prolonged inflammation are occurs and dentin regenerative processes are blocked, copious amounts of pro-inflammatory mediators are produced from odontoblasts and infiltrating inflammatory cells activate various molecular and cellular signaling pathways that lead to the breakdown of dental pulp tissue [2]. This review focuses on the roles of odontoblasts in the innate immunity of dental pulp tissues, especially the expression profiles and functions of PRRs expressed in odontoblasts and the innate immune responses of odontoblasts triggered by the interaction between these PRRs and PAMPs shared by microorganisms.
2. Pattern recognition receptors (PRRs), their ligands and pathogen-associated molecular patterns (PAMPs)
The innate immune system is the major contributor to acute inflammation and effective defense induced by microbial infection, and it is also important in activating acquired immune responses [3], [4]. Generally, the rapid initial sensing of invading microbial pathogens is mediated by specialized PRRs for PAMPs, which are structures conserved among microbial species. Recent studies have demonstrated that PRRs also recognize damage-associated molecular patterns (DAMPs), which include endogenous molecules released from damaged cells [4]. PRR families can be divided into 4 different classes including Toll-like receptors (TLRs), nucleotide-oligomerization binding domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs), and retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) (Table 1). TLRs and CLRs localize to the plasma membrane or endolysosome, whereas NLRs and RLRs are cytoplasmic proteins and serve as a second line of defense against pathogens that have evaded cell-surface-associated or endolysosomal PRRs. These receptors are expressed at different levels in various tissues, and they activate different signaling pathways and host immune responses after engagement with their ligands.
Table 1.
PRRs, their localization, ligands and origins.
| PRRs | Localization | Ligand | Origin of the ligand | |
|---|---|---|---|---|
| TLR | TLR1 | Plasma membrane | Triacyl lipoprotein | Bacteria |
| TLR2 | Plasma membrane | Lipoprotein | Bacteria, viruses, parasites, self (DAMPs) | |
| HMGB1 | ||||
| TLR3 | Endolysosome | dsRNA, PolyI:C | Virus | |
| TLR4 | Plasma membrane | LPS, Lipid A | Bacteria, viruses, self (DAMPs) | |
| HMGB1 | ||||
| TLR5 | Plasma membrane | Flagellin | Bacteria | |
| TLR6 | Plasma membrane | Diacyl lipoprotein | Bacteria, viruses | |
| TLR7/8 | Endolysosome | ssRNA, imiquimod, imidazoquinoline, loxoribine, R-848 | Bacteria, viruses, self (DAMPs) | |
| TLR9 | Endolysosome | CpG-DNA | Bacteria, virus, protozoa, self (DAMPs) | |
| TLR10 | Endolysosome | Unknown | Unknown | |
| TLR11 | Plasma membrane | Profilin-like molecule | Protozoa | |
| NLR | NOD1 | Cytoplasm | iE-DAP | Bacteria |
| NOD2 | Cytoplasm | MDP | Bacteria | |
| NLRC3 | ||||
| NLRC4 | Cytoplasm | Flagellin | ||
| NLRC5 | Cytoplasm | |||
| CLR | Dectin-1 | Plasma membrane | β-Glucan | Fungi |
| Dectin-2 | Plasma membrane | α-Mannan | Fungi | |
| MINCLE | Plasma membrane | TDM, SAP130 | Fungi, self (DAMPs) | |
| RLR | RIG-I | Cytoplasm | Short dsRNA, 5′ triphosphate dsRNA | RNA, Viruses, |
| DNA virus | ||||
| MDA5 | Cytoplasm | Long dsRNA | RNA viruses | |
| LGP2 | Cytoplasm | Unknown | RNA viruses | |
| NLRP | NLRP1-14 | Cytoplasm | Silica, asbestos, ATP, uric acid, beta-amyloid, cholesterol crystal | |
| NLRP3 | ||||
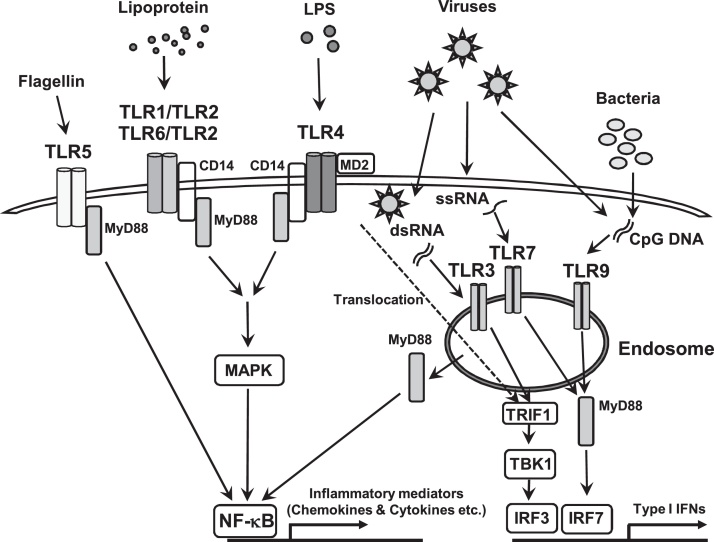
The best-characterized human PRR family is the TLRs. To date, 10 TLRs have been identified in humans and 13 in mice. TLRs were first identified as PRRs for various molecules derived from pathogens, such as bacteria, fungi, viruses, and parasites, and they form heterodimers, including TLR1/TLR2 and TLR6/TLR2, or homodimers, such as TLR3-TLR3 [5]. All TLRs are single-spanning type I transmembrane proteins with an ectodomain composing of leucine-rich repeat motifs that mediate the recognition of PAMPs, a transmembrane domain, and an intracellular Toll–interleukin 1 (IL-1) receptor (TIR) domain, which contains the sites to maintain dimeric interactions between TLR subunits and to recruit cytoplasmic adaptor proteins required for downstream signal transduction [5], [6]. TLRs are localized differentially within the cells. TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed on the plasma membrane to sense components of bacteria and fungi present in the extracellular environment, whereas TLR3, TLR7, TLR8, and TLR9 are present on endosomal membranes in intracellular compartments to sense nucleic acids from microorganisms [7] (Fig. 1). TLR2 is crucial for sensing various components from bacteria, such as lipoteichoic acid (LTA), which is one of the cell wall components of Gram-positive bacteria, lipopeptides, and peptidoglycan, as well as mycoplasma, fungi, and viruses, and it recognizes its ligands by forming a heterodimer with either TLR1 or TLR6 [8]. These heterodimer complexes, TLR1/TLR2 and TLR6/TLR2, recognize distinct ligands comprising triacetylated and diacetylated lipoproteins, respectively. TLR4 recognizes lipopolysaccharide (LPS), the major outer membrane component of Gram-negative bacteria [8], mediated by myeloid differentiation factor 2 (MD2) on the cell surface in association with LPS binding protein (LBP) and CD14 [4]. TLR5 recognizes flagellin from flagellated motile bacteria. TLR3, TLR7, and TLR8 sense RNA from viruses, and TLR9 binds to unmethylated cytidine-phosphate-guanosine (CpG) DNA derived from bacteria and is cleavaged by cellular proteases to activate downstream signal transduction pathway [6]. The engagement of most TLRs with their specific ligands activates a mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) via the myeloid differentiation primary response 88 (MyD88)-dependent pathway to produce pro-inflammatory mediators, however, TLR4 translocates to the endosome and TLR3 activates TANK-binding kinase 1 (TBK1) via a TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent pathway and then TBK1 phosphorylates interferon (IFN)-regulatory factor (IRF)3, which translocates into the nucleus to produce type I IFNs and pro-inflammatory cytokines [6], [7]. TLR7 and TLR9 can also activate NF-κB to induce pro-inflammatory cytokines and chemokines [6].
Figure 1.
Toll-like receptor (TLR) signaling pathways. TLR1, TLR2, TLR4, TLR5 and TLR6, are expressed on the plasma membrane to sense the components of bacteria and fungus present in extracellular environments, whereas TLR3, TLR7, TLR8 and TLR9 are present on endosomal membranes in intracellular compartments to sense nucleic acids from microorganisms. TLR engagement activation recruites some adaptoer molecules, such as the myeloid differentiation primary response 88 (MyD88), and then activates mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) to produce pro-inflammatory mediators and in another way, TLR4 translocated to the endosome and TLR3 activate TANK-binding kinase 1 (TBK1) via TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent pathway and then TBK1 phosphorylates interferon (IFN)-regulatory factor (IRF)3, which translocate into the nucleus to produce type I IFNs. TLR5 recognizes flagellin and activates NF-κB through MyD88. TLR 7 and TLR9 activations lead to the phosphorylation of IRF7 via MyD88-dependent pathway and phosphorylated IRF7 also translocates into the nucleus to up-regulate the expression of type I IFNs. TLR7 and TLR9 can also activate NF-κB to induce pro-inflammatory cytokines and chemokines.
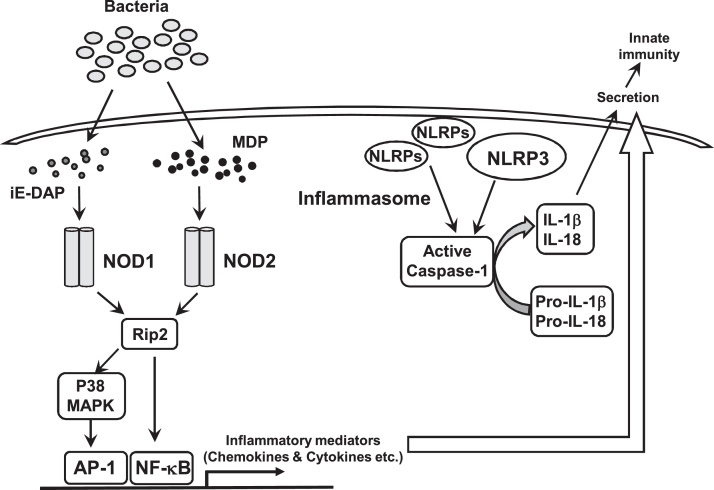
Although many NLRs are not still fully characterized, NOD proteins, NOD1 and NOD2, which are also known as caspase recruitment domain-containing protein 15 (CARD15), are two well-characterized NLR family members that sense fragments conserved in the cell wall of many types of bacteria [9]. NOD1 and NOD2 respectively detect γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP), a motif present in many Gram-negative bacteria and certain Gran-positive bacteria, and muramyl dipeptide (MDP), a peptidoglycan motif widely distributed in the cell walls of both Gram-positive and -negative bacteria. Both NODs can activate NF-κB signaling via Rip2, also known as receptor-interacting protein kinase 2 (RIPK2), to produce pro-inflammatory cytokines, such as IL-1β [10] (Fig. 2). Among NLR-related proteins (NLRPs), NLRP3, known as cryopyrin or NALP3, which was identified as a member of the intracellular NLR family, mediates immune responses to various PAMPs of cytosolic pathogens, such as LPS, MDP, and bacterial and viral RNA, including poly I:C, a dsRNA analog, as well as the imidazoquinoline antiviral compounds [10], and danger signals [11], [12]. NLRP3 assembles a large multiprotein complex named the inflammasome in the presence of microbial components, thereby leading to the activation of caspase-1 and subsequent processing and secretion of the pro-inflammatory cytokines, IL-1β and IL-18 (Fig. 2).
Figure 2.
Functions of NOD-like receptors (NLRs). NOD1 and NOD2 sense intracellular γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), respectively, and then activate NF-κB and/or AP-1 via MAPK signaling pathwys. NLRs, such as NLRP3, activated by various microbial or endogeneous molecules, including PAMPs and DAMPs, in the intracellular compartment form inflammasomes, which is a large multiprotein complex, and lead to the activation of caspase-1 and subsequent processing of the pro-inflammatory cytokines precursors, pro-IL-1β and pro-IL-18, and secretion of those mature pro-inflammatory mediators.
CLRs can sense the structures of carbohydrates, such as mannose, fucose, and glucan, present in bacteria, fungi, and viruses, activating distinct signaling pathways to induce the expression of specific cytokines and regulate both innate and adaptive immune responses. Moreover, some CLRs can induce signaling pathways that directly activate NF‐κB, but other CLRs affect signaling by TLRs, suggesting that CLR and TLR signaling includes cross-talk between these pathways [13].
RLRs are present in the cytoplasm and sense RNA viruses. After the engagement of RLRs, transcription factors, such as NF-κB, are activated to express IFN-α/β, which plays a role in anti-viral defense by triggering the expression of antiviral proteins and promoting the destruction of infected cells [14].
3. Innate immune responses to dental caries
Oral bacterial invasion accompanying the progression of dental caries as well as the physical and chemical stimuli to the exposed dentinal surface sequentially initiates acute and chronic activation of the innate immune responses in dentin-pulp complex to develop pulpitis [15], [16]. As the carious infection progresses to the pulp-dentin interface, changes occur in the microflora, characterized by a decrease in the proportion of Gram-positive aerobic bacteria and an increase in that of Gram-negative anaerobic bacteria [17]. Therefore, as the environment at deep carious lesion becomes more anaerobic, the polymicrobial infections become more comlex with a high bacterial diversity. Odontoblasts located in the outermost layer in dental pulp first recognize caries-related pathogens, such as Streptococcus mutans, which frequently dominates shallow lesions and has a major impact on the initial lesion and pulpal pathology, and play important roles in the innate immune system of dental pulp tissues [18], [19], [20]. Given a transition from Gram-positive aerobic bacteria in early caries to anaerobic Gram-negative bacteria in deep carious lesions, odontoblasts are also required to possess some weapons to fight various Gram-negative bacteria. In addition to odontoblasts, as the caries progresses following by the initial invasion of oral microbes, various resident pulpal cells including pulpal fibroblasts, stem cells, endothelial cells and dendritic cells, and infiltrated immunocompetent cells orchestrate the rapid inflammatory response by detecting bacterial components, PAMPs, via PRRs. Numerous inflammatory mediators, such as chemokines and cytokines, produced from these cells, are also involved in the initial pulpal immune reactions to dental caries through molecular and cellular signaling triggered by autocrine and paracrine actions [16], [21].
4. Pattern recognition receptors (PRRs) in odontoblasts
Several PRRs, including TLRs and NODs, are expressed in odontoblasts from humans and rodents, including mouse [22] and rat [18], [23] (Table 2). However, the detected expression patterns of PRRs seem to be different depending on the analysis methods, origins and human donors.
Table 2.
PRRs in odontoblasts.
| PRRs | Origin | Methods | References | |
|---|---|---|---|---|
| TLR | TLR1 | Human | Real-time PCR | [24] |
| TLR2 | Human | Real-time PCR | [24] | |
| RT-PCR & IHC | [31] | |||
| Real-time PCR | [25] | |||
| Real-time PCR & flow cytometry | [32] | |||
| Immunohistochemistry | [27] | |||
| Mouse | Immunohistochemistry | [22] | ||
| TLR3 | Human | Real-time PCR | [24] | |
| Real-time PCR | [25] | |||
| Real-time PCR & DNA microarray | [33] | |||
| TLR4 | Human | Real-time PCR | [24] | |
| RT-PCR, western blotting & immunohistochemistry | [26] | |||
| RT-PCR & immunohistochemistry | [31] | |||
| Real-time PCR | [25] | |||
| Real-time PCR & flow cytometry | [32] | |||
| Immunohistochemistry | [30] | |||
| Mouse | Immunohistochemistry | [22] | ||
| TLR5 | Human | Real-time PCR | [24] | |
| TLR6 | Human | Real-time PCR | [24] | |
| TLR7 | Human | Real-time PCR | [33] | |
| TLR8 | Human | Real-time PCR, DNA microarray & immunohistochemistry | [33] | |
| TLR9 | Human | Real-time PCR | [24] | |
| Real-time PCR & DNA microarray | [33] | |||
| Mouse | Real-time PCR | [34] | ||
| Rat | RT-PCR | [23] | ||
| TLR10 | ||||
| NLR | NOD1 | Human | Immunohistochemistry | [35] |
| Rat | Flow cytometry | [18] | ||
| NOD2 | Human | Real-time PCR & flow cytometry | [37] | |
| Human | RT-PCR & immunohistochemistry | [36] | ||
| NLRP3 | Human | RT-PCR, western blotting & immunohistochemistry | [38] | |
| Immunofluorescence staining | [39] | |||
4.1. Toll-like receptors (TLRs)
In vitro-differentiated odontoblasts constitutively expressed TLR1-6 and TLR9 genes, but not TLR7, TLR8, or TLR10, and demonstrated that LTA, an TLR2 ligand, triggers the activation of odontoblasts and up-regulates the expression of its own receptor, TLR2, as well as the production of several chemokines [24]. These findings suggested that odontoblasts can sense a variety of PAMPs, including triacetylated and diacetylated lipoproteins (by TLR1/TLR2 and TLR6/TLR2), viral dsRNA (via TLR3), LPS (via TLR4), flagellin (via TLR5), and unmethylated CpG motif-containing DNA (via TLR9) and fight various pathogens invading the tooth. Staquet et al. demonstrated using real-time polymerase chain reaction (PCR) that odontoblast-like cells express TLR2, TLR3 and TLR4 by culturing pulp explants from healthy non-erupted human third molars [25], and Jiang et al. also showed the expression, production, and distribution of TLR4 in odontoblasts by reverse transcription (RT)-PCR, western blotting, and immunohistochemistry [26]. Regarding the expression level of TLR2 in odontoblasts, Farges et al. reported that TLR2, as well as CCL2 and CXCL1, were up-regulated in odontoblast located under caries lesions, whereas that under healthy dentin was not [27]. Odontoblast TLR2 functionally activated by LTA up-regulates the levels of the TLR2 gene and protein as well as chemokines, such as CCL2 and IL-8, via the induction of nuclear translocation of NF-κB [25], [27], [28] and the recruitment of immature dendritic cells [24], [25], but it does not increase TLR4-mediated odontoblast responses [29]. Of note, TLR2 engagement with LTA also up-regulates the expression of TLR3, TLR5, and TLR9 to a lesser extent [24]. These findings suggested that the up-regulation of TLR2 in odontoblasts by the recognition of Gram-positive bacteria augments their innate immune responses to a wide variety of by-products from oral pathogens in the pathological process of pulpitis. Recently, an immunohistochemical study by Liu et al. showed that TLR4 expressed in the odontoblast layer as well as areas that colocalize with blood vessels had greater staining in teeth with deep caries than in healthy teeth, suggesting that TLR4 in odontoblasts can be activated during microorganism invasion [30]. Veerayutthwilai et al. established organotypic tooth crown cultures to maintain human odontoblasts within their dentin scaffold and demonstrated that TLR4 was immunolocalized in human odontoblasts and their dentinal processes in situ. In addition, cells in the odontoblast layer expressed mRNAs for various innate immunity markers, such as TLR2 and TLR4, as well as the odontoblastic marker dentin sialophosphoprotein (DSPP). They also showed that odontoblasts express PRRs in situ, thus allowing differential responses to Gram-positive and -negative bacteria, suggesting that pro-inflammatory cytokines and innate immune responses in decayed teeth may result from TLR4 signaling [31]. Furthermore, Horst et al. demonstrated that odontoblast-like cells differentiated from pulp progenitor cells show heterogeneity in their TLR2 and TLR4 cell-surface expression. These variations were significantly affected by the odontoblast response to oral bacteria, including periodontitis-related pathogens Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, which mainly activate TLR4 signaling in these cells [32]. Besides TLR2 and TLR4, another previous study using DNA microarrays and quantitative PCR reported that TLR3, TLR7, TLR8, and TLR9 mRNAs were detected both in odontoblasts and pulp tissues. However, TLR8 expression levels were higher in odontoblasts, suggesting that TLR8, which can recognize viral ssRNA, expressed in odontoblasts participates in innate immune responses of the dentin-pulp complex [33]. Mouse and rat odontoblast-like cell lines also constitutively expressed TLR9 [23], [34].
4.2. Nucleotide-oligomerization binding domain (NOD) receptors
Weak NOD1 expression in human dental pulp tissues without caries is observed in the cytoplasm of odontoblasts as well as vascular endothelial cells. Of note, significantly up-regulation of NOD1 expression in the odontoblast layer was found in specimens with carious lesions, and positive expression of NOD1 in the odontoblastic layer of radicular dental pulp distant from the coronal carious lesion was also observed [35]. A recent in vitro study demonstrated that NOD1 expressed in odontoblasts also transmits signals to the nucleus via the p38-AP-1 pathway and suggested that NOD1 may play important roles in the initiation and progression of pulpitis [18]. NOD2 mRNA and protein were present in normal human dental pulp tissues, with most NOD2 protein expression being localized to odontoblasts and some pulp vascular endothelial cells. In contrast, human dental pulp cells (HDPCs) showed a low level of NOD2 protein expression [36]. Another study reported that significant up-regulation of NOD2 gene as well as tumor necrosis factor (TNF)-α occurs in acute inflamed human dental pulps compared to healthy ones. In particular, a study confirmed that TLR2 ligands, including LTA and Pam3CSK4, up-regulate the levels of NOD2 gene expression and protein levels in odontoblast-like cells, suggesting interplay between the NOD2 and TLR2 pathways [37], with Pam2CSK4, a TLR2/TLR6 ligand, having a more potent effect on up-regulating NOD2 expression than Pam3CSK4, which is a TLR1/TLR2 ligand [29]. Therefore, NOD2 up-regulation is part of the odontoblast immune response to Gram-positive bacteria, which gain access to the dental pulp from infected dentin during the caries process and might play important roles in protecting dental pulp from the harmful effects of cariogenic pathogens [37].
4.3. Nucleotide-oligomerization binding domain-like receptor-related protein 3 (NLRP3)
Among NLRPs, a previous study demonstrated that the mRNA expression and protein production of NLRP3 are detected in normal human dental pulp tissues, and this protein is mainly expressed in odontoblasts as well as pulp vascular endothelial cells [38]. A recent immunofluorescence staining study also reported that NLRP3 and caspase-1 showed positive staining in the odontoblast layer of normal dental pulp tissue. In addition, extensive staining of NLRP3 and caspase-1 was found in a disrupted odontoblastic layer of tissue derived from irreversible pulpitis, suggesting that the NLRP3/caspase-1 pathway may play an important biological role in the innate immune response of human dental pulp [39].
5. Innate immune responses in odontoblasts and subodontoblast area
As described above, the binding with bacterial ligands, PAMPs, to PRRs including TLRs and NODs expressed by odontoblast, activates key intracellular signaling pathways involving MAP kinases, such as p38 MAPK, and transcription factors including NF-κB and AP-1. Activated odontoblasts secrete pro-inflammatory and immunomodulatory mediators, such as chemokiens and cytokines, which diffuse into subodontoblast pulp area. Therefore, these mediators activate adjacent innate immune cells, including pulpal fibroblast, stem cells and dental pulp tissue-resident immune cells, and also up-regulate and attract various populations of immune effector cells towards the dentin-pulp interface and beneath the odontoblast layer [40]. In subodontoblat pulp area, the activated adjacent innate immune cells also extracellularly produce chemokines and pro-inflammatory cytokines to recruit more immune/inflammatory cells and activate resident cells in tissues [41]. With regard to cellular and/or molecular mechanisms in subodontoblast area, these secreted mediators have potent autocrine and paracrine inflammatory actions and generate more intricate signaling network leading to amplification of the inflammatory response within dental pulp tissue [40].
5.1. Chemokines and cytokines regulated by pattern recognition receptor (PRR) signaling
Farges et al. reported that odontoblast-like cells produce the pro-inflammatory cytokines IL-6 and IL-8 as well as the immunosuppressive cytokine IL-10, in response to TLR2 agonists, LTA and Pam3CSK4. However, granulocyte-macrophage colony-stimulating factor, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-7, IL-12(p70), IL-13, and TNF-α were not detected or only at extremely low concentrations, suggesting that TLR2 activation in human odontoblasts selectively induces the production of mediators known to positively or negatively influence inflammatory and immune responses in pathogen-challenged dental pulp tissues. Therefore, these molecules may be important in regulating the fine tuning of the pulp response to Gram-positive bacteria that enter dentin during the caries process [42]. A similar study showed that IL-8 and TNF-α are up-regulated in odontoblast-like cells upon LTA stimulation [28]. Another study showed that an increased amount of CCL2 and CXCL10 was detected in the supernatants from LTA-stimulated odontoblasts, and these supernatants augmented the migration of immature dendritic cells in vitro compared with controls. The clinical relevance of these observations came from immunohistochemical analysis showing that CCL2 was expressed in vivo by odontoblasts and blood vessels present under active carious lesions but not in healthy dental pulps. In contrast with this inflammatory response, gene expression of major dentin matrix components (type I collagen and DSPP) and transforming growth factor (TGF)-β1, a known inducer of dentin formation, was sharply down-regulated in odontoblasts by LTA. Taken together, these data suggest that odontoblasts activated through TLR2 by Gram-positive bacterial LTA are able to initiate an innate immune response by secreting chemokines that recruit immature dendritic cells, which accumulated locally in the para-odontoblastic region corresponding to the pulpal end of carious dentinal tubules as a rapid, active response to carious stimuli [43], while down-regulating their specialized functions of dentin matrix synthesis and mineralization [24].
Bacterial DNA, as a widely recognized PAMP, is characterized by a high content of unmethylated CpG motifs and has immunostimulatory activity [44]. He et al. demonstrated that CpG DNA induces pro-inflammatory responses to up-regulate IL-6, IL-8, and TNF-α in a rat odontoblast-like cell line via the TLR9/MyD88/NF-κB signaling pathway, suggesting that CpG DNA-mediated immune responses in odontoblasts play an important role in the dental pulp defense system and/or the process of dental pulp inflammatory progression [23], [45].
As to the modulating factors for TLR activation, a recent study showed that LBP, which is able to bind LPS, is clearly present in most pulp cells, including odontoblasts in inflamed pulp tissues, but it was not detected in healthy pulp. LBP reduced the TLR2-dependent production of inflammatory cytokines as well as TLR2 expression in odontoblast-like cells by inhibiting the p38 and NF-κB signaling pathways [46], suggesting that LBP might inhibit LTA binding to CD14 and/or the interaction of CD14/LTA with TLR2 expressed on odontoblasts. Given the fact that dentin formation is down-regulated in odontoblast-like cells by TLR2 activation [24], LBP might be useful to protect dental pulp against cariogenic microorganisms and modulate host defense in human dental pulp. Regarding other accessory receptors of TLR2, it has been suggested that CD36, which is a member of the scavenger receptor type B family and recognizes oxidized LDL particles, acts as a facilitator or co-receptor for di-acylglyceride recognition through the TLR2/TLR6 complex in response to LTA. Therefore, CD36 serves a function analogous to CD14, which activates LPS signal via TLR4 pathway [47]. Staquet et al. also reported that LTA can slightly but significantly up-regulate CD36 expression [29].
Regarding the interaction with NOD2 and other PRRs, recent studies showed that NOD2 plays roles in NLRP1 inflammasome assembly and caspase-1 activation to secrete IL-1β because of its direct interaction with pro-caspase-1 and NLRP1 [48], [49]. However, NLRP3 and caspase-1-dependent mechanisms are not involved in the synergistic effect on IL-1β production between NOD2 and TLR4 [50]. An in vitro study using odontoblast-like cells demonstrated that LTA, a TLR2 ligand, up-regulates NOD2 gene expression and protein levels and this increase is higher in odontoblast-like cells than that in dental pulp fibroblasts [37]. These findings suggested that odontoblasts have modulatory mechanisms to sense microorganisms and provide complex innate immune responses, such as crosstalk between some PRR pathways, against bacterial invasion.
5.2. Antimicrobial effects regulated by pattern recognition receptor (PRR) signaling
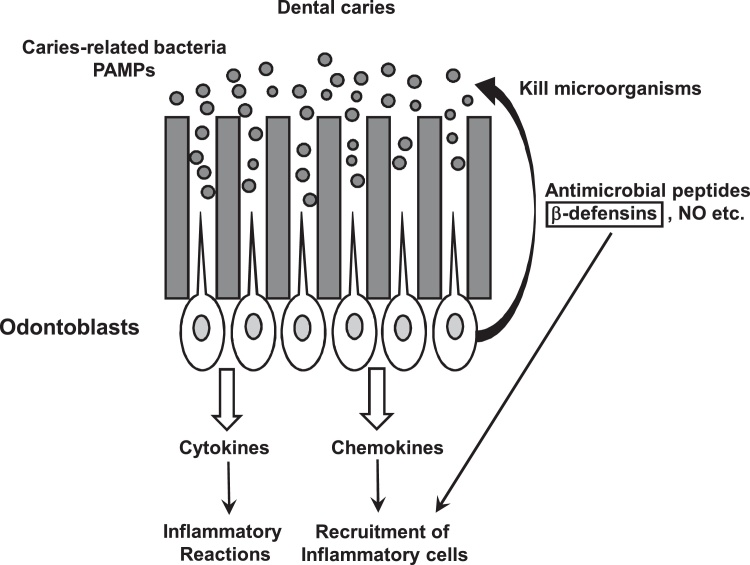
In addition to these pro-inflammatory mediators, small, cationic, broad-spectrum antimicrobial peptides, such as defensins, are considered to be important antibiotic-like effectors of innate immunity and are induced in odontoblasts [31], [40], [51], [52]. These peptides form channel-like micropores, to disrupt the membrane integrity and then induce leakage of the cell content to kill microbes, such as bacteria, viruses, parasites, and fungi [53], [54]. Human defensins are classified into 3 types, α-, β- and θ-defensins, depending on the spacing between the cysteine residues and the topology of the disulfide bridges [55]. In addition to displaying potent antimicrobial properties in innate immunity, β-defensins also play a role in adaptive immunity [56]. Among 3 types, the most studied β-defensins in the human genome are human β-defensins (hBDs)-1, -2, -3 and -4, which are expressed by odontoblasts and in the dental pulp [31], [52], [57]. These hBDs have been shown to possess antibacterial activity against caries-related microorganisms found in mature biofilms [58], [59], [60], [61] and also to play important roles in pulpal defense [40] (Fig. 3). Previous in vivo studies using RT-PCR and immunohistochemistry demonstrated that hBD-1 is distinctly expressed in the cytoplasm of odontoblasts in caries-free teeth, but the cytoplasmic expression level of hBD-2 in the odontoblast layer is weak [52], [57]. Another study showed that tooth crown odontoblast ex vivo cultures had differential responses to PRR-specific agonists, Pam3CSK4 for TLR2 and Escherichia coli LPS for TLR4 [31], and demonstrated that TLR4 activation by LPS up-regulated hBD-2 as well as IL-1β, TNF-α, CCL20, IL-8, TLR2, and TLR4. However, TLR2 activation by Pam3CSK4 could not modify hBD-2 gene expression and down-regulated hBD-1 and -3 mRNA levels, suggesting that pro-inflammatory cytokines and innate immune responses in decayed teeth may result from TLR4 signaling. Thus, hBDs produced in odontoblasts are differentially regulated through TLRs by Gram-positive and −negative bacteria. Besides their microbicidal activity, BDs also play a role in recruiting T cells, immature dendritic cells, monocytes, and macrophages at lower concentrations than required for their microbicidal activity, suggesting that they have a functional overlap with chemokines [51]. Conversely, some chemokines have defensin-like microbicidal activities. For example, a C-terminal truncated version of neutrophil-activating protein 2 (NAP-2)/CXCL7 in platelets, platelet factor 4 (PF4)/CXCL4, macrophage inflammatory protein (MIP)-3α/CCL20, monokine induced by IFN-γ (Mig)/CXCL9, IFN-inducible protein (IP)-10/CXCL10, and IFN-inducible T cell α-chemoattractant (I-TAC)/CXCL11 have microbicidal activities [62], [63]. Of note, hBDs can also up-regulate the expression of chemokines in cells. Odontoblast-like cells stimulated with rhBD-2 down-regulated the gene expression of hBD-1, but showed up-regulation of the mRNA expression levels of IL-6 and IL-8 [64]. Regarding wound healing, a previous study showed that hBD-2 significantly increases the mRNA expression level of DSPP, a tooth-specific protein expressed mostly by odontoblast cells, and osteopontin. This finding suggested that hBD-2 has the ability to stimulate odontoblast differentiation in addition to the traditional immune regulatory roles in inflammation and has potential in the removal of bacteria from infected dental pulp tissues [58].
Figure 3.
Potential functions of hBDs in response to caries-related pathogens and their PAMPs. Once the PRRs expressed in odontoblatsts sense caries-pathogens and their PAMPs diffused into dentinal tubules, odontoblasts produce and release antimicrobial peptides, such as defensins and NO, which kill microbes, such as bacteria, viruses, parasites and fungi. BDs are also important antibiotic-like effectors of innate immunity and induce the production of chemokines to attract inflammatory cells to the infection site.
Based on the antimicrobial effects on the growth and survival of cariogenic bacteria, such as S. mutans [65], the another antibacterial agent nitric oxide (NO) produced by odontoblasts has also received special attention [66]. NO is a highly diffusible free radical produced by NO synthases (NOSs), which use molecular oxygen and L-arginine as substrates to produce NO and citrulline [67]. Three isoforms of NOS have been identified. In general, endothelial NOS (eNOS) and neuronal NOS (nNOS) are constitutively expressed in various cells in physiological conditions, but an inducible NOS (iNOS) plays roles in host defense [68], [69], [70], [71], [72]. Previous research has shown that the mRNA and protein levels of iNOS are enhanced in acute inflammatory dental pulp, but not in healthy pulp tissue, and iNOS immunopositivity can be observed in the external stratum of the pulp, in the odontoblasts as well, mainly near accumulated leukocytes [73]. Another quantitative immunohistochemical study also reported that only single odontoblasts were weakly positive for iNOS in healthy dental pulp, but higher signal intensities of iNOS were detected in odontoblasts of inflamed dentin-pulp complex in comparison with healthy odontoblasts [74]. These different expression levels and localization of NOSs suggested that eNOS maintains pulpal homeostasis, whereas iNOS plays a role only in inflammatory pathological processes of dental pulp. Regarding NO production in response to PRRs activation in odontoblasts, iNOS protein synthesis and activity were augmented in odontoblasts that differentiated in vitro in response to stimulation with TLR2 ligand, and extracellularly released NO reduced S. mutans growth [66]. These findings suggested that antimicrobial molecules, such as hBDs and NO, may be useful to combat intradentinal invading caries-related microorganisms and as agents for dental pulp healing and regeneration.
5.3. Pulp wound healing and regeneration through pattern recognition receptors (PRRs)
TGF-β family proteins play various roles in the formation and repair of the dentin-pulp complex as well as odontoblast differentiation [75], [76], [77], [78], Horst et al. reported that TGF-β1 inhibits TLR2 and TLR4 expression and attenuates odontoblast responses, suggesting that the balance between TLR-mediated inflammation and TGF-β1 anti-inflammatory activity plays an important role in pulpal inflammation [32].
With respect to the mechanism of pulp wound healing and regeneration through PRRs, Nomiyama et al. showed that: alkaline phosphatase (ALP) activity; the expression of DSPP, runt-related transcription factor 2 (Runx2), and osteocalcin; and the extracellular formation of mineralized nodules of rat odontoblastic cell line are suppressed in an LPS dose-dependent manner, suggesting that Gram-negative bacterial infection might down-regulate odontoblast function via the PRR pathways of the innate immune response [79]. Taken together, after recognizing caries-related pathogen by PRRs, odontoblasts down-regulate their characteristic functions, such as dentin matrix synthesis, and mineralization but instead initiate innate immune responses, including chemokine secretion to recruit immature dendritic cells [80].
5.4. The interaction between damage-associated molecular patterns (DAMPs) and pattern recognition receptors (PRRs) in odontoblasts
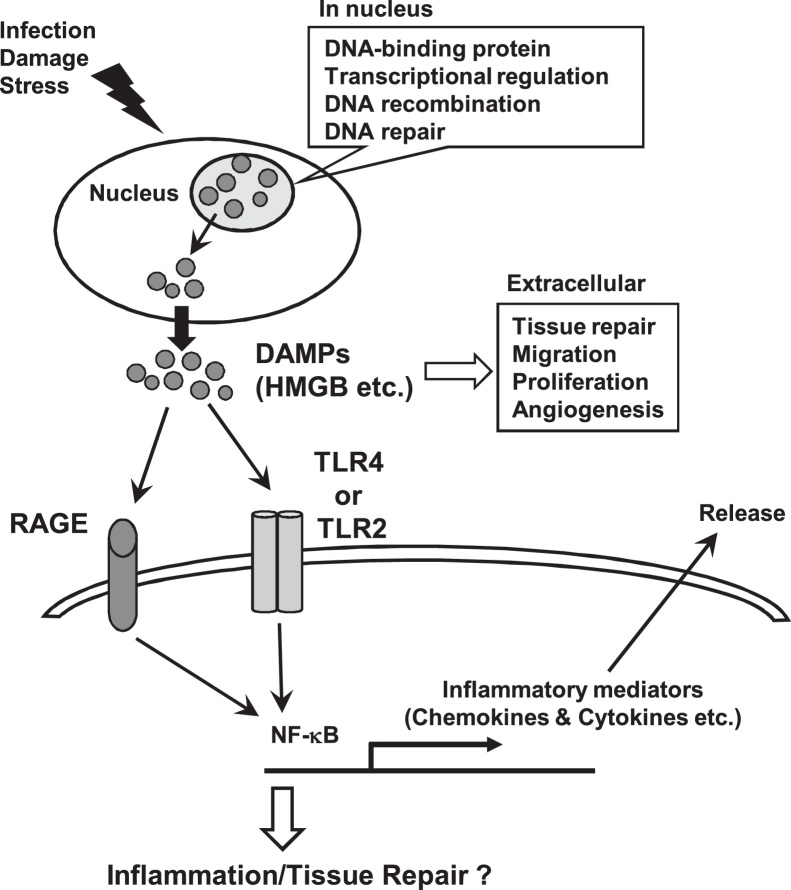
Immune cells are recruited by chemokines up-regulated through PRR activation to dental pulp tissues, and their antimicrobial activities can also trigger significant collateral host tissue damage [16]. In particular, during dental pulp inflammation, these immune cells produce proteases, such as metalloproteinase, as well as reactive oxygen species (ROS) and various enzymes to combat invading pathogens. However, these molecules can also cause damage to dental pulp cells and lead to host cell death. Some intracellular molecules released from dying cells can work as damage signals, known as damage-associated molecular patterns (DAMPs), to exacerbate inflammatory responses and activate innate immunity to stop damage [81]. Many DAMPs, such as high mobility group box 1 (HMGB1), histones, ATP, heat shock proteins (HSPs), S100s, and uric acid crystals, have been identified and their roles and receptors have been investigated. Some DAMPs can bind TLRs to induce cellular activation and trigger the inflammatory responses. Among DAMPs, HMGB1 is a DNA-binding protein with various biological roles including transcriptional regulation, DNA recombination and its repair in nucleus [82]. HMGB1 can bind to the receptor for advance glycation endproducts (RAGE) and also interact with TLR2 and TLR4 to activate NF-κB via these signaling pathways to participate in inflammatory diseases [83], [84] (Fig. 4). Extracellular HMGB1 can accelerate the delivery of CpG DNA to TLR9, and TLR9 signaling activated by HMGB1-DNA complexes can lead to the secretion of cytokines [85], [86]. These findings suggested that different signaling pathways, such as the interplay between the RAGE and TLR signaling pathways, may play a crucial role in NF-κB activation [87]. HMGB1 is expressed in 98% of the HDPCs, especially at low levels in both the nucleus and cytoplasm of odontoblasts in human healthy dental pulp tissue. However, its expression levels increased significantly in both the cytoplasm and nucleus in odontoblasts from inflamed pulp tissues compared with healthy tissues [88], [89]. The expression levels of its receptor, RAGE, have been shown to be up-regulated in clinically inflamed dental pulp tissues, especially in odontoblast, compared with healthy dental pulp tissues [90]. A recent in vitro study has shown that LPS stimulation dose-dependently up-regulated both HMGB1 and RAGE in odontoblast-like cells [90]. It has also been shown that extracellular HMGB1 secreted from cells can up-regulate ALP, dentin matrix protein-1 (DMP-1), DSPP, and RAGE, and thereby promoting the odontoblastic proliferation and odontoblast differentiation of HPDCs as well as mineralized nodule formation [88]. Moreover, extracellular HMGB1 induces the migration of HPDCs in a dose-dependent manner [89]. Collectively, HMGB1 is also involved in the recruitment of HPDCs, which promote the repair and regeneration of dental pulp tissues, suggesting that HMGB1 and PRRs, including TLR and RAGE, play important roles in the dental pulp immune response triggered by oral pathogens.
Figure 4.
Potential effetcs of HMGB1 on odontoblasts. In nucleus, HMGB1, a DNA-binding protein, plays important biological roles including transcriptional regulation, DNA recombination and its repair. In response to infection, damage and stress, HMGB1 is passively released by dead cells or actively secreted by stressed cells. Extracellular HMGB1 binds to the receptor for advance glycation endproducts (RAGE), TLR2 and TLR4 and then activates NF-κB to secrete cytokines and chemokines and the process of tissue repair.
5.5. Sensory role of odontoblasts for external stimuli
In addition to the defense system of odontoblasts as the first barrier of dentin-pulp complex against the invasion of exogenous pathogens, odontoblasts also are the first cells to recognize external stimuli, such as thermal variations, mechanical and chemical stresses [80]. Therefore, odontoblasts play sensory role for detecting nociceptive signals by various external stimuli. Odontoblasts express several classes of ion channels involving in nociception and signal propagation, such as voltage-gaged Na+ channels [91], [92]. The dental pulp tissue has sensory nerve fibers reaching the odontoblast layer and odontoblasts transduce the nociceptive signals to nearby nerve cells [80]. Previous studies demonstrated that nervous fibers appeared accompanying 30–70% of the odontoblastic prolongations and their synapsis-like relation with the odontotoblast processes by electron microscopy [93]. However, the detailed association between odontoblasts and nerve fibers still remains unclear and the precise mechanisms how odontoblasts transduce the noxious signal to surrounding nerve fibers are not yet completely understood.
6. Conclusion
Odontoblasts first recognize caries-related pathogens by the engagement of PRRs and subsequently initiate innate immune responses in the dental pulp. These innate inflammatory events play important roles on fighting pathogens invading into dentin, but they can be detrimental to dental pulp and then lead to irreversible damage, such as dental pulp necrosis. However, the PRRs involved in responses to PAMPs in odontoblasts have not been fully clarified. Therefore, to maintain dental pulp tissues in a healthy condition for as long as possible, a deeper understanding is needed of the mechanisms underlying PAMP-induced innate immune responses and the roles of PRRs in odontoblasts, which may lead to the development of novel therapeutic strategies and treatments for pulpitis.
Conflict of interest
None declared.
References
- 1.Yoshida S., Ohshima H. Distribution and organization of peripheral capillaries in dental pulp and their relationship to odontoblasts. Anat Rec. 1996;245:313–326. doi: 10.1002/(SICI)1097-0185(199606)245:2<313::AID-AR14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Cooper P.R., Takahashi Y., Graham L.W., Simon S., Imazato S., Smith A.J. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent. 2010;38:687–697. doi: 10.1016/j.jdent.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 7.Kumar H., Kawai T., Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Caruso R., Warner N., Inohara N., Nunez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G., Shaw M.H., Kim Y.G., Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 11.Anand P.K., Malireddi R.K., Kanneganti T.D. Role of the nlrp3 inflammasome in microbial infection. Front Microbiol. 2011;2:12. doi: 10.3389/fmicb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C.S., Shin D.M., Jo E.K. The role of NLR-related protein 3 inflammasome in host defense and inflammatory diseases. Int Neurourol J. 2012;16:2–12. doi: 10.5213/inj.2012.16.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geijtenbeek T.B., Gringhuis S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehwinkel J. Exposing viruses: RNA patterns sensed by RIG-I-like receptors. J Clin Immunol. 2010;30:491–495. doi: 10.1007/s10875-010-9384-7. [DOI] [PubMed] [Google Scholar]
- 15.Hirao K., Yumoto H., Takahashi K., Mukai K., Nakanishi T., Matsuo T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J Dent Res. 2009;88:762–767. doi: 10.1177/0022034509341779. [DOI] [PubMed] [Google Scholar]
- 16.Cooper P.R., Holder M.J., Smith A.J. Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. J Endod. 2014;40:S46–S51. doi: 10.1016/j.joen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Jang J.H., Shin H.W., Lee J.M., Lee H.W., Kim E.C., Park S.H. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediators Inflamm. 2015;2015:794143. doi: 10.1155/2015/794143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosokawa Y., Hirao K., Yumoto H., Washio A., Nakanishi T., Takegawa D. Functional roles of NOD1 in odontoblasts on dental pulp innate immunity. BioMed Res Int. 2016;2016:9325436. doi: 10.1155/2016/9325436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love R.M., Jenkinson H.F. Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med. 2002;13:171–183. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 20.Hahn C.L., Best A.M., Tew J.G. Cytokine induction by Streptococcus mutans and pulpal pathogenesis. Infect Immun. 2000;68:6785–6789. doi: 10.1128/iai.68.12.6785-6789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn C.L., Liewehr F.R. Innate immune responses of the dental pulp to caries. J Endod. 2007;33:643–651. doi: 10.1016/j.joen.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Mutoh N., Tani-Ishii N., Tsukinoki K., Chieda K., Watanabe K. Expression of toll-like receptor 2 and 4 in dental pulp. J Endod. 2007;33:1183–1186. doi: 10.1016/j.joen.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 23.He W., Yu Q., Zhou Z., Wang P. CpG oligonucleotides induce an immune response of odontoblasts through the TLR9, MyD88 and NF-kappaB pathways. Biochem Biophys Res Commun. 2010;399:274–278. doi: 10.1016/j.bbrc.2010.07.068. [DOI] [PubMed] [Google Scholar]
- 24.Durand S.H., Flacher V., Romeas A., Carrouel F., Colomb E., Vincent C. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176:2880–2887. doi: 10.4049/jimmunol.176.5.2880. [DOI] [PubMed] [Google Scholar]
- 25.Staquet M.J., Durand S.H., Colomb E., Romeas A., Vincent C., Bleicher F. Different roles of odontoblasts and fibroblasts in immunity. J Dent Res. 2008;87:256–261. doi: 10.1177/154405910808700304. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H.W., Zhang W., Ren B.P., Zeng J.F., Ling J.Q. Expression of toll like receptor 4 in normal human odontoblasts and dental pulp tissue. J Endod. 2006;32:747–751. doi: 10.1016/j.joen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Farges J.C., Keller J.F., Carrouel F., Durand S.H., Romeas A., Bleicher F. Odontoblasts in the dental pulp immune response. J Exp Zool B Mol Dev Evol. 2009;312B:425–436. doi: 10.1002/jez.b.21259. [DOI] [PubMed] [Google Scholar]
- 28.Keller J.F., Carrouel F., Colomb E., Durand S.H., Baudouin C., Msika P. Toll-like receptor 2 activation by lipoteichoic acid induces differential production of pro-inflammatory cytokines in human odontoblasts, dental pulp fibroblasts and immature dendritic cells. Immunobiology. 2010;215:53–59. doi: 10.1016/j.imbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Staquet M.J., Carrouel F., Keller J.F., Baudouin C., Msika P., Bleicher F. Pattern-recognition receptors in pulp defense. Adv Dent Res. 2011;23:296–301. doi: 10.1177/0022034511405390. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Gao Y., Zhan X., Cui L., Xu S., Ma D. TLR4 activation by lipopolysaccharide and Streptococcus mutans induces differential regulation of proliferation and migration in human dental pulp stem cells. J Endod. 2014;40:1375–1381. doi: 10.1016/j.joen.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Veerayutthwilai O., Byers M.R., Pham T.T., Darveau R.P., Dale B.A. Differential regulation of immune responses by odontoblasts. Oral Microbiol Immunol. 2007;22:5–13. doi: 10.1111/j.1399-302X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 32.Horst O.V., Tompkins K.A., Coats S.R., Braham P.H., Darveau R.P., Dale B.A. TGF-beta1 Inhibits TLR-mediated odontoblast responses to oral bacteria. J Dent Res. 2009;88:333–338. doi: 10.1177/0022034509334846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paakkonen V., Rusanen P., Hagstrom J., Tjaderhane L. Mature human odontoblasts express virus-recognizing toll-like receptors. Int Endod J. 2014;47:934–941. doi: 10.1111/iej.12238. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Zhu Q.L., Huang P., Yu Q., Wang Z.H., Cooper P.R. CpG ODN-induced matrix metalloproteinase-13 expression is mediated via activation of the ERK and NF-kappaB signalling pathways in odontoblast cells. Int Endod J. 2013;46:666–674. doi: 10.1111/iej.12043. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y.Y., Chan C.H., Hung S.L., Chen Y.C., Lee Y.H., Yang S.F. Up-regulation of nucleotide-binding oligomerization domain 1 in inflamed human dental pulp. J Endod. 2011;37:1370–1375. doi: 10.1016/j.joen.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Lin Z.M., Song Z., Qin W., Li J., Li W.J., Zhu H.Y. Expression of nucleotide-binding oligomerization domain 2 in normal human dental pulp cells and dental pulp tissues. J Endod. 2009;35:838–842. doi: 10.1016/j.joen.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 37.Keller J.F., Carrouel F., Staquet M.J., Kufer T.A., Baudouin C., Msika P. Expression of NOD2 is increased in inflamed human dental pulps and lipoteichoic acid-stimulated odontoblast-like cells. Innate Immun. 2011;17:29–34. doi: 10.1177/1753425909348527. [DOI] [PubMed] [Google Scholar]
- 38.Song Z., Lin Z., He F., Jiang L., Qin W., Tian Y. NLRP3 is expressed in human dental pulp cells and tissues. J Endod. 2012;38:1592–1597. doi: 10.1016/j.joen.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W., Lv H., Wang H., Wang D., Sun S., Jia Q. Activation of the NLRP3/caspase-1 inflammasome in human dental pulp tissue and human dental pulp fibroblasts. Cell Tissue Res. 2015;361:541–555. doi: 10.1007/s00441-015-2118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farges J.C., Alliot-Licht B., Renard E., Ducret M., Gaudin A., Smith A.J. Dental pulp defence and repair mechanisms in dental caries. Mediators Inflamm. 2015;2015:230251. doi: 10.1155/2015/230251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Farges J.C., Carrouel F., Keller J.F., Baudouin C., Msika P., Bleicher F. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement. Immunobiology. 2011;216:513–517. doi: 10.1016/j.imbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai K., Okiji T., Suda H. Co-increase of nerve fibers and HLA-DR- and/or factor-XIIIa-expressing dendritic cells in dentinal caries-affected regions of the human dental pulp: an immunohistochemical study. J Dent Res. 1999;78:1596–1608. doi: 10.1177/00220345990780100401. [DOI] [PubMed] [Google Scholar]
- 44.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 45.He W., Zhang Y., Zhang J., Yu Q., Wang P., Wang Z. Cytidine-phosphate-guanosine oligonucleotides induce interleukin-8 production through activation of TLR9, MyD88, NF-kappaB, and ERK pathways in odontoblast cells. J Endod. 2012;38:780–785. doi: 10.1016/j.joen.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Carrouel F., Staquet M.J., Keller J.F., Baudouin C., Msika P., Bleicher F. Lipopolysaccharide-binding protein inhibits toll-like receptor 2 activation by lipoteichoic acid in human odontoblast-like cells. J Endod. 2013;39:1008–1014. doi: 10.1016/j.joen.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Hoebe K., Georgel P., Rutschmann S., Du X., Mudd S., Crozat K. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 48.Stutz A., Golenbock D.T., Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu L.C., Ali S.R., McGillivray S., Tseng P.H., Mariathasan S., Humke E.W. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferwerda G., Kramer M., de Jong D., Piccini A., Joosten L.A., Devesaginer I. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur J Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- 51.Yang D., Biragyn A., Kwak L.W., Oppenheim J.J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 52.Paris S., Wolgin M., Kielbassa A.M., Pries A., Zakrzewicz A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. J Endod. 2009;35:520–523. doi: 10.1016/j.joen.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Sahl H.G., Pag U., Bonness S., Wagner S., Antcheva N., Tossi A. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol. 2005;77:466–475. doi: 10.1189/jlb.0804452. [DOI] [PubMed] [Google Scholar]
- 54.Mansour S.C., Pena O.M., Hancock R.E. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35:443–450. doi: 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Pazgier M., Hoover D.M., Yang D., Lu W., Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semple F., Dorin J.R. beta-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dommisch H., Winter J., Acil Y., Dunsche A., Tiemann M., Jepsen S. Human beta-defensin (hBD-1, -2) expression in dental pulp. Oral Microbiol Immunol. 2005;20:163–166. doi: 10.1111/j.1399-302X.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 58.Shiba H., Mouri Y., Komatsuzawa H., Ouhara K., Takeda K., Sugai M. Macrophage inflammatory protein-3alpha and beta-defensin-2 stimulate dentin sialophosphoprotein gene expression in human pulp cells. Biochem Biophys Res Commun. 2003;306:867–871. doi: 10.1016/s0006-291x(03)01075-1. [DOI] [PubMed] [Google Scholar]
- 59.Song W., Shi Y., Xiao M., Lu H., Qu T., Li P. In vitro bactericidal activity of recombinant human beta-defensin-3 against pathogenic bacterial strains in human tooth root canal. Int J Antimicrob Agents. 2009;33:237–243. doi: 10.1016/j.ijantimicag.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Lee S.H., Baek D.H. Antibacterial and neutralizing effect of human beta-defensins on Enterococcus faecalis and Enterococcus faecalis lipoteichoic acid. J Endod. 2012;38:351–356. doi: 10.1016/j.joen.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Lee J.K., Chang S.W., Perinpanayagam H., Lim S.M., Park Y.J., Han S.H. Antibacterial efficacy of a human beta-defensin-3 peptide on multispecies biofilms. J Endod. 2013;39:1625–1629. doi: 10.1016/j.joen.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Krijgsveld J., Zaat S.A., Meeldijk J., van Veelen P.A., Fang G., Poolman B. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem. 2000;275:20374–20381. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 63.Cole A.M., Ganz T., Liese A.M., Burdick M.D., Liu L., Strieter R.M. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 64.Dommisch H., Winter J., Willebrand C., Eberhard J., Jepsen S. Immune regulatory functions of human beta-defensin-2 in odontoblast-like cells. Int Endod J. 2007;40:300–307. doi: 10.1111/j.0143-2885.2007.01228.x. [DOI] [PubMed] [Google Scholar]
- 65.Silva Mendez L.S., Allaker R.P., Hardie J.M., Benjamin N. Antimicrobial effect of acidified nitrite on cariogenic bacteria. Oral Microbiol Immunol. 1999;14:391–392. doi: 10.1034/j.1399-302x.1999.140612.x. [DOI] [PubMed] [Google Scholar]
- 66.Farges J.C., Bellanger A., Ducret M., Aubert-Foucher E., Richard B., Alliot-Licht B. Human odontoblast-like cells produce nitric oxide with antibacterial activity upon TLR2 activation. Front Physiol. 2015;6:185. doi: 10.3389/fphys.2015.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Law A.S., Baumgardner K.R., Meller S.T., Gebhart G.F. Localization and changes in NADPH-diaphorase reactivity and nitric oxide synthase immunoreactivity in rat pulp following tooth preparation. J Dent Res. 1999;78:1585–1595. doi: 10.1177/00220345990780100301. [DOI] [PubMed] [Google Scholar]
- 68.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 69.MacMicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 70.Coleman J.W. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 71.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 72.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Di Nardo Di Maio F., Lohinai Z., D'Arcangelo C., De Fazio P.E., Speranza L., De Lutiis M.A. Nitric oxide synthase in healthy and inflamed human dental pulp. J Dent Res. 2004;83:312–316. doi: 10.1177/154405910408300408. [DOI] [PubMed] [Google Scholar]
- 74.Korkmaz Y., Lang H., Beikler T., Cho B., Behrends S., Bloch W. Irreversible inflammation is associated with decreased levels of the alpha1-, beta1-, and alpha2-subunits of sGC in human odontoblasts. J Dent Res. 2011;90:517–522. doi: 10.1177/0022034510390808. [DOI] [PubMed] [Google Scholar]
- 75.Smith A.J. Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J Dent Educ. 2003;67:678–689. [PubMed] [Google Scholar]
- 76.Begue-Kirn C., Smith A.J., Ruch J.V., Wozney J.M., Purchio A., Hartmann D. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992;36:491–503. [PubMed] [Google Scholar]
- 77.Begue-Kirn C., Smith A.J., Loriot M., Kupferle C., Ruch J.V., Lesot H. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int J Dev Biol. 1994;38:405–420. [PubMed] [Google Scholar]
- 78.Ruch J.V., Lesot H., Begue-Kirn C. Odontoblast differentiation. Int J Dev Biol. 1995;39:51–68. [PubMed] [Google Scholar]
- 79.Nomiyama K., Kitamura C., Tsujisawa T., Nagayoshi M., Morotomi T., Terashita M. Effects of lipopolysaccharide on newly established rat dental pulp-derived cell line with odontoblastic properties. J Endod. 2007;33:1187–1191. doi: 10.1016/j.joen.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 80.Bleicher F. Odontoblast physiology. Exp Cell Res. 2014;325:65–71. doi: 10.1016/j.yexcr.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Venereau E., Ceriotti C., Bianchi M.E. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 83.Park J.S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J.Y., Strassheim D. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 84.Klune J.R., Dhupar R., Cardinal J., Billiar T.R., Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian J., Avalos A.M., Mao S.Y., Chen B., Senthil K., Wu H. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 86.Ivanov S., Dragoi A.M., Wang X., Dallacosta C., Louten J., Musco G. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Beijnum J.R., Buurman W.A., Griffioen A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 88.Qi S.C., Cui C., Yan Y.H., Sun G.H., Zhu S.R. Effects of high-mobility group box 1 on the proliferation and odontoblastic differentiation of human dental pulp cells. Int Endod J. 2013;46:1153–1163. doi: 10.1111/iej.12112. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X., Jiang H., Gong Q., Fan C., Huang Y., Ling J. Expression of high mobility group box 1 in inflamed dental pulp and its chemotactic effect on dental pulp cells. Biochem Biophys Res Commun. 2014;450:1547–1552. doi: 10.1016/j.bbrc.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 90.Tancharoen S., Tengrungsun T., Suddhasthira T., Kikuchi K., Vechvongvan N., Tokuda M. Overexpression of receptor for advanced glycation end products and high-mobility group box 1 in human dental pulp inflammation. Mediators Inflamm. 2014;2014:754069. doi: 10.1155/2014/754069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allard B., Magloire H., Couble M.L., Maurin J.C., Bleicher F. Voltage-gated sodium channels confer excitability to human odontoblasts: possible role in tooth pain transmission. J Biol Chem. 2006;281:29002–29010. doi: 10.1074/jbc.M601020200. [DOI] [PubMed] [Google Scholar]
- 92.Ichikawa H., Kim H.J., Shuprisha A., Shikano T., Tsumura M., Shibukawa Y. Voltage-dependent sodium channels and calcium-activated potassium channels in human odontoblasts in vitro. J Endod. 2012;38:1355–1362. doi: 10.1016/j.joen.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Carda C., Peydro A. Ultrastructural patterns of human dentinal tubules, odontoblasts processes and nerve fibres. Tissue Cell. 2006;38:141–150. doi: 10.1016/j.tice.2006.01.002. [DOI] [PubMed] [Google Scholar]