Abstract
Objective: This study aimed to assess and compare the distribution of cardiovascular risk factors and the estimated 10-year risk of fatal or non-fatal acute myocardial infarction (AMI) or cerebral stroke (CS) among the Sami and non-Sami populations of Northern Norway. Methods: The SAMINOR 2 Clinical Survey is a cross-sectional survey conducted in 10 municipalities in the counties of Finnmark, Troms and Nordland in rural Northern Norway in 2012–2014. All inhabitants aged 40–79 years were invited to participate, and 6004 (48.2%) accepted. The NORRISK 2 model was used to estimate the 10-year risk of fatal or non-fatal AMI or CS. Sex and age were included in the model, as well as the following risk factors for cardiovascular disease (CVD): serum total cholesterol, serum high-density lipoprotein cholesterol, systolic blood pressure, smoking habits and anti-hypertensive treatment. Results: Only minor ethnic differences were observed between Sami and non-Sami populations in a number of individual risk factors for CVDs. Overall, the NORRISK 2 model revealed no ethnic differences in the 10-year risk of AMI or CS. Conclusions: There were no differences in 10-year risk of AMI or CS between the Sami and non-Sami populations in 10 selected municipalities in Northern Norway.
Keywords: Cardiovascular mortality, cardiovascular morbidity, indigenous, native, aboriginal, ethnic, risk model, NORRISK, Norway
Introduction
Indigenous populations often have poorer health than their respective majority reference populations [1], and recent reviews have indicated poorer cardiovascular health in Inuit populations and other indigenous populations in North America [2–4]. Population health is sculptured by many factors: level of education, wealth, environmental quality and protection, diet, behaviour traits such as physical activity and smoking, occupational and domestic stresses and genetics. Varying exposures to such factors over time can generate ethnic differences in health [5].
The Sami are an indigenous people whose traditional settlement area, Sápmi, stretches from the Kola Peninsula in the north to Engerdal and Idre in the south of Norway and Sweden, respectively [6]. Finnmark is the northernmost county in Norway and the region most influenced by Sami language and culture [6]. The Kven are descendants of Finnish-speaking settlers who emigrated from Sweden and Finland to the northern parts of Norway in the 1700s and 1800s [7].
In 1974–1975, a cardiovascular risk score among Sami and Kven/Finnish men aged 35–49 years in Finnmark was about 40% higher than that among Norwegian men [8]. The score only included systolic blood pressure, serum total cholesterol and smoking habits. However, later studies restricted to Finnmark County in the period 1974–1989 [9–12] and the SAMINOR 1 Survey from 2003–2004 [13] also including the southern regions of Sápmi showed none or only minor differences in the distribution of cardiovascular risk factors and in the burden of cardiovascular disease (CVD) between Sami and non-Sami populations in Norway. In terms of differences in CVD mortality between these ethnic groups, there have been conflicting results [14,15]. Furthermore, in Northern Norway, risk-factor levels and CVD mortality have declined over the last decades [16]. Whether this decline has been the same in Sami and non-Sami populations is unknown. Hence, updated knowledge concerning the distribution of CVD risk factors and a comprehensive CVD risk assessment of the Sami and non-Sami populations settled in Sápmi is needed.
CVD is multifactorial. Thus, guidelines for prevention recommend assessing the impact of several risk factors simultaneously [17]. The NORRISK 2 model is a cardiovascular risk model based on Norwegian data. It estimates the 10-year risk of fatal or non-fatal acute myocardial infarction (AMI) or cerebral stroke (CS) by combining information from several risk factors [18]. Using this model and data from the second survey of the Population-based Study on Health and Living Conditions in Regions with Sami and Norwegian Populations (the SAMINOR 2 Clinical Survey), the primary objective of the present study was to compare the distribution of risk factors included in the NORRISK 2 model and the estimated 10-year risk of AMI or CS in Sami and non-Sami populations. Furthermore, to give a comprehensive cardiovascular risk profile to Sami and non-Sami populations, we also present the distribution of other established risk factors and education attainment. The 10-year risk for AMI or CS has not previously been computed and compared for Sami and non-Sami populations.
Methods
The Population-based Study on Health and Living Conditions in Regions with Sami and Norwegian Populations – the SAMINOR Study – is run by the Centre for Sami Health Research at UiT – The Arctic University of Norway. The present analyses are based on cross-sectional data from the second survey, the SAMINOR 2 Clinical Survey (hereafter referred to as SAMINOR 2), conducted in 2012–2014. Invitation to SAMINOR 2 included all inhabitants (n=12,455) aged 40–79 years residing in the municipalities of Evenes in Nordland County; Skånland, Kåfjord, Storfjord and Lyngen in Troms County; and Karasjok, Kautokeino, Porsanger, Tana and Nesseby in Finnmark County. In total, 6004 inhabitants accepted. The overall response rate was 48.2% (i.e. 45.8% men and 54.2% women) and varied from 41% in Evenes to 56% in Kautokeino. The participants completed a self-administered questionnaire that was mailed with the invitation letter and returned at the clinical examination. Participants aged 40–69 years completed an eight-page questionnaire; those aged 70–79 years completed a four-page questionnaire, with fewer questions and larger fonts. Questionnaires were prepared in Norwegian and translated into Northern Sami. Both the Sami and the Norwegian versions of the questionnaire were distributed to participants in Kautokeino, Karasjok, Nesseby and Tana, and the Sami version was available upon request to participants in Kåfjord, Storfjord, Porsanger and Lyngen. Invitees in Skånland and Evenes received the Norwegian questionnaire only. The SAMINOR Study was accredited by the Norwegian Data Inspectorate and approved by the Regional Committee for Medical and Health Research Ethics. The committee also approved this study, for which all participants gave written informed consent.
Clinical examination and blood-sample collection
Waist circumference was measured at the umbilicus with the participant standing, and abdominal obesity was determined by using the thresholds >102 cm and >88 cm for men and women, respectively. At least 15 minutes after participant arrival, blood pressure was measured with an automatic device (CARESCAPE™ V100 monitor), with the participants in a seated position with their arms resting at heart level. Following a two-minute rest, three measurements were taken at one-minute intervals. The average of the last two measurements was used in the statistical analysis.
Non-fasting blood samples were collected with participants in a seated position. Tubes with anticoagulant were used for glycated haemoglobin (HbA1c) measurements conducted on-site within 15 minutes of blood collection using DCA Vantage™ (Siemens Medical Solutions Diagnostics, Tarrytown, NY), which uses an agglutination inhibition immunoassay method. Tubes for serum samples were centrifuged within two hours of blood collection. Serum was separated and stored at −20°C, transported for further storage at −70°C and later used to analyse high-density lipoprotein (HDL) cholesterol, triglycerides and total cholesterol at the University Hospital of North Norway using an enzymatic colorimetric test run with Cobas 8000B (Roche Diagnostics GmbH, Mannheim, Germany). Thresholds for triglycerides and HbA1c were taken from European [17] and World Health Organization guidelines [19], respectively.
Questionnaire
Ethnicity was ascertained by the questions: ‘What language(s) do/did you, your parents and your grandparents use at home?’, ‘What is your, your father’s and your mother’s ethnic background?’ and ‘What ethnicity do you consider yourself to be?’ On all items, the response options were ‘Norwegian’, ‘Sami’, ‘Kven’ and ‘other’. The questions were answered separately for each relative (11 questions in total), and multiple answers were allowed. Participants were defined as Sami if they considered themselves to be Sami or reported a Sami ethnic background and at least one of their grandparents, parents or they themselves spoke a Sami language at home. All others were categorised as non-Sami.
We identified current smokers and non-smokers from the questions: ‘Have you ever smoked daily?’ (yes/no) and ‘Are you currently a daily smoker?’ (yes/no). Previous smokers were categorised as non-smokers.
Anti-hypertensive treatment was ascertained from the question: ‘Are you taking medication for high blood pressure?’ The response options were ‘yes, currently’, ‘in the past, but not currently’ and ‘no’. Participants reporting former use were merged with non-users, and missing values were ad hoc imputed as non-users.
Both questionnaire information and HbA1c measurements were applied to identify participants with diabetes mellitus. The question was: ‘Have you ever been diagnosed with diabetes (high blood sugar)?’ (yes/no). Missing values were classified as ‘no’. In addition, all participants with HbA1c values ≥6.5% (48 mmol/mol) were classified as having diabetes mellitus, regardless of their reply on the questionnaire.
Physical activity was measured by asking: ‘Please indicate your levels of physical activity at the ages of 14, 30 and at your current age, on a scale from 1 to 10. “Physical activity” includes household chores and professional activities, as well as regular exercise and other physical activity, such as walking/hiking. Please mark (with an ‘X’) below the number that most accurately denotes your physical activity levels’. In this study, we used physical activity at current age, an instrument validated in middle-aged women living in Tromsø, Norway [20]. We recoded the 10-level physical activity scale into three categories: low (levels 1–3), moderate (levels 4–7) and high physical activity (levels 8–10).
Years of education was measured with the question: ‘How many years of education have you completed? (Include any and all years in which you attended school or studied)’. We categorised this item into three levels: 0–9 years, 10–12 years and ≥13 years, which roughly corresponds to compulsory primary and lower secondary school, upper secondary school and higher education, respectively.
The NORRISK 2 model
The NORRISK 2 model is validated and intended to be used in primary prevention of CVD in the Norwegian population aged 40–79 years [18]. The model is used to estimate the 10-year risk (%) of hospitalisation with AMI (International Classification of Diseases [ICD-10] codes I21–22) as main or secondary diagnosis, death from ischaemic heart diseases (ICD-10 codes I20–25) as the underlying cause, or hospitalisation with CS (ICD-10 codes I60–61 and I63–64 except I63.6) as main or secondary diagnosis or death from CS as underlying cause [18]. The 10-year risk estimations are based on sex, age, total cholesterol, HDL-cholesterol, systolic blood pressure, smoking habits, anti-hypertensive treatment and family history of premature coronary heart disease [18]. All but family history of premature coronary heart disease were included in SAMINOR 2. In the model development, Selmer et al. treated death from other causes as competing risk. We used the age-specific, high-risk thresholds suggested by Selmer et al. [18] to identify individuals at high 10-year risk of fatal or non-fatal AMI or CS events who should be offered pharmacological treatment: ≥5% in the age group 45–54, ≥10% in the age group 55–64 and ≥15% in the age group 65–74 years. For those aged 40–44 and 75–79 years included in our study, we used the high risk threshold ≥5% and ≥15%, respectively.
Statistical analyses
Statistical analyses were performed using Stata v14.0 (StataCorp, College Station, TX). Ethnic differences in characteristics and age-specific cardiovascular risks were tested by two-sample t-tests with equal variance for continuous variables and Pearson’s chi-square test (Tables I and II) and Fisher’s exact test (Tables III and IV) for categorical variables. Test of trends across ordered groups (Tables I and II) was done with Wilcoxon’s rank-sum test. Age standardisation of means and proportions was done separately in men and women using the direct method and having the sex-specific invited SAMINOR 2 sample in five-year age groups as the standard population. Comparisons of age-standardised means and proportions were done with a two-sample t-test with equal variance and a two-sample test for proportions, respectively, using the age-standardised estimates of means, proportions and standard deviations. We considered p-values <0.05 to be statistically significant.
Table I.
Unadjusted sample characteristics of non-Sami and Sami men (n=2346): The SAMINOR 2 Clinical Survey (2012–2014).
| Variables | Non-Sami, n = 1372 | Sami, n = 974 | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 59.4 (10.2) | 58.8 (10.1) | 0.11 |
| 40–54 years, % (n) | 32.9 (451) | 36.3 (353) | 0.03 |
| 55–64 years, % (n) | 31.4 (431) | 32.4 (316) | |
| 65–79 years, % (n) | 35.7 (490) | 31.3 (305) | |
| Total cholesterol (mmol/L), mean (SD) | 5.42 (1.0) | 5.52 (1.1) | 0.03 |
| Total cholesterol >5.0 mmol/L, % (n) | 67.6 (928) | 69.7 (679) | 0.29 |
| HDL-cholesterol (mmol/L), mean (SD) | 1.29 (0.4) | 1.24 (0.4) | <0.001 |
| HDL-cholesterol <1.0 mmol/L, % (n) | 17.9 (246) | 21.6 (210) | 0.03 |
| Triglycerides (mmol/L), mean (SD) | 1.80 (1.1) | 1.94 (1.1) | 0.002 |
| Triglycerides >1.7 mmol/L, % (n) | 44.1 (605) | 51.2 (499) | 0.001 |
| Systolic blood pressure (mmHg), mean (SD) | 134.9 (17.2) | 134.3 (18.1) | 0.39 |
| Systolic blood pressure ≥140 mmHg, % (n) | 34.4 (472) | 33.8 (329) | 0.75 |
| Diastolic blood pressure (mmHg), mean (SD) | 78.1 (9.4) | 77.3 (9.9) | 0.04 |
| Diastolic blood pressure ≥90 mmHg, % (n) | 11.0 (151) | 11.4 (111) | 0.77 |
| Anti-hypertensive treatment (yes), % (n) | 26.7 (366) | 23.4 (228) | 0.07 |
| Current smoking (yes), % (n) | 16.0 (220) | 19.1 (186) | 0.05 |
| HbA1ca (%), mean (SD) | 5.69 (0.6) | 5.79 (0.8) | <0.001 |
| Diabetes mellitusb (yes), % (n) | 8.3 (114) | 11.0 (107) | 0.03 |
| Waist circumferencec (cm), mean (SD) | 100.0 (10.6) | 98.5 (10.8) | 0.001 |
| Waist circumference >102 cm, % (n) | 35.0 (479) | 31.8 (309) | 0.11 |
| Physical activityd, mean (SD) | 5.3 (2.0) | 5.2 (2.2) | 0.08 |
| Low (1–3), % (n) | 19.6 (263) | 23.4 (220) | 0.43 |
| Moderate (4–7), % (n) | 66.3 (892) | 60.6 (569) | |
| High (8–10), % (n) | 14.1 (190) | 16.0 (150) | |
| Years of educatione, mean (SD) | 12.0 (3.6) | 11.7 (3.7) | 0.14 |
| 0–9 years, % (n) | 27.7 (369) | 32.3 (302) | 0.04 |
| 10–12 years, % (n) | 33.1 (441) | 31.0 (290) | |
| ≥13 years, % (n) | 39.2 (524) | 36.7 (343) |
Missing values: non-Sami, n = 3; Sami, n = 3.
Diabetes mellitus: self-reported diabetes or HbA1c ≥6.5%.
Missing values: non-Sami, n = 3; Sami, n = 2.
Missing values: non-Sami, n = 27; Sami, n = 35.
Missing values: non-Sami, n = 38; Sami, n = 39.
SD: standard deviation.
Table II.
Unadjusted sample characteristics of non-Sami and Sami women (n=2972): The SAMINOR 2 Clinical Survey (2012–2014).
| Variables | Non-Sami, n=1777 | Sami, n=1195 | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 58.6 (10.6) | 57.9 (10.2) | 0.08 |
| 40–54 years, % (n) | 37.0 (657) | 37.7 (451) | 0.10 |
| 55–64 years, % (n) | 30.4 (541) | 34.0 (406) | |
| 65–79 years, % (n) | 32.6 (579) | 28.3 (338) | |
| Total cholesterol (mmol/L), mean (SD) | 5.59 (1.0) | 5.61 (1.1) | 0.55 |
| Total cholesterol >5.0 mmol/L, % (n) | 71.1 (1264) | 72.1 (861) | 0.59 |
| HDL-cholesterol (mmol/L), mean (SD) | 1.55 (0.5) | 1.45 (0.4) | <0.001 |
| HDL-cholesterol <1.3 mmol/L, % (n) | 25.3 (450) | 32.7 (391) | <0.001 |
| Triglycerides (mmol/L), mean (SD) | 1.53 (0.9) | 1.66 (0.9) | <0.001 |
| Triglycerides >1.7 mmol/L, % (n) | 33.6 (597) | 39.7 (475) | 0.001 |
| Systolic blood pressure (mmHg), mean (SD) | 130.6 (18.3) | 129.6 (19.3) | 0.14 |
| Systolic blood pressure ≥140 mmHg, % (n) | 27.0 (479) | 26.5 (317) | 0.80 |
| Diastolic blood pressure (mmHg), mean (SD) | 72.4 (9.0) | 71.8 (9.3) | 0.08 |
| Diastolic blood pressure ≥90 mmHg, % (n) | 3.6 (64) | 3.8 (45) | 0.82 |
| Anti-hypertensive treatment (yes), % (n) | 26.7 (475) | 26.2 (313) | 0.75 |
| Current smoking (yes), % (n) | 20.1 (357) | 22.5 (269) | 0.11 |
| HbA1ca (%), mean (SD) | 5.66 (0.6) | 5.71 (0.5) | 0.05 |
| Diabetes mellitusb (yes), % (n) | 8.3 (148) | 10.0 (120) | 0.11 |
| Waist circumferencec (cm), mean (SD) | 92.7 (12.1) | 93.4 (12.0) | 0.13 |
| Waist circumference >88 cm, % (n) | 61.7 (1093) | 66.1 (789) | 0.01 |
| Physical activityd,mean (SD) | 5.6 (2.1) | 5.3 (2.1) | <0.001 |
| Low (1–3), % (n) | 15.5 (257) | 22.8 (261) | <0.001 |
| Moderate (4–7), % (n) | 64.8 (1076) | 60.7 (696) | |
| High (7–10), % (n) | 19.7 (328) | 16.5 (189) | |
| Years of educatione,mean (SD) | 12.4 (4.0) | 12.7 (4.4) | 0.09 |
| 0–9 years, % (n) | 26.0 (445) | 27.8 (316) | 0.28 |
| 10–12 years, % (n) | 30.3 (517) | 23.2 (263) | |
| ≥13 years, % (n) | 43.7 (747) | 49.0 (556) |
Missing values: non-Sami, n=1; Sami, n=6.
Diabetes mellitus: self-reported diabetes or HbA1c ≥6.5%.
Missing values: non-Sami, n=5; Sami, n=2.
Missing values: non-Sami, n=116; Sami, n=49.
Missing values: non-Sami, n=68; Sami, n=60.
SD: standard deviation.
Table III.
Mean 10-year risk and proportion of participants at high risk of acute myocardial infarction (AMI) or cerebral stroke (CS) in non-Sami and Sami men aged 40–79 years, n=2346: The SAMINOR 2 Clinical Survey (2012–2014).
| Age | Non-Sami (n=1372) |
Sami (n=974) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | n | p-value | ||||||
| 40–54 years | Mean (SD) | 451 | 3.6 | (2.6) | 353 | 4.0 | (3.1) | 0.06 |
| ≥5% (n) | 21.5% | (97) | 23.2% | (82) | 0.61 | |||
| 55–64 years | Mean (SD) | 431 | 8.8 | (4.2) | 316 | 9.5 | (5.0) | 0.06 |
| ≥10% (n) | 26.7% | (115) | 36.1% | (114) | 0.006 | |||
| 65–79 years | Mean (SD) | 490 | 18.0 | (6.4) | 305 | 18.1 | (6.7) | 0.81 |
| ≥15% (n) | 63.5% | (311) | 61.3% | (187) | 0.55 | |||
| Total | ||||||||
| Crude mean (SE) | 1372 | 10.4 | (0.21) | 974 | 10.2 | (0.25) | 0.55 | |
| Age-standardised mean (SE) | 1372 | 9.5 | (0.10) | 974 | 9.8 | (0.13) | 0.05 | |
| % with high riska (n) | 38.1% | (523) | 39.3% | (383) | 0.56 | |||
| Age-standardised % with high risk | 35.6% | 37.5% | 0.35 | |||||
Proportions with 10-year risk of AMI or CS ≥5%, ≥10% and ≥15% in age groups 40–54, 55–64 and 65–79 years, respectively.
SD: standard deviation.
Table IV.
Mean 10-year risk and proportion of participants at high risk of acute myocardial infarction (AMI) or cerebral stroke (CS) in non-Sami and Sami women aged 40–79 years, n=2972: The SAMINOR 2 Clinical Survey (2012–2014).
| Age |
Non-Sami (n=1777) |
Sami (n=1195) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | n | p-value | ||||||
| 40–54 years | Mean (SD) | 657 | 1.3 | (1.2) | 451 | 1.4 | (1.3) | 0.24 |
| ≥5% (n) | 2.3% | (15) | 2.7% | (12) | 0.70 | |||
| 55–64 years | Mean (SD) | 541 | 5.0 | (3.0) | 406 | 4.7 | (2.8) | 0.27 |
| ≥10% (n) | 5.2% | (28) | 5.4% | (22) | 0.88 | |||
| 65–79 years | Mean (SD) | 579 | 12.0 | (5.4) | 338 | 12.2 | (5.3) | 0.68 |
| ≥15% (n) | 24.7% | (143) | 28.1% | (95) | 0.28 | |||
| Total | ||||||||
| Crude mean (SE) | 1777 | 5.9 | (0.14) | 1195 | 5.6 | (0.16) | 0.12 | |
| Age-standardised mean (SE) | 1777 | 5.8 | (0.07) | 1195 | 5.9 | (0.08) | 0.44 | |
| % with high riska (n) | 10.5% | (186) | 10.8% | (129) | 0.78 | |||
| Age-standardised % with high risk | 11.0% | 12.6% | 0.19 | |||||
Proportions with 10-year risk of AMI or CS ≥5%, ≥10% and ≥15% in age groups 40–54, 55–64 and 65–79 years, respectively.
SD: standard deviation.
In sensitivity analyses, three ethnic categories were compared with regard to mean risk scores and proportions at high risk: (1) those who reported ‘Sami’ on all 11 questions on ethnicity, (2) those who reported ‘Sami’ on at least one question and (3) those not reporting ‘Sami’ on any of the questions. We assessed whether region of residence modified mean risk scores and proportions at high risk (using the original ethnic categorisation) by merging municipalities that may be perceived as similar in terms of number of Sami inhabitants, Sami language users and geographical location: (1) Kautokeino and Karasjok; (2) Nesseby, Tana and Porsanger; and (3) Skånland, Evenes, Storfjord, Lyngen and Kåfjord. Additionally, in a separate set of analyses, we excluded participants on anti-hypertensive treatment (n=1382) to explore whether there were ethnic differences in non-users of anti-hypertensive medication.
Results
Of the 6004 SAMINOR 2 participants, 21 did not answer the questionnaire, 75 did not answer the questions on ethnicity, 26 had missing cholesterol values, three had missing systolic blood pressure, 193 reported angina pectoris, 244 reported AMI and 124 had missing information on smoking habits and were excluded. Thus, our analyses included 5318 individuals (44.1% of the sample were men and 40.8% were Sami), representing 42.7% of the invited sample.
In men, statistically significant differences were found in mean total cholesterol, the distribution of HDL-cholesterol and triglycerides, mean diastolic blood pressure, HbA1c, proportions with diabetes mellitus, mean waist circumference and the level of education (Table I). Compared with non-Sami men, Sami men had a more unfavourable distribution (except for waist circumference and diastolic blood pressure).
In women, Sami had statistically significantly lower HDL-cholesterol and physical activity level and higher triglycerides and higher proportions with waist circumference >88 cm (Table II).
In the total analytical population, disregarding ethnicity, the age-standardised mean 10-year risk of AMI or CS and the proportion of participants at high risk were 9.6% and 36.4%, respectively, for men, and 5.8% and 11.6%, respectively, for women (results not shown).
The mean 10-year risk of AMI or CS by age for Sami and non-Sami men and women is displayed in Figure 1. Overall, there were no ethnic differences in the 10-year risk of AMI or CS (Tables III and IV, and Figure 1). This was due to only minor ethnic differences in the distribution of the cardiovascular risk factors included in the NORRISK 2 model. However, among men aged 55–64 years, more Sami than non-Sami had a high 10-year risk of AMI or CS (36.1% vs. 26.7%; p=0.006). Sami men in this age group had a somewhat unfavourable distribution in most of the risk factors included in the model (results not shown). Additionally, the age-standardised 10-year mean risk for non-Sami and Sami men was borderline significant (9.5 vs. 9.8; p=0.05).
Figure 1.
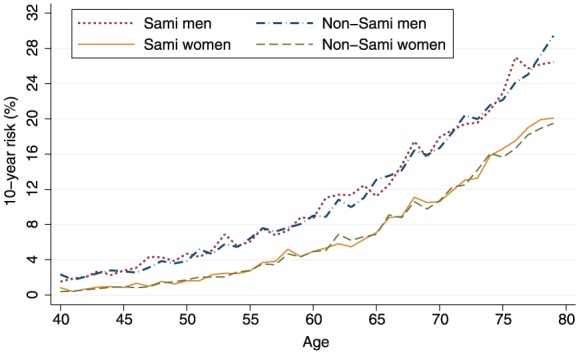
Mean 10-year risk of fatal or non-fatal acute myocardial infarction or cerebral stroke by age for non-Sami and Sami men (n=2346) and women (n=2972): The SAMINOR 2 Clinical Survey (2012–2014).
Overall, the sensitivity analyses did not show markedly different results by ethnicity or region of residence. Among non-users of anti-hypertensive medication, Sami women had lower systolic (125.9 vs. 127.9 mmHg; p=0.008) and diastolic (71.0 vs. 71.9 mmHg; p=0.02) blood pressure than non-Sami women (results not shown).
Discussion
We observed no overall differences in the 10-year risk of AMI or CS, as estimated by the NORRISK 2 model, between Sami and non-Sami participants. This was due to minor ethnic differences in the distribution of the cardiovascular risk factors included in the model. Overall, 36.4% of men and 11.6% of women had a high 10-year risk of AMI or CS.
Our results of an overall similar distribution of cardiovascular risk factors between Sami and non-Sami participants agree with previous studies from Norway, Sweden and Finland [9-10, 13, 21-25]. However, we found a somewhat unfavourable distribution of triglycerides, physical activity (women only), waist circumference (women only) and diabetes mellitus (men only) in Sami compared to non-Sami participants. To what extent ethnic discrepancies in these risk factors may contribute to a different risk of AMI or CS in Sami relative to non-Sami populations, is uncertain.
A publication with data from 1974/1975–1989 found that Sami men in Finnmark had a similar risk of AMI to Norwegian men, but indicated a 50% increased incidence of cerebrovascular disease [12]. In 1970–1998, Sami men and women (based on census data) living north of the Arctic Circle in Norway had higher mortality from cerebrovascular diseases, that is, 14% and 28% higher mortality rates in men and women, respectively. However, male reindeer herders had a lower risk of death from ischaemic heart disease than other Sami and non-Sami populations, and this was also the case, to some extent, for cerebrovascular disease [14]. In a follow-up study from 1977/1978–1992, including men aged 35–52 years in Finnmark, 67% and 52% lower mortality rates were found for ischaemic heart disease and total CVD, respectively, in Sami compared to non-Sami men [15].
Among men in Sweden, the overall incidence of hospitalisations due to cerebrovascular disease was higher in Sami populations but lower in Sami reindeer herders than in a regional non-Sami reference population [26]. Death from AMI was also higher in Sami women but not in female Sami reindeer herders [26,27], among whom the incidence of AMI was lower and cerebrovascular diseases higher [26]. In Finland, no difference in mortality from cerebrovascular disease between Sami and non-Sami populations have been reported [28], whereas a lower mortality from ischaemic heart disease has been observed in Sami women compared to a non-Sami female reference population [28,29].
Overall, it seems like Sami men and women may have a somewhat increased risk of ischaemic heart disease and cerebrovascular disease that does not necessarily apply to male reindeer herders, who might have reduced risk due to assumed higher physical activity levels [30]. This seems to contrast with what we found in our study, that is, no overall ethnic difference in 10-year risk of AMI and CS in men and women. We did find that Sami men aged 55–64 years had a higher 10-year risk of AMI or CS than their non-Sami counterparts. However, this might be a chance finding.
The inferior cardiovascular health observed among indigenous peoples relative to non-indigenous peoples in North America may be due to lower socio-economic status and poorer access to quality health care among the former [4]. A publication from Norway found that differences in smoking habits, systolic blood pressure, serum cholesterol and body mass index explained 72% and 56% of the absolute and relative educational gradients, respectively, in CVD mortality [31]. Assuming that the educational gradient in Sami and non-Sami populations is the same, the most plausible explanation for the similar levels of risk factors and risk of AMI or CS observed in our study may be that Sami and non-Sami populations in Norway differ little with regard to education levels (Tables I and II). However, Sjölander et al. found a somewhat higher incidence of cerebrovascular diseases and AMI mortality in Sami compared to non-Sami women in Sweden, which was not explained by education or income [26]. Thus, variables other than traditional socio-economic ones may also be relevant in explaining the disparities in the risk of CVD between Sami and non-Sami populations. Equal access to health care has also been put forward as a plausible explanation for the small differences in health and risk factors between Sami and non-Sami populations [32]. The fact that we observed no difference in anti-hypertensive treatment indicates that ethnic discrepancies in access to CVD treatment may not be an issue.
This study has some strengths. The relatively large sample of Sami and non-Sami participants from both coastal and inland regions, individual information on ethnic background and several biological markers and clinical measurements enabled an in-depth analysis of the total risk of AMI or CS. The NORRISK 2 model has a major advantage, as it takes into account competing risk and incorporates both fatal and non-fatal end points. Furthermore, the NORRISK 2 model was designed and validated for those aged 40–79 years in the general population of Norway [18], which is the same age range as that included in SAMINOR 2.
There are also some limitations. We assumed a similar CVD aetiology among Sami and non-Sami participants, which may not be correct [15]. Moreover, our risk estimations are most likely overestimated, as risk factor levels are expected to decrease in the future [16]. We did not have data on family history of premature ischaemic heart diseases and thus could not include it in the model. However, unpublished results from the SAMINOR 1 Survey, restricted to the same municipalities as SAMINOR 2, showed no ethnic differences in this regard (results not shown). We were not able to assess the 10-year risk of AMI or CS among Sami reindeer herders alone due to lack of information on occupation and association with reindeer husbandry. Finally, the NORRISK 2 model does not take into account all measured cardiovascular risk factors (e.g. triglycerides, physical activity, waist circumference and diabetes mellitus).
Only 10 municipalities were included in SAMINOR 2. Generalisations to the entire Sami or non-Sami populations in Norway are therefore not justified. Our analyses included 42.7% of the invited sample, with more female than male participants, though participation in both sexes increased with age, as in other studies [33]. Therefore, results for the youngest age group, particularly for men, are uncertain. We do not know the response rate by ethnic group due to lack of ethnic information in national registries. However, participation was highest in Kautokeino, Nesseby and Tana, where Sami are in the majority, and low in some municipalities where they are in the minority. This may have led to an over-representation of Sami participants from majority areas compared to Sami from minority areas and non-Sami. Whether this affected the results of this study is, however, unknown.
This study provides updated knowledge about the cardiovascular health of Sami and non-Sami populations in rural Northern Norway, which is needed, as the majority of comparable studies were conducted in Finnmark during 1974–1989. Furthermore, a cardiovascular risk assessment that combines the impact of several risk factors simultaneous and estimates the 10-year risk of AMI or CS has not been conducted previously in Sami and non-Sami populations.
Conclusion
We observed only minor ethnic differences in several risk factors for CVDs. Based on results from the NORRISK 2 model, we observed no overall differences in the 10-year risk of AMI or CS between the Sami and non-Sami populations living in 10 rural municipalities in Northern Norway. Applying the thresholds of Selmer et al. [18], 36.4% of men and 11.6% of women in this population were at high risk of AMI or CS.
Acknowledgments
The authors like to thank senior researcher Randi Selmer, Norwegian Institute of Public Health, for guidance on the use and development of the NORRISK 2 model. Additionally, the authors thank the participants of the SAMINOR 2 and the principal investigator of the SAMINOR Study, Ann Ragnhild Broderstad.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The SAMINOR 2 was financed by the Norwegian Ministry of Health and Care Services; the Northern Norway Regional Health Authority; the Regional Research Fund of Northern Norway; the Sami Parliament; the Sami Norwegian National Advisory Unit on Mental Health and Substance Use; and Finnmark, Troms, and Nordland county councils. Siri’s PhD funding is provided by the Centre for Sami Health Research. The centre is primarily financed by the Norwegian Ministry of Health and Care Services.
ORCID iDs: Susanna R.A.siri  https://orcid.org/0000-0003-3231-8139
https://orcid.org/0000-0003-3231-8139
References
- [1]. Anderson I, Robson B, Connolly M, et al. Indigenous and tribal peoples’ health (The Lancet–Lowitja Institute Global Collaboration): a population study. Lancet 2016;388:131–57. [DOI] [PubMed] [Google Scholar]
- [2]. Tvermosegaard M, Dahl-Petersen IK, Nielsen NO, et al. Cardiovascular disease susceptibility and resistance in circumpolar Inuit populations. Can J Cardiol 2015;31:1116–23. [DOI] [PubMed] [Google Scholar]
- [3]. Harris R, Nelson LA, Muller C, et al. Stroke in American Indians and Alaska natives: a systematic review. Am J Public Health 2015;105:e16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Hutchinson RN, Shin S. Systematic review of health disparities for cardiovascular diseases and associated factors among American Indian and Alaska Native populations. PLoS One 2014;9: e80973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Bhopal RS. Migration, ethnicity, race, and health in multicultural societies. 2nd ed. Oxford: Oxford University Press, 2014. [Google Scholar]
- [6]. Solbakk J. The Sámi people: a handbook. Karasjok: Davvi Girji, 2006. [Google Scholar]
- [7]. Niemi E. The Finns in Northern Scandinavia and minority policy. In: Tägil S. (ed.) Ethnicity and nation building in the Nordic world. London: Hurst, 1995, pp.145–78. [Google Scholar]
- [8]. Åkerblom H. (ed.). The cardiovascular study in Finnmark 1974–1975. Report 25. Nordic Council for Arctic Medical Research, Oulu, 1979. [Google Scholar]
- [9]. Thelle DS, Førde OH. The cardiovascular study in Finnmark County: coronary risk factors and the occurrence of myocardial infarction in first degree relatives and in subjects of different ethnic origin. Am J Epidemiol 1979;110:708–15. [DOI] [PubMed] [Google Scholar]
- [10]. Utsi E, Bønaa KH. [Coronary heart diseases among Lapps and Norwegians in Finnmark]. Tidsskr Nor Laegeforen 1998;118:1358–62. [PubMed] [Google Scholar]
- [11]. Njølstad I, Arnesen E, Lund-Larsen PG. Body height, cardiovascular risk factors, and risk of stroke in middle-aged men and women. A 14-year follow-up of the Finnmark study. Circulation 1996;94:2877–82. [DOI] [PubMed] [Google Scholar]
- [12]. Njølstad I, Arnesen E, Lund-Larsen PG. Cardiovascular diseases and diabetes mellitus in different ethnic groups: the Finnmark study. Epidemiology 1998;9:550–6. [PubMed] [Google Scholar]
- [13]. Nystad T, Utsi E, Selmer R, et al. Distribution of apoB/apoA-1 ratio and blood lipids in Sami, Kven and Norwegian populations: the SAMINOR study. Int J Circumpolar Health 2008;67:67–81. [PubMed] [Google Scholar]
- [14]. Tynes T, Haldorsen T. Mortality in the Sami population of North Norway, 1970–98. Scand J Public Health 2007;35:306–312. [DOI] [PubMed] [Google Scholar]
- [15]. Tverdal A. Cohort study of ethnic group and cardiovascular and total mortality over 15 years. J Clin Epidemiol 1997;50:719–23. [DOI] [PubMed] [Google Scholar]
- [16]. Selmer R, Hovda G, Graff-Iversen S, et al. Cardiovascular disease in Norway. Public health Report 2014, https://www.fhi.no/en/online-publications/public-health-report-2014/health–disease/cardiovascular-disease-in-norway—/ (2014, accessed 5 January 2016).
- [17]. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Selmer R, Igland J, Ariansen I, et al. NORRISK 2: A Norwegian risk model for acute cerebral stroke and myocardial infarction. Eur J Prev Cardiol 2017;24:773–82. [DOI] [PubMed] [Google Scholar]
- [19]. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated Report of a WHO Consultation. WHO/NMH/CHP/CPM/11.1.2011. Geneva: World Health Organization. [PubMed] [Google Scholar]
- [20]. Borch KB, Ekelund U, Brage S, et al. Criterion validity of a 10-category scale for ranking physical activity in Norwegian women. Int J Behav Nutr Phys Act 2012;9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Edin-Liljegren A, Hassler S, Sjölander P, et al. Risk factors for cardiovascular diseases among Swedish Sami – a controlled cohort study. Int J Circumpolar Health Suppl 2004;63:292–7. [DOI] [PubMed] [Google Scholar]
- [22]. Laitinen J, Näyhä S, Sikkilä K, et al. Diet and cardiovascular risk factors among Lapp and Finnish reindeer herders. Nutr Res 1996;16:1083–93. [Google Scholar]
- [23]. Sjölander P. What is known about the health and living conditions of the indigenous people of northern Scandinavia, the Sami? Glob Health Action 2011;4: 8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Näyhä S, Jarvelin MR. Health trends in northern Finland. Int J Circumpolar Health 1998;57:94–103. [PubMed] [Google Scholar]
- [25]. Luoma PV, Näyhä S, Sikkilä K, et al. High serum alpha-tocopherol, albumin, selenium and cholesterol, and low mortality from coronary heart disease in northern Finland. J Intern Med 1995;237:49–54. [DOI] [PubMed] [Google Scholar]
- [26]. Sjölander P, Hassler S, Janlert U. Stroke and acute myocardial infarction in the Swedish Sami population: incidence and mortality in relation to income and level of education. Scand J Public Health 2008;36:84–91. [DOI] [PubMed] [Google Scholar]
- [27]. Hassler S, Johansson R, Sjölander P, et al. Causes of death in the Sami population of Sweden, 1961–2000. Int J Epidemiol 2005;34:623–9. [DOI] [PubMed] [Google Scholar]
- [28]. Soininen L, Pukkola E. Mortality of the Sami in northern Finland 1979–2005. Int J Circumpolar Health 2008;67:43–55. [PubMed] [Google Scholar]
- [29]. Näyhä S. Low mortality from ischaemic heart disease in the Sámi district of Finland. Soc Sci Med 1997;44:123–31. [Google Scholar]
- [30]. Nilsson LM, Dahlgren L, Johansson I, et al. Diet and lifestyle of the Sami of southern Lapland in the 1930s–1950s and today. Int J Circumpolar Health 2011;70:301–18. [DOI] [PubMed] [Google Scholar]
- [31]. Ariansen I, Graff-Iversen S, Stigum H, et al. Do repeated risk factor measurements influence the impact of education on cardiovascular mortality? Heart 2015;101:1889–94. [DOI] [PubMed] [Google Scholar]
- [32]. Brustad M. Helse i samisk befolkning- en kunnskapsoppsummering av publiserte resultater fra befolkningsundersøkelser i Norge. In: Todal J, Brustad M, Broderstad EG, et al. (eds.) Samiske tall forteller 2 [Commented Sami statistics 2]. Guovdageaidnu: Sámi University College, 2009, pp.16–73. [Google Scholar]
- [33]. Langhammer A, Krokstad S, Romundstad P, et al. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol 2012;12: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]


