Abstract
Previous animal studies have indicated that coupling restraint stress load with activation of the masticatory organs (chewing) causes a reduction in the systemic and central nervous system stress response. However, the brain mechanism underlying this effect is unknown. Therefore, in this review, we summarize the literature regarding brain regions involved in the attenuating effects of chewing and the systemic stress response attenuation effects induced by those brain regions. In addition, we also focusing on the amygdala, as the emotional control center, and the hypothalamic–pituitary–adrenal axis, as one of the outputs of the systemic response. In particular, we will report on one of the chewing-related stress attenuation mechanisms within the brain brought about by the activation of the inhibition pathway accompanying the activation of the amygdala’s GABAergic function.
Keywords: Stress, Chewing, Masticatory organ, Amygdala, GABA
1. Introduction
In this article, we will review the literature on how chewing can alleviate stress responses. As one of the first studies to investigate this, in 1983, Vincent et al. reported that, in animals subjected to restraint and water inundation stress, those that could chew on a nylon brush developed fewer and smaller gastric lesions, and were better able to maintain body temperature when compared to animals exposed only to stress [1]. This suggests that increased afferent information input, mainly from the trigeminal nerve, via chewing under conditions of stress, induces a stress attenuation mechanism. However, the pathways by which this occurs has yet to be fully elucidated.
Similar animal experiments have since been conducted, including gene analysis studies and studies investigating the expression of specific proteins in regions of the brain that exhibit chewing-related stress responses (Table 1). To study systemic responses, serum levels of stress hormones and immune system parameters accompanying hypothalamic–pituitary–adrenal (HPA) axis activation have also been investigated [2]. In addition, researchers have investigated heart rate increases and the incidence of arrhythmia accompanying activation of the autonomic nervous system [3]. These studies indicate that chewing under stress has an inhibitory effect on stress responses, which involve the HPA axis, the sympathoadrenal system, and hyperactivation of the immune system (Table 1).
Table 1.
Research results to date regarding the effects of chewing on stress-induced brain and systemic responses.
| Brain region | Sub-nucleus | Marker | Stress | Stress with chewing | Refs. |
|---|---|---|---|---|---|
| Hippocampus | BDNF mRNA | ↓ | ↑ | [17] | |
| NT-3 mRNA & protein | ↑ | ↓ | [17] | ||
| CA1 | MR | ↑ | ↓ | [51] | |
| CA1 | GR | ↓ or ± | ↑ | [30], [51] | |
| CA1 | NMDA receptor | ↓ | ↑ | [42] | |
| CA1 | H1 receptor | ↓ | ↑ | [42] | |
| DG | Cell birth | ↓ | ↑ | [83] | |
| Hypothalamus | Free radical | ↑ | ↓ | [11] | |
| PVN | c-fos | ↑ | ↓ | [25] | |
| PVN | CRF protein | ↑ | ↓ | [20] | |
| PVN | nNOS mRNA & protein | ↑ | ↓ | [88] | |
| PVN | pERK1/2 | ↑ | ↓ | [22] | |
| Periaqueductal gray | dl | pERK1/2 | ↑ | ↓ | [60] |
| vl | pERK1/2 | ↑ | ↓ | [60] | |
| Insular cortex | Ant | pERK1/2 | ↑ | ↓ | [72] |
| Post | pERK1/2 | ↑ | ↓ | [72] | |
| Amygdala | CeA | GABA | ± | ± | [34] |
| BLA | GABA | ↑ | ↑↑ | [34] |
| Target area | Representation or behavior | Stress | Stress with chewing | Refs. |
|---|---|---|---|---|
| Hippocampus | Goal in time on Morris water maze | ↓ | ↑ | [30] |
| LTP | ↓ | ↑ | [38] | |
| LTD | ↑ | ↓ | [84] | |
| Blood level | ACTH | ↑ | ↓ | [17] |
| Corticosterone | ↑ | ↓ | [17] | |
| IL-1β | ↑ | ↓ | [85] | |
| IL-6 | ↑ | ↓ | [85] | |
| Leptin | ↑ | ↓ | [85] | |
| TSH | ↓ | ↑ | [85] | |
| Noradrenaline | ↑ | ↓ | [3] | |
| Hypothalamus | PO2 | ↓ | ± | [86] |
| Blood-flow | ± | ↑ | [86] | |
| NO | ↑ | ↓ | [87] | |
| Amygdala | PO2 | ↓ | ↑ | [86] |
| Blood-flow | ± | ↑ | [86] | |
| Cardiovascular system | Arrhythmias | ↑ | ↓ | [3] |
| Blood pressure | ↑ | ↓ | [85] | |
| Body-wide | Sympathetic activity | ↑ | ↓ | [3] |
| Core temperature | ↑ | ↓ | [85] |
Abbreviations: BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MR: mineralocorticoid receptor; GR: glucocorticoid receptor; NMDA: N-methyl-d-aspartate receptor; H1 receptor: histamine receptor 1; CRF: corticotropin-releasing factor; nNOS: neuronal nitric oxide synthase; pERK1/2: phosphorylated extracellular signal-regulated kinase; GABA: gamma-aminobutyric acid; LTP: long-term potentiation; LTD: long-term depression; ACTH: adrenocorticotropic hormone; IL-1β: interleukin-1β; IL-6: interleukin-6; TSH: thyroid-stimulating hormone; PO2: partial pressure of oxygen; NO: nitric oxide.
Restraint stress is commonly used in such experiments, and involves stress-related behavioral, biochemical, and physiological changes in laboratory animals. Restraint stress has been used in the context of a mental and physical stress experiment system in our previous studies [4], [5], [6], the basis of which is the expression of affective reactions resulting from the activated output accompanying the integration and evaluation of external stimulation as sensory information input. This sensory information follows a direct pathway from the thalamus to the cerebral neocortex and an indirect pathway from the thalamus through the archicortex, which includes the hippocampus, to the amygdala [2], [7], [8], [9]. The amygdala integrates this information and interconnects with the neocortex, hippocampus, hypothalamus, and brainstem. This reduces physical and emotional reactions and allows the animal to adapt to the external environment [10].
In this review, we will report the results of stress response behavior tests, focusing on their influence on brain regions previously found to be involved in the attenuation of stress via chewing, in particular, the amygdala and the HPA axis. In addition, we will discuss the importance of the activation of gamma-aminobutyric acid (GABA)ergic function within the amygdala in the context of a chewing-induced stress inhibition mechanism.
2. Ex vivo and in vivo analysis of the effects of chewing-induced stress attenuation in the hypothalamus
In 2005, Miyake et al. [11] measured the effects of stress on the amount of free radicals and active oxygen within the entire brain in mice. They performed their experiments in a control group, a 30-min stress-only group, and a 30-min stress-with-chewing group. To obtain ex vivo measurements of the effects of chewing on stress, the authors injected a blood-brain barrier-permeable nitroxyl spin probe, 3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine-1-oxyl (MC-PROXYL), into the tail vein. They then used the L-band electron spin resonance technique to measure the decay rate of MC-PROXYL within the brain. While the stress-only group showed a significant increase in the levels of active oxygen and free radicals within the hypothalamic region when compared to the control group, the stress-with-chewing group exhibited significantly lower levels of active oxygen and free radicals than the stress-only group. While free radicals and active oxygen are necessary for life, the balance between their formation and the elimination by antioxidants is crucial, as excessive oxidative stress has harmful systemic effects and is considered to be involved in brain ischemia, acceleration of aging, and neurodegenerative disease [12], [13], [14], [15].
In a similar experimental series using small-animal positron-emission tomography with F-fluorodeoxyglucose injected via the tail vein, Ono et al. [16] carried out a whole-brain analysis. They found that glucose uptake in the hypothalamic region was significantly increased under stress conditions, and that chewing during stress counteracted this effect, reducing glucose uptake to control levels. Simultaneous measurements of plasma corticosterone changes have confirmed previous research findings [17] that chewing significantly counteracts stress-induced corticosterone elevation. Detailed analysis of region-of-interest-based glucose uptake has revealed that there is a significant reduction of uptake in the paraventricular hypothalamic nucleus (PVN) [16].
These results indicate that stress rapidly and significantly increases the levels of local active oxygen and free radicals, and glycometabolism within the hypothalamus, which is the stress response center. These changes caused by stress suggest that stress increases neural activity within the hypothalamic region. Getting together, both ex vivo and in vivo experiments have shown that chewing inhibits this neural activity. Given that glucose metabolism within the PVN, the control center of the HPA axis, was reduced and neural activity was inhibited, it is thought that secretion of the stress hormone, namely, plasma corticosterone was significantly inhibited as a consequence. The aforementioned results suggest that chewing may act as some kind of inhibitive mechanism for stress-related neural activation within the PVN, which weakens the HPA axis response and ultimately results in a reduced secretion of stress hormones.
3. Chewing-induced suppression of the stress response in the PVN
The hypothalamus is crucial in biological reactions to external stressors. In particular, the PVN, located upstream of the HPA axis, is an important center of higher-order integration between the neuroendocrine and autonomic nervous systems, and is crucial for maintaining homeostasis of the body [18]. The stress response of the PVN has been confirmed by numerous studies, and we ourselves have also investigated the phenomenon of chewing-induced stress response reduction (Table 1). The expression of corticotropin releasing factor (CRF) within the PVN is underscored as a central modulator of the stress system. CRF is released into the pituitary portal vein, stimulating the pituitary to secrete adrenocorticotropic hormone (ACTH), which in turn causes the secretion of cortisol from the adrenal cortex. Following this, the release of CRF and ACTH is regulated through negative feedback via steroid receptors of the pituitary gland, hypothalamus, hippocampus, and amygdala. Moreover, CRF neurons in the PVN induce the release of adrenaline and noradrenaline through activation of the autonomic nervous system via the locus coeruleus [19].
According to Hori et al. [20], chewing reduces the number of CRF-positive cells in the PVN, which increases following stress. Moreover, there is a similar response in the levels of c-fos-positive (an immediate-early gene) [21] and phosphorylated extracellular signal-regulated kinase (p-ERK1/2)-positive [22] cells in the PVN. Intracellular changes in these proteins are thought to occur prior to CRF protein expression. Studies of neuronal activation as reflected by glucose uptake, histochemical analyses, and measurements of serum stress hormones such as ACTH and corticosterone under stress-only and stress with chewing conditions (Table 1) have confirmed that the PVN is an essential brain regions for the ameliorative effects of chewing. Proprioception in the maxillofacial region is generally is relayed through the mesencephalic trigeminal nucleus, tactile pressure is relayed via the principal sensory nucleus of the trigeminal nerve, and pain and temperature information is relayed through the spinal sensory nucleus of the trigeminal nerve. The above described sensations thus travel through the medial lemniscus and are relayed in the ventral posterior medial nucleus of the thalamus [23], [24]. The PVN is thought to be regulated by inputs from various regions of the brain, as well as by humoral factors, such as hormones and cytokines. The numbers of p-ERK1/2 immunopositive cells in the PVN, which increase following stress, are suppressed by chewing within as little as 5 min [22]. This rapid response suggests that chewing does not only affect humoral factors, but also affects the projections of the thalamus, which is the relay nucleus, via trigeminal nerves to response regions of the brain. The latter neural pathway may be via the action of neurotransmitters.
4. Chewing-induced suppression of the stress response in the paraventricular hypothalamic nucleus via GABAergic mechanisms in the basolateral amygdala
Stress-induced neural mechanisms in the PVN involve projections from the excitatory posterior hypothalamus, the ventrolateral region of the bed nucleus of the stria terminalis (BNST), the inhibitory medial preoptic nucleus, the dorsomedial hypothalamic nucleus, interneurons in the PVN, and the posterior BNST. Neurotransmission in these projections is suppressed by the amygdala, which is located upstream of these connections in the suppression system [25]. Amygdala ablation in laboratory animals has been shown to influence the HPA axis stress response, and leads to changes in the levels of serum stress hormones, CRF, vasopressin, and c-fos mRNA in the PVN, and the numbers of immunopositive cells for vasopressin [26], [27], [28]. The amygdala can be divided into sub-nuclei, such as the medial, central, cortical, basomedial, and basolateral nuclei [29]. These sub-nuclei are interconnected and form input synapses onto the central amygdala (CeA), which is a major output region of the amygdala. The CeA is modulated by excitatory glutamatergic afferent fibers, phasic and tonic GABAergic afferent fibers, and local interneurons. The CeA is known to project to the PVN [30], [31]. When stimulated electronically, the CeA activates the HPA axis [32]. Based on these neural connections, destruction of the CeA is thought to influence the activation of the HPA axis, the higher center of which is the hypothalamus.
Reznikov et al. [33] have conducted an acute stress experimental series similar to our own wherein they measured the efflux of GABA (the main mediator of inhibitory neurotransmission in the central nervous system) in the CeA and the basolateral amygdala (BLA) using in vivo microdialysis. The results of the above study indicated that although no fluctuation was found in the CeA, GABA efflux was significantly increased in the BLA following stress. Using the same method, we examined indicators of the influence of chewing on GABA efflux in the amygdala under stress. In the stress-only condition, GABA efflux increased in the BLA. GABA efflux was further increased in the stress-with-chewing condition [34]. This suggests that increased input from the trigeminal nerve due to chewing increases GABA levels in the BLA, which magnifies the effects of the GABAergic system. This is turn increases inhibitory input to the CeA and ameliorates stress-induced p-ERK1/2 expression within the PVN [34]. Anti-GABA transporter-1 saporine (conjugates of saporin with a rabbit antiserum raised against a peptide in the extracellular domain of GABA transporter-1) was used to facilitate the specific immunotoxic destruction [35], [36] of amygdala GABAergic neurons in one or both sides of the brain. This allowed us to investigate the effects of chewing on the PVN, which receives projections from the amygdala, by counting the numbers of pERK1/2-immunopositive cells in the PVN and measuring serum stress hormone levels. In animals that underwent unilateral destruction of BLA GABAergic cells, the chewing-induced suppression effect on the increase in the number of p-ERK1/2-immunopositive cells was significantly attenuated in the ipsilateral PVN. In animals with bilateral destruction, the chewing-induced decreases in the levels of stress-induced serum stress hormones ACTH and corticosterone were similarly suppressed [34]. These results suggest that the stress reduction effects of the activation of the masticatory organ during stress in the brain are induced by the activation of BLA GABAergic neurons. This activation is then relayed via the CeA and attenuates the stress response in the PVN. Our findings imply that BLA GABAergic neurons mediate the chewing-induced reduction in the levels of stress hormones via the hypothalamus.
5. Chewing-induced suppression mechanisms in stress response regions of the amygdala and in other areas
The hippocampus is functionally and structurally vulnerable to stress starting at the early stages of development [37]. We measured long-term potentiation (LTP), which is a major cellular mechanisms of learning and memory, to investigate the effects of chewing during stress on the hippocampus. In the stress-with-chewing condition, the stress-induced reduction in hippocampal LTP was significantly recovered within 24 h when compared to the stress-only group [38]. We used the Morris water maze to test spatial memory and found that stress-induced cognitive decline was reduced by chewing [39]. However, in both experiments, we found no significant difference in serum corticosterone levels. This suggests that the neural pathways that mediate the stress-reducing effects of chewing exist outside of the amygdala and the HPA axis.
Proprioception of the oral cavity is mediated by the mesencephalic trigeminal nucleus, which activates histamine neurons in the tuberomammillary nucleus (TMN) [40], [41]. While the cell bodies of the histamine neurons are located in the TMN, their axons are distributed throughout the brain and are present in the hippocampus. We treated the animals with pyrilamine, which is an H1 receptor antagonist, before and after a stress task to examine the impact of stress on hippocampal LTP [42]. The administration of pyrilamine prior to the stress load reduced the recovery effects of chewing on hippocampal LTP. This suggests that chewing under stress activates the histamine neuron system. Histamine facilitates N-methyl d-aspartate (NMDA) receptor function by stimulating the activity of phospholipase C, which results in an increase in the excitability of NMDA receptors [43], [44], which is essential in the formation of LTP. Therefore, NMDA receptor functions, which are reduced due to stress, were improved via the action of H1 histamine receptors in the hippocampus. This in turn led to the recovery of hippocampal LTP.
The amygdala directly and indirectly projects to the hippocampus, and thus contributes to hippocampal function [45], [46], [47], [48]. Kim et al. [49] investigated the role of GABAergic neurons in attenuating the effects of stress on hippocampal LTP. Muscimol, which is a GABAA receptor agonist, was delivered to the amygdala both before and after a stress task, and hippocampal LTP was examined 90 min later. When muscimol binds to GABAA receptors, it opens chloride channels, which in turn increases the permeability of chloride ions [50]. This inhibits neuronal firing. The reduction in hippocampal LTP was inhibited in animals treated with muscimol prior to the stress load. This suggests that muscimol suppresses stress-induced excitation of the amygdala and alters the inhibition of amygdalo-hippocampal activity during stress. This compound thus reverses the stress-induced reduction in hippocampal LTP. Similarly, in an investigation using the Morris water maze to carry out acquisition and single retention tests, rats were treated with muscimol prior to the stress load. Animals in the stress-with-chewing group had similar goal-in times to those observed during the training trials, showed no indications of stress-related impairments, and maintained the acquired spatial memory [49].
In the majority of cases, chewing exerts inhibitory control on the reactions within the stress-response regions of the brain. However, based on the above series of results, we postulated that the attenuation of the inhibitory effect of stress on hippocampal LTP was due to the synergistic effects of the activation of hippocampal histamine H1 receptors and TMN histamine neuron activation [42], as well as the suppression of excitatory neuronal input from the amygdala to the hippocampus [34] due to the chewing-induced activation of GABAergic neurons in the BLA. Chewing-induced changes in the concentration-dependent affinity and expression of hippocampal mineralocorticoid receptors and glucocorticoid receptors [51], [52], [53], [54], [55] and variation in noradrenaline volume within the brain [56], [57], including the amygdala, have been considered contributory factors to hippocampal LTP. Chewing may lead to the rapid amelioration of stress-induced hippocampal LTP attenuation via the synergic effects of stress hormone negative feedback function changes, which accompany the inhibition of HPA axis activation via the amygdala.
Stress is known to activate the sympathetic nervous system and to increase blood pressure and pulse rate. The periaqueductal gray (PAG) is one of the control centers for the circulatory system. The PAG surrounds the midbrain aqueduct and is divisible into four standard regions: dorsomedial, dorsolateral, lateral, and ventrolateral. These regions have pathways that project to the cerebral hemispheres, brainstem, marginal nucleus of the spinal cord (lamina I), nociceptive neurons of the trigeminal nerve nucleus, etc. [58]. Input from the cerebral hemispheres primarily originates from the medial prefrontal area, anterior cingulate gyrus, orbitofrontal cortex, insular cortex, central nucleus of the amygdala, and hypothalamus. This input mediates a range of functions, including autonomic nervous system responses such as pain modulation, defense reactions, and blood pressure increases, as well as emotional behavior, reproductive behavior, eating behavior, and consciousness [59]. In an experiment examining the effects of chewing on p-ERK1/2-positive cell expression in the PAG, stress led to a significant increase in the numbers of p-ERK1/2-positive cells in the dorsolateral PAG and the ventrolateral PAG [60]. However, the expression of p-ERK1/2 was suppressed and returned to control levels 15 min after stress with chewing. The amygdala projects to the PAG, which is involved in the descending pain modulatory system and contributes to stress- and fear-induced analgesia [61]. In addition, according to de Abreau et al. [62], when the GABAA receptor agonist muscimol is microinjected into the BLA, it suppresses the increases in mean arterial pressure and heart rate induced by NMDA administration into the lateral/dorsolateral PAG. Moreover, our prior research indicates that chewing suppresses stress-related premature ventricular contractions caused by acute restraint stress-induced increases in peripheral blood noradrenaline concentration. Chewing also suppresses increases in low to high frequency ratio, which is an indicator of cardiac sympathetic activity [3]. Based on the above findings, we postulated that chewing during stress controls the reactions of the sympathetic nervous system and the circulatory system by suppressing stress-induced excitatory input to the PAG following the activation of GABAergic neurons in the BLA.
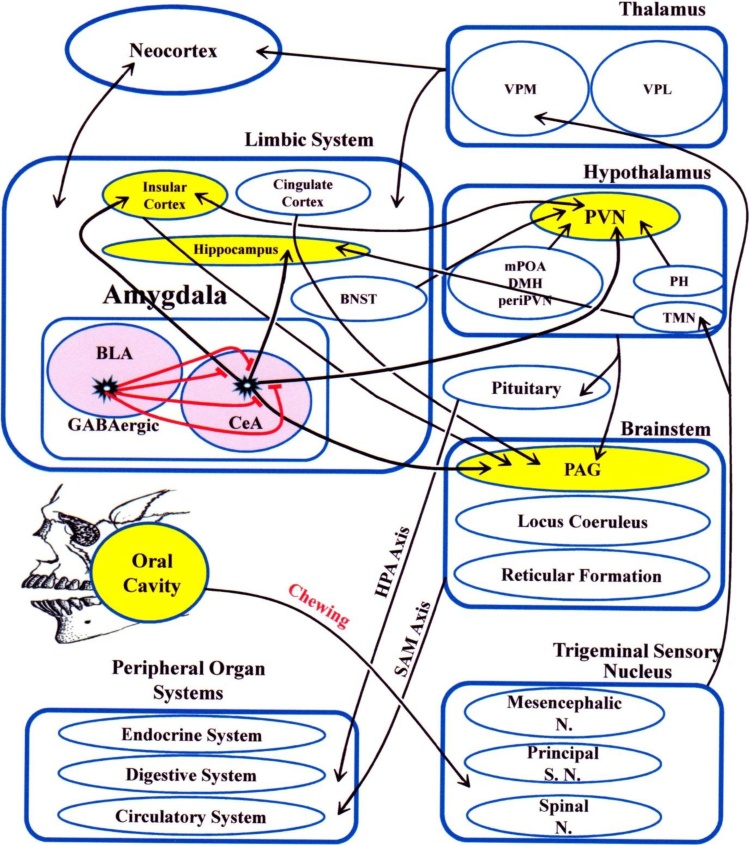
Cardiac responses to stress are controlled by another brain region, namely the insular cortex [63], [64]. The insular cortex receives taste, smell, pain, and somatosensory perception inputs [64], [65], [66], [67]. The involvement of this region to control the circulatory, respiratory, and digestive systems [68], [69] suggests the possibility of its involvement in the integration between various sensory and emotional inputs [70], [71]. Our recent research indicates that chewing significantly suppresses the stress-induced increase in the number of p-ERK1/2-immunopositive cells, even in the insular cortex [72]. This implies that chewing may play a role in neural activity. Inhibition of synaptic transmission of the insular cortex by bilateral microinjection of the nonselective synaptic blocker cobalt chloride (CoCl2, 1 mM/100 nL) [73] and suppression of localized noradrenergic neurotransmission in the insular cortex by administration of an α-1 or α-2 adrenoreceptor antagonist [74], both of experiments resulted in the suppression of acute stress-induced increases in blood pressure and pulse rate, and tachycardia. Given that the insular cortex shares rich interconnections with the limbic system, including the amygdala and hypothalamus [75], [76], the chewing-induced activation of GABAergic neurons in the amygdala during stress suppress stress-induced neural activity in the insular cortex that receives amygdala projections. Chewing also suppresses stress-induced noradrenaline release within the brain [56], [57], [77], [78], [79], [80], [81]. Given that the central autonomic network, which is essential for life, consists of the insular cortex, amygdala, hypothalamus, PAG, parabrachial complex, solitary nucleus, and ventrolateral medulla [82], chewing may attenuate the stress responses of the circulatory system and the autonomic nervous system by suppressing the secretion of catecholamines and stress information transmission in the brain. We have summarized neural pathways potentially involved in the ameliorative effects of chewing in Fig. 1.
Fig. 1.
Potential neural pathways underlying chewing-induced stress response amelioration.
Red lines indicate BLA GABAergic inhibitory nerve fibers. Thick lines indicate essential nerve connections and thin lines indicate the associated nerve connections. Arrow lines indicate projections and double arrow lines indicate reciprocal projections. Abbreviations: BLA: basolateral amygdala; BNST: bed nucleus of the stria terminalis; CeA: central amygdala; DMH: dorsomedial hypothalamus; HPA: hypothalamic–pituitary–adrenal; mPOA: medial preoptic hypothalamic nucleus; N: nucleus; PAG: periaqueductal gray; periPVN: interneurons around the PVN; PH: posterior hypothalamus; PVN: periventricular hypothalamic nucleus; S: sensory; SAM: sympathoadrenal; TMN: tuberomammillary nucleus; VPL: ventral posterior lateral nucleus of the thalamus; VPM: ventral posterior medial nucleus of the thalamus.
6. Conclusions and future studies
Activation of the masticatory organ (chewing) during stress has been shown to reduce the numbers of stomach ulcers and their durations. Chewing also suppresses the secretion of plasma stress hormones and catecholamines, preserves spatial reasoning, facilitates the rapid recovery of hippocampal LTP, and reduces blood pressure, heart rate, and the incidence of arrhythmia. However, until now, the brain mechanisms underlying these effects have remained unidentified. Our aim was thus to identify the mechanisms underlying the effects of chewing on stress. We present evidence from research on: (1) the amygdala, which is the emotional center that carries out the integration and evaluation of sensory information; (2) the HPA axis, which is the site of output projections carrying information regarding emotional expression from the amygdala (especially p-ERK1/2, which is a neural activity indicator within the PVN); and (3) plasma stress hormones, which are downstream outputs of the stress response. We found that the stress-reduction effects of chewing during stress were due to BLA-induced GABA efflux, which initiates GABAergic activity within the amygdala. This in turn controls stress response-related neural activity in each brain region that the amygdala projects to and suppresses the activities of response organs and humoral factors downstream of the HPA axis. Future research should be carried out to investigate the role of the prefrontal area, as it has strong interconnections with the amygdala. Other stress-related brain regions should also be investigated.
Conflicts of interest
There are no conflicts of interest associated with this review.
Acknowledgements
The authors acknowledge the excellent technical assistance of E. Aso, S. Okada, and Y. Saito. This work was supported by JSPS KAKENHI under Grant numbers 26463124 and 15K20459.
References
- 1.Vincent G.P., Paré W.P., Prenatt J.E., Glavin G.B. Aggression, body temperature, and stress ulcer. Physiol Behav. 1984;32(2):265–268. doi: 10.1016/0031-9384(84)90140-9. [DOI] [PubMed] [Google Scholar]
- 2.Ono Y., Yamamoto T., Kubo K.Y., Onozuka M. Occlusion and brain function: mastication as a prevention of cognitive dysfunction. J Oral Rehabil. 2010;37(8):624–640. doi: 10.1111/j.1365-2842.2010.02079.x. Epub 2010 Mar 2. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi S., Minamisawa S., Sasaguri K., Onozuka M., Sato S., Ono Y. Chewing reduces sympathetic nervous response to stress and prevents poststress arrhythmias in rats. Am J Physiol Heart Circ Physiol. 2011;301(4):H1551–H1558. doi: 10.1152/ajpheart.01224.2010. Epub 2011 Aug 5. [DOI] [PubMed] [Google Scholar]
- 4.İzgüt-Uysal V., Bülbül M., Tan R., Derin N., Üstünel İ., Ağar A. Effect of chronic stress l-carnitine on rat stomach. J Physiol Sci. 2007;57:187–192. doi: 10.2170/physiolsci.RP004707. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura T., Takimur R., Yamaguchi M., Ichinose M. Estimation of restraint stress in rats using salivary amylase activity. J Physiol Sci. 2012;62:421–427. doi: 10.1007/s12576-012-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izgüt-Uysal V.N., Gemici B., Birsen I., Acar N., Üstünel I. The protective effect of apelin against water-immersion and restraint stress-induced gastric damage. J Physiol Sci. 2014;64:279–289. doi: 10.1007/s12576-014-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mátyás F., Lee J., Shin H.S., Acsády L. The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur J Neurosci. 2014;39(11):1810–1823. doi: 10.1111/ejn.12610. Epub 2014 May 12. [DOI] [PubMed] [Google Scholar]
- 8.Hoover W.B., Vertes R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 9.Becerra L., Morris S., Bazes S., Gostic R., Sherman S., Gostic J. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26(42):10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pessoa L. A network model of the emotional brain. Trends Cognit Sci. 2017;21(5):357–371. doi: 10.1016/j.tics.2017.03.002. Epub 2017 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake S., Sasaguri K., Hori N., Shoji H., Yoshino F., Miyazaki H. Biting reduces acute stress-induced oxidative stress in the rat hypothalamus. Redox Rep. 2005;10(1):19–24. doi: 10.1179/135100005X21417. [DOI] [PubMed] [Google Scholar]
- 12.Chan P.H. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman E.R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 14.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 15.Smith M.A., Harris P.L., Sayre L.M., Perry G. Iron accumulation in Alzheimer disease is a source of redox generated free radicals. Proc Natl Acad Sci U S A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono Y., Lin H.C., Tzen K.Y., Chen H.H., Yang P.F., Lai W.S. Active coping with stress suppresses glucose metabolism in the rat hypothalamus. Stress. 2012;15(2):207–217. doi: 10.3109/10253890.2011.614296. [DOI] [PubMed] [Google Scholar]
- 17.Lee T., Saruta J., Sasaguri K., Sato S., Tsukinoki K. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res. 2008;1195(February):43–49. doi: 10.1016/j.brainres.2007.12.013. Epub 2007 Dec 15. [DOI] [PubMed] [Google Scholar]
- 18.Herman J.P., Prewitt C.M., Cullinan W.E. Neuronal circuit regulation of the hypothalamo–pituitary–adrenocortical stress axis. Crit Rev Neurobiol. 1996;10(3–4):371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 19.Binder E.B., Nemeroff C.B. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. Epub 2009 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori N., Yuyama N., Tamura K. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 2004;83(2):124–128. doi: 10.1177/154405910408300208. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko M., Hori N., Yuyama N., Sasaguri K., Slavicek R., Sato S. Biting suppresses Fos expression in various regions of the rat brain—further evidence that the masticatory organ functions to manage stress. Stomatologie. 2004;101:151–156. [Google Scholar]
- 22.Sasaguri K., Kikuchi M., Hori N., Yuyama N., Onozuka M., Sato S. Suppression of stress immobilization-induced phosphorylation of ERK 1/2 by biting in the rat hypothalamic paraventricular nucleus. Neurosci Lett. 2005;383(1–2):160–164. doi: 10.1016/j.neulet.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Steindler D.A. Trigeminocerebellar, trigeminotectal, and trigeminothalamic projections: a double retrograde axonal tracing study in the mouse. J Comp Neurol. 1985;237:155–175. doi: 10.1002/cne.902370203. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida A., Dostrovsky J.O., Sessle B.J., Chiang C.Y. Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol. 1991;307(4):609–625. doi: 10.1002/cne.903070408. [DOI] [PubMed] [Google Scholar]
- 25.Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R. Regulation of the hypothalamic–pituitary–adrenocortical stress response. Compr Physiol. 2016;6(2):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van de Kar L.D., Piechowski R.A., Rittenhouse P.A., Gray T.S. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. 1991;54(2):89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 27.Prewitt C.M., Herman J.P. Lesion of the central nucleus of the amygdala decreases basal CRH mRNA expression and stress-induced ACTH release. Ann N Y Acad Sci. 1994;746:438–440. doi: 10.1111/j.1749-6632.1994.tb39279.x. [DOI] [PubMed] [Google Scholar]
- 28.Prewitt C.M., Herman J.P. Hypothalamo–pituitary–adrenocortical regulation following lesions of the central nucleus of the amygdala. Stress. 1997;1(4):263–280. doi: 10.3109/10253899709013746. [DOI] [PubMed] [Google Scholar]
- 29.Knapska E., Radwanska K., Werka T., Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87(4):1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- 30.Gray T.S., Carney M.E., Magnuson D.J. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 31.Tsubouchi K., Tsumori T., Yokota S., Okunishi H., Yasui Y. A disynaptic pathway from the central amygdaloid nucleus to the paraventricular hypothalamic nucleus via the parastrial nucleus in the rat. Neurosci Res. 2007;59:390–398. doi: 10.1016/j.neures.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Feldman S., Weidenfeld J. The excitatory effects of the amygdala on hypothalamo–pituitary–adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 33.Reznikov L.R., Reagan L.P., Fadel J.R. Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res. 2009;1256:61–68. doi: 10.1016/j.brainres.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Yamada K, Narimatsu Y, Onuki M, Okada N, Kubo K, Sasaguri K, et al. GABAergic neurons in the basolateral amygdala are essential to ameliorative effects of chewing. Submitted.
- 35.Roland J.J., Stewart A.S., Janke K.L., Giolow M.R., Kostek J.A., Savage L.M. Medial septum-diagonal band of Broca (MSDB) GABAergicregulation of hippocampal acetylcholine efflux is dependent on cognitivedemands. J Neurosci. 2014;34(2):506–514. doi: 10.1523/JNEUROSCI.2352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radley J.J., Gosselink K.L., Sawchenko P.E. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen B.S. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1–2):172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 38.Ono Y., Kataoka T., Miyake S., Cheng S.J., Tachibana A., Sasaguri K.I. Chewing ameliorates stress-induced suppression of hippocampal long-term potentiation. Neuroscience. 2008;154(4):1352–1359. doi: 10.1016/j.neuroscience.2008.04.057. Epub 2008 May 3. [DOI] [PubMed] [Google Scholar]
- 39.Miyake S., Yoshikawa G., Yamada K., Sasaguri K., Yamamoto T., Onozuka M. Chewing ameliorates stress-induced suppression of spatial memory by increasing glucocorticoid receptor expression in the hippocampus. Brain Res. 2012;1446:34–39. doi: 10.1016/j.brainres.2012.01.011. Epub 2012 Jan 16. [DOI] [PubMed] [Google Scholar]
- 40.Ericson H., Blomqvist A., Köhler C. Brainstem afferents to the tuberomammillary nucleus in the rat brain with special reference to monoaminergic innervation. J Comp Neurol. 1989;281(2):169–192. doi: 10.1002/cne.902810203. [DOI] [PubMed] [Google Scholar]
- 41.Elliott E.M., Sapolsky R.M. Corticosterone enhances kainic acid-induced calcium elevation in cultured hippocampal neurons. J Neurochem. 1992;59(3):1033–1040. doi: 10.1111/j.1471-4159.1992.tb08345.x. [DOI] [PubMed] [Google Scholar]
- 42.Ono Y., Kataoka T., Miyake S., Sasaguri K., Sato S., Onozuka M. Chewing rescues stress-suppressed hippocampal long-term potentiation via activation of histamine H1 receptor. Neurosci Res. 2009;64(4):385–390. doi: 10.1016/j.neures.2009.04.011. Epub 2009 Apr 22. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald J.F., Kotecha S.A., Lu W.Y., Jackson M.F. Convergence of PKC-dependent kinase signal cascades on NMDA receptors. Curr Drug Targets. 2001;2:299–312. doi: 10.2174/1389450013348452. [DOI] [PubMed] [Google Scholar]
- 44.Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of hippocampal neuronal responses to NMDA. J Physiol. 1992;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krettek J.E., Price J.L. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and subiculum in the rat and cat. J Comp Neurol. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- 46.Aggleton J.P. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res. 1986;64:515–526. doi: 10.1007/BF00340489. [DOI] [PubMed] [Google Scholar]
- 47.Pikkarainen M., Ronkko S., Savander V., Insausti R., Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- 48.Kim J.J., Diamond D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 49.Kim J.J., Koo J.W., Lee H.J., Han J.S. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25(6):1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldman R.S., Meyer J., Quenzer L.F. Sinauer Associates Inc.; Sunderland, Mass U.S.A: 1997. Principles of neuropsychopharmacology. ISBN 0-87893-175-9. [Google Scholar]
- 51.Sasaguri K., Yoshikawa G., Yamada K., Miyake S., Kubo K.Y., Yamamoto T. Combination of chewing and stress up-regulates hippocampal glucocorticoid receptor in contrast to the increase of mineralocorticoid receptor under stress only. Neurosci Lett. 2012;519(1):20–25. doi: 10.1016/j.neulet.2012.04.080. [DOI] [PubMed] [Google Scholar]
- 52.Pavlides C., Watanabe Y., Magarinos A.M., McEwen B.S. Opposing roles of type I and type II adrenal steroid receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- 53.Kim J.J., Yoon K.S. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 54.Smriga M., Nishiyama N., Saito H. Mineralocorticoid receptor-mediated enhancement of neuronal excitability and synaptic plasticity in the dentate gyrus in vivo is dependent on the beta-adrenergic activity. J Neurosci Res. 1998;51:593–601. doi: 10.1002/(SICI)1097-4547(19980301)51:5<593::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 55.Pavlides C., McEwen B.S. Effects of mineralocorticoid and glucocorticoid receptors on long-term potentiation in the CA3 hippocampal field. Brain Res. 1999;851:204–214. doi: 10.1016/s0006-8993(99)02188-5. [DOI] [PubMed] [Google Scholar]
- 56.Tsuda A., Tanaka M., Ida Y., Shirao I., Gondoh Y., Oguchi M. Expression of aggression attenuates stress-induced increases in rat brain noradrenaline turnover. Brain Res. 1988;474(1):174–180. doi: 10.1016/0006-8993(88)90680-4. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T., Yoshida M., Yokoo H., Tomita M., Tanaka M. Expression of aggression attenuates both stress-induced gastric ulcer formation and increases in noradrenaline release in the rat amygdala assessed by intracerebral microdialysis. Pharmacol Biochem Behav. 1998;59(1):27–31. doi: 10.1016/s0091-3057(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 58.Keay K.A., Bandler R. Parallel circuits mediating distinct emotional coping reactions to different type of stress. Neurosci Biobehav Res. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 59.Benarroch E.E. Periaqueductal gray: an interface for behavioral control. Neurology. 2012;78:210–217. doi: 10.1212/WNL.0b013e31823fcdee. [DOI] [PubMed] [Google Scholar]
- 60.Yamada K., Narimatsu Y., Ono Y., Sasaguri K., Onozuka M., Kawata T. Chewing suppresses the stress-induced increase in the number of pERK-immunoreactive cells in the periaqueductal grey. Neurosci Lett. 2015;599:43–48. doi: 10.1016/j.neulet.2015.05.023. Epub 2015 May 14. [DOI] [PubMed] [Google Scholar]
- 61.Jaggi A.S., Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011;1381:187–201. doi: 10.1016/j.brainres.2011.01.002. Epub 2011 Jan 14. [DOI] [PubMed] [Google Scholar]
- 62.de Abreu A.R., Abreu A.R., Santos L.T., de Souza A.A., da Silva L.G., Jr, Chianca D.A., Jr Amygdalar neuronal activity mediates the cardiovascular responses evoked from the dorsolateral periaqueductal gray in conscious rats. Neuroscience. 2015;284:737–750. doi: 10.1016/j.neuroscience.2014.10.055. Epub 2014 Nov 4. [DOI] [PubMed] [Google Scholar]
- 63.Alves F.H., Crestani C.C., Resstel L.B., Correa F.M. Cardiovascular effects of noradrenaline microinjected into the insular cortex of unanesthetized rats. Auton Neurosci. 2011;160:90–98. doi: 10.1016/j.autneu.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Nagai M., Hoshide S., Kario K. The insular cortex and cardiovascular system: a new insight into the brain–heart axis. J Am Soc Hypertens. 2010;4:174–182. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Mesulam M.M., Mufson E.J. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [PubMed:7174907] [WorldCat.org] [DOI]. [DOI] [PubMed] [Google Scholar]
- 66.Jasmin L., Burkey A.R., Granato A., Ohara P.T. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- 67.Accolla R., Bathellier B., Petersen C.C., Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruggiero D.A., Mraovitch S., Granata A.R., Anwar M., Reis D.J. A role of insular cortex in cardiovascuclar function. J Comp Neurol. 1987;257:189–207. doi: 10.1002/cne.902570206. [DOI] [PubMed] [Google Scholar]
- 69.Yasui Y., Breder C.D., Saper C.B., Cechetto D.F. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- 70.Craig A.D. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [PubMed:12965300] [WorldCat.org]. [DOI] [PubMed] [Google Scholar]
- 71.Singer T., Critchley H.D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci (Regul Ed) 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [PubMed:19643659] [WorldCat.org] [DOI]. [DOI] [PubMed] [Google Scholar]
- 72.Onuki M., Yamamoto T., Sasaguri K., Yamada K., Kawata T. Chewing ameliorates the effects of restraint stress on pERK immunoreactive neurons in the rat insular cortex. Neurosci Lett. 2018;674:60–65. doi: 10.1016/j.neulet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Alves F.H., Crestani C.C., Correa F.M. The insular cortex modulates cardiovascular responses to acute restraint stress in rats. Brain Res. 2010;1333:57–63. doi: 10.1016/j.brainres.2010.03.077. [DOI] [PubMed] [Google Scholar]
- 74.Alves F.H., Crestani C.C., Resstel L.B., Corrêa F.M. Both α1- and α2-adrenoceptors in the insular cortex are involved in the cardiovascular responses to acute restraint stress in rats. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0083900. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi C.J., Cassell M.D. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. J Comp Neurol. 1998;399(4):469–491. doi: 10.1002/(sici)1096-9861(19981005)399:4<469::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 76.Shi C.J., Cassell M.D. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399(4):440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 77.Joels M., Baram T.Z. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cecchi M., Khoshbouei H., Javors M., Morilak D.A. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 79.Ma S., Morilak D.A. Chronic intermittent cold stress sensitises the hypothalamic–pituitary–adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- 80.Pardon M.C., Gould G.G., Garcia A., Phillips L., Cook M.C., Miller S.A. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 81.Cecchi M., Khoshbouei H., Morilak D.A. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 82.Benarroch E.E. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68(10):988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 83.Kubo K.Y., Sasaguri K., Ono Y., Yamamoto T., Takahashi T., Watanabe K. Chewing under restraint stress inhibits the stress-induced suppression of cell birth in the dentate gyrus of aged SAMP8 mice. Neurosci Lett. 2009;466(December (3)):109–113. doi: 10.1016/j.neulet.2009.08.030. Epub 2009 Aug 15. [DOI] [PubMed] [Google Scholar]
- 84.Ono Y., Koizumi S., Onozuka M. Chewing prevents stress-induced hippocampal LTD formation and anxiety-related behaviors: a possible role of the dopaminergic system. Biomed Res Int. 2015;2015:294068. doi: 10.1155/2015/294068. Epub 2015 May 17. PMID: 26075223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okada S., Hori N., Kimoto K., Onozuka M., Sato S., Sasaguri K. Effects of biting on elevation of blood pressure and other physiological responses to stress in rats: biting may reduce allostatic load. Brain Res. 2007;1185(December):189–194. doi: 10.1016/j.brainres.2007.09.030. Epub 2007 Sep 21. [DOI] [PubMed] [Google Scholar]
- 86.Miyake S., Wada-Takahashi S., Honda H., Takahashi S.S., Sasaguri K., Sato S. Stress and chewing affect blood flow and oxygen levels in the rat brain. Arch Oral Biol. 2012;57(November (11)):1491–1497. doi: 10.1016/j.archoralbio.2012.06.008. Epub 2012. [DOI] [PubMed] [Google Scholar]
- 87.Miyake S., Takahashi S.S., Yoshino F., Todoki K., Sasaguri K., Sato S. Nitric oxide levels in rat hypothalamus are increased by restraint stress and decreased by biting. Redox Rep. 2008;13(1):31–39. doi: 10.1179/135100008X259132. [DOI] [PubMed] [Google Scholar]
- 88.Hori N., Lee M.C., Sasaguri K., Ishii H., Kamei M., Kimoto K. Suppression of stress-induced nNOS expression in the rat hypothalamus by bitinRg. J Dent Res. 2005;84(7):624–628. doi: 10.1177/154405910508400708. [DOI] [PubMed] [Google Scholar]