Abstract
Aim:
This study aimed to understand the association of gene-specific methylation of the promoter region of methylenetetrahydrofolate reductase (MTHFR) in the causation of recurrent miscarriages (RMs) both independently and also in light of MTHFR C677T polymorphism, hyperhomocysteinemia, folate, and Vitamin B12 deficiency.
Settings and Design:
This was a hospital-based, case–control, observational study.
Methods:
The proposed study included a total of 85 RM cases and 121 nonpregnant controls. Biochemical (homocysteine, folate, and Vitamin B12) investigations, MTHFR polymorphism (C677T), and MTHFR allele-specific methylation were done on all the samples.
Results:
Methylation-specific polymerase chain reaction of MTHFR gene revealed that methylated allele (single dose) was found to pose a significant 3.6-fold increased risk for RM. The degree of risk of methylated allele for RM was found to be aggravated from the normal genotype CC (2.8 folds) to CT (7.5 folds) individuals. Vitamin B12 deficiency and folate repletion were found to be posing an increased risk in association with methylated allele for recurrent miscarriages as compared to the respective controls.
Conclusion:
Recurrent miscarriage cases were found to be hypermethylated with respect to MTHFR gene-specific methylation as compared to the controls. High prevalence of folate repletion causing imbalance between folate and Vitamin 12 levels may lead to hypermethylation among recurrent miscarriage cases. The present study highlights the significance of the epigenetic mechanisms in the causation of the recurrent miscarriages.
KEYWORDS: Folate, gene-specific methylation, methylenetetrahydrofolate reductase, recurrent miscarriages, Vitamin B12 and hyperhomocysteinemia
INTRODUCTION
Recurrent miscarriage (RM) is a multifactorial disorder. The etiology is unknown in a significant number of women with RM. RM affects approximately 2%–4% of reproductive-aged women worldwide. Researches in the last decade found that many genetic and environmental factors contribute to the etiology of RM. One of these factors is hyperhomocysteinemia (HHcy), a key intermediate phenotype of the one-carbon metabolic pathway. HHcy has been reported to be an independent risk factor for many pregnancy complications such as preeclampsia, neural tube defects, placental abruption or infarction, and unexplained recurrent miscarriage.[1,2,3,4] The key factors of one-carbon metabolism, that is folate and Hcy, are vital to the maintenance and progression of pregnancy.[5] Methylenetetrahydrofolate reductase (MTHFR) C677T mutation causing reduced enzymatic activity is a known influencing factor of HHcy.[6] Methylation of the genome (germ cell) is vital both for normal spermatogenesis and embryo development following fertilization.[7] Major functions at the feto–maternal interface, such as nutrient transport, trophoblast proliferation, invasion, and angiogenesis, are found to be regulated by correctly imprinted maternal and paternal genes.[8]
The present study is an attempt to understand the association (if any) between methylation of MTHFR promoter region and RMs in light of folate, Vitamin B12, and homocysteine levels, and MTHFR C677T status.
METHODS
The participants (RM cases and controls) were recruited from Department of Obst and Gynae Lady Hardinge Medical College and Smt. Sucheta Kriplani hospital, New Delhi. The case group comprised of 85 nonpregnant women with three or more consecutive unexplained pregnancy losses before 24 weeks of gestation. To rule out for RM cases with explained reasons, all the women with recurrent pregnancy losses were subjected to antiphospholipid antibodies workup, lupus anticoagulants, β-microglobulin test, glucose tolerance test (GTT), ultrasonography for ruling out for uterine anomalies, polycystic ovaries, antral follicle count for ovarian reserve, hysterosalpingography/hysteroscopy for ruling out uterine anomaly, and intrauterine adhesions. Further, dilated test for ruling out cervical incompetence, hormonal profile including day 2 follicle-stimulating hormone, luteinizing hormone, prolactin, and thyroid profile test were performed. Premenstrual endometrium biopsy for ruling out tuberculosis is also performed. The period of gestation of RM cases ranged from 5 to 14 weeks (mean gestation; 7.52 weeks ± 2.19). RM case group comprises of 92.19% of the women with miscarriage before 12 weeks of gestation (early miscarriage). The control group comprised of 121 nonpregnant women with two or more consecutive normal successful pregnancies and were matched for age, geography, social class, and ethnicity. The controls were healthy parous women who had presented to the hospital for tubal ligation. Ethical clearance was obtained from Ethical committee, Lady Hardinge Medical College and Smt. Sucheta Kriplani hospital, New Delhi.
After obtaining informed written consent, 5 ml fasting intravenous blood sample was drawn from each woman and was subjected to serum folate, Vitamin B12, and plasma homocysteine level estimations using Immulite 1000 instrument (Siemens- Diagnostic- Products, and Flanders, NJ, USA) based on chemiluminescence. The buffy coat was subjected to DNA isolation[9] followed by MTHFR C677T detection.[10] The cutoffs for HHcy, serum folate, and Vitamin B12 deficiencies used were ≥15 umol/L, ≤3 ng/ml, and ≤220 pg/ml, respectively.[11] The genomic DNA was subjected to bisulfite conversion using Qiagen bisulfite conversion kits (EpiTect bisulfite kit, Qiagen, Hilden, Germany) for the analysis of MTHFR gene allele-specific methylation status among cases as well as controls. The primers were designed as per the protocol given by Khazamipour et al.,[12] specific for methylated and unmethylated regions of 1st CpG island in MTHFR promoter region. Genotyping of polymerase chain reaction products revealed two bands for methylated and unmethylated (MU) for heterozygous condition and one each for methylated homozygous and unmethylated homozygous condition, i.e., MM and UU, respectively.
RESULTS
In the present study, MTHFR gene-specific methylation of 85 RM cases and 121 controls was done. Further, distribution of methylated and unmethylated alleles was observed among RM cases and controls with respect to the MTHFR C677T genotypes, HHcy, Vitamin B12 deficiency, and folate deficiency.
The selected locus was found to be polymorphic in the studied women, wherein the methylated allele in homozygous state (MM) was found to be absent among control group as compared to a very low frequency (1.18%) among RM cases [Figure 1]. Further, the frequency of MU genotype was higher (32.94%) in RM cases compared to controls (12.40%). These observed differences with respect to the distribution of methylated allele are found to be statistically significant (P = 0.0002). Further odds ratio (OR) analysis revealed more than 3-fold significant increased risk of methylated allele in single dose for RM (OR = 3.66 [1.81–7.39]).
Figure 1.
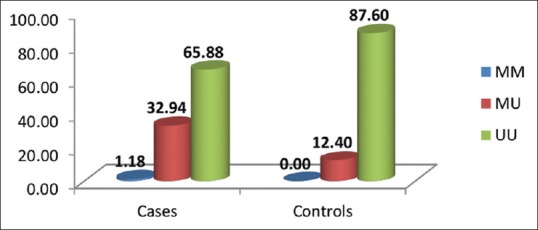
Distribution of methylenetetrahydrofolate reductase allele-specific methylation genotypes among recurrent miscarriage cases and controls
When distribution pattern of methylated allele between RM cases and controls was compared with respect to the MTHFR C677T genotypic status [Table 1], there seems to be a significant difference between RM cases and controls, wherein the frequency of methylated allele was found to be significantly higher among RM cases carrying CC and CT genotypes of MTHFR C677T polymorphism as compared to the controls.
Table 1.
Distribution of MTHFR methylation- specific genotype with respect to MTHFR C677T gene polymorphism among recurrent miscarriage cases and controls
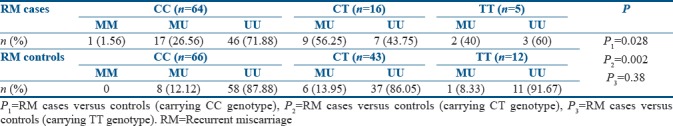
The OR analysis revealed a significant (P = 0.02) increased risk of 2.8 ([confidence interval [CI]: 1.13–7.12], P = 0.03) folds of methylated allele for CC carrying RM cases, followed by 7.9 folds ([CI: 2.1–29.4], P = 0.002) increased risk for CT carrying RM cases. Methylated allele was found to pose 7.33-fold increased risk ([CI: 0.48–111.2], P = 0.19) for TT carrying RM cases as compared to the respective controls, although the risk was not found to be significant because of less number of individuals with TT genotype.
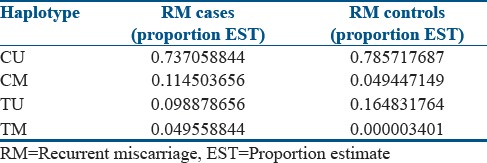
Haplotypic distribution of MTHFR C677T polymorphism and MTHFR gene-specific methylation suggests that methylated allele in association with C allele (0.11) or T allele (0.04) was found to be more frequent among RM cases than RM controls [Table 2]. The combination of alleles (C and U) for both the polymorphisms was comparatively higher among RM controls than RM cases. Haplotypic combination T-M, i.e., mutated T allele and methylated allele, seems to be quite frequent among cases as compared to that of controls, where it is almost negligible.
Table 2.
Haplotypic distribution of MTHFR C677T and methylation-specific polymerase chain reaction alleles among recurrent miscarriage cases and recurrent miscarriage controls
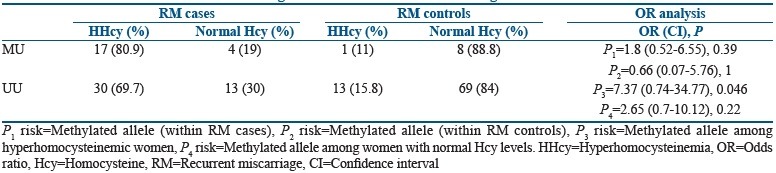
The distribution of RM cases and controls is analyzed on the basis of raised levels of homocysteine (HHcy) and normal homocysteine levels [Table 3]. Majority of the RM cases (80.9%) carrying methylated allele were found to be hyperhomocysteinemic, whereas only 11% of RM controls carrying methylated allele were found to be hyperhomocysteinemic. When the association of methylated allele with elevated homocysteine levels was analyzed among cases and controls independently, no association could be found. Further, an attempt was made to understand the association of methylated allele with RMs among women with elevated levels of homocysteine and normal levels of homocysteine. The analysis revealed that the methylated allele was posing a significant increased risk of more than 7 folds for RMs as compared to controls with elevated homocysteine levels [Table 3], whereas methylated allele was not found to be posing any significant risk for RM cases as compared to controls with normal homocysteine levels.
Table 3.
Distribution of methylated and unmethylated allele with respect to hyperhomocysteinemia among recurrent miscarriage cases and recurrent miscarriage controls
The distribution pattern of RM cases carrying methylated allele with Vitamin B12 deficiency was found to be significantly high (80.6%), as against only 33% among RM controls [Table 4]. Further, 45.4% of the RM cases carrying unmethylated allele (UU) were found to be Vitamin B12 deficient as compared to controls which had only 12%.
Table 4.
Distribution of methylated and unmethylated allele with respect to Vitamin B12 deficiency among recurrent miscarriage cases and recurrent miscarriage controls
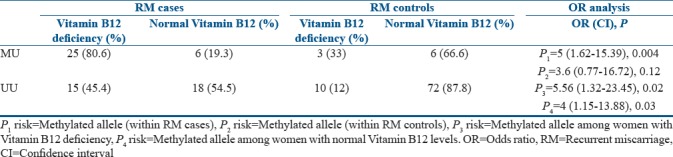
When the association of methylated allele with Vitamin B12 deficiency was analyzed in cases and controls independently, Vitamin B12 deficiency was found to be associated with methylated allele among RM cases as compared to controls. This is attributed to more of Vitamin B12 deficiency found among RM cases. Further, an attempt was made to understand the association of methylated allele with RMs among women with Vitamin B12 deficiency and normal levels. The analysis revealed that the methylated allele with Vitamin B12 deficiency was posing a significant increased risk of more than 5 folds for RMs as compared to controls with Vitamin B12 deficiency [Table 4] and a significant increased risk of 4 folds for RMs as compared to controls with normal Vitamin B12 levels.
The number of women with methylated allele and folate deficiency is found to be more (77.78%) among control group as compared to none among the RM cases, and hence the risk of folate deficiency for RM was not calculated [Table 5].
Table 5.
Distribution of methylated and unmethylated allele with respect to folate deficiency among recurrent miscarriage cases and recurrent miscarriage controls
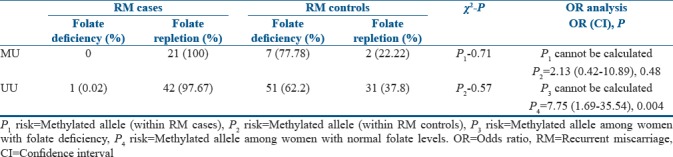
When the association of methylated allele with folate deficiency was analyzed among control group, no association could be found. Further, an attempt was made to understand the association of methylated allele with RMs among women with folate repletion and deficiency. The analysis revealed that the methylated allele was posing a significant increased risk of more than 7 folds for RMs as compared to controls with folate repletion [Table 5].
DISCUSSION
DNA methylation is an integral component in epigenetics playing a crucial role in cellular development and differentiation and also forms a basis for numerous human diseases. The results found in the present study are indicative of the vital role played by MTHFR promoter region hypermethylation in RMs. The present study hints toward the involvement of hypermethylation of promoter region of MTHFR C677T allele in the process of implantation, resulting in early rejection of the fetus leading to RMs. Similar results were obtained in an earlier study conducted by the authors wherein MTHFR gene-specific methylation (performed by Sequenom) was found to be significantly higher among RM cases (communicated elsewhere). Sequenom method was used to capture gene specific methylation using mass array technique, MALDI-TOF Sequenom (ACE - PROBE) which captures 17CpG sites in the 1st Island of the MTHFR gene promoter region. Genomic DNA (50 ng/ μL) was bisulfite-treated using the EZ DNA methylation™ Kit (Zymo Research, California, USA). Moreover, the results are also in concordance with another study which hinted toward the association of MTHFR gene promoter region hypermethylation in sperm DNA of recurrent spontaneous abortion couples.[13]
However, MTHFR 677T allele was not found to be associated with RMs in a study previously conducted by the authors.[14] Moreover, the degree of risk of methylation in combination with MTHFR C677T status was found to be aggravated on moving from the normal genotype CC (2.8 folds) to CT (7.5 folds). The significance of MTHFR C677T polymorphism has been suggested in a meta-analysis[15] wherein polymorphism at this particular locus was found to be associated with recurrent pregnancy loss risk. Indeed, epigenetic modifications in the promoter region and the consequent downregulation of gene expression have been shown to act in the same way and in some cases parallel to genetic mutations in a number of pathologies.[16]
The risk caused for RMs by the methylated allele (3.6 folds) gets increased (7.35 folds) among women with raised levels of homocysteine as previously reported,[14] wherein hyperhomocysteinemia was found to be an independent risk factor for RMs.
The risk caused by methylated allele was found to be enhanced in the presence of Vitamin B12 deficiency. RM cases in the present study were found to be folate replete (irrespective of their methylated allele status) due to folate supplementation as a part of routine antenatal care in consecutive miscarriages. Further, high levels of folate along with methylated allele were found to be a causal risk factor for RM. These results are supported by a study which reported the reduced global DNA methylation patterns in the presence of excessive folic acid.[17] The results are also in concordance with a recent report by Kok et al.,[18] wherein long-term supplementation with folic acid and Vitamin B12 in elderly patients resulted in alterations in DNA methylation of several genes, especially the genes involved in developmental processes as well as carcinogenesis.
Epigenetic profile of the genome is reprogrammed dynamically during the early development of the fetus.[19] It is suggested that hypermethylation of MTHFR promoter region among women with RM hints toward epigenetic silencing, which may result in a reduced availability of methyl groups for the epigenetic processes that are important for normal development, resulting in miscarriages.
Epigenetics plays an important role in multiple developmental diseases and processes, especially dealing with infertility issues. DNA methylation should be looked at as a novel factor. This study opens up ways of research on larger sample size cohorts along with follow-up studies before drawing any concrete conclusions and further provides a new insight in identifying the underlying epigenetic markers causing RM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wouters MG, Boers GH, Blom HJ, Trijbels FJ, Thomas CM, Borm GF, et al. Hyperhomocysteinemia: A risk factor in women with unexplained recurrent early pregnancy loss. Fertil Steril. 1993;60:820–5. [PubMed] [Google Scholar]
- 2.Goddijn-Wessel TA, Wouters MG, van de Molen EF, Spuijbroek MD, Steegers-Theunissen RP, Blom HJ, et al. Hyperhomocysteinemia: A risk factor for placental abruption or infarction. Eur J Obstet Gynecol Reprod Biol. 1996;66:23–9. doi: 10.1016/0301-2115(96)02383-4. [DOI] [PubMed] [Google Scholar]
- 3.Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: A systematic review. Placenta. 1999;20:519–29. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- 4.Wenstrom KD, Johanning GL, Owen J, Johnston KE, Acton S, Tamura T, et al. Role of amniotic fluid homocysteine level and of fetal 5, 10-methylenetetrahydrafolate reductase genotype in the etiology of neural tube defects. Am J Med Genet. 2000;90:12–6. doi: 10.1002/(sici)1096-8628(20000103)90:1<12::aid-ajmg3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Yajnik CS, Deshmukh US. Fetal programming: Maternal nutrition and role of one-carbon metabolism. Rev Endocr Metab Disord. 2012;13:121–7. doi: 10.1007/s11154-012-9214-8. [DOI] [PubMed] [Google Scholar]
- 6.Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl 1):S56–64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 7.Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the Germline and preimplantation embryos. Genes Dev. 2014;28:812–28. doi: 10.1101/gad.234294.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgiades P, Watkins M, Burton GJ, Ferguson-Smith AC. Roles for genomic imprinting and the zygotic genome in placental development. Proc Natl Acad Sci U S A. 2001;98:4522–7. doi: 10.1073/pnas.081540898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 11.Sukla KK, Raman R. Association of MTHFR and RFC1 gene polymorphism with hyperhomocysteinemia and its modulation by Vitamin B12 and folic acid in an Indian population. Eur J Clin Nutr. 2012;66:111–8. doi: 10.1038/ejcn.2011.152. [DOI] [PubMed] [Google Scholar]
- 12.Khazamipour N, Noruzinia M, Fatehmanesh P, Keyhanee M, Pujol P. MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: The role of epigenetics in male infertility. Hum Reprod. 2009;24:2361–4. doi: 10.1093/humrep/dep194. [DOI] [PubMed] [Google Scholar]
- 13.Rotondo JC, Bosi S, Bazzan E, Di Domenico M, De Mattei M, Selvatici R, et al. Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion. Hum Reprod. 2012;27:3632–8. doi: 10.1093/humrep/des319. [DOI] [PubMed] [Google Scholar]
- 14.Puri M, Kaur L, Walia GK, Mukhopadhhyay R, Sachdeva MP, Trivedi SS, et al. MTHFR C677T polymorphism, folate, vitamin B12 and homocysteine in recurrent pregnancy losses: A case control study among North Indian women. Journal of perinatal medicine. 2013;41:549–54. doi: 10.1515/jpm-2012-0252. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Yang X, Lu M. Methylenetetrahydrofolate reductase gene polymorphisms and recurrent pregnancy loss in China: A systematic review and meta-analysis. Arch Gynecol Obstet. 2016;293:283–90. doi: 10.1007/s00404-015-3894-8. [DOI] [PubMed] [Google Scholar]
- 16.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000;16:168–74. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni A, Dangat K, Kale A, Sable P, Chavan-Gautam P, Joshi S, et al. Effects of altered maternal folic acid, Vitamin B12 and docosahexaenoic acid on placental global DNA methylation patterns in Wistar rats. PLoS One. 2011;6:e17706. doi: 10.1371/journal.pone.0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kok DE, Dhonukshe-Rutten RA, Lute C, Heil SG, Uitterlinden AG, van der Velde N, et al. The effects of long-term daily folic acid and Vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin Epigenetics. 2015;7:121. doi: 10.1186/s13148-015-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin LJ, Zhang Y, Lv PP, He WH, Wu YT, Liu AX, et al. Insufficient maintenance DNA methylation is associated with abnormal embryonic development. BMC Med. 2012;10:26. doi: 10.1186/1741-7015-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]


