Abstract
Stem cell (SC) therapy has come up enormously, particularly for indications where not much can be done medically or surgically to improve the condition. SCs are the foundation cells for every organ, tissue, and cell in the body, and it can either reproduce into a SC or differentiate into specialized types of cells. Premature ovarian insufficiency (POI) is a clinical syndrome defined by loss of ovarian activity before the age of 40 years. POI is characterized by menstrual disturbance (amenorrhea or oligomenorrhea) with raised gonadotropins, low anti-Mullerian hormone (AMH), and estradiol level. Autologous SCs were tried in POI to increase the follicular recruitment and avoiding the need for oocyte donation program. This review analyzes the causes, etiopathogenesis, and role of autologous bone marrow-derived SC therapy (ABMDSCT) in POI. It also highlights the recent studies and summarizes the current understanding and future directions for SCs in POI. Here, we also report the first successful birth of a baby from India, where autologous SC therapy in a 45-year-old perimenopausal single woman helped in procuring a pregnancy and delivery of a healthy 2.7 kg female baby through assisted reproduction. In the absence of SC therapy, accepting oocyte donor program or adoption would have been the only viable options for this patient for which she was not ready. This may be the world's first successful case of application of ABMDSCT in a 45-year-old female to give successful birth to a healthy baby.
KEYWORDS: Anti-mullerian hormone, autologous bone marrow-derived stem cell therapy, perimenopausal women, premature ovarian insufficiency
INTRODUCTION
The trend of delayed motherhood over the last decades can be explained by several factors such as lifestyle changes, social causes, work, and economic. However, the reality is that biology does not always allow for this. The best reproductive time for women is when they are in their 20s, when not so many women consider motherhood yet. At that age, women produce the best oocytes, thus increasing the chances of a safe pregnancy that will result in a healthy baby. However, it has been estimated that premature ovarian failure (POF) or early menopause affects 10% of the female population, who despite the young age, would have problems to conceive.[1]
Premature ovarian insufficiency (POI) is a common cause of infertility in women and is characterized by amenorrhea, oligomenorrhea, hypoestrogenism, low-Anti-Mullerian Hormone (AMH), and elevated gonadotrophin levels in women under the age of 40.[2,3] It indicates a reduction in quantity and quality of oocytes in women of reproductive age group. Evaluating ovarian reserve and individualizing the therapeutic strategies are very important for optimizing the success rate for treatment of infertility.
When POI patients desire pregnancy, the majority of the time the only option is to receive donor oocyte program. However, early detection and active management can substantially minimize the need for egg donation in these women. But many women, due to religious, cultural, or ethical issues, would like to use their own eggs. Autologous stem cell therapy (ASCT) for ovarian rejuvenation and regeneration has now opened new doors for women with POI.
“We are envisaging new methods for activating those follicles that do not develop and the results so far are promising. This treatment gives cause for hope for patients who would otherwise not be able to conceive with their own egg,” said Prof. Antonio Pellicer, copresident and founder of the La Fe Hospital, Valencia.
There are many case reports and small series reporting the use of various medical therapies in an attempt to induce fertility in women with POI; however, the few randomized therapeutic trials that are available fail to demonstrate any significant improvement in ovulation and pregnancy rates. In a systematic review of the various therapeutic interventions thought to restore ovarian function in POI, the authors concluded that the interventions were equally ineffective and unlikely to be an improvement on expectant management. SCs derived from different sources may have some effect on the rescue of ovary function, such as recovering ovarian sex hormone function, reducing apoptosis of germ cells, and increasing the number of follicles.[4] However, the improved ovary after SC transplantation is a complex mix of many unclear factors requiring further investigation. The transplanted SCs have been proven to differentiate into granulosa cell (GC)-like cells much more easily than into oocytes.[5]
Along with the multifactorial pathogenesis and genetics of POI, in this review, we will briefly comment the major approaches for the diagnosis and treatment of infertility in POI patients and will revisit the literature supporting the process of ovarian rejuvenation by autologous bone marrow-derived SC therapy (ABMDSCT). We will also discuss in detail about our approach and the experience of delivering world's first baby born with ovarian ASCT in a perimenopausal woman of 45 years. On extensive research, we found that all studies so far are for POI and ours is the first successful birth with ABMDSCT at 45 years' age.
CASE REPORT
A 45-year-old perimenopausal female, who was infrequently menstruating for the last 3 years, came to our fertility clinic. Her AMH level was low 0.4 ng/mL. On ultrasonography, ovaries were unremarkable with antral follicle count of one. Being a single mother, she did not wanted to take the option of assisted reproduction with the donor oocyte program.
Several researchers have confirmed the presence of ovarian SCs, as well as bone marrow-derived SCs that have been able to colonize the ovaries and initiate folliculogenesis. Also few independent groups have shown the ability of autologous SCs to differentiate into primordial germ cells, which may form functional haploid gametes. Considering the above study, it was thought to use ABMDSC for the rejuvenation of functioning ovarian tissue and optimizing the success rate of achieving pregnancy through assisted reproduction.
With the patient's written, audiovisual informed consent about the nature of the procedure to be undertaken and after explaining the pros and cons of this procedure, the patient was taken for the procedure. Her bone marrow aspiration was done from posterior iliac crest under local anesthesia maintaining strict asepsis. Aspiration was done using Jamshidi needle (13G) and 20 ml syringe prewashed with heparin. Around 120 ml of bone marrow was aspirated. 16 ml BMDSC were separated using the sepax (fully automated closed capability system) which uses optical sensor technology and simultaneous application of centrifugation and sedimentation. Considering the small size of ovaries which were not well approachable with the vaginal route and were difficult to fix for instillation, we preferred laparoscopic instillation of ABMDSC in ovaries. The patient was given general anesthesia; preparation for laparoscopy was done. Four-puncture laparoscopy with 5-mm telescope was performed. Ovaries were held at the cranial and caudal position with forcep and intraovarian instillation of about 1–2 ml of ABMDSC at 3–4 sites performed bilaterally as shown in Figure 1.
Figure 1.
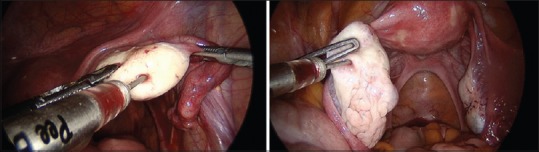
Laparoscopic instillation of autologous bone marrow derived stem cells
After 8 weeks ABMDSCT, the ultrasound revealed two follicles in each ovary. In addition, the AMH improved to 0.9 ng/mL. Considering the age of the patient, the first cycle of egg pooling was planned immediately.
We retrieved three eggs and one Grade A compacting embryo was frozen on day 3. On patient's insistance, we went ahead with frozen embryo transfer in subsequent cycle instead of second sitting of ABMDSCT. Her beta- human chorionic gonadotropin was 1280 on day 14 of embryo transfer, and a single intrauterine viable gestation was seen at 6 weeks on ultrasound. Noninvasive prenatal testing was done at 11 weeks which showed normal karyotype. The pregnancy was uneventful. A 2.7 kg female baby was delivery by cesarean birth at 38 weeks. The baby cried well after birth, had a good Apgar score, and has had an uneventful neonatal course so far.
This is not only India's first baby with this novel therapy but also may be the world's first baby with ABMDSC therapy at 45 years of age. All published reports have worked in cases of POI/POF, where patient age is below 40 years.
DISCUSSION
Premature ovarian insufficiency or “POI” also known as POF.[6] In POI, there is premature loss of ovarian follicles before the age of 40. The ovaries stop releasing eggs and stop producing hormones (estrogen, progesterone, and testosterone). The diagnosis of POI is made by evaluating the frequency of periods (over at least 4 months) and by measuring the hormone levels in the blood (two measurements at least 4 weeks apart both showing elevated levels of follicle stimulating hormone [FSH]) in menopausal range (>40 IU/L) and/or AMH <0.9 hypoestrogenism (estradiol levels <50 pg/ml).[7,8] However, all POI women do not always stop menstruating neither do their ovaries shutdown completely; hence, it is different from menopause which is permanent cessation of menstruation due to ovarian follicular activity loss.[9,10] POI has varying degrees of ovarian functions, and about 5%–10% can even conceive and deliver a child after the diagnosis and treatment.[10]
POI has important physical and psychological consequences due to both the symptoms of hypoestrogenism and the long-term sequelae of sex steroid deficiency. It also results in an age-specific increase in mortality rate.[11]
POI is a continuum of disorders with four clinical states as follows:[12]
Occult POI presents as unexplained infertility with normal baseline serum FSH levels
Biochemical POI presents as unexplained infertility with elevated basal serum FSH levels
Overt POI previously known as POF is characterized by elevated serum FSH levels with associated menstrual disorders such as oligomenorrhea, polymenorrhea, and metrorrhagia
POI is an extreme state of total primordial follicle depletion; an irreversible state characterized by anovulation, amenorrhea, infertility, and elevated gonadotropin levels.
It affects around 1%–3% of women in the reproductive age below 40 years and around 0.1% in women below 30 years of age.[13] Only around 5%–10% of POI women conceive spontaneously due to fluctuations in ovarian functions.[14,15]
In the present scenario of limited treatment options, treatment is intended for two proposes: the first being hormone replacement therapy to reduce complications due to the impaired endocrine function of ovaries, and the second for fertility concerns. Infertility treatments available for POI which may be used before or during ovarian failure, especially in cancer patients, include fertility preservation such as ovarian cortex, oocyte and embryo cryopreservation, oocyte or embryo donation, and adoption in women without any ovarian function.[16] However, exploration of these options is not feasible always; hence, newer modalities such as SCs transplantation needs to be explored.
SCs are the foundation cells for every tissue in the body. They are undifferentiated cells that have the potential to develop into specific functioning self-sustain cells.[17] Pluripotent SCs, including human embryonic stem cells and human-induced pluripotent stem cells, are useful cell sources to study and to dissect the molecular mechanisms underlying in vitro differentiation into specialized cell types. They are capable of developing into germ cells and GCs.[17] Extensive epigenetic programming of gamete precursors and the complex interactions between somatic and germ cells required for human oogenesis likely represent the main obstacles in SC-based neooogenesis. In addition, resuming oogenesis in vivo in adulthood still appears a distant hypothesis, as there is still a lack of consensus about the existence and functionality of adult ovarian SC.[18] In the presence of all these limitations, autologous bone marrow-derived mesenchymal SC for follicular recruitment in POI to attain pregnancy has been under trial in various centers globally.
Recently, Herraiz et al.[19] have conducted a study to assess if infusion of human BMDSCs could promote follicle development in the patients with impaired ovarian functions by experimenting in mice and obtained promising results and raised the possibility that promoting ovarian angiogenesis by BMDSC infusion could be an alternative approach to improve follicular development in women with impaired ovarian function. However, these studies are still in nascent and experimental stages, and its human application has not yet been developed. Few more research-like rejuvenation of POF with SCs to determine the efficacy of bone marrow-derived SC therapy on ovarian function recovery in patients with idiopathic and other types of POF still underway, and results are awaited.
Considering the above studies, we decided to try ABMDSCT in our needy perimenopausal single woman of 45-year age. We found studies in the patients of POI that is under age of 40 years, while ours is the only successful case performed at the age of 45 years, which may be the world's first baby with ABMDSC at the age of 45 years. Our successful case at 45 age not only gives a ray of hope to a needy woman even after 40 years but also make us think, can we arrest/delay menopause?
Pathogenesis of premature ovarian insufficiency
According to pathogenesis, there are two types of POI: one has a limited number of remaining follicles, and the other has an abundant number of follicles with maturation defects. The POF pathophysiology is believed to differ from normal menopause. The declined ovarian function in the first type of POF is reversible whereas in the latter one the changes are permanent.[20,21]
Although ovarian surgery is one of the common cause of POI, the pathogenesis of POF requires further investigation. Three major causes for POF include as follows: X chromosome-linked genetic defects, autoimmune disorders and iatrogenic causes secondary to surgery, and chemotherapy and radiotherapy.
Genetics
Genetic pathogenesis is among the most commonly known cause. The X chromosome-linked defects play major roles in these genetic pathogeneses, which include X monosomy (also called Turner's syndrome), trisomy, mosaicism, and X chromosome deletions.[22,23,24]
Many genes have been identified over the past few years that contribute to the development of POF. However, few genes have been identified that can explain a substantial proportion of cases of POF. The unbiased approaches of genome-wide association studies and next-generation sequencing technologies have identified several novel genes implicated in POF. As only a small proportion of genes influencing idiopathic POF have been identified, thus far, it remains to be determined how many genes and molecular pathways may influence idiopathic POF development. However, owing to POI's diverse etiology and genetic heterogeneity, we expect to see the contribution of several new and novel molecular pathways that will greatly enhance our understanding of the regulation of ovarian function. Future genetic studies in large cohorts of well-defined, unrelated, and idiopathic POI patients will provide a great opportunity to identify the missing heritability of idiopathic POI.[25]
Immunological
Immunological pathogenesis has also been studied in POF etiology. Autoimmune disorders associated with humoral and cellular immunity results in antibody creation or T-cell mediated injury of ovarian cells such as GCs, oocytes, and the zona pellucida.[26,27,28,29]
Ongoing research is focused on the development of more accurate diagnostic tools for the determination of the real prevalence of autoimmune etiology in ovarian disease, detection of concomitant or future associated autoimmune disorders, as well as a selection of patients in whom immune-modulating therapy may restore, at least temporarily, and ovarian function and fertility.
Iatrogenic
Iatrogenic causes of POI are becoming more common as laparoscopy has given more access to surgical management options. Various surgeries such as laparoscopic ovarian drilling and surgery for benign ovarian diseases such as endometrioma are among the common iatrogenic causes of POI.
One in 49 women will be diagnosed with cancer before the age of 40 years. Chemotherapeutic agents have been widely used to treat a cancer patient. Chemical agents can impair follicular stock by driving ovarian cell apoptosis, leading to a finite number of primordial follicles, and ensuing POF. Second, some chemical agents interfere with local hormonal regulation related to either follicular recruitment or rest. Third, some chemical agents may interrupt interactions between the oocytes and GCs, which are crucial for follicular growth and maturation.[30] For the above reasons, follicular storage decreases or the follicles do not fully mature, increasing the POF risk. The risk of nonsurgical POI is 13-fold higher in cancer survivors compared with their noncancer affected siblings.[14]
Idiopathic
In many commentaries, unexplained POI is the most common diagnosis with reported incidence figures as high as 90%. This may reflect the nature of investigations performed or the referral source of the women.[14]
TREATMENT FOR PREMATURE OVARIAN INSUFFICIENCY
Process of ovarian rejuvenation – autologous bone marrow-derived stem cell therapy
It is still acceptable to note that the primordial follicle population remains limited during female reproductive life. Once the storage is depleted, females are considered to have entered reproductive senescence or menopause. People have been trying to use SC therapy for POI-induced infertility based on the possibility of long-term replenishment for damaged oocytes. These transplanted SCs could reside in the ovarian tissue and rescue ovarian function, as seen in the preclinical mouse model of chemotherapy-induced POI; however, these mechanisms require further investigation.[31]
One agrees to the fact that the failed ovary needs rejuvenation. Successful ovarian rejuvenation, this is the activation of follicles in premature stages, regardless of the influence of gonadotropins, is being researched by IVI through two techniques: ovarian fragmentation for follicular activation and the infusion of SCs into the ovarian artery.[32]
Both techniques allow for a partial reversion of the aging process of the ovary, the organ of ovulation, and they activate the dormant follicles, which would otherwise stay in the ovary without maturing, not even with medication.
These are the last resort treatment options for women with POF desiring pregnancy. The technique of SC therapy has obtained promising results, as even spontaneous pregnancies in women with low ovarian reserve after undergoing a bone marrow transplant have been achieved.
Large studies related to SCs that are capable of generating oocytes have been undertaken in mice. This outcome brings some hope for new POI treatments. SCs derived from different sources may have some effect on the rescue of ovary function, such as recovering ovary sex hormone function, reducing apoptosis of GCs, and increasing the number of primordial follicles.[4] The transplanted SCs have been proven to differentiate into GC-like cells much more easily than into oocytes. The improved ovary after SC transplantation is a complex mix of many unclear factors requiring further investigation.
Other studies showed that SCs could inhibit stromal cell apoptosis through the secretion of stanniocalcin-1 and some other paracrine factors.[4] Thus, the recovery of damaged ovarian function in the POI after SC transplantation is complex, with transplanted SCs salvaging the sufficient number of existing oocytes and also helping to repair the damaged ovarian niches. There are several SC types that have been investigated in POF treatment.
In a recent benchmark study, 30 patients with POF were included in the study. Inclusion criteria were as follows: (1) patients with normal karyotype spontaneous POF and (2) patients between 18–40 years old. Exclusion criteria were as follows: (1) Patients with secondary ovarian failure (e. g., hypothalamic causes), (2) Autoimmune diseases, (3) Those with major medical problems such as malignancy, and hepatitis, and (4) Abnormal karyotyping (e.g., Turner syndrome). 26 out of the 30 patients included (86.7%) showed fall in FSH levels and rise in estrogen and AMH levels after 4 weeks of injection, and this change was maintained throughout the 48 week follow-up period. About 18 patients (60%) showed ovulation with ovum sizes ranging from 12 to 20 mm. Only one patient had a spontaneous pregnancy, while three patients were subjected to in vitro fertilization cycles. This study shows that autologous MSC may improve the conditions in patients with POI.[33]
One has to also think of the increase in the possibility of ovarian granulosa cell tumor (GCT) occurrence arising from sex-cord stromal cells of the ovary, even though it is uncommon cancer after ABMDSC therapy.[5]
All previously documented literature have included criteria of POI, i.e., age <40 years in their studies. The most probably our study is the first of its kind, where autologous SC transplantation is used for ovarian rejuvenation in a perimenopausal woman of age 45 years. This baby born with ASCT is a ray of hope not only for women with POI but also for perimenopausal women, who do not wish to go for assisted reproduction with donor oocyte program. It also made us rethink that we can arrest the menopause. Further larger trials may be needed, to get healthy pregnancy in the patient with POI using SC procedure.
A search was conducted in Research gate, Google Scholar, Pubmed database and various other research engines with MeSH terms pregnancy, autologous stem cells, ovarian rejuvenation, perimenopausal yielding no matching results.
CONCLUSION
Clinical applications of SC therapy have become popular for treating POI. Oocyte and GCs regeneration along with the reestablishment of hormone or cytokine profiles supporting SC follicular development may be involved in the improvement of both the damaged ovary function and fertility recovery. Increased understanding of this mechanism will promote its wide clinical application and give a ray of hope to the infertile couples who do not wish to opt for donor-assisted reproductive techniques.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Arora P, Polson DW. Diagnosis and management of premature ovarian failure. Obstet Gynaecol. 2011;13:67–72. [Google Scholar]
- 2.Alzubaidi NH, Chapin HL, Vanderhoof VH, Calis KA, Nelson LM. Meeting the needs of young women with secondary amenorrhea and spontaneous premature ovarian failure. Obstet Gynecol. 2002;99:720–5. doi: 10.1016/s0029-7844(02)01962-2. [DOI] [PubMed] [Google Scholar]
- 3.Shelling AN. Premature ovarian failure. Reproduction. 2010;140:633–41. doi: 10.1530/REP-09-0567. [DOI] [PubMed] [Google Scholar]
- 4.Dan S, Haibo L, Hong L. Pathogenesis and stem cell therapy for premature ovarian failure. OA Stem Cells. 2014;2:4. [Google Scholar]
- 5.Sekkate S, Kairouani M, Serji B, M'Rabti H, El Ghissassi I, Errihani H, et al. Granulosa cell tumors of the ovary. Bull Cancer. 2014;101:93–101. doi: 10.1684/bdc.2013.1879. [DOI] [PubMed] [Google Scholar]
- 6.Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:9. doi: 10.1186/1750-1172-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar N, Manesh I. Premature ovarian insufficiency: Aetiology and long-term consequences. Women Health Open J. 2017;3:45–58. [Google Scholar]
- 8.Jin M, Yu Y, Huang H. An update on primary ovarian insufficiency. Sci China Life Sci. 2012;55:677–86. doi: 10.1007/s11427-012-4355-2. [DOI] [PubMed] [Google Scholar]
- 9.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996;866:1–07. [PubMed] [Google Scholar]
- 11.Taylor AE. Systemic adversities of ovarian failure. J Soc Gynecol Investig. 2001;8:S7–9. doi: 10.1016/s1071-5576(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 12.Maiti GD. Premature ovarian failure: A chronic debilitating condition of womanhood. In: Talwar P, editor. Manual of Cytogeneticsin Reproductive Biology. London, UK: J.P. Medical Ltd; 2014. pp. 119–20. [Google Scholar]
- 13.Pouresmaeili F, Fazeli Z. Premature ovarian failure: A critical condition in the reproductive potential with various genetic causes. Int J Fertil Steril. 2014;8:1–2. [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton AJ. Premature ovarian insufficiency: Pathogenesis and management. J Midlife Health. 2015;6:147–53. doi: 10.4103/0976-7800.172292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE guideline: Management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–37. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 16.Blumenfeld Z. Fertility treatment in women with premature ovarian failure. Expert Rev Obstet Gynecol. 2011;6:321–30. [Google Scholar]
- 17.Edessy M, Hosni HN, Wafa Y, Bakry S, Shady Y, Kamel M. Stem cells transplantation in premature ovarian failure. World J Med Sci. 2014;10:12–6. [Google Scholar]
- 18.Vanni VS, Viganò P, Papaleo E, Mangili G, Candiani M, Giorgione V, et al. Advances in improving fertility in women through stem cell-based clinical platforms. Expert Opin Biol Ther. 2017;17:585–93. doi: 10.1080/14712598.2017.1305352. [DOI] [PubMed] [Google Scholar]
- 19.Herraiz S, Buigues A, Díaz-García C, Romeu M, Martínez S, Gómez-Seguí I, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109:908–18.e2. doi: 10.1016/j.fertnstert.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Woad KJ, Watkins WJ, Prendergast D, Shelling AN. The genetic basis of premature ovarian failure. Aust N Z J Obstet Gynaecol. 2006;46:242–4. doi: 10.1111/j.1479-828X.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalantaridou SN, Davis SR, Nelson LM. Premature ovarian failure. Endocrinol Metab Clin North Am. 1998;27:989–1006. doi: 10.1016/s0889-8529(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 22.Zinn AR, Page DC, Fisher EM. Turner syndrome: The case of the missing sex chromosome. Trends Genet. 1993;9:90–3. doi: 10.1016/0168-9525(93)90230-f. [DOI] [PubMed] [Google Scholar]
- 23.Zinn AR, Ross JL. Turner syndrome and haploinsufficiency. Curr Opin Genet Dev. 1998;8:322–7. doi: 10.1016/s0959-437x(98)80089-0. [DOI] [PubMed] [Google Scholar]
- 24.Goswami R, Goswami D, Kabra M, Gupta N, Dubey S, Dadhwal V, et al. Prevalence of the triple X syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders. Fertil Steril. 2003;80:1052–4. doi: 10.1016/s0015-0282(03)01121-x. [DOI] [PubMed] [Google Scholar]
- 25.Chapman C, Cree L, Shelling AN. The genetics of premature ovarian failure: Current perspectives. Int J Womens Health. 2015;7:799–810. doi: 10.2147/IJWH.S64024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Weissenbruch MM, Hoek A, van Vliet-Bleeker I, Schoemaker J, Drexhage H. Evidence for existence of immunoglobulins that block ovarian granulosa cell growth in vitro. A putative role in resistant ovary syndrome? J Clin Endocrinol Metab. 1991;73:360–7. doi: 10.1210/jcem-73-2-360. [DOI] [PubMed] [Google Scholar]
- 27.Shivers CA, Dunbar BS. Autoantibodies to zona pellucida: A possible cause for infertility in women. Science. 1977;197:1082–4. doi: 10.1126/science.70076. [DOI] [PubMed] [Google Scholar]
- 28.Rhim SH, Millar SE, Robey F, Luo AM, Lou YH, Yule T, et al. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest. 1992;89:28–35. doi: 10.1172/JCI115572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith S, Hosid S. Premature ovarian failure associated with autoantibodies to the zona pellucida. Int J Fertil Menopausal Stud. 1994;39:316–9. [PubMed] [Google Scholar]
- 30.Tuttle AM, Stämpfli M, Foster WG. Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum Reprod. 2009;24:1452–9. doi: 10.1093/humrep/dep023. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: A new treatment for premature ovarian failure? Curr Opin Obstet Gynecol. 2016;28:217–22. doi: 10.1097/GCO.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabr H, Elkheir WA, El-Gazzar A. Autologous stem cell transplantation in patients with idiopathic premature ovarian failure. J Tissue Sci Eng. 2016;7(3(Suppl)) DOI: 10.4172/2157-7552.C1.030. [Google Scholar]


