Abstract
Background
Granulocyte colony-stimulating factors are effective at reducing the risk and duration of neutropenia. The current meta-analysis compared the neutropenia-related efficacy and safety of lipegfilgrastim to those of pegfilgrastim and filgrastim.
Methods
Embase was searched for trials examining the efficacy/safety of lipegfilgrastim, pegfilgrastim, or filgrastim. Outcomes included febrile neutropenia, severe neutropenia, duration of severe neutropenia, time to recovery of absolute neutrophil count, and incidence of bone pain. Direct comparisons were made using random-effects models. No trials directly compared lipegfilgrastim and filgrastim. Indirect comparisons were made between lipegfilgrastim and filgrastim with pegfilgrastim as the common comparator.
Results
This meta-analysis included a total of 5769 patients from 24 studies. Over all cycles, lipegfilgrastim showed a lower, nonsignificant risk of febrile neutropenia compared with pegfilgrastim. Lipegfilgrastim has a lower risk of febrile neutropenia versus filgrastim but was also not statistically significant. The risk ratio for severe neutropenia in cycle 1 was 0.80, a 20% reduction in favor of lipegfilgrastim. For cycles 2–4, the risk ratio was 0.53 (0.35, 0.79) for lipegfilgrastim versus pegfilgrastim. The risk of severe neutropenia in cycles 2–4 was also significantly lower for lipegfilgrastim (risk ratio 0.45, 0.27, 0.75, respectively). No significant differences were found for febrile neutropenia and severe neutropenia in cycle 1. However, in cycles 2–4, lipegfilgrastim was associated with significant and clinically meaningful reductions in risk of severe neutropenia versus either pegfilgrastim or filgrastim.
Conclusions
Compared with pegfilgrastim or filgrastim, lipegfilgrastim has a statistically significantly lower absolute neutrophil count recovery time; however, differences in duration of severe neutropenia and bone pain were nonsignificant.
Keywords: Neutropenia, lipegfilgrastim, pegfilgrastim, filgrastim, meta-analysis
Introduction
Neutropenia is a common yet serious complication experienced by cancer patients treated with myelosuppressive chemotherapy.1 Neutropenia is the primary cause for chemotherapy delays and dose reductions, potentially compromising patient outcomes, including survival and complete response rates.2–4 Of particular concern are severe neutropenia (SN; absolute neutrophil count (ANC) <0.5 × 109/L) and febrile neutropenia (FN; SN with fever), which is defined as an oral temperature of >38.3℃ or two consecutive readings of >38.0℃ for 2 h and an ANC of <0.5 × 109/L or expected to fall below 0.5 × 109/L.5 FN is associated with prolonged hospitalization, serious infections and the use of broad-spectrum antibiotics, decreased quality of life, and increased mortality.6,7 The average mortality rate associated with episodes of FN ranges from 5% to 13.7%; however, it can reach 50% or higher in selected populations.7–9 Additionally, FN is associated with substantial economic consequences related to hospitalization and employment losses.3
One of the primary treatment strategies to reduce the risk of SN and FN is the prophylactic use of granulocyte colony-stimulating factor (G-CSF). G-CSF is a biological growth factor that supports the proliferation, differentiation, and activation of hematopoietic cells.6,10 US and European guidelines10,11 suggest that G-CSF be used as primary prophylaxis after chemotherapy when the risk of FN is >20%. Prophylactic use of G-CSFs is associated with a reduction in the incidence, severity, and duration of SN (DSN) and FN, a reduction in FN-related hospitalizations, and a lower mortality rate due to infection.12–14 In addition, the use of G-CSFs is associated with fewer dose reductions and delays, leading to greater relative dose intensity and increasing the probability of receiving a full dose of chemotherapy within a cycle.13,15,16 The most frequent patient-reported adverse event (AE) associated with G-CSF use is mild-to-moderate bone pain.13
Two of the most widely used G-CSFs available are short-acting filgrastim (FIL; Neupogen®; Amgen Inc., Thousand Oaks, CA)17 and long-acting pegfilgrastim (PEG; Neulasta®; Amgen Inc.).18 Short-acting FIL is administered subcutaneously or intravenously once daily for up to 14 days or until the ANC has reached 10,000/mm3 following its chemotherapy-induced nadir. Previous clinical trials12,13,19–21 have indicated that 8 to 14 days of FIL treatment produce the most optimal results, with 11 injections as the average.19,22 Long-acting PEG is administered once per chemotherapy cycle,17 and previous randomized controlled trials (RCTs) and observational studies suggest that a single dose of PEG is equivalent, and in some instances superior, to a 10 - to 14-day daily course of FIL.23–26
Lipegfilgrastim (LIP) (Lonquex®; Teva Pharmaceuticals Industries Ltd, Petach Tikva, Israel) is a long-acting, once-per-cycle G-CSF that was approved by the European Medicines Agency in 2013. Phase 3 trials of chemotherapy-naïve patients with breast cancer reported that LIP was non-inferior to PEG with respect to DSN, and the incidence and duration of FN-related dose reductions, hospitalizations, and antibiotic use were similar to those of PEG.27,28 The safety profile of LIP is also similar to that of PEG and bone pain-related symptoms were similar in patients receiving LIP or PEG.27,29
This paper describes a meta-analysis of recombinant human G-CSF studies in patients receiving chemotherapy in order to assess the relative benefit of available long-acting agents (LIP versus PEG) and similarly compare LIP to the short-acting agent (FIL). Outcomes of interest were neutropenia-related efficacy and safety.
Methods
Data sources and search strategy
A systematic literature review was performed to identify trials examining the efficacy and safety of LIP, PEG, or FIL, either head-to-head or versus placebo/non-treatment (PLA/NT). Elsevier’s Embase Biomedical Answers website was used to identify published literature in the MEDLINE and Embase databases on the use of G-CSFs and/or antibiotic therapy in the prevention and treatment of chemotherapy-induced neutropenia and FN. The search crossed relevant disease and drug terms with additional terms related to epidemiology, humanistic and economic burden, and regional identifiers (see Supplemental material). The search was restricted to English-language reports published between 2005 and 2015. Additional records were identified by hand-searching other relevant sources, including product label information and the reference lists of key published studies.
Design
A direct meta-analysis comparing the efficacy of LIP versus PEG was chosen because randomized head-to-head trials have been conducted between the compounds. For the comparison of LIP versus FIL, indirect analytical techniques were employed because no head-to-head trials were identified. Potential paths of comparison and contrast of interest are outlined in Figure 1. We selected two common comparators (or links) for the indirect treatment comparison of LIP to FIL: via PEG and via PLA/NT. Refinements of the analytical methods were made based on the findings of the search and assessment of the completeness of data.
Figure 1.
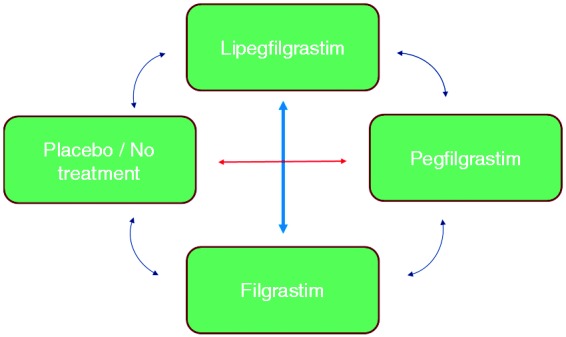
Multipath meta-analysis plan.
Study selection and data extraction
To be included in the analysis, studies needed to meet the following criteria: prospective design, randomized treatment assignment, double-blind design (or, at minimum, blinding of outcome assessment), pre-specified eligibility criteria, adequate sample size, report of the number of participants randomized, adequate details of treatment groups at baseline, comparable treatment groups at baseline, and similar co-interventions (that could affect results) across groups. A follow-up primary outcome assessment rate of 80% was required for the first cycle of treatment. Studies of G-CSFs for stem cell mobilization in bone marrow or peripheral blood stem cell transplantation and studies in children were excluded from consideration.
Abstracts of articles identified in the search were screened by two independent reviewers. Full reports were obtained for the studies selected in the first stage and evaluated by the two reviewers for final inclusion. Data related to the outcomes of interest were extracted independently by the reviewers into structured spreadsheets. At each step, disagreements between the reviewers were resolved by discussion or the inclusion of a third reviewer.
Data analysis
The pre-selected efficacy outcomes were incidence of FN, incidence of SN, hospitalization for FN or SN, time to recovery of ANC, and DSN. Because data on hospitalization incidence were missing in some reports and inconsistently reported in others, this outcome could not be analyzed. In addition to these pre-specified efficacy outcomes, incidence of bone pain was also examined. Random-effects meta-analysis was used to synthesize results from direct (head-to-head) trials using RevMan 5.2 software.
Meta-analysis methods for continuous outcome measures varied due to differences in the availability of reported results. Generic inverse variance methods based on estimates of mean treatment differences and standard errors were used for time to ANC recovery. Analysis of DSN employed inverse variance based on the means and standard deviations of each treatment group. Indirect estimates of treatment differences were conducted using the Bucher method30 with two iterations: PEG as the common comparator and PLA/NT as the common comparator.
Heterogeneity was assessed using forest plots that graphically represent between-study variability alongside the ratio measures. The inconsistency index (I2) was used to quantify the percentage of variability due to heterogeneity rather than sampling error. This index can be interpreted on a scale from 0–40% (might not be important) to 75–100% (considerable heterogeneity).
Where available, intention-to-treat analyses were used instead of per-protocol (PP) analyses. No imputation for missing data was performed for binary outcome measures. For continuous outcomes, missing standard deviations were calculated from the standard error or the 95% confidence interval (CI) or vice versa. The crossover study by Shi et al.31 was inadequately reported with statistical methods suitable for parallel-group studies. In the case of dose-finding studies, only arms with therapeutic levels of G-CSF were used in analyses (e.g., 6 mg of LIP or PEG); consequently, the Kosaka et al.32 study was not considered in the analyses.
Results
Study characteristics
After duplicates were removed, 91 unique abstracts were identified and screened (Figure 2). Of those, 35 full-text articles were obtained and reviewed for eligibility. Ultimately 24 studies involving 5769 patients were deemed eligible for inclusion and were subjected to data extraction. Characteristics of these 24 studies are presented in Table 1.
Figure 2.
Flow diagram of publication selection process.
Table 1.
Study characteristics.a
| Study | Tumor type | Definition of fever (for FN) | Frequency of neutrophil counts |
|---|---|---|---|
| LIP vs. PEG | |||
| Buchner et al.35 | Breast | >38.5℃ for 1 h | Cycle 1: 24 h before chemo, daily from day 2 to 15 until ANC recovery; Cycles 2–4: daily from day 5 |
| Bondarenko et al.27 | Breast | >38.5℃ for 1 h | Cycle 1: 24 h before chemo, daily up to day 15 or until ANC recovery; Cycles 2–4: 24 h before chemo, day 1 and 3, daily from day 5 to 15 of each cycle until ANC recovery |
| LIP vs. PLA/NT | |||
| Volovat et al.33 | NSCLC | >38.5℃ for 1 h | Cycle 1: 24 h before chemo, daily up to day 15 or until ANC recovery; Cycles 2–4: 24 h before chemo, day 3, daily from day 5 to 15 of each cycle until ANC recovery, end of study visit |
| PEG vs. FIL | |||
| Green et al.25 | Breast | ≥38.2℃ | Daily |
| Grigg et al.36 | NHL | >38.2℃ | Cycle 1: day 1 and 3, daily from day 7 to 14 until ANC recovery, then 3 times per week to end of cycle; Cycles 2, 4, 5: day 3, 2 times per week to end of cycle; Cycles 3 and 6: day 3, 3 times per week to end of cycle |
| Holmes et al.37 | Breast | ≥38.2℃ | Screening, before each cycle and 1 time per week in cycle 1 |
| Holmes et al.24 | Breast | ≥38.2℃ | Screening, before each cycle and 1 time per week in cycle 1 |
| Park et al.38 | Breast | Not defined | NR |
| Shi et al.31 | Solid, NHL | >38.0℃ | Day 0, 3, 5, 7, 9, 11, 13, 17, and 21 |
| Vose et al.34 | NHL, HD | ≥38.2℃ | Day 1, daily after day 6, and at follow–up visits |
| PEG vs. PLA/NT | |||
| Balducci et al.39 | Solid, NHL | ≥38.0℃ on same day | Day 1 of each cycle; cycle 1 day 8, once between day 11 and 13, and day 15; and at nadir following cycles |
| Hecht et al.40 | Colorectal | ≥38.2℃ | NR |
| Kosaka et al.32 | Breast | ≥37.5℃ on same or following day | Cycle 1: day 1, 2, 8, 11, and 15; Remaining cycles: day 1, 2, 8, and 11; Open label: day 1 and 2 |
| Vogel et al.41 | Breast | ≥38.2℃ | NR |
| FIL vs. PLA/NT | |||
| Crawford et al.20,42 | SCLC | ≥38.2℃ | NR |
| del Giglio et al.43 | Breast | >38.5℃ for more than 1 h | 24 h from chemo and daily from day 2 to 15 or until ANC recovery |
| Doorduijn et al.44 | NHL | >38.5℃ | NR |
| Fossa et al.45 | Germ cell | >38.0℃ | Day 1 of each cycle and weekly thereafter |
| Muhonen et al.46 | Breast | NR | NR |
| Osby et al.47 | NHL | >38.5℃ once or >38.0℃ twice in 4 h | Day 8 or 9, 11 or 12, 14 or 15, and 22 |
| Pettengell et al.48 | NHL | ≥37.5℃ for 1 h | Weekly; daily if FN occurred |
| Romieu et al.49 | Breast | >38.0℃ | Day 1 and every 3 days until ANC ≥ 2 × 109/L (at least day 14 ± 2) |
| Trillet-Lenoir et al.50 | SCLC | ≥38.2℃ | 3 times per week |
| Zinzani et al.51 | NHL | NR | NR |
ANC: absolute neutrophil count; FIL: filgrastim; FN: febrile neutropenia; HD: Hodgkin’s disease; LIP: lipegfilgrastim; NHL: non-Hodgkin’s lymphoma; NR: not reported; NSCLC: non-small-cell lung cancer; NT: no treatment; PEG: pegfilgrastim; PLA: placebo; SCLC: small cell lung cancer; SN: severe neutropenia.
Also stipulated protocol-defined SN as the administration of systemic antibiotics (not used in meta-analysis). Grade 3 neutropenia = ANC between 0.5 × 109/L and 1.0 × 109/L; Grade 4 neutropenia = ANC < 0.5 × 109/L.
Most studies included data from phase 2 or phase 3 RCTs with a mix of double-blind and open-label trials. Parallel-group comparisons were most common, although for two studies the comparator was existing treatment protocols or physician decision versus active treatment. In both cases, the first cycle of chemotherapy was treated as a placebo-controlled period. Trials included three types of control groups: active control, placebo control, or no treatment.
Eleven reports provided data from trials of breast cancer tumors, one study involved non small-cell lung disease, two studies were for small cell lung cancer, one was for colorectal cancer, eight involved non-Hodgkin’s lymphoma, and one was for germ cell tumors. Across trials, the percentage of females ranged from 31% to 100% (in breast cancer). Three studies included patients with stage I cancer; the remainder included only more advanced cancer stages (II–IV). Full descriptions of chemotherapy regimens and definitions of the efficacy outcomes are provided in Table 1.
The inclusion criteria of one pivotal LIP study design had a significant impact on the analysis. Due to ethical considerations, the LIP versus PLA study33 was limited to patients not at high risk for FN. As might be expected, the incidence of FN in the PLA group of this study was low (8.0%) compared to those in the PLA group of the older FIL versus PLA trials (12.5% to 59.6% per trial; 43.0% across the combined PLA population). For this reason, the older FIL versus PLA studies could not be considered “similar” to the LIP versus PLA study, and therefore the planned indirect analysis through PLA as a common comparator was deemed invalid, and only indirect analyses of LIP versus FIL through PEG are presented.
Direct and indirect comparisons
Incidence of FN
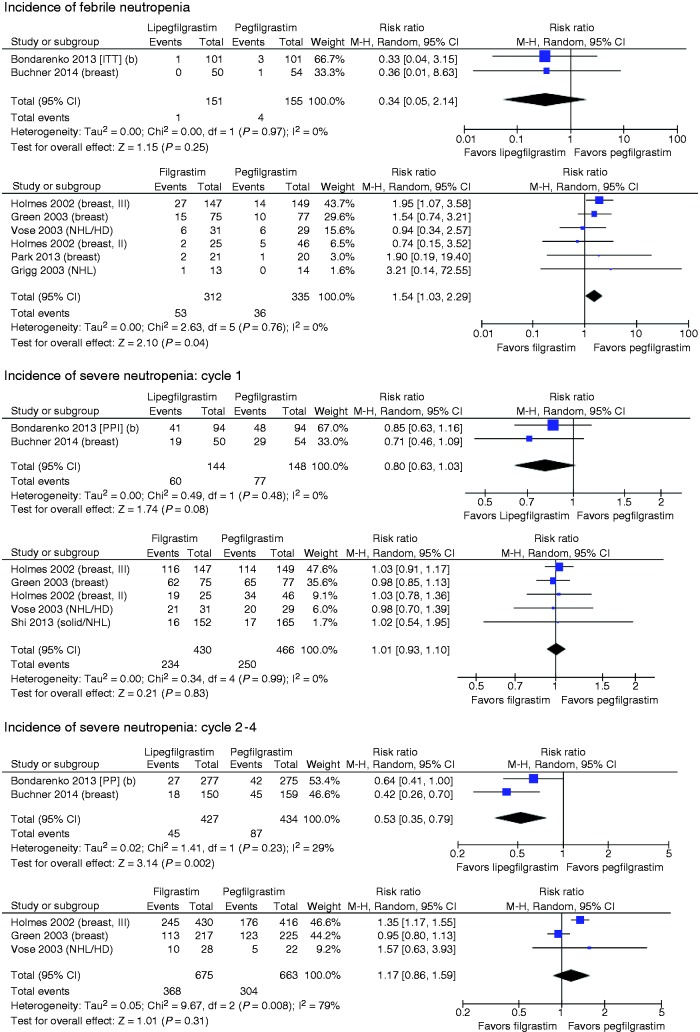
The incidence of FN was low across the two studies (see Figure 3), with only five events occurring among 306 patients. In the pooled comparison, the risk of FN was lower with LIP than PEG, but did not reach statistical significance (risk ratio (RR) = 0.34, 95% CI: 0.05, 2.14). In the FIL versus PEG head-to-head trials, the pooled comparison suggested that PEG had lower risk of FN relative to FIL (RR = 1.54, 95% CI: 1.03, 2.29). In the indirect comparison of LIP versus FIL via PEG, LIP had nonstatistically significant lower risk of FN in the overall analyses (RR = 0.22, 95% CI: 0.03, 1.51). Similar results were seen in two sensitivity analyses (LIP versus FIL via PEG): one limited to studies of breast cancer patients (RR = 0.20, 95% CI: 0.03, 1.41) and one limited to reports published in the past five years (RR = 0.18, 95% CI: 0.02, 1.51).
Figure 3.
Forest plots of LIP versus PEG and FIL versus PEG.
Incidence of SN
Across all studies considered, the incidence of SN was substantially higher in the first cycle of chemotherapy than in subsequent cycles. Therefore, we report cycle 1 separately from cycles 2–4. LIP data from each cycle were available for all studies (see Table 2). Among FIL versus PEG studies, Holmes et al.24 reported only cycle 1 incidence, Vose et al.34 reported only cycles 1-2, and Shi et al.31 was a crossover study for which only cycle 1 data (prior to the crossover) were included (see Figure 3).
Table 2.
Comparison of incidence of FN and SN.
| Direct comparison |
Indirect comparison |
||
|---|---|---|---|
| Outcome | LIP vs. PEG RR (95% CI) | FIL vs. PEG RR (95% CI) | LIP vs. FIL (via PEG) RR (95% CI) |
| % FN | 0.34 (0.05, 2.14) | 1.54 (1.03, 2.29) | 0.22 (0.03, 1.51) |
| % SN cycle 1 | 0.80 (0.63, 1.03) | 1.01 (0.93, 1.10) | 0.79 (0.61, 1.03) |
| % SN cycles 2–4 | 0.53 (0.35, 0.79) | 1.17 (0.86, 1.59) | 0.45 (0.27, 0.75) |
CI: confidence interval; FIL: filgrastim; FN: febrile neutropenia; LIP: lipegfilgrastim; PEG: pegfilgrastim; RR: risk ratio; SN: severe neutropenia.
SN in cycle 1
The RR for SN in cycle 1 for the LIP versus PEG trials was 0.80 (95% CI: 0.63, 1.03), a 20% relative risk reduction in favor of LIP which did not reach statistical significance (p = 0.08). In cycle 1, the incidence of SN in the PEG-treated arms of FIL versus PEG studies ranged from 51.1% to 84.4% with one outlying result at 10.3%.31 In studies of FIL versus PEG, no appreciable difference was seen in the incidence of SN in cycle 1 of chemotherapy. (Note that the Bondarenko et al.27 report included only PP analyses.)
The indirect comparison of LIP and FIL produced a similar RR for SN in cycle 1: 0.79 (95% CI: 0.61, 1.03). Sensitivity analyses limited to breast cancer studies or excluding the outlying Shi et al.’s study did not change the estimate for the indirect analysis.
SN in cycles 2–4
The risk of SN per cycle in cycles 2–4 was statistically significantly lower for LIP versus PEG (RR = 0.53, 95% CI: 0.35, 0.79). In studies of PEG versus FIL, the overall RR was 1.17 (95% CI: 0.86, 1.59), a nonsignificant difference in favor of PEG. In the indirect comparison, the risk of SN in cycles 2–4 was significantly lower for LIP compared to FIL (RR = 0.45, 95% CI: 0.27, 0.75). The size and significance of this association remained in a sensitivity analysis excluding the study by Vose et al.34 (RR = 0.46, 95% CI: 0.27, 0.79), which reported only cycles 1 and 2.
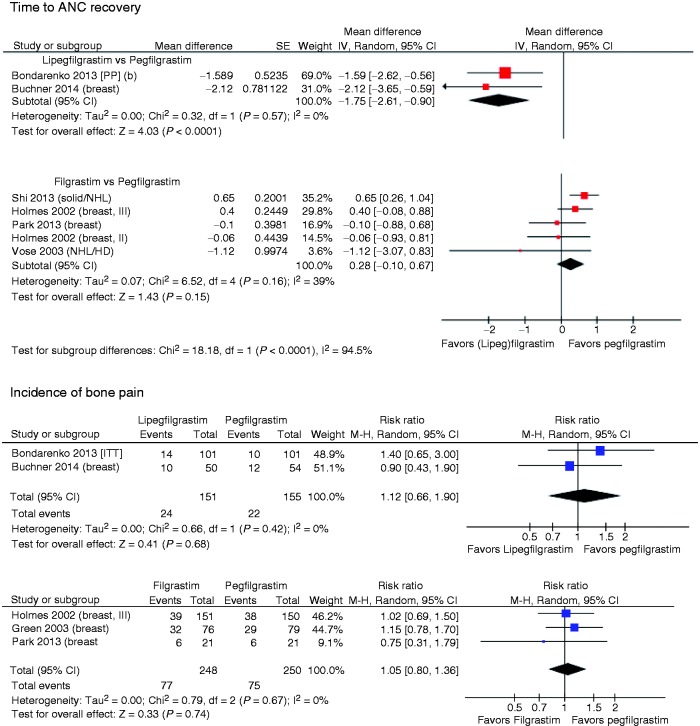
Time to ANC recovery
The mean time to ANC recovery was 1.75 days (95% CI: 0.90, 2.61) earlier in the LIP arms versus the PEG arms (Table 3). Five studies compared the efficacy of FIL versus PEG, with the two largest studies reflecting shorter time to recovery for PEG and the three smaller ones with estimates in the opposite direction (Figure 3).
Table 3.
Comparison of time to ANC recovery, duration of SN, and incidence of bone pain.a
| Direct comparison |
Indirect comparison |
||
|---|---|---|---|
| Outcome | LIP vs. PEG Mean difference (95% CI) | FIL vs. PEG Mean difference (95% CI) | LIP vs. FIL (via PEG) Mean difference (95% CI) |
| Time to ANC recovery, days | −1.75 (−2.61, −0.90) | 0.28 (−0.10, 0.60) | −1.88 (−2.82, −0.95) |
| Duration of SN, days | −0.17 (−0.39, 0.04) | 0.00 (−0.28, 0.28) | −0.17 (−0.53, 0.19) |
| % Bone pain | 1.12 (0.66, 1.90) | 1.05 (0.80, 1.36) | 1.07 (0.59, 1.93) |
ANC: absolute neutrophil count; CI: confidence interval; FIL: filgrastim; LIP: lipegfilgrastim; PEG: pegfilgrastim; RR: risk ratio; SN: severe neutropenia.
RR reported for bone pain.
Mean recovery time was shorter for LIP versus FIL by 1.88 days (95% CI: 0.95, 2.82). For the main indirect comparison, the Shi et al.’s crossover study31 was excluded due to unknown intrapatient correlation in cycles 2–4. The size and direction of the difference between LIP and FIL remained in a sensitivity analyses limited to breast cancer patients, with a mean difference of 1.95 days (95% CI: 1.02, 2.88) in favor of LIP. When the Shi’s study was included with various assumptions regarding intrapatient correlation across the cycles, similar results were found (point estimates from 2.03 to 2.04 days).
Duration of severe neutropenia
For the LIP versus PEG trials, which involved daily neutrophil counts, the mean DSN did not differ significantly between LIP and PEG, with a mean difference of 0.17 days (95% CI: −0.39, 0.04) in favor of LIP (see Table 3). The differences between treatment arms within the FIL versus PEG studies were quite small: less than one day in either direction. The overall estimated effect was 0.0 days. The indirect analysis of LIP versus FIL reflects primarily the difference between LIP and PEG, with the extra comparison of FIL versus PEG not changing the estimate.
Incidence of bone pain
Combining the two studies that compared LIP with PEG, the rates of bone pain were 15.9% and 14.2%, respectively (see Table 3 and Figure 3). The direct comparison of LIP with PEG produced a RR of 1.12 (95% CI: 0.66, 1.90), indicating that the difference did not reach statistical significance. The indirect comparison of LIP versus FIL via PEG was similar to that of the direct comparison and was also nonsignificant: 1.07 (95% CI: 0.59, 1.93).
Discussion
Myeloid growth factors are critical to reduce the incidence of chemotherapy-induced neutropenia and FN and allow full dosing of chemotherapy on schedule. Commercially available agents include FIL, which is administered daily; and the long-acting, once-per-chemotherapy cycle drugs LIP and PEG. This meta-analysis applied a mixed treatment comparison approach with direct and indirect comparisons to make it possible to compare the efficacy of LIP versus PEG (through a direct meta-analysis) and LIP versus FIL (through an indirect meta-analysis with PEG as the common comparator). Four efficacy outcomes and one AE were included to provide a comprehensive comparison of these agents.
In trials that compared LIP to PEG, the risk of FN was numerically lower for LIP but not statistically significant. This may be due to the low incidence of FN in the two studies. The overall low incidence of FN in the Bondarenko et al.27 study may be explained by the protocol-specified strict definition for FN and the use of prophylactic systemically active antibiotics for patients at high risk for infection in this non-inferiority trial, which may have reduced the FN event rate. This prophylactic treatment creates exchangeability problems for the indirect analysis of LIP versus FIL. Although FN occurred in 1.9% to 3.0% of patients in the PEG arm of the LIP versus PEG trials, the incidence among PEG-treated patients was as high as 20.7% in the PEG versus FIL trials. The myelosuppressive intensity of the chemotherapy regimens used in each study as well as the presence of other risk factors may have impacted the base rate of FN.
In chemotherapy cycles 2–4, LIP was associated with significant and clinically meaningful reductions in risk of SN versus either PEG (RR = 0.53, 95% CI: 0.35, 0.79) or FIL (RR = 0.45, 95% CI: 0.27, 0.75). In cycle 1, differences between LIP/PEG and LIP/FIL were smaller and did not reach statistical significance.
Time to ANC recovery, which has a longer duration than DSN overall, was significantly shorter for LIP than for both PEG (1.75 days, 95% CI: 0.90, 2.61) and FIL (1.88 days, 95% CI: 0.95, 2.82). Bone pain, the most commonly reported AE across trials, was numerically higher for LIP but not to a statistically significant extent.
For the outcome of FN, comparisons of LIP to PEG and FIL were hampered by the low incidence of FN in the two pivotal LIP studies: a non-inferiority study which had patients at high risk for infection use prophylactic systemically active antibiotics per protocol,27 and the other which was limited to patients not at high risk for FN.33 Although FN occurred in only 1.9% to 3.0% of patients in the PEG arm of the LIP versus PEG trials, the incidence among PEG-treated patients was as high as 20.7% in the PEG versus FIL trials.
The study also shares the limitations of most meta-analyses: differences in the baseline patient population (e.g., cancer type), differences in study design (e.g., crossover between groups and the timing of assessments of SN and ANC), differences in the definition of FN, and changes in concomitant treatment strategies over time (e.g., prophylactic antibiotic use).
The clinical trial data have clearly demonstrated the safety and efficacy of LIP. Unlike most of the previous studies, the current meta-analysis provided a comprehensive assessment of the efficacy and safety of LIP, PEG, and FIL by including the incidence of FN, SN, time to ANC recovery, DSN, and bone pain. Compared with either PEG or FIL, LIP was associated with lower risk of FN and SN in cycle 1, but the RRs were not statistically significant. In chemotherapy cycles 2–4, LIP was associated with significant and clinically meaningful reductions in risk of SN versus either PEG or FIL. Compared with PEG or FIL, LIP has a statistically significantly shorter ANC recovery time. Differences in DSN and bone pain were not significant. This meta-analysis supports the conclusion that LIP is more effective than either PEG or FIL for the prevention of SN in cycles 2–4 of chemotherapy and that patients treated with LIP had a shorter time to ANC recovery. More head-to-head clinical studies and real-world data analyses are suggested to validate the comparative findings.
Supplementary Material
Acknowledgments
All authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Editorial assistance in the preparation of this manuscript was provided by Jason Allaire, PhD of Generativity Solutions Group, Cary, NC, USA.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bond received funding from Teva Pharmaceuticals to develop the meta-analysis. Dr Schwartzberg has received consulting fees from Amgen Pharmaceuticals. Dr Klastersky has nothing to disclose. The remaining authors are employees of Teva Pharmaceuticals.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Teva Pharmaceuticals.
References
- 1.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 2004; 100: 228–237. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 2003; 21: 4524–4531. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Kuderer N, Greene J, et al. The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer 1998; 34: 1857–1864. [DOI] [PubMed] [Google Scholar]
- 4.Webster J, Lyman GH. Use of G-CSF to sustain dose intensity in breast cancer patients receiving adjuvant chemotherapy: a pilot study. Cancer Control 1996; 3: 519–523. [PubMed] [Google Scholar]
- 5.Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 2016; 27: v111–v118. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Djulbegovic B, Norris LB, et al. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med 2013; 368: 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006; 106: 2258–2266. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch BR, Lyman GH. Pharmacoeconomics of the myeloid growth factors: a critical and systematic review. PharmacoEconomics 2012; 30: 497–511. [DOI] [PubMed] [Google Scholar]
- 9.Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exper Ther Med 2011; 2: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011; 47: 8–32. [DOI] [PubMed] [Google Scholar]
- 11.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 2011; 52: e56–e93. [DOI] [PubMed] [Google Scholar]
- 12.Clark OA, Lyman GH, Castro AA, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol 2005; 23: 4198–4214. [DOI] [PubMed] [Google Scholar]
- 13.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 2007; 25: 3158–3167. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Dale DC, Culakova E, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 2013; 24: 2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almenar Cubells D, Bosch Roig C, Jimenez Orozco E, et al. Effectiveness of daily versus non-daily granulocyte colony-stimulating factors in patients with solid tumours undergoing chemotherapy: a multivariate analysis of data from current practice. Eur J Cancer Care 2013; 22: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shayne M, Culakova E, Wolff D, et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer 2009; 115: 5319–5328. [DOI] [PubMed] [Google Scholar]
- 17.Neupogen® (filgrastim) [prescribing information]. Thousand Oaks, CA: Amgen Inc, 2012.
- 18.Neulasta® (pegfilgrastim) [prescribing information]. Thousand Oaks, CA: Amgen Inc, 2012.
- 19.Cooper KL, Madan J, Whyte S, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 2011; 11: 404–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 1991; 325: 164–170. [DOI] [PubMed] [Google Scholar]
- 21.Heil G, Hoelzer D, Sanz MA, et al. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. The International Acute Myeloid Leukemia Study Group. Blood 1997; 90: 4710–4718. [PubMed] [Google Scholar]
- 22.Weycker D, Barron R, Edelsberg J, et al. Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis. BMC Health Serv Res 2014; 14: 189–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto L, Liu Z, Doan Q, et al. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin 2007; 23: 2283–2295. [DOI] [PubMed] [Google Scholar]
- 24.Holmes FA, Jones SE, O'Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 2002; 13: 903–909. [DOI] [PubMed] [Google Scholar]
- 25.Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003; 14: 29–35. [DOI] [PubMed] [Google Scholar]
- 26.Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer 2013; 13: 11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondarenko I, Gladkov OA, Elsaesser R, et al. Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 2013; 13: 386–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladkov OA, Buchner A, Bias P, et al. Chemotherapy-associated treatment burden in breast cancer patients receiving lipegfilgrastim or pegfilgrastim: secondary efficacy data from a phase III study. Support Care Cancer 2016; 24: 395–400. [DOI] [PubMed] [Google Scholar]
- 29.Bondarenko IM, Bias P, Buchner A. Incidence of bone pain in patients with breast cancer treated with lipegfilgrastim or pegfilgrastim: an integrated analysis from phase II and III studies. Support Care Cancer 2016; 24: 267–273. [DOI] [PubMed] [Google Scholar]
- 30.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–691. [DOI] [PubMed] [Google Scholar]
- 31.Shi YK, Chen Q, Zhu YZ, et al. Pegylated filgrastim is comparable with filgrastim as support for commonly used chemotherapy regimens: a multicenter, randomized, crossover phase 3 study. Anti-Cancer Drugs 2013; 24: 641–647. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka Y, Rai Y, Masuda N, et al. Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 2015; 23: 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volovat C, Bondarenko IM, Gladkov OA, et al. Phase III, randomized, double-blind, placebo-controlled, multicenter study of lipegfilgrastim in patients with non-small cell lung cancer receiving myelosuppressive therapy. SpringerPlus 2015; 4: 316-015-1067-1067–316-015-1067-1067 eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vose JM, Crump M, Lazarus H, et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol 2003; 21: 514–519. [DOI] [PubMed] [Google Scholar]
- 35.Buchner A, Elsasser R, Bias P. A randomized, double-blind, active control, multicenter, dose-finding study of lipegfilgrastim (XM22) in breast cancer patients receiving myelosuppressive therapy. Breast Cancer Res Treat 2014; 148: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigg A, Solal-Celigny P, Hoskin P, et al. Open-label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin's lymphoma. Leuk Lymphoma 2003; 44: 1503–1508. [DOI] [PubMed] [Google Scholar]
- 37.Holmes FA, O'Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 2002; 20: 727–731. [DOI] [PubMed] [Google Scholar]
- 38.Park KH, Sohn JH, Lee S, et al. A randomized, multi-center, open-label, phase II study of once-per-cycle DA-3031, a biosimilar pegylated G-CSF, compared with daily filgrastim in patients receiving TAC chemotherapy for early-stage breast cancer. Invest New Drugs 2013; 31: 1300–1306. [DOI] [PubMed] [Google Scholar]
- 39.Balducci L, Al-Halawani H, Charu V, et al. Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist 2007; 12: 1416–1424. [DOI] [PubMed] [Google Scholar]
- 40.Hecht JR, Pillai M, Gollard R, et al. A randomized, placebo-controlled phase ii study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer 2010; 9: 95–101. [DOI] [PubMed] [Google Scholar]
- 41.Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 2005; 23: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 42.Crawford J, Glaspy JA, Stoller RG, et al. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther 2005; 3: 36–46. [DOI] [PubMed] [Google Scholar]
- 43.del Giglio A, Eniu A, Ganea-Motan D, et al. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer 2008; 8: 2407–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doorduijn JK, van der Holt B, van Imhoff GW, et al. CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin's lymphoma. J Clin Oncol 2003; 21: 3041–3050. [DOI] [PubMed] [Google Scholar]
- 45.Fossa SD, Kaye SB, Mead GM, et al. Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy. European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party, Cambridge, United Kingdom. J Clin Oncol 1998; 16: 716–724. [DOI] [PubMed] [Google Scholar]
- 46.Muhonen T, Jantunen I, Pertovaara H, et al. Prophylactic filgrastim (G-CSF) during mitomycin-C, mitoxantrone, and methotrexate (MMM) treatment for metastatic breast cancer. a randomized study. Am J Clin Oncol 1996; 19: 232–234. [DOI] [PubMed] [Google Scholar]
- 47.Osby E, Hagberg H, Kvaloy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood 2003; 101: 3840–3848. [DOI] [PubMed] [Google Scholar]
- 48.Pettengell R, Gurney H, Radford JA, et al. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin's lymphoma: a randomized controlled trial. Blood 1992; 80: 1430–1436. [PubMed] [Google Scholar]
- 49.Romieu G, Clemens M, Mahlberg R, et al. Pegfilgrastim supports delivery of FEC-100 chemotherapy in elderly patients with high risk breast cancer: a randomized phase 2 trial. Crit Rev Oncol/Hematol 2007; 64: 64–72. [DOI] [PubMed] [Google Scholar]
- 50.Trillet-Lenoir V, Green J, Manegold C, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 1993; 29A: 319–324. [DOI] [PubMed] [Google Scholar]
- 51.Zinzani PL, Pavone E, Storti S, et al. Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin's lymphoma. Blood 1997; 89: 3974–3979. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.