Abstract
Background & objectives:
Peripheral T cell lymphomas (PTCLs) are a heterogeneous group of non-Hodgkin's lymphomas (NHLs), with universally poor outcome. This study was undertaken to provide data on demographics and outcomes of patients with PTCL who underwent treatment in a single tertiary care centre in southern India.
Methods:
Retrospective study was done on all patients (age ≥18 yr) diagnosed with PTCL from January 2007 to December 2012. The diagnosis of PTCL was made according to the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues.
Results:
A total of 244 adult patients were diagnosed with PTCL (non-cutaneous). The most common subtype was PTCL-not otherwise specified (35.7%), followed by anaplastic large cell lymphoma (ALCL), ALK negative (21.3%), natural killer/T cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL), ALCL, ALK positive, hepatosplenic T cell lymphoma (HSTCL) and adult T cell leukaemia/lymphoma followed in frequency with 13.1, 11.5, 8.6, 8.2 and 1.6 per cent cases, respectively. The three-year Kaplan-Meier overall survival (OS) and event-free survival (EFS) for the patients who received chemotherapy (n=122) were 33.8±5.0 and 29.3±4.7 per cent, respectively. Various prognostic indices developed for T cell lymphomas were found to be useful.
Interpretation & conclusions:
Except for ALCL, ALK positive, all other PTCLs showed poor long-term outcome with CHOP-based chemotherapy. Novel therapies are needed to improve the outcome.
Keywords: CHOP-based therapy, histological subtypes, non-cutaneous, non-Hodgkin lymphoma, overall survival, peripheral T cell lymphomas
Peripheral T cell lymphomas (PTCL) are a heterogeneous group of non-Hodgkin's lymphomas (NHLs) which are of mature T cell origin1, accounting for only 10-15 per cent of all lymphoid malignancies2. A higher incidence has been described from Asia, especially natural killer T cell lymphoma (NKTCL) and adult T cell leukaemia, lymphoma (ATLL) subtypes2.
The International Peripheral T Cell Lymphoma Project (IPTCLP)2 is the largest series describing pathological findings and outcomes in this group of patients. International Prognostic Index (IPI) was found to be useful for prognostication of PTCL, similar to that for B cell NHLs; however, certain PTCLs such as NKTCL are associated with poor outcome even with low IPI. Various new scoring systems, either subtype specific or non-specific with better risk stratification, have been compared; however, no single scoring system has been considered unanimously superior3. CHOP-based regimens have shown poor results in T cell lymphomas, with the notable exception of anaplastic large cell lymphoma (ALCL), ALK positive4. Molecular signatures by gene expression profiling have added to the understanding of these diverse groups of disorders4. With advances in understanding of disease biology and development of newer treatment options, the outlook towards PTCL, which was referred to as ‘poor step child’ of lymphomas1, is changing and the future seems to be hopeful for improved outcomes in this universally fatal group of disorders. The aim of the present study was to review the incidence of various PTCLs in a single centre from south India and to study the outcomes for the patients who underwent treatment from January 2007 to December 2012.
Material & Methods
All adult (age ≥18) patients diagnosed with PTCL (non-cutaneous) presenting to the Haematology department at Christian Medical College (CMC), Vellore, India, between January 2007 and December 2012 and whose data could be retrieved were included in this retrospective study. The study was approved by the Institutional Review Board. The diagnosis of PTCL was made in the department of Pathology, CMV, Vellore, according to the WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues, Fourth Edition (2008)5. The immunohistochemistry markers used for diagnosis were CD3, CD4, CD8, ALK-1, CD56, CD30, EBV LMP-1, TIA-1, EMA, Ki-67, Granzyme B, CD43, CD138, CD20, PD-1, TDT, LCA, CD5, CD7, CD15, CD10, PAX-5, CD117, CD99 and CD21. T cell receptor clonality assay was performed in doubtful cases.
Results were analyzed in terms of the clinical characteristics and laboratory parameters at diagnosis and response to the different treatment regimens. Response to treatment was assessed by standard criteria6. All patients started on treatment were evaluated for response and outcome. Overall survival (OS) was measured from the start of therapy up to the date of death (from any cause). For the purpose of this analysis, patients who had relapsed or had progressive disease during therapy and then subsequently lost to follow up or opted for palliative care were considered as dead, 30 days after the last follow up. Event-free survival (EFS) was calculated from the start of therapy up to the first adverse event, i.e. relapse, progression, secondary malignancy or death.
Descriptive statistics were calculated for all variables. Differences in proportions were assessed using the Chi-square test. Survival curves were drawn by the Kaplan-Meier method and compared by the log-rank test. SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for the analysis.
Results
Between January 2007 and December 2012, a total of 972 adult patients were diagnosed with NHLs, of whom 244 patients were diagnosed with PTCL (non-cutaneous) (25.1%). The most common subtype was PTCL-not otherwise specified (NOS) (n=83, 35.7%), followed by ALCL ALK negative (n=52, 21.3%). Others included NK-TCL (n=32, 13.2%), angioimmunoblastic T-cell lymphoma (AITL) (n=28, 11.5%), ALCL ALK positive (n=21, 8.6%), hepatosplenic T cell lymphoma (HSTCL) (n=20, 8.2%) and ATLL (n=4, 1.6%).
The clinical characteristics of all patients and those with different histological subtypes are shown in the Table. The median age was 45 yr (range 18-80 yr) with male predominance in all subtypes (n=202, 82.8%). The median age of patients with HSTCL and ALCL, ALK positive was found to be one decade earlier than the median age of the total patients. The majority of the patients had Stage III/IV disease except patients with NKTCL, where only 25 per cent cases (n=8) had advanced stage disease. Three patients had bone lesions. Bone marrow involvement was seen in 37.5 per cent (n=75 of 200) of cases and almost all patients with HSTCL (n=18, 90%) and ATLL (n=4, 100%) showed bone marrow involvement. Hemophagocytosis was seen in nine cases of 200 (4.5%). B symptoms and elevated LDH were also found in 81 (n=189 of 233) and 88 (n=177 of 199) per cent of patients, respectively. Median duration of symptoms before diagnosis was three months (range 1-48 months). Autoimmune manifestations were seen in six patients (one had immune thrombocytopenia and five had autoimmune haemolysis), all these patients had AITL. Hypergammaglobulinemia was found in five of 28 AITL patients (17.9%).
Table.
Clinical characteristics of patients with peripheral T cell lymphomas (PTCL) by histological subtype
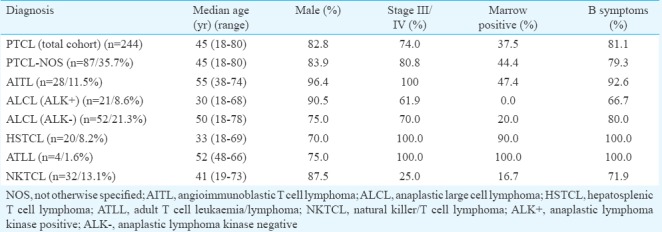
One hundred and twenty two of the total 244 patients proceeded with treatment at our centre. The distribution of all the patients is shown in Fig. 1. The majority of patients received CHOP-based chemotherapy (n=116, 95%). Sixty two per cent (n=76) of patients required admission during treatment for either febrile neutropenia or other chemotherapy-related side effects. The three-year Kaplan-Meier overall survival (OS) and EFS for the total patients on treatment (n=122) were 33.8±5.0 per cent (median follow up duration of 14 months) and 29.3±4.7 per cent (median follow up duration of 8 months), respectively (Fig. 2A and B). In the group of patients who completed therapy (n=64), the median follow up was 24 months (range 7-92 months). This group comprised 23 (35.9%) patients with ALCL and 23 (35.9%) patients with PTCL-NOS. The three-year Kaplan-Meier of OS and EFS for the PTCL-NOS (n=43), AITL (n=6), ALK-positive ALCL (n=18), ALK-negative ALCL (n=24), HSTCL (n=7), ATLL (n=3) and NK/T cell lymphoma (n=18) histological group were 27.4±7.8 and 62.5±21.3 per cent, 59.0±11.9 and 47.2±11.9 per cent, 0 and 33.3±27.2 per cent, 8.0±7.6 and 22.7±7.5 per cent, 36.5±2.0 and 53.1±12.1 per cent, 37.7±11.4 and 0 per cent, 33.3±27.2 and 8.2±7.8 per cent, respectively.
Fig. 1.
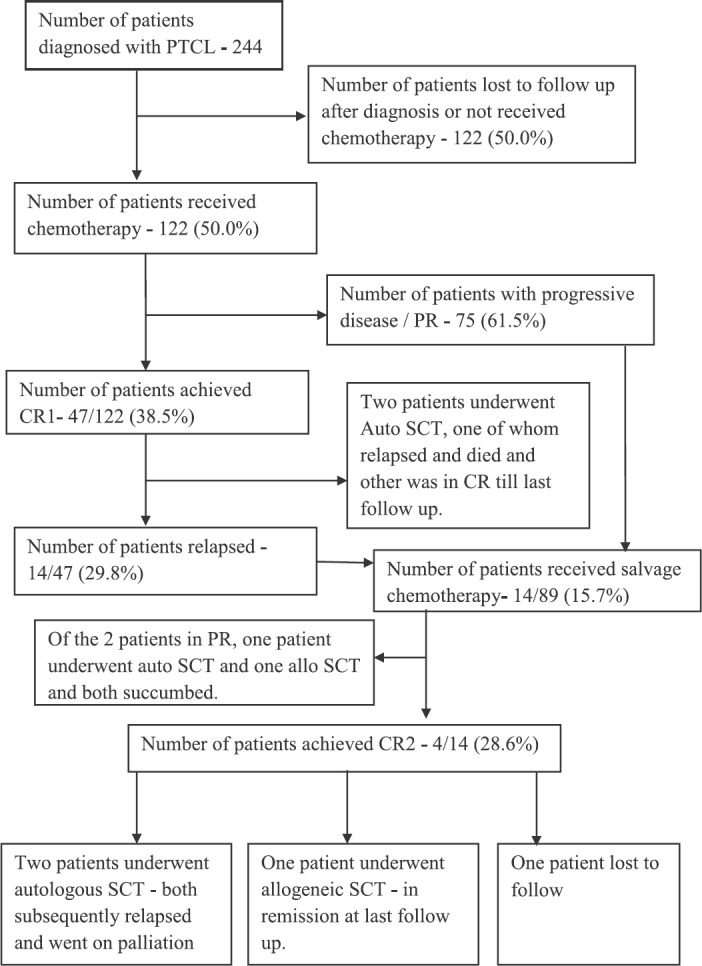
Flowchart showing outcomes in patients with peripheral T cell lymphomas (PTCL) studied. CR, complete remission; Allo, allogeneic; Auto, autologous; SCT, stem cell transplant, PR, partial response.
Fig. 2.
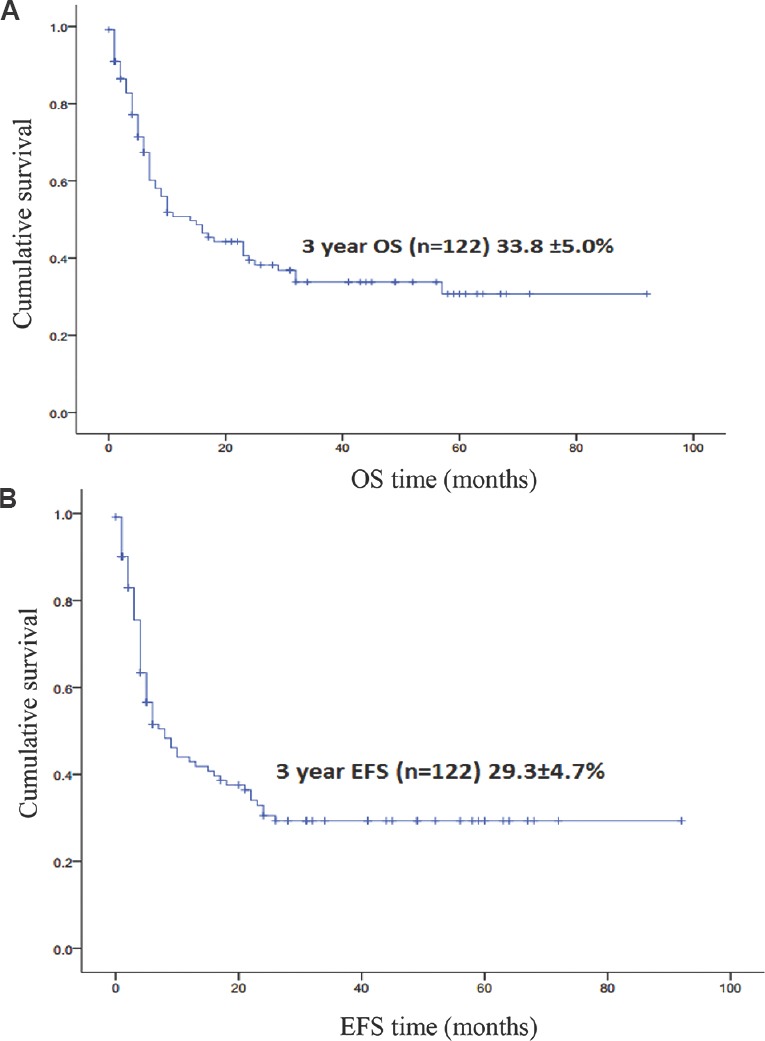
Kaplan-Meier curve showing overall survival (OA) (A) and event-free survival (EFS) (B) in the total treated patients (n=122) who received chemotherapy..
Various prognostic scores were applied for risk stratification. In the case of NK/T cell lymphoma when the IPI was used as prognostic score, 57.1 per cent (n=18) of the cases were in lower risk categories (low and low intermediate), while 42.9 per cent (n=14) of the cases were in higher risk (high intermediate and high risk).
The three-year Kaplan-Meier OS and EFS for treated patients (n=116) when prognosticated by IPI score into low risk (n=25), low intermediate risk (n=20), high intermediate risk (n=50) and high risk (n=21) categories were 52.5±11.6 and 51.0±12.7 per cent, 27.8±7.2 and 6.9±6.6 per cent, 52.5±12.6 and 41.8±10.7 per cent, 22.5±6.7 and 8.2±7.6 per cent, respectively (Fig. 3A and B). The three-year Kaplan-Meier OS and EFS for treated patients (n=117) when prognosticated by prognostic Index for T-cell lymphoma (PIT) score into low risk (n=7), low intermediate risk (n=28), high intermediate risk (n=48) and high risk (n=34) categories were 63.3±21.0 and 51.1±10.4 per cent, 31.2±7.8 and 15.4±7.4 per cent, 57.1±18.7 and 40.3±10.0 per cent, 30.0±7.6 per cent versus 12.3±7.2 per cent, respectively (Fig. 4A and B).
Fig. 3.
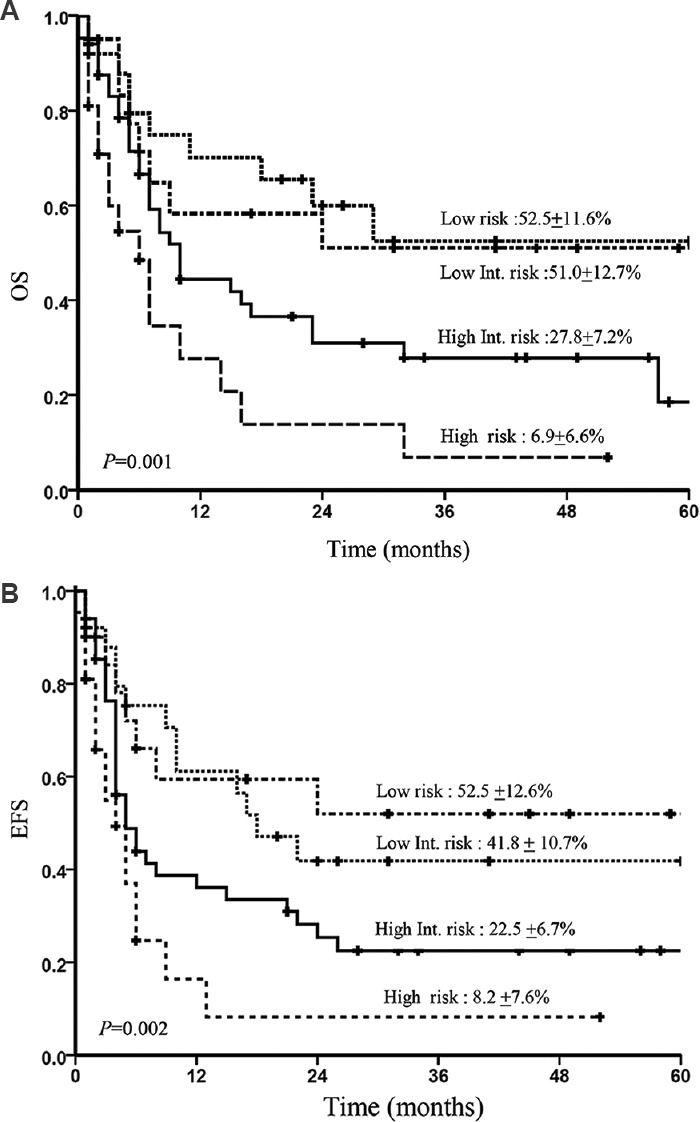
Kaplan-Meier curve showing overall survival (OA) (A) and event-free survival (EFS) (B) according to the International Prognostic Index (IPI) score in all treated patients.
Fig. 4.
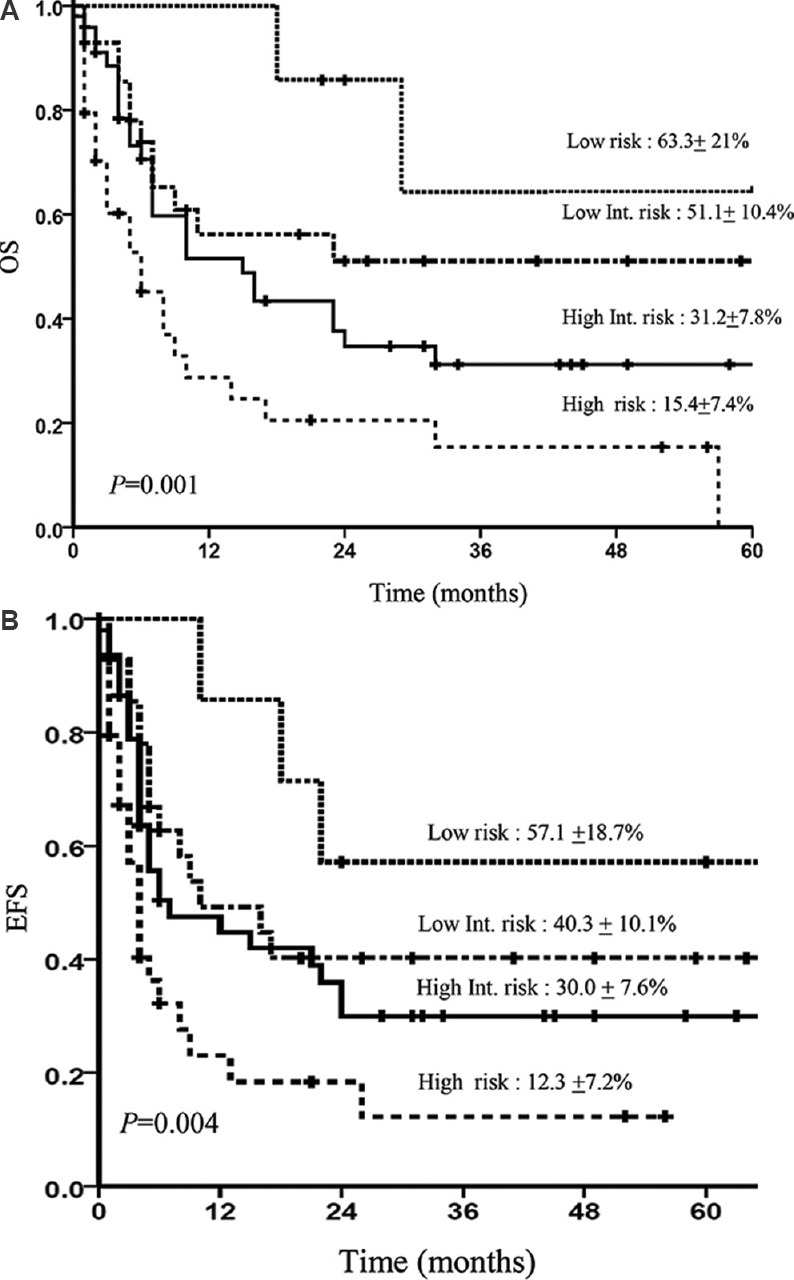
Kaplan-Meier curve showing overall survival (OA) (A) and event-free survival (EFS) (B) according to the prognostic index for T cell lymphoma (PIT) in all treated patients.
Discussion
Although PTCL accounts for only 10 per cent of all NHL in the Western world2, the incidence of T cell lymphomas is higher in Asia, with an incidence of 20 per cent noted in a recent retrospective study from India7. As per the IPTCLP2, PTCL-NOS was the most common subtype, followed by AITL (Western countries) and ATLL and NK/T cell lymphoma in Far Eastern countries. The most common histological subtype in our study was found to be PTCL-NOS (35.7%) followed by ALCL, ALK negative (21.3%). The median age of diagnosis was concordant with published literature for ALCL, ALK positive and HSTCL while the diagnosis was one decade earlier for rest of the subtypes2.
Bony lesions were noted in only three patients (1.3%); however, bone marrow involvement was seen in 37.5 per cent of patients. Data on specific extranodal sites involvement were not available in all cases. HSTCL had bone marrow involvement in 90 per cent of cases (n=18) and bone marrow involvement was least common with ALCL (ALK positive 0% and ALK negative 20%). This was comparable with published literature2. All patients with AITL, HSTCL and ATLL had Stage III/IV disease, whereas only 25 per cent cases of patients with NK/T cell lymphoma had Stage III/IV disease. The incidence of advanced stage disease was higher when compared to IPTCLP, possibly a reflection of differences in health-seeking behaviour.
Associated autoimmune manifestations in the form of autoimmune haemolytic anaemia and immune thrombocytopenia were found in six patients and all had a histological diagnosis of AITL. Hypergammaglobulinemia was found in 17.9 per cent of patients with AITL, compared with an earlier published study where it was seen in 30 per cent of cases8.
Prognostic scores such as IPI, IPTCLP score, PIT and mPIT when applied showed significantly poorer outcomes in high-risk groups9,10,11,12. In case of NK/T cell lymphoma when NKPI was used as a prognostic score, the majority of the patients (71.4%) were assigned to higher risk categories, compared to 42.9 per cent in the high-risk group when prognosticated by IPI. Considering the poor prognosis even with localized disease, NKPI is suggested to be used as a prognostic scoring system for this condition. Using PIAI as prognostic score for AITL, 84 per cent were in a high-risk category which was consistent with the previous study8. In view of a small number of patients with AITL and NK/T cell lymphoma receiving treatment, survival difference depending on PIAI and NKPI could not be assessed in the present study.
Chemotherapy was administered to 122 patients, and majority received CHOP-based chemotherapy. Almost half of the patients required admission due to febrile neutropenia and other chemotherapy-related side effects. Growth factor support was used to augment neutrophil recovery in 59.3 per cent patients. Median follow up was only eight months. In view of only a few patients undergoing stem cell transplant, statistical correlation of the transplant data could not be done.
Our study showed the overall poor long-term outcome with CHOP-based chemotherapy in this group of patients (excluding ALCL, ALK positive), emphasizing the need for more intensive chemotherapeutic regimens. Median follow up duration was only eight months for the patients who were treated. In the group of patients who completed therapy (n=64), the median follow up was 24 months (range 7-92 months). This group comprised 23 (35.9%) patients with ALCL and 23 (35.9%) patients with PTCL-NOS. The OS and EFS of patients including all subgroups by IPI, PIT, mPIT and IPTCLP were significantly inferior in high-risk groups.
Even under ideal conditions, intensification of therapy has yielded only marginal benefits13,14, with the exception of the addition of etoposide15 and possibly, autologous stem cell transplant16. Recent advances in the treatment of relapsed PTCL have included the use of romidepsin and pralatrexate17,18, but the addition of these agents to upfront therapy has not shown promising results19,20.
PTCL is a diverse group of NHL with poor overall outcome compared to their B cell counterparts. Failure of CHOP-based chemotherapy warrants search for novel and affordable agents for treatment.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Armitage JO. The aggressive peripheral T-cell lymphomas: 2013. Am J Hematol. 2013;88:910–8. doi: 10.1002/ajh.23536. [DOI] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez-García G, García-Herrera A, Cardesa T, Martínez A, Villamor N, Ghita G, et al. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol. 2011;22:397–404. doi: 10.1093/annonc/mdq359. [DOI] [PubMed] [Google Scholar]
- 4.Dearden CE, Johnson R, Pettengell R, Devereux S, Cwynarski K, Whittaker S, et al. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma) Br J Haematol. 2011;153:451–85. doi: 10.1111/j.1365-2141.2011.08651.x. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. 4th ed. Lyon, France: IARC Press; 2008. WHO classification of tumours of haematopoietic and lymphoid tissues. [Google Scholar]
- 6.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 7.Arora N, Manipadam MT, Nair S. Frequency and distribution of lymphoma types in a tertiary care hospital in South India: Analysis of 5115 cases using the World Health Organization 2008 classification and comparison with world literature. Leuk Lymphoma. 2013;54:1004–11. doi: 10.3109/10428194.2012.729056. [DOI] [PubMed] [Google Scholar]
- 8.Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: Analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240–6. doi: 10.1200/JCO.2011.37.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: A prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612–8. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 10.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 11.Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–9. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 12.Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E, et al. Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24:2472–9. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- 13.Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–23. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan D, Unger JM, Spier CM, Persky DO, Young F, LeBlanc M, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119:371–9. doi: 10.1002/cncr.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German high-grade non-Hodgkin lymphoma study group. Blood. 2010;116:3418–25. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 16.d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–9. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 17.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–6. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–9. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Advani RH, Ansell SM, Lechowicz MJ, Beaven AW, Loberiza F, Carson KR, et al. A phase II study of cyclophosphamide, etoposide, vincristine and prednisone (CEOP) alternating with pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): Preliminary results from the T-cell consortium trial. Blood. 2013;122:3034–44. doi: 10.1111/bjh.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupuis J, Morschhauser F, Ghesquières H, Tilly H, Casasnovas O, Thieblemont C, et al. A phase Ib/II trial of romidepsin in association with CHOP in patients with peripheral T-cell lymphoma (PTCL): The Ro-CHOP study. Hematol Oncol. 2013;31(Suppl 1):136–42. [Google Scholar]


