Abstract
Background & objectives:
Chronic venous insufficiency (CVI) is a common clinical problem among obese patients. This study was conducted to evaluate the impact of body mass index (BMI) and associated morbidities such as diabetes, hypertension and hypothyroidism on venous disease clinical scores as per Clinical, Etiological, Anatomical, Pathological (CEAP) classification.
Methods:
In this study, adult patients with BMI more than 30 kg/m2 with signs of CVI were evaluated clinically and by using Duplex ultrasonography of venous system. The patients with C0, C1, C2, C3 and C4, C5, C6 clinical scores in CEAP classification were grouped as lower and higher clinical scores of CVI, respectively.
Results:
Of the 200 enrolled patients, 147 (73.5%) were males and were associated with higher grades of clinical scores (P=0.051). Superficial venous system was involved in 96 per cent patients and 91 per cent patients had reflux in the sapheno-femoral junction. A negative association was observed between hypertension and male gender (P=0.001). Higher BMI was associated with higher clinical scoring (P=0.053). BMI >40 kg/m2 was associated with primary aetiology (P=0.007) of CVI. There was no correlation between superficial, deep or perforator incompetence with BMI (P=0.506). Duplex-confirmed significant reflux was observed in patients with higher BMI (P=0.006). Age and BMI were positively correlated with clinical score (r=0.176; P=0.013 & r=0.140; P=0.049), respectively.
Interpretation & conclusions:
Our findings indicated that elderly male patients with high BMI seemed to be at a higher risk of advanced clinical grades of CVI. The impact of comorbid conditions such as diabetes, hypertension and hypothyroidism on CVI could not reach at significance in the present study.
Keywords: Body mass index, CEAP classification, chronic venous insufficiency, clinical score, elderly male, obesity
Chronic venous disease of the lower limbs is one of the major problems observed in vascular surgery practice; however, it remains an underdiagnosed condition in general clinical practice because of many factors which can broadly be divided as patient- and healthcare provider-related factors. Patients usually neglect the disease till it impairs the quality of life or limits the overall functioning of lower extremity1. Primary care physicians often tend to ignore or overlook the problem because of the underappreciation of the consequences2. Impaired return of the blood causes increase in the pressure in the veins of lower extremity. Venous hypertension secondary to higher reflux or obstruction is an important cause of chronic venous insufficiency (CVI). Venous insufficiency is strongly associated with venous ulceration3 and hence chronic CVI can result in difficult-to-heal and recurrent venous ulcers2. The complications of CVI impair the ability of a person to involve in social or routine occupational activities and also result in economic burden on the patient and family due to loss of work hours2.
Several factors are known to increase the risk of CVI. Important risk factors include age, gender, pregnancy, history of leg injury, family history, tall stature, professional demand to stand for long hours as in bus conductors, teachers, policeman on duty, iatrogenic as in venous interventions and resultant deeper venous obstruction4,5. Factors contributing to the development of deep vein thrombosis as per Virchow's triad of hypercoagulability, stasis and venous injury contribute to the development of CVI5. Hormonal supplemental usage in women and smoking in men adds to the risk of deep vein thrombosis and resultant CVI6. Clinical history, detailed physical examination and non-invasive vascular investigations are the three cornerstones in assessing the incompetence of venous system and venous function. Diabetes, hypertension and hypothyroidism have a variable association with both high body mass index (BMI) and CVI6. The information on impact of BMI with respect to comorbidities such as diabetes, hypertension and hypothyroidism on CVI in Indian patients is limited and worth addressing from a demographic standpoint.
The present study was conducted to evaluate the impact of BMI and associated comorbidities such as diabetes, hypertension and hypothyroidism on venous disease clinical scores as per Clinical, Etiological, Anatomical, Pathological (CEAP) classification for evaluation of CVI.
Material & Methods
In this cross-sectional study, consecutive adult patients (≥18 yr of age) with BMI more than 30 kg/m2 presenting to the Outpatient Department of Vascular Surgery, Nizam's Institute of Medical Sciences, Hyderabad, India, between March 2015 and March 2016, with signs of CVI without peripheral arterial disease, were enrolled. Eligible patients were evaluated clinically and with duplex scan for the presence and higher clinical scores of chronic venous diseases. Duration of reflux more than 500 msec was considered significant. Deep venous thrombosis indicative of obstructive venous return was diagnosed by non-compressibility of deep veins without augmentation and respiratory phasicity with an echogenic thrombus in the deep veins of the lower extremity.
The evaluation was done using well-established basic CEAP system7,8. This descriptive classification system describes CVI using a coding system with four subject headings. The heading C describes seven clinical classes, ranging from C0 to C6, defined as follows: C0: No visible or palpable sign of venous disease (i) C1: Telangiectasias or reticular veins (ii) C2: Varicose veins (iii) C3: Oedema (iv) C4: Skin changes ascribed to venous disease: pigmentation, venous eczema, hypodermic inflammation (v) C5: Skin changes as defined in C4 with healed ulceration (vi) C6: Skin changes as defined in C4 with active, unhealed ulceration.
In this study, the basic CEAP was used describing only the highest clinical class. The aetiology heading (E) differentiates three types of causes of CVI (Ec for congenital, Ep for primary and Es for secondary). Primary or idiopathic venous disorders develop independent of other disease and account for the vast majority of cases. Secondary venous disorders develop as a consequence of pathology, such as following deep venous thrombosis or trauma. The anatomical heading (A) codifies CVI according to the anatomic distribution of venous disease (As stands for superficial venous network, Ad for deep venous network and Ap for perforator veins). Finally, the pathophysiological heading (P) differentiates CVI associated with reflux (Pr), obstruction (Po) or a combination of the two mechanisms7,8.
The pathological conditions such as diabetes, hypertension and hypothyroidism were recorded and evaluated against the age, gender, BMI and clinical scores (low or high) of CEAP classification for evaluation for CVI. The low clinical scores include C0, C1, C2, C3 clinical stages and the high-grade clinical scores include C4, C5 and C6 clinical stages of CEAP classification7.
The association of age, BMI and comorbid conditions with the higher clinical scores of CVI was studied. Higher clinical scores of CVI were assessed with clinical examination and non-invasive imaging method. For clinical assessment, the CEAP classification system8 was used. In this study, only the highest clinical class was recorded. In addition, duplex ultrasonography was used to assess the reflux secondary to incompetence of venous valves or chronic obstruction of the deeper venous system. Duplex sonography has been found to be useful for assessment of the leg veins9,10.
Statistical analysis: Categorical variables are presented in numbers and percentages. Chi-square test was used to find association between gender and BMI. Spearman's correlation was used to find the association between age and BMI versus clinical scores. The statistical software namely SAS 9.2 (SAS Institute Inc., Cary, NC, USA), SPSS 15.0 (SPSS Inc, Chicago, IL, USA), Stata 10.1 (Stata Corp., College Station, Texas, USA), MedCalc 9.0.1 (MedCalc software, Ostend, Belgium), Systat 12.0 (SYSTAT software Inc., USA) and R environment ver. 2.11.1 (https://www.r-project.org/about.html) were used for the analysis of the data.
Results
A total of 200 consecutive patients were enrolled in this study, of whom 147 (73.5%) and 53 (26.5%) were male and female, respectively. Sixty per cent of patients were between 30 and 50 yr of age. Forty two per cent of patients had BMI at least 40 kg/m2. Gender-wise distribution of patients according to BMI is shown in Table I. Involvement of left leg was observed in 85.7 per cent (126 of 147) male patients. There was association (P=0.098) between male gender and BMI though that could not attain significance.
Table I.
Distribution of patients according to body mass index (BMI)
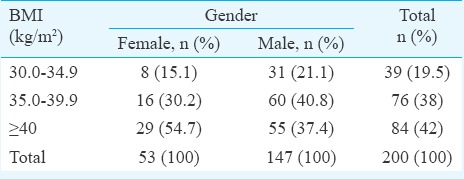
Table II shows distribution of patients according to the CEAP classification. There was a strong association between male gender and higher clinical scores in patients of morbid obesity (P=0.051) aetiologically. In our study, the primary aetiology of CVI (Ep) was observed in 90 per cent (n=180) of all patients. Superficial venous system (As) was involved in the disease pathology in 192 cases (96%). A total of 91 per cent patients had reflux (Pr) in the sapheno-femoral junction.
Table II.
Clinical, Etiological, Anatomical, Pathological (CEAP) distribution with respect to gender
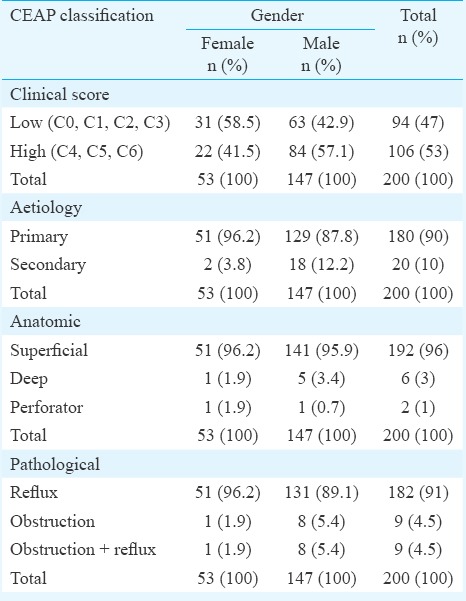
Only 17.7 per cent male patients (26 of the 147), were found to be hypertensive (P <0.001); a negative correlation implying that less number of morbidly obese patients were found to be hypertensive. Eighty six per cent of patients (n=172) presenting with CVI did not have diabetes. There was no history of hypertension of hypothyroidism in 76.5 (n=153) and 95.5 per cent (n=191) of patients, respectively Table III.
Table III.
Distribution of diabetic, hypertensive and hypothyroid patients with respect to gender
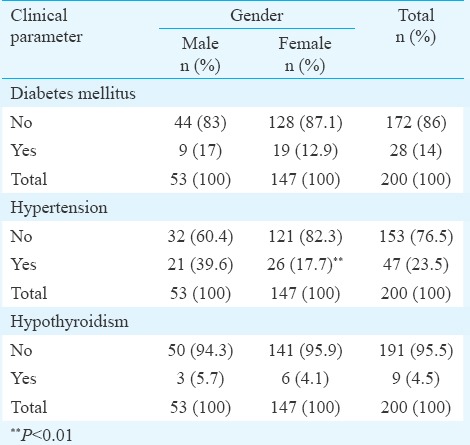
Higher BMI was associated with higher grades of clinical scoring (P=0.053). The primary or the idiopathic aetiology showed significant association with BMI >40 kg/m2 (P=0.007). There was no correlation between superficial, deep or perforator incompetence with BMI (P=0.506). Duplex-confirmed significant reflux was observed in patients with higher BMI (P=0.006) (Table IV). Age and BMI were positively correlated with higher clinical score (r=0.176; P=0.013 and r=0.140; P=0.049), respectively.
Table IV.
Clinical, Etiological, Anatomical, Pathological (CEAP) distribution of patients with respect to body mass index
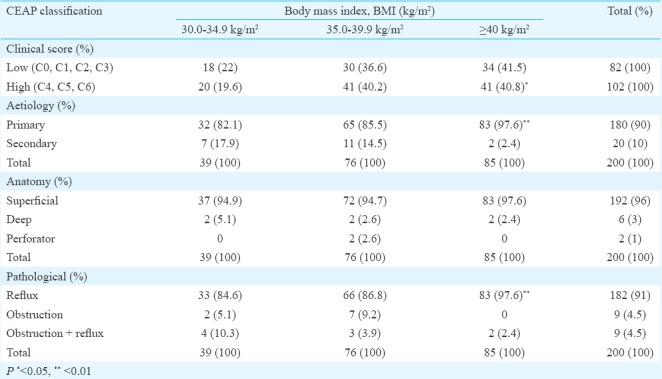
Discussion
Higher BMI (i.e., obesity) is an important risk factor for chronic venous disease. We included patients with BMI of ≥30 kg/m2 which is considered as the cut-off for defining obesity11. Higher BMI was associated with higher clinical scores of CVI in our study. Danielsson et al12 showed significant association between mean BMI of 28.9 kg/m2 and clinical higher clinical scores of chronic venous disease. All patients in our study had BMI more than 30 kg/m2. Our findings are in accordance with the published literature showing significant association between BMI and higher clinical scores12,13. Another study involving Indian patients with BMI <30 kg/m2 might be useful to see if similar significant association still exists.
There is also a contradictory evidence for obesity. Multivariate analysis by French epidemiological study in male patients showed that obesity was not an aggravating factor for chronic venous disease. The authors mentioned that obesity was probably a reflection of age because obese people in their trial were more aged compared to others14. Age was associated with higher clinical scores of chronic venous disease in the French study14. Thus, our findings are similar to French study with regard to association between age and higher clinical scores of disease. The San Diego population study also showed independent association of moderate venous disease with age15. Another study demonstrated a strong relationship between the cross-sectional areas of veins of lower extremity and rising BMI16. This might contribute to increased clinical scores of CVI in obese people. The Edinburgh Vein study showed significant relations between increasing height and varicose veins17. Association between height and CVI was not evaluated in our study. It would be worthwhile to examine the relation of height and higher clinical scores of CVI in Indian patients.
Diabetes and hypertension can be comorbidities in patients with chronic venous disease6. Hyperglycaemia-induced pathological changes may cause vascular dysfunction in patients with diabetes18. Reduced peripheral blood flow and local angiogenesis are some of the known vascular problems in diabetes. In our study the relation between diabetes and higher clinical scores of CVI was evaluated and no influence of diabetes was observed on the higher clinical scores of CVI. This could be because of limited number of patients with diabetes in our study. Larger studies comparing higher clinical scores of CVI in diabetics versus non-diabetic individuals might give more insights in this regard.
Relation between obesity and hypertension is well known, but the effect of obesity on venous function is not clearly established. In an animal study, impaired in vivo venous pressor response has been shown19. We did not find primary influence of hypertension or hypothyroidism on the higher clinical scores of CVI in this study.
Our study, being cross-sectional, bears a primary limitation that the exposure and outcome are simultaneously assessed and hence, there is generally no temporal relationship between exposure and outcome. Without longitudinal study design, it is not possible to establish the true cause and effect relationship. The results of the present study, being a single institutional experience, cannot be generalized to reflect the entire Indian population. CEAP classification provides a descriptive statistical analysis of the prevalence of the disease burden, with higher clinical scores pointing to advanced form of disease. The more useful scores such as Venous Clinical Severity Score, Venous Segmental Disease Score and Venous Disability Score can provide data on clinical severity, anatomo-pathophysiologic severity and disability of chronic venous disease in morbidly obese patients7.
Overall the findings of our study suggested that reflux pattern and BMI correlated with the clinical presentation. Similarly, it seemed that elderly male patients with high BMI and primary venous reflux were at an increased risk of the clinical progression of CVI from varicose veins, with trophic skin changes and venous ulcers.
Acknowledgment
Authors acknowledge Dr K.P. Suresh (Biostatistician, Bengaluru) for helping with statistical analyses.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Dekiwadia DB, Jindal R, Varghese R, Bedi HS, Padaria S, Patel MD, et al. Executive summary: A consensus statement – Part I: Recommendations for the management of chronic venous disease (CVD) in India and key role of primary care doctors. J Assoc Physicians India. 2016;64:53–6. [PubMed] [Google Scholar]
- 2.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130:333–46. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi PY, Kiemele L, Cha SS, Chandra A. A cross-sectional evaluation of the association between lower extremity venous ulceration and predictive risk factors. Wounds. 2009;21:290–6. [PubMed] [Google Scholar]
- 4.Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: A dual case-control study. J Vasc Surg. 1995;22:622–8. doi: 10.1016/s0741-5214(95)70050-1. [DOI] [PubMed] [Google Scholar]
- 5.Jawien A. The influence of environmental factors in chronic venous insufficiency. Angiology. 2003;54(Suppl 1):S19–31. doi: 10.1177/0003319703054001S04. [DOI] [PubMed] [Google Scholar]
- 6.Matic P, Jolic S, Tanaskovic S, Soldatovic I, Katsiki N, Isenovic E, et al. Chronic venous disease and comorbidities. Angiology. 2015;66:539–44. doi: 10.1177/0003319714541988. [DOI] [PubMed] [Google Scholar]
- 7.Rabe E, Pannier F. Clinical, aetiological, anatomical and pathological classification (CEAP): Gold standard and limits. Phlebology. 2012;27(Suppl 1):114–8. doi: 10.1258/phleb.2012.012s19. [DOI] [PubMed] [Google Scholar]
- 8.Porter JM, Moneta GL. Reporting standards in venous disease: An update. International consensus committee on chronic venous disease. J Vasc Surg. 1995;21:635–45. doi: 10.1016/s0741-5214(95)70195-8. [DOI] [PubMed] [Google Scholar]
- 9.Stapff M, Betzl G, Küffer GV, Hahn D, Spengel FA. The value of duplex sonography in the diagnosis of deep leg and pelvic vein thrombosis. Bildgebung. 1987;56:52–6. [PubMed] [Google Scholar]
- 10.Eichlisberger R, Frauchiger B, Jäger K. Assessment of the leg veins using duplex ultrasonography. Ther Umsch. 1991;48:697–707. [PubMed] [Google Scholar]
- 11.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 12.Danielsson G, Eklof B, Grandinetti A, Kistner RL. The influence of obesity on chronic venous disease. Vasc Endovascular Surg. 2002;36:271–6. doi: 10.1177/153857440203600404. [DOI] [PubMed] [Google Scholar]
- 13.Padberg F, Jr, Cerveira JJ, Lal BK, Pappas PJ, Varma S, Hobson RW., 2nd Does severe venous insufficiency have a different etiology in the morbidly obese? Is it venous? J Vasc Surg. 2003;37:79–85. doi: 10.1067/mva.2003.61. [DOI] [PubMed] [Google Scholar]
- 14.Benigni JP, Cazaubon M, Tourneroche A, Achhammer I, Mathieu M. Is obesity an aggravating factor in chronic venous disease. Results of a French epidemiological study in male patients? Int Angiol. 2006;25:297–303. [PubMed] [Google Scholar]
- 15.Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: The San Diego Population Study. J Vasc Surg. 2007;46:331–7. doi: 10.1016/j.jvs.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kröger K, Ose C, Rudofsky G, Roesener J, Weiland D, Hirche H, et al. Peripheral veins: Influence of gender, body mass index, age and varicose veins on cross-sectional area. Vasc Med. 2003;8:249–55. doi: 10.1191/1358863x03vm508oa. [DOI] [PubMed] [Google Scholar]
- 17.Lee AJ, Evans CJ, Allan PL, Ruckley CV, Fowkes FG. Lifestyle factors and the risk of varicose veins: Edinburgh vein study. J Clin Epidemiol. 2003;56:171–9. doi: 10.1016/s0895-4356(02)00518-8. [DOI] [PubMed] [Google Scholar]
- 18.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Song D, Hutchings SR, Pang CC. Impaired in vivo venous constriction in conscious obese Zucker rats with metabolic syndrome. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:451–6. doi: 10.1007/s00210-006-0088-8. [DOI] [PubMed] [Google Scholar]


