Abstract
Background:
An external ventricular drain (EVD) treats hydrocephalus in patients with aneurysmal subarachnoid hemorrhage (aSAH). This study examines the utility of cerebrospinal fluid (CSF) lactate collected from an EVD as a proposed biomarker to predict patient outcome and vasospasm/delayed cerebral ischemia.
Methods:
Consecutive adults admitted to Wake Forest Baptist Medical Center from 2010 to 2015 with aSAH were identified through the electronic medical record, and clinical variables were collected and analyzed for correlation with incidence of vasospasm and discharge outcome.
Results:
In all, 51 patients with aSAH and an EVD had CSF lactate measured which ranged from 1.9 to 6.2 mmol/L, with a median value of 3.2 mmol/L. Vasospasm based on transcranial Doppler assessment occurred in 29 patients (57%), of which 20 (45%) were clinically symptomatic. Good outcome (discharge to home/acute rehab) occurred in 35 patients (69%). Sixteen patients (31%) had an unfavorable outcome (died/discharged to nursing homes/long-term acute care facility). In multivariate regression analysis, unfavorable outcome at discharge (P = 0.02), elevated CSF protein (P = 0.04), and admission Hunt and Hess score 3–5 (P = 0.05) were significantly associated with higher CSF lactate. The risk of symptomatic vasospasm increased with lactate in univariate analysis, but did not reach statistical significance (P = 0.077).
Conclusion:
The measurement of the CSF biochemical markers using an EVD is feasible and safe. We found that elevated CSF lactate correlates with patient outcome. Larger prospective studies are needed to test the validity of this finding and for understanding the underlying pathophysiologic mechanisms.
Keywords: Cerebrospinal fluid lactate, intracranial aneurysm, lactate, outcome, subarachnoid hemorrhage
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is a complex disorder with initial treatment centered on securing the aneurysm, followed by a prolonged phase of treating and monitoring a variety of potential neurologic and medical complications,[1,5,20] in an effort to prevent secondary brain insult. Vasospasm and delayed cerebral ischemia (DCI), in particular, contribute to nearly 30% of morbidity and mortality.[4,12]
Early detection and treatment of vasospasm and DCI is the cornerstone of neurocritical care in patients with aSAH. A detailed neurological examination is indispensable; however, clinical examination alone may not be sufficient to rule out DCI, especially in patients with altered consciousness. Various tools such as transcranial Doppler ultrasound (TCD), computed tomography angiogram (CTA), computed tomography perfusion scan, electroencephalogram, and catheter angiography complement the bedside examination.
Measuring the biochemical milieu may be an adjunct to imaging for detection of vasospasm and impending DCI. Cerebral microdialysis enables bedside analysis of the chemistry of the extracellular space.[15] The analysis of the absolute values and ratios of lactate and pyruvate can help in early detection of metabolic crisis;[16,18] however, microdialysis is an invasive technique, and data obtained are truly representative only of the local tissue milieu. An external ventricular drain (EVD) is often already in place in patients at high risk for the development of symptomatic vasospasm due to concomitant development of hydrocephalus from larger volumes of SAH and intraventricular hemorrhage.[3,23] The EVD represents an alternative to microdialysis, allowing collection of cerebrospinal fluid (CSF) for analysis, which may represent the metabolic environment on a more global scale.[6,7,8,14] CSF lactate may be a useful marker for impending DCI as it is created during the glycolytic process during brain insult. The objective of this retrospective study is to assess whether ventricular CSF lactate measured during aSAH is related to the risk of vasospasm and/or outcome.
MATERIALS AND METHODS
Following approval from the Institutional Review Board, consecutive adults (18 years of age or older) admitted to Wake Forest Baptist Medical Center from August 2010 to April 2015 with aSAH were identified through the electronic medical record. Charts were manually reviewed, and those who had CSF lactate levels drawn through an EVD within the first 10 days of symptom onset were included in the study. At our center, lactate is routinely measured in the CSF sample of all patients to monitor for ventriculitis. Usual triggers for CSF analysis are unexplained fever, leukocytosis, and/or unexplained change in mentation; however, some providers also send CSF for analysis every 3 days for monitoring purposes. Additional clinical information collected included admission Fisher's score, Hunt and Hess score, radiographic findings, aneurysm location and treatment method, transcranial Doppler velocities, CSF analysis, and clinical outcomes. Screening for vasospasm and DCI was done with regular neurologic examinations, daily screening TCD, and, when necessary, CTA and catheter angiogram. Sonographic vasospasm was defined as a velocity of >120 cm/s in the middle cerebral artery or anterior cerebral artery on TCD. CSF cultures were analyzed to evaluate for the presence of ventriculitis. Cerebral infarction was confirmed with diagnostic imaging, which consisted of diffusion-weighted and apparent diffusion coefficient magnetic resonance imaging (MRI) of the brain in the majority of cases and computed tomography imaging of the brain if MRI was not performed. Clinical outcomes were graded based on discharge disposition. The outcome was defined as good if discharged home or to acute rehabilitation and unfavorable if discharged to nursing home, long-term acute care, hospice, or death before discharge.
Statistical analysis
All statistical analysis was done using SAS software JMP Pro version 13.0. The results were analyzed using descriptive statistics and P values ≤ 0.05 were considered statistically significant. Means and medians as appropriate with corresponding standard deviations and interquartile ranges, respectively, were used as measurements of central tendencies and dispersion. Next, a series of univariate analyses were performed to investigate the relationship between CSF lactate and several independent variables. P values were calculated using nonparametric tests (Kruskal–Wallis and Wilcoxon rank sum) for categorical variables and Pearson or Spearman correlation coefficients for continuous and ordinal data. All variables with univariate P values < 0.10 were then included in anautomated backward stepwise regression analysis using probability to enter 0.25 and probability to leave 0.1. The variables retained by this approach were then included in a logistic regression model using standard least squares approach and P values were calculated.
RESULTS
In all, 51 patients with aSAH requiring an EVD were included in the final analysis [Table 1]; 33 (65%) were women. On presentation, the most common Hunt and Hess grade was 3 (39%) and Fisher's score 4 (88%). Intracerebral hemorrhage occurred in 11 (22%) patients. Ruptured aneurysms were most commonly seen in the anterior circulation (n = 33, 65%). Coiling was a slightly more common method of treatment (n = 29, 57%). The median CSF lactate was drawn at 4 days [interquartile range (IQR) 3–6, range 2–9) following symptom onset. Lactate values ranged from 1.9 to 6.2 mmol/L, with a median value of 3.2 mmol/L (IQR 2.4–3.7).
Table 1.
Patient characteristics
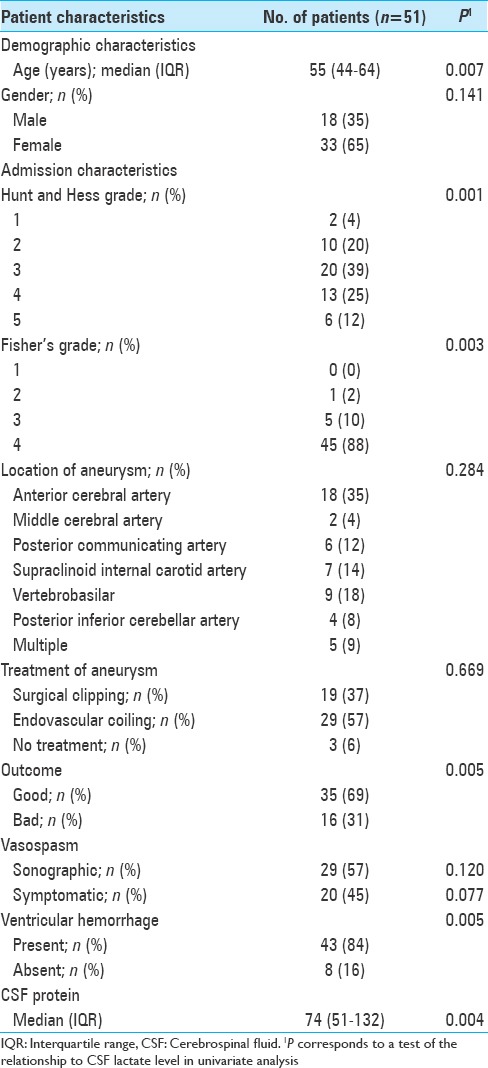
CSF cultures confirmed ventriculitis in five patients. Alpha hemolytic streptococcus was the most commonly isolated organism. CSF lactate in these patients ranged from 2 to 3.7 mmol/L, with a median value of 2.5 mmol/L. Three of these five patients went on to develop symptomatic vasospasm. During the hospital course, sonographic evidence of vasospasm (based on TCD criteria) was found in 29 patients (57%), with 20 (39%) becoming symptomatic. Good outcome occurred in 35 (69%) patients, defined as discharge to home or acute rehabilitation. Sixteen patients (31%) either died or were discharged to nursing homes/long-term acute care facility and considered to have an unfavorable outcome.
In univariate analyses, higher levels of CSF lactate were significantly related to older age (r = 0.37, P = 0.007), higher admission Fisher's grade (r = 0.41, P = 0.003), higher admission Hunt and Hess score (r = 0.45, P = 0.001), and higher levels of CSF protein (r = 0.41, P = 0.004). Individuals with intraventricular hemorrhage (P = 0.005) and those with unfavorable outcomes at discharge (P = 0.005) had higher median levels of CSF lactate. The risk of symptomatic vasospasm increased with lactate; however, it did not reach statistical significance (P = 0.077). Clinical severity of hemorrhage at admission was estimated using Hunt and Hess score and the patients were dichotomized into two groups, one group with scores from 0 to 2 and the other with scores from 3 to 5 for inclusion in multivariate model. The correlation of CSF lactate with admission Hunt and Hess score is outlined in Figure 1. These seven variables, namely, age, admission Fisher's grade, admission Hunt and Hess score, CSF protein, ventricular hemorrhage, outcome, and symptomatic vasospasm were then analyzed using an automated backward stepwise regression analysis as outlined earlier in statistical analysis section. Four variables retained by this approach were then included in a logistic regression model using standard least squares approach and P values were reported as follows: unfavorable outcome at discharge (P = 0.02), CSF protein (P = 0.04), admission Hunt and Hess score 3–5 (P = 0.05), and older age (P = 0.056). CSF lactate was not significantly related to vasospasm in multivariate analysis.
Figure 1.
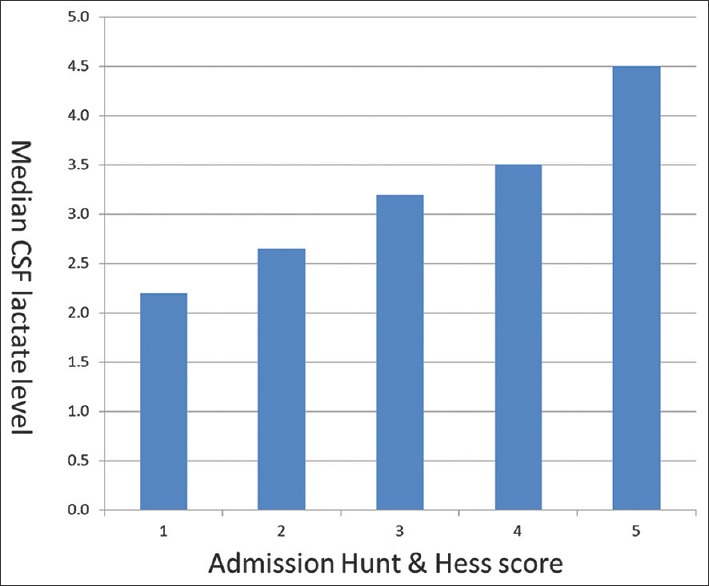
Median CSF lactate levels increase along with increasing the clinical severity of subarachnoid hemorrhage on presentation as measured using admission Hunt and Hess score
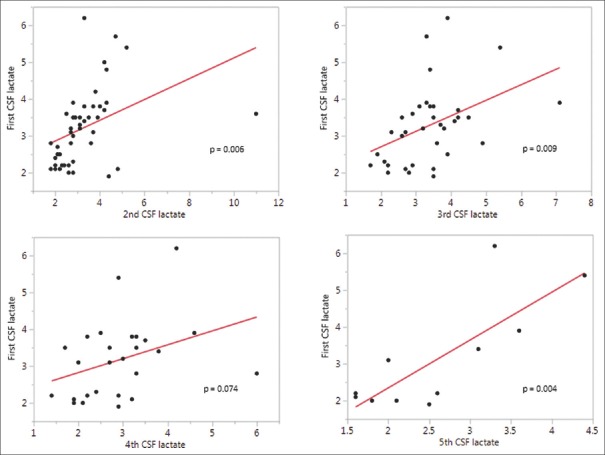
For a subset of patients, we had more than one CSF lactate level measured. As these values were not available on all patients, we could not include them in multivariate analysis. To determine how the initial levels of CSF lactate were related to later levels, we performed a series of correlations. [Figure 2] displays the correlation between CSF lactate levels drawn at five separate time points as follows: for second lactate level drawn at a median of 7 days from symptom onset, IQR 6–9, n = 44, P = 0.006, r = 0.40; for third lactate level drawn at a median of 10 days from symptom onset, IQR 8–12, n = 36, P = 0.009, r = 0.43; for fourth lactate level drawn at a median of 11 days from symptom onset, IQR 10–16, n = 26, P = 0.074, r = 0.36; and for fifth lactate level drawn at a median of 13 days from symptom onset, IQR 11–19, n = 9, P = 0.004, r = 0.79.
Figure 2.
First CSF lactate level against subsequent levels drawn during the same admission in a subset of patients demonstrating positive correlation, which supports the validity of the first measurement to be used as a single predictive marker in downstream analyses
DISCUSSION
This is the largest study examining the utility of ventricular fluid CSF lactate for predicting vasospasm and outcome in patients with aSAH. We found that elevated CSF lactate measured using the EVD within the first 10 days following symptom onset correlates with unfavorable outcome at discharge. However, we did not find any correlation with vasospasm nor infection in our multivariate model although there was a trend toward significance in univariate analysis with regard to vasospasm.
Biomarkers predictive of vasospasm and patient outcome represent an important area of research in aSAH.[2] Cerebral microdialysis as a technique allows continuous measurements of the brain extracellular milieu. Since its conception by Ungerstedt and Pycock in the 1970s, the procedure has undergone significant refinement and there is a growing body of literature regarding its use in patients with trauma and aSAH.[11] Lactate, pyruvate, glycerol, and glutamate are the major molecules of interest. Elevated lactate and lactate/pyruvate (LP) ratio have been shown to correlate with poor outcome and predict the development of vasospasm in patients with aSAH.[17,22] Microdialysis has two major pitfalls: first is that it only measures the local tissue milieu and may not represent the metabolism of the entire brain and second is that it is an invasive technique.
Since poor grade aSAH likely already have an EVD and are the population of patients at highest risk for vasospasm, we hypothesized that measuring the CSF lactate using EVD may represent the milieu of the whole brain and avoid an additional invasive procedure of placing the probe for microdialysis. Although we found a correlation with unfavorable outcome, we did not find an association with vasospasm although we suspect it to be the missing link explaining the poor outcome. Sample size, measuring a single lactate value, and retrospective design may be some of the factors not allowing us to adequately test a correlation with vasospasm.
Lactate is produced in the CNS through aerobic glycolysis by astrocytes and is transferred to neurons to act as an alternative substrate for glucose during cerebral insult.[16] Structural changes within endothelium during vasospasm have been proposed to correlate with a failure of cell energy metabolism which leads to anaerobic glycolysis, glycogenolysis, and subsequent lactate accumulation in brain parenychema.[21] There remains uncertainty whether the observed increase in lactate is subsequent to a hyperglycolytic process, neurons using this alternative energy source, rather than a pathologic hypoxic environment, and therefore elevated lactate may be an indirect byproduct of vasospasm as a marker of the brain's adaptive response for an energy source rather than a driver of pathologic signaling.[16] In a prospective study by Oddo et al., the extracellular concentration of lactate and pyruvate, LP ratio, and brain tissue O2 content were measured and they found that brain tissue lactate is frequently elevated in aSAH due to a hyperglycolytic state (high pyruvate and normal O2 content) and not often hypoxia. Patients with elevate lactate from a hyperglycolytic state had a good outcome, and for those patients elevated lactate is simply a marker of an adaptive metabolic response to subarachnoid hemorrhage. Only those with brain tissue hypoxia and elevated LP ratio (representing true energy failure) had an unfavorable outcome.
The literature regarding the clinical utility of CSF lactate measured from the ventricular fluid is sparse. In a study of 38 patients by Shimoda et al., elevated ventricular CSF lactate was found to correlate with the World Federation of Neurosurgical Societies score and prognosis.[19] In this study, poor prognosis group had a significant tendency of lactic acidosis, especially between days 5 and 7 after aSAH. Ventricular CSF lactate increased even without CSF acidosis in poor grade group. Mori et al. reported similar findings in a series of 20 patients with aSAH. The authors measured ventricular CSF lactate, pyruvate, and LP ratio and found that CSF lactate levels, pyruvate, and LP ratios were higher on post-rupture days 5–7 in patients with symptomatic vasospasm.[14] Using this previous work, we chose a cutoff of <10 days from symptom onset to interpret the CSF lactate measurement.
Using EVD to measure the CSF biochemical milieu is an attractive alternative to placing probes for cerebral microdialysis. Our study provides preliminary data and a potential path for further research in this direction. We demonstrate that collecting CSF from EVD for biochemical analysis is feasible and the complication rates are no higher than what is otherwise expected in this patient population. We had a ventriculitis rate of about 10% which is consistent with previous studies.[10,13] In addition, CSF lactate values did not correlate with ventriculitis as previous work suggests lactate may be a maker for bacterial meningitis,[9] which may represent an area of further investigation in an aneurysmal patient population.
We acknowledge several limitations with this study. First, the retrospective design and the use of data from a single tertiary care referral center can lead to a selection bias of complex and unusual cases. Second, our study design included only patients with an EVD, limiting the applicability of this potential biomarker to a subset of subarachnoid hemorrhage patients. While we acknowledge this limitation, we believe that our study has adequately focused on the at-risk population for developing vasospasm. Third, CSF lactate in this study was measured once within the first 10 days after subarachnoid hemorrhage. Lactate values following subarachnoid hemorrhage likely vary depending on the time from symptom onset and the development of vasospasm, making serial measurement of both lactate and pyruvate in all patients valuable yet not captured in this study due to the lack of such data. However, a subset of records contained serial lactate measurements and CSF lactate demonstrated a positive correlation between the first and subsequent measurements mitigating the possibility of missing lactate elevation with a single measurement. Fourth, only 20 patients in the study developed symptomatic vasospasm, which limited the power to detect vasospasm correlation with elevated CSF lactate. In a cohort of 51 patients, 20 of 51 (45%) symptomatic vasospasm are within the expected frequency, and a larger initial sample would be the best way to overcome this limitation. Finally, we do not have long-term follow-up data and therefore report outcomes at discharge.
CONCLUSION
In spite of the limitations noted above, our study provides useful information regarding the utility of ventricular CSF lactate measurement in aSAH. We demonstrate that the measurement of CSF biochemical markers using an already available EVD in aSAH patients is feasible. As opposed to microdialysis, which truly reflects local tissue milieu, ventricular CSF may perhaps represent the whole brain milieu and is an attractive avenue that needs further exploration. Our study is not adequately powered to detect significant difference in CSF lactate levels between patients with and without vasospasm. Future prospective studies are needed measuring lactate, pyruvate, and LP ratio at frequent intervals using ventricular CSF and correlating these biomarkers with vasospasm and long-term outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Jaclyn J. Renfrow, Email: jrenfrow@wakehealth.edu.
Casey D. Frey, Email: cfrey@wakehealth.edu.
Madison Arnel, Email: marnel@wakehealth.edu.
Stacey Q. Wolfe, Email: sqwolfe@wakehealth.edu.
Christopher McLouth, Email: cmclouth@wakehealth.edu.
Sudhir Datar, Email: sdatar@wakehealth.edu.
REFERENCES
- 1.Bonita R, Thomson S. Subarachnoid hemorrhage: Epidemiology, diagnosis, management, and outcome. Stroke. 1985;16:591–4. doi: 10.1161/01.str.16.4.591. [DOI] [PubMed] [Google Scholar]
- 2.Burrell C, Avalon NE, Siegel J, Pizzi M, Dutta T, Charlesworth MC, et al. Precision medicine of aneurysmal subarachnoid hemorrhage, vasospasm and delayed cerebral ischemia. Expert Rev Neurother. 2016;16:1251–62. doi: 10.1080/14737175.2016.1203257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The Fisher scale revisited. Stroke. 2001;32:2012–20. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 4.Datar S, Rabinstein AA. Postinterventional critical care management of aneurysmal subarachnoid hemorrhage. Curr Opin Crit Care. 2017;23:87–93. doi: 10.1097/MCC.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 5.Egge A, Waterloo K, Sjoholm H, Ingebrigtsen T, Forsdahl S, Jacobsen EA, et al. Outcome 1 year after aneurysmal subarachnoid hemorrhage: Relation between cognitive performance and neuroimaging. Acta Neurol Scand. 2005;112:76–80. doi: 10.1111/j.1600-0404.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 6.Fujishima M, Nakatomi Y, Tamaki K, Ishitsuka T, Kawasaki T, Omae T. Cerebrospinal fluid lactate and pyruvate concentrations in patients with malignant hypertension. J Neurol. 1984;231:71–4. doi: 10.1007/BF00313719. [DOI] [PubMed] [Google Scholar]
- 7.Fujishima M, Sugi T, Choki J, Yamaguchi T, Omae T. Cerebrospinal fluid and arterial lactate, pyruvate and acid-base balance in patients with intracranial hemorrhages. Stroke. 1975;6:707–14. doi: 10.1161/01.str.6.6.707. [DOI] [PubMed] [Google Scholar]
- 8.Gigante P, Hwang BY, Appelboom G, Kellner CP, Kellner MA, Connolly ES. External ventricular drainage following aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 2010;24:625–32. doi: 10.3109/02688697.2010.505989. [DOI] [PubMed] [Google Scholar]
- 9.Grille P, Torres J, Porcires F, Bagnulo H. Value of cerebrospinal fluid lactate for the diagnosis of bacterial meningitis in postoperative neurosurgical patients. Neurocir (Asturias, Spain) 2012;23:131–5. doi: 10.1016/j.neucir.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien) 2008;150:209–14. doi: 10.1007/s00701-007-1458-9. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson PJ, Jalloh I, Helmy A, Carpenter KLH, Rostami E, Bellander B-M, et al. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med. 2015;41:1517–28. doi: 10.1007/s00134-015-3930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyrouz SG, Diringer MN. Clinical review: Prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11:220. doi: 10.1186/cc5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo CH, Spelman D, Bailey M, Cooper DJ, Rosenfeld JV, Brecknell JE. External ventricular drain infections are independent of drain duration: An argument against elective revision. J Neurosurg. 2007;106:378–83. doi: 10.3171/jns.2007.106.3.378. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Nakajima K, Maeda M. Long-term monitoring of CSF lactate levels and lactate/pyruvate ratios following subarachnoid haemorrhage. Acta Neurochir (Wien) 1993;125:20–6. doi: 10.1007/BF01401823. [DOI] [PubMed] [Google Scholar]
- 15.Nyberg C, Karlsson T, Hillered L, Ronne Engström E. Metabolic pattern of the acute phase of subarachnoid hemorrhage in a novel porcine model: Studies with cerebral microdialysis with high temporal resolution. PLoS One. 2014;9:e99904. doi: 10.1371/journal.pone.0099904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oddo M, Levine JM, Frangos S, Maloney-Wilensky E, Carrera E, Daniel RT, et al. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke. 2012;43:1418–21. doi: 10.1161/STROKEAHA.111.648568. [DOI] [PubMed] [Google Scholar]
- 17.Sarrafzadeh A, Haux D, Küchler I, Lanksch WR, Unterberg AW. Poor-grade aneurysmal subarachnoid hemorrhage: Relationship of cerebral metabolism to outcome. J Neurosurg. 2004;100:400–6. doi: 10.3171/jns.2004.100.3.0400. [DOI] [PubMed] [Google Scholar]
- 18.Sarrafzadeh AS, Sakowitz OW, Kiening KL, Benndorf G, Lanksch WR, Unterberg AW. Bedside microdialysis: A tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit Care Med. 2002;30:1062–70. doi: 10.1097/00003246-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda M, Yamada S, Yamamoto I, Tsugane R, Sato O. Time course of CSF lactate level in subarachnoid haemorrhage. Correlation with clinical grading and prognosis. Acta Neurochir (Wien) 1989;99:127–34. doi: 10.1007/BF01402321. [DOI] [PubMed] [Google Scholar]
- 20.Spetzler RF, McDougall CG, Albuquerque FC, Zabramski JM, Hills NK, Partovi S, et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg. 2013;119:146–57. doi: 10.3171/2013.3.JNS12683. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Muramatsu M, Kojima T, Taki W. Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2003;34:2796–800. doi: 10.1161/01.STR.0000103743.62248.12. [DOI] [PubMed] [Google Scholar]
- 22.Unterberg AW, Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001;94:740–9. doi: 10.3171/jns.2001.94.5.0740. [DOI] [PubMed] [Google Scholar]
- 23.Wilson T, Stetler W, Davis M, Giles D, Khan A, Chaudhary N, et al. Intraventricular hemorrhage is associated with early hydrocephalus, symptomatic vasospasm, and poor outcome in aneurysmal subarachnoid hemorrhage. J Neurol Surg Part A Cent Eur Neurosurg. 2014;76:126–32. doi: 10.1055/s-0034-1394189. [DOI] [PubMed] [Google Scholar]