Abstract
The management of chronic myeloid leukemia with BCR-ABL1 tyrosine kinase inhibitors has evolved chronic myeloid leukemia into a chronic, manageable disease. A patient-centered approach is important for the appropriate management of chronic myeloid leukemia and optimization of long-term treatment outcomes. The pharmacist plays a key role in treatment selection, monitoring drug–drug interactions, identification and management of adverse events, and educating patients on adherence. The combination of tyrosine kinase inhibitors with unique safety profiles and individual patients with unique medical histories can make managing treatment difficult. This review will provide up-to-date information regarding tyrosine kinase inhibitor-based treatment of patients with chronic myeloid leukemia. Management strategies for adverse events and considerations for drug–drug interactions will not only vary among patients but also across tyrosine kinase inhibitors. Drug–drug interactions can be mild to severe. In instances where co-administration of concomitant medications cannot be avoided, it is critical to understand how drug levels are impacted and how subsequent dose modifications ensure therapeutic drug levels are maintained. An important component of patient-centered management of chronic myeloid leukemia also includes educating patients on the significance of early and regular monitoring of therapeutic milestones, emphasizing the importance of adhering to treatment in achieving these targets, and appropriately modifying treatment if these clinical goals are not being met. Overall, staying apprised of current research, utilizing the close pharmacist–patient relationship, and having regular interactions with patients, will help achieve successful long-term treatment of chronic myeloid leukemia in the age of BCR-ABL1 tyrosine kinase inhibitors.
Keywords: Chronic myeloid leukemia, BCR-ABL fusion protein, protein kinase inhibitors, patient-centered care
Introduction
The main treatment option for patients with chronic myeloid leukemia (CML) is monotherapy with one of the following BCR-ABL1 tyrosine kinase inhibitors (TKIs): imatinib, dasatinib, nilotinib, bosutinib, or ponatinib (Table 1).1–5 The BCR-ABL1 TKI chosen largely depends on the patient’s CML phase, previous treatment, and response; however, with multiple TKIs available, there are several opportunities to tailor treatment to each individual patient’s characteristics while still achieving an optimal response to therapy. For any cancer treatment, an early and durable response and minimal adverse events (AEs) are desired. Pharmacists are knowledgeable healthcare providers, access points for patients to gain information, and key components to maintaining an individualized, patient-centered approach to treatment. In this review, we discuss important considerations for the selection of TKIs and the management of CML, providing information to assist pharmacists in making well-informed decisions regarding management of this chronic disease.
Table 1.
| Imatinib1 | Dasatinib2 | Nilotinib3 | Bosutinib4 | Ponatinib5 | |
|---|---|---|---|---|---|
| Dosing schedule | Once daily | Once daily | Twice daily, 12 h apart | Once daily | Once daily |
| Pills/day (dosage) | 1 (400 mg) | 1 (100 mg) | 4 (150 mg) | 1 (500 mg) | 1 (45 mg) |
| Dose modification requirement for pre-existing conditions | |||||
| Hepatic | Yes (severe only) | No | Yes | Yes | Yes |
| Renal | Yes | No | No | Yes | N/A |
| Meal requirement | Should be taken with a meal and a large glass of water | No requirement | Should NOT be taken with a meal; fasting 2 h before and 1 h after each dose | Should be taken with food | No requirement |
| Alternative administrationa | Dissolve tablets in water or apple juice | Dissolve tablets in lemonade, apple juice, or orange juice | Disperse capsule in 5 mL of applesauce | N/A | N/A |
| Pharmacology | |||||
| Kinases inhibited | BCR-ABL, PDGF, SCF, c-KIT | BCR-ABL (active), SRC family, c-KIT, EPHA2, PDGFRβ | BCR-ABL, PDGFR, c-KIT, CSF-1R, DDR1 | BCR-ABL, SRC family | BCR-ABL, VEGFR, PDGFR, FGFR, EPHR, SRC family, c-KIT, RED, TIE2, FLT3 |
| Time to Cmax (h) | 2–41,6 | 0.5–62 | 33; 2–46 | 4–6 | 6 |
| Bioavailability | 98%6 | Unknown in humans (14%–51% in mice)6 | 31%6 | 34% | Unknown |
| pH-dependent absorptionb | No | Yes | Yes | Yes | No |
| Prescribing information recommendation | No change in acid-suppressive therapy necessary | Avoid PPI use; antacid 2 h before/after dasatinib; avoid H2 antagonists/PPIs | Avoid PPI use; antacid 2 h before/after nilotinib; H2 antagonists 10 h before or 2 h after nilotinib | Avoid PPI use; antacids and H2 blockers 2 h before or after bosutinib | No change in acid-suppressive therapy necessary |
| Alternative literature | H2 antagonists 2 h after dasatinib7; Cmax of H2 or PPI received/not received = 0.858 | PPIs can be used concomitantly6 | |||
| CML treatment approvalsc | CP: first line CP/AP/BP: after IFNα failure | CP: first line CP/AP/MPB/LBP: second line | CP: first line AP: second line | AP/BP: second line | AP/BP: second line |
AP: accelerated phase; BP: blast phase; CML: chronic myeloid leukemia; CP: chronic phase; INFα: interferon-α; LBP: lymphoid blast phase; MBP: myeloid blast phase; N/A: not available; TKI: tyrosine kinase inhibitor.
Prescribing information for TKIs warns against crushing or cutting the medication for administration due to health risks.
Based on recommendations available in package inserts for each medication and co-administration with drugs that elevate gastric pH.
Treatment of other phases of CML may require dose modifications.
Review of the literature
Online searches were performed between September 2015 and March 2017 for results published between January 2000 and March 2017. PubMed, Google, and websites for annual meetings of the American Society of Clinical Oncology and American Society of Hematology were accessed to obtain available literature on dasatinib, imatinib, nilotinib, bosutinib, and ponatinib treatment of CML. Clinical trials were identified using the term “chronic myeloid leukemia” with “dasatinib,” “imatinib,” “nilotinib,” “bosutinib,” or “ponatinib” on the ClinicalTrials.gov website. The terms “discontinuation” or “treatment-free remission” were included with each of the TKI names in a separate search on ClinicalTrials.gov. For safety and efficacy data, search results with references reporting clinical trial data from prospective studies were used, focusing on studies with long-term results for each TKI; references describing data from retrospective analyses of these long-term trials were allowed for subsequent data reporting (e.g. mutation analysis, adherence). Other prospective and retrospective analyses included are of clinical studies with available adherence data. Prescribing information and references/recommendations from the latest guidelines released by the National Comprehensive Cancer Network® (NCCN®) were used as a guide for additional searches using a combination of each individual TKI name with the term of interest (e.g. “pleural effusion,” “myelosuppression,” “cardiovascular events,” “pharmacology,” “adherence,” “antacid”).
The evolving CML treatment landscape
BCR-ABL1 TKIs for the treatment of CML-CP
The characteristic trait of CML is a chromosomal translocation that generates the constitutively active tyrosine kinase BCR-ABL1,10 resulting in malignant growth of cells. Prior to the advent of BCR-ABL1 TKIs, CML in chronic phase (CP) was treated with interferon-α plus cytarabine.11 TKIs were introduced for treatment of advanced and newly diagnosed CML with the approval of imatinib for these indications in 2001 and 2002, respectively.1 First-line approval of imatinib was granted after the phase III International Randomized Study of Interferon and STI571 (IRIS) trial demonstrated superior efficacy of imatinib (n = 553) versus interferon-α plus cytarabine (n = 553), including greatly improved progression-free survival (PFS) at 18 months (92.1% vs 73.5%).11 After imatinib, second-generation BCR-ABL1 TKIs, dasatinib and nilotinib, were approved for second-line treatment of CML in patients who are either resistant to or intolerant of imatinib.2,3 The efficacy and tolerability of dasatinib or nilotinib as frontline therapy for patients with newly diagnosed CML-CP was established in two separate phase III clinical trials. The phase III DASISION (DASatinib versus Imatinib Study In treatment-Naive CML patients) and phase III ENESTnd (Evaluating Nilotinib Efficacy and Safety in clinical Trials-newly diagnosed patients) trials led to the approval of dasatinib and nilotinib, respectively, as first-line agents for CML in 2010.2,3,12,13 Alternative second- and third-generation TKIs approved in 2012 for subsequent treatment of CML-CP include bosutinib and ponatinib, respectively.4,5,14,15
Treatment with BCR-ABL1 TKIs beyond CML-CP
In addition to use in patients with CML-CP, each BCR-ABL1 TKI also has unique approval for use in treatment of advanced CML in accelerated or blast phases and Philadelphia chromosome–positive acute lymphoblastic leukemia.1–5 Progression to blast crisis is associated with the spread of leukemic cells outside of the bone marrow, including to the central nervous system (CNS).10 Imatinib has limited ability to penetrate the blood–brain barrier and enter the cerebrospinal fluid to treat these relocalized cancer cells.16 Dasatinib and nilotinib have also both been found only in low concentrations in cerebrospinal fluid; however, the higher potency of the second-generation TKIs and their ability to overcome imatinib-resistant mutations has led to preliminary evidence for successful use of dasatinib17 and nilotinib18 in treating CNS-associated CML.
The benefit of early response to TKI therapy
Establishment of molecular response milestones
Early response is an important treatment goal for CML patients receiving TKIs. The achievement of early cytogenetic19–24 and molecular responses22,24–28 after initiating BCR-ABL1 TKI therapy has been shown to correlate with positive long-term outcomes. Molecular responses are measured by comparing the number of BCR-ABL1 transcripts or the ratio of these transcripts to a control gene (e.g. ABL1, BCR, or β-glucuronidase)29 before and after initiating therapy. Molecular response parameters were first described in the IRIS clinical trial, after which a standardized baseline of BCR-ABL1 levels and responses were defined (International Scale [IS]) using the standardized baseline as 100%.29,30 Use of the IS allows for a uniform system of tracking molecular milestones and provides critical information for the clinical decision-making process. Significant therapeutic milestones were defined as complete cytogenetic response (CCyR; no Philadelphia chromosome–positive [Ph+] metaphases) or major molecular response (MMR; reduction in standardized BCR-ABL1 transcript levels of at least three logs) at 12 months.29 In the IRIS trial, after 5 years of follow-up, all patients who achieved these milestones on imatinib had maintained CML-CP and had not progressed.19
Early molecular response with second-generation BCR-ABL1 TKIs
The DASISION study went on to demonstrate the long-term benefits and positive outcomes correlated with an early response to TKI therapy after 5 years of follow-up.31 Specifically, dasatinib-treated patients who attained an early molecular response (BCR-ABL1 ≤10% [IS] at 3 months) demonstrated statistically significantly higher response rates than patients with BCR-ABL1 transcripts >10% at 3 months for PFS (89% vs 72%; p = 0.0014), overall survival (OS; 94% vs 81%; p = 0.0028), and transformation-free survival (97% vs 83%; p = 0.0004).31 A similar trend between response and early molecular response was also observed for imatinib-treated patients in DASISION.31
Although the estimated 5-year PFS and OS rates were comparable between the dasatinib and imatinib cohorts in DASISION,31 early responses were higher for patients taking dasatinib. The percentage of patients who achieved BCR-ABL1 ≤10% at 3 months was 84% and 64% (p < 0.0001) for those on dasatinib and imatinib, respectively.24 The benefits of an early molecular response with dasatinib were also observed in a second-line setting in the 7-year follow-up of the phase III dose-optimization trial.32 In that study, patients with CML-CP who were administered dasatinib 100 mg once a day (QD) after imatinib failure/intolerance, and achieved BCR-ABL1 ≤10% versus >10% at 3 months, demonstrated significantly improved PFS and OS.32
Improvements in PFS and OS were also observed in the ENESTnd trial.28 Patients who received the recommended dose of nilotinib for newly diagnosed CML-CP (300 mg BID) and achieved an early molecular response had a 95.2% estimated 4-year PFS compared with 82.9% in nonresponders (p = 0.0061). Similarly, the 4-year OS rates were 96.7% and 86.7% (p = 0.0116) for patients who achieved or did not achieve an early molecular response, respectively.
Management of the individual CML patient
Response-assessment schedule
To ensure patients are responding appropriately to BCR-ABL1 TKI therapy, several tests to monitor medication efficacy are recommended by both NCCN and the European Society for Medical Oncology (ESMO) (Table 2).10,33 Early and regular response monitoring is critical to the management of CML to identify medication resistance and prepare both the healthcare team and patients for adjustments to therapy if a patient is not responding to frontline therapy (Table 3).10,33
Table 2.
| Test | Sample source | Performed to confirm diagnosis | Related response | NCCN timing of testing | NCCN additional testing | ESMO testing guidelines |
|---|---|---|---|---|---|---|
| Complete blood count (CBC) | Peripheral blood | Yes | Normal platelet and blood counts; no blasts or immature cells in blood | Per physician recommendation | Per physician recommendation | Every 15 days until CHR, then every 3 months |
| Bone marrow cytogenetics | Bone marrow | Yes | CCyR; 0 Ph+ metaphases | At 3 and 6 months if qPCR (IS) is unavailable; or there is no CCyR or MMR at 12 monthsa | If BCR-ABL1 transcript levels increase ≥1-log without MMR | At 3 and 6 months, every 6 months until CCyR, then every 12 months if MMR unavailable |
| qPCR (IS) | Peripheral blood or bone marrow | Yes | CMR; no detectable BCR-ABL1 transcripts by qPCR (IS) using an assay with a sensitivity of ≥4.5 logs below standardized baseline | If CCyR achieved, every 3 months for 2 years; every 3–6 months thereafter | If BCR-ABL1 transcript levels increase ≥1-log with MMR | Every 3 months until MMR, then every 6 months; qualitative PCR at diagnosis |
| BCR-ABL1 mutation analysis | Peripheral blood or bone marrow | No | N/A | If BCR-ABL1/ABL1 >10% by qPCR (IS) after 3–6 months of treatment; if CCyR is not present at any time after 12 months | Any loss of response; 1-log increase in BCR-ABL1 transcript levels and loss of MMR; or disease progression to blast phase | Defined failureb at 3, 6, or 12 months or loss of CHR or CCyR at any time |
CCyR: confirmed cytogenetic response; CHR: complete hematologic response; CML: chronic myeloid leukemia; CMR: complete molecular response; ESMO: European Society for Medical Oncology; IS: International Scale; MMR: major molecular response; N/A: not applicable; qPCR: quantitative polymerase chain reaction; TKI: tyrosine kinase inhibitor.
Absence of MMR in the presence of a CCyR is not considered a treatment failure. bFailure by ESMO guidelines is defined as Ph+ metaphases >95% or BCR-ABL1 >10% at 3 months, Ph+ metaphases >65% or BCR-ABL1 >10% at 6 months, or Ph+ metaphases ≥1% or BCR-ABL1 >1% at 12 months. Table adapted from NCCN Tests and Treatment Responses in Chronic Phase CML, accessed November 2016; NCCN CML Guidelines v1.2016, accessed October 2015; and ESMO Clinical Practice Guidelines 2012.
Table 3.
| Follow-up period | Response | NCCN recommendationa |
|---|---|---|
| 3 months | BCR-ABL1 transcripts >10% or lack of PCyRb | Primary treatment: Imatinib: Switch to alternate TKI or, if alternate is not possible, escalate imatinib dose to ≤800 mg, as toleratedc |
| Dasatinib/nilotinib: Continue or switch to alternate TKI (other than imatinib) | ||
| 6 months | BCR-ABL1 transcripts >10% or lack of PCyR | Switch to alternate TKI (other than imatinib)c,d |
| PCyR or BCR-ABL1 transcripts ≤10%, but >1% (IS) | Continue or switch to alternate TKI (other than imatinib), or if alternate TKI or omacetaxined are not possible, escalate imatinib dose to ≤800 mg, as tolerated | |
| 12 months | <PCyR or BCR-ABL1 transcripts >10% (IS) | Switch to alternate TKI (other than imatinib)c,d |
| Cytogenetic relapse | Switch to alternate TKI (other than imatinib), or if alternate TKI or omacetaxined are not possible, escalate imatinib dose to ≤800 mg, as toleratedc |
IS: International Scale; NCCN: National Comprehensive Cancer Network; PCyR: partial cytogenetic response; TKI: tyrosine kinase inhibitor.
If response milestones are not being achieved, please evaluate patient adherence and BCR-ABL1 mutation status.
Bone marrow cytogenetics identifies 1%–35% of cells with a Ph chromosome.
Evaluation for an allogenic hematopoietic cell transplant or enrollment in a clinical trial are additional options.
Omacetaxine is a treatment option for patients who are intolerant of or not responding to two or more TKIs.
A confirmed diagnosis of CML requires cytogenetic analysis of bone marrow cells to identify Ph+ metaphases.10 This cytogenetic test also helps establish the disease phase of the patient, which could affect treatment options. Another regularly performed test recommended in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) is quantitative polymerase chain reaction (qPCR) using IS. Molecular analysis through qPCR (IS) will measure levels of BCR-ABL1 transcripts to determine if NCCN-defined molecular response milestones are being met.10 A baseline level of transcripts is determined at diagnosis from peripheral blood or bone marrow samples.10 The guidelines from ESMO slightly vary from NCCN Guidelines® in that they require qualitative RT-PCR to be performed at diagnosis and qPCR at checkups after treatment initiation (Table 3).33 Qualitative PCR does not focus on quantifying the level of BCR-ABL1 transcripts as with qPCR, but rather determines if the BCR-ABL1 transcript present is considered rare or common, which could affect the treatment plan.33 The ESMO guidelines also recommend continued analysis of blood cell counts from baseline, every 15 days, until a complete hematologic response is reached, and then every 3 months after response is achieved.33
A concern of many patients is how frequently bone marrow samples are required for response-assessment testing. Typically, patients can have the majority of their monitoring tests performed on peripheral blood samples as long as the laboratory is using qPCR (IS).10 Regardless of whether qPCR (IS) is used, visiting the same laboratory for testing each time is preferred to reduce chances of result variability across testing sites.35 Bone marrow cytogenetics are required if qPCR (IS) is not available or if there is no MMR (0.1% BCR-ABL1 transcripts or ≥3-log drop from baseline) after 12 months of therapy (Table 2).10 Knowledge of early cytogenetic responses can serve as an indicator for future therapeutic success, as several studies have reported that early responses are associated with improved OS and PFS in both first-line13,20,23,26,31 and second-line32 settings. Once CCyR and MMR are achieved, qPCR (IS) is recommended every 3 months for 2 years and every 3–6 months thereafter.10
Pharmacology of BCR-ABL1 TKIs
It is important to understand the pharmacology of the BCR-ABL1 TKIs, especially when discussing resistant mutations and subsequent therapy beyond first line. The BCR-ABL1 fusion protein consists of an N-terminal lobe and a larger C-terminal lobe.36 When ATP binds to the N-terminal lobe, a glycine-rich loop, known as the P-loop, encloses the ATP molecule and allows the C-terminal loop to serve as a catalytic base.36 The available BCR-ABL1 inhibitors are ATP competitors and have different binding properties.36,37 Imatinib, nilotinib, and ponatinib have stronger binding affinity to the inactive protein conformation, whereas dasatinib36,38 and bosutinib39 can bind both the active and inactive conformations. Many hydrogen bonds and hydrophobic interactions between Abl1 and imatinib or nilotinib are the same; however, nilotinib is 20-fold more potent than imatinib.36,38 Nilotinib’s higher potency is likely due to additional interaction sites with trifluoromethyl and imidazole substituents of Abl1, which contribute to its increased binding affinity.36 Dasatinib is smaller and has fewer binding interactions with BCR-ABL1 than imatinib but is 325-fold more potent38; the higher potency of dasatinib is likely due to its ability to bind multiple BCR-ABL1 conformations.36 Ponatinib binding of BCR-ABL1 is similar to that of imatinib and nilotinib, but it is able to overcome the steric interference created by the T315I mutation and maintains this hydrogen bond, which is lost by the other BCR-ABL1 inhibitors.36
Absorption kinetics also differs for each of these drugs, affecting their plasma concentrations and bioavailability (Table 1). Concomitant dietary restrictions may impact TKI choice due to the varied effect food has on the bioavailability of some TKIs over others. For example, while no statistically significant differences in bioavailability were observed when imatinib or dasatinib were administered with a meal,6 the Cmax of nilotinib increased by 112% when given with a high-fat meal.6,40 When administered with a meal, the Cmax of bosutinib increased 1.8-fold4; however, ponatinib exposure levels are unaffected by food.5
Concomitant medications and drug–drug interactions
The risk of developing CML increases with age41 and elderly patients are also at a higher risk of polypharmacy,42 making use of concomitant medication(s) with BCR-ABL1 TKIs very likely. In a retrospective analysis of electronic medical records of 248 senior oncology patients, the average number of medications (prescription, nonprescription, and herbal) used was 9 (range 1–30).43 This study also reported the majority of co-administered medications were prescriptions (mean: 6), the most common being those that targeted the cardiovascular system, treated high cholesterol, or were gastrointestinal, diuretic, or endocrine-related medications.43 This study is somewhat limited due to a small sample size from a single institution, lack of information on excessive medication use, hospitalizations, and AEs; however, it consists solely of data from pharmacist-led medication assessments versus relying on patient self-reporting or generalized extraction of data from medical records. Medication assessments are recommended by NCCN Guidelines for Older Adult Oncology patients, in part, to identify and discontinue nonessential medications and evaluate drug–drug interactions.43,44 In the study by Nightingale et al., 34% and 12% of patients were identified to be prescribed 1 and 2 potentially inappropriate medications, respectively.43
As people age and require treatment of comorbid conditions, some concomitant medications are inevitable. If a significant drug–drug interaction is identified, it can possibly be remedied through dose modification of the TKI as directed by the medication prescribing information,1–5 or by switching to either an alternative BCR-ABL1 TKI or different medication to treat the concomitant medical condition.10
Patients with gastrointestinal conditions that require suppression of gastric acid are often prescribed H2 antagonists and/or proton-pump inhibitors (PPIs), which may affect drug absorption, primarily if solubility of the medication is pH dependent. The solubility of dasatinib decreases dramatically as pH increases above 4.0.7 The prescribing information recommends avoiding H2 antagonists and PPIs while taking dasatinib and restricting antacid co-administration to 2 h before or after dasatinib.2 One study with 24 healthy patients investigated dasatinib pharmacokinetics in the presence of gastric acid-regulating drugs and reported a decrease in dasatinib exposure if the H2 antagonist famotidine was taken 10 h prior to dasatinib.7 Interestingly, no change in dasatinib exposure was observed if famotidine was administered 2 h after dasatinib. This study, however, did administer dasatinib 50 mg twice a day, as the 100 mg once a day dose had not yet been established.7 The Cmax of dasatinib was reduced by 24% in patients who received H2 antagonists/PPIs in conjunction with dasatinib in a phase III dose optimization study,8 suggesting the use of acid blockers with dasatinib may need further exploration. The prescribing information for nilotinib recommends use of H2 antagonists 10 h before or 2 h after nilotinib3; however, no significant changes in the nilotinib Cmax were observed in a small study of 22 patients taking nilotinib concomitantly with the PPI esomeprazole.6 Prescribing information for nilotinib and bosutinib recommend adjusting administration of antacids containing aluminum or magnesium hydroxide to either 2 h before or 2 h after the TKI to help counter negative effects on BCR-ABL1 TKI concentration.3,4
Patients concomitantly taking strong CYP3A4 inhibitors and inducers should be prescribed BCR-ABL1 TKIs with caution. CYP3A is involved in the metabolism of almost half of drugs, including TKIs used to treat CML, and can lead to reduced concentrations of concomitant medications.45 Use of dasatinib and other approved BCR-ABL1 TKIs should be avoided in conjunction with strong CYP3A4 inducers (e.g. phenytoin, carbamazepine, rifampin, rifabutin, phenobarbital, St. John’s Wort).1–5 Additionally, concomitant use of CYP3A inhibitors (e.g. ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir) should also be avoided in CML patients taking TKIs, since it could increase the amount of drug present.1–5 If co-administration of CYP3A inhibitors cannot be prevented, dose reductions and close monitoring are recommended for dasatinib, nilotinib, and ponatinib.2,3,5
Use of each of the approved BCR-ABL1 TKIs has been shown to increase the chance of a patient developing thrombocytopenia1–5; therefore, patients taking anticoagulants should inform their healthcare team to determine if additional monitoring is required. In the ENESTnd trial, 3.6% of patients who received nilotinib 300 mg BID experienced some form of significant bleeding,13 8% of dasatinib-treated patients in DASISION reported experiencing some form of hemorrhage (including gastrointestinal bleeding),2 and grade 3/4 hemorrhage (of any type) was reported by 1.8% of imatinib-treated patients in the IRIS trial.1 With the potential for increased bleeding, patients taking anticoagulants should use BCR-ABL1 TKIs with caution (Table 4). Prothrombin time and international normalized ratio should be monitored in imatinib- and nilotinib-treated patients taking warfarin, while dasatinib-treated patients taking warfarin, heparin, enoxaparin, nadroparin, and dalteparin may have an elevated risk of bleeding.46 Also, because there is an increased risk of hemorrhage with dasatinib intake, patients with a history of ulcerative colitis/gastrointestinal ulcers should be carefully monitored while taking this BCR-ABL1 TKI.2
Table 4.
Drug–drug interactions between frontline BCR-ABL1 TKIs and anticoagulants.46
| Imatinib | Dasatinib | Nilotinib | |
|---|---|---|---|
| Acenocoumarol | CYP 2C9 inhibition → increased anticoagulationa | Increased risk of bleedingb | CYP 2C9 inhibition → increased anticoagulation |
| Clopidogrel (antiplatelet) | CYP 3A4 and 2C19 inhibition → decreased clopidogrel bioactivation | • Increased risk of bleeding • CYP 3A4 inhibition → decreased clopidogrel bioactivation | CYP 3A4 inhibition → decreased clopidogrel bioactivation |
| Dalteparin | NR | Increased risk of bleeding | NR |
| Enoxaparin | NR | Increased risk of bleeding | NR |
| Heparin | PgP inhibition → increased imatinib exposure | • Increased risk of bleeding • PgP inhibition → increased dasatinib exposure | NR |
| Nadroparin | NR | Increased risk of bleeding | NR |
| Phenprocoumon | CYP 2C9 inhibition → increased anticoagulation | Increased risk of bleeding | CYP 2C9 inhibition → increased anticoagulation |
| Warfarin | CYP 2C9 inhibition → increased anticoagulation | Increased risk of bleeding | CYP 2C9 inhibition → increased anticoagulation |
NR: no reported or expected interaction.
With increased anticoagulation monitor prothrombin time with international normalized ratio.
Increased risk of bleeding is due to thrombocytopenic effect that is generally caused by TKIs and usually of no clinical relevance.
An uncommon, but serious, cardiovascular event that can be initially caused or exacerbated by pharmacodynamic drug–drug interactions is a prolonged QT interval, which can occur during treatment with dasatinib and is included as a boxed warning in the nilotinib prescribing information.2,3 Symptomatic QT prolongation was observed in approximately 2% of patients receiving nilotinib in the 5-year report of the ENESTnd study.13 Guidelines for nilotinib suggest withholding the medication when QT intervals exceed 480 ms and not to resume at the prior dose until QT intervals are <450 ms.3 Across dasatinib clinical trials (N = 2440), <1% of patients had a QT prolongation of >500 ms.2 Although the reported number of overall events is low, healthcare providers should be aware of patients taking medications that prolong QT intervals, such as antiarrhythmic medicines (e.g. quinidine, procainamide, disopromide, dofetilide, ibutilide, sotalol),2,3,47 antiemetics (e.g. metoclopramide, serotonin receptor antagonists), antifungals, and several medications targeting the nervous system (e.g. haloperidol, risperidone, methadone),46 as these could increase the potential for this AE.
Furthermore, it is recommended that patients be screened for conditions that are associated with prolonged QT interval (e.g. hypokalemia, hypomagnesemia, and congenital long QT syndrome), the diagnosed conditions be monitored with an electrocardiogram,47 and any electrolyte imbalances be corrected prior to initiating dasatinib or nilotinib.2,3 Prescription guidelines for nilotinib recommend that electrocardiograms be conducted at baseline, 7 days after initiation of medication, and after any dose adjustment.3
Comorbid conditions
The frequent success of CML treatment with TKIs has resulted in patients experiencing favorable long-term outcomes, including high OS rates.20,31,48 As patients with CML age, the management of comorbidities will play an increasingly significant role in long-term survival. The effect of comorbidities on survival was assessed in 1519 imatinib-treated patients in the CML IV study over a median follow-up of 67.5 months.49 The CML IV study reported that comorbidities in patients taking imatinib did not affect achievement of early responses (cytogenetic or molecular), remission rates, or PFS; however, OS was negatively affected by comorbidities and patients with CML were more likely to die from their preexisting conditions than from CML itself.49
Comorbidities of each patient should be taken into consideration when choosing an initial TKI, both throughout the course of therapy and if subsequent therapy is being considered.10 For example, NCCN Guidelines® describe that dasatinib may be preferential to nilotinib for patients with a history of arrhythmias, heart disease, pancreatitis, or hyperglycemia; nilotinib may be preferred for patients with a history of lung disease or at risk for pleural effusion.10 A recent study of real-world data from patients with CML (N = 2296) used records from MarketScan® Commercial and Medicare databases to assess how comorbid conditions influenced the choice of TKI.50 Jabbour et al. reported that 41% of patients had at least one comorbid condition, which influenced the choice of the prescribed BCR-ABL1 TKI and that the most common conditions identified were heart disease, diabetes, and lung disease.50
Each of the first-line BCR-ABL1 TKIs differs in regard to the recommendations for use in patients with impaired liver function (Table 1).1–5 Differences in exposure to concentrations of dasatinib were not significantly altered in patients with hepatic impairment; therefore, dose adjustments are not currently deemed necessary.2 In contrast, patients with impaired liver function who are taking nilotinib should be prescribed a reduced dose.3 Patients taking imatinib do not require a dose reduction for hepatic impairment, unless the condition is considered severe.1,6 Regardless of medication, it is recommended that liver function of patients be tested prior to initiation of imatinib and also be monitored monthly (or as clinically indicated) for changes.1
The BCR-ABL1 TKIs have not specifically been investigated in patients with impaired kidney function and there are no primary guidelines or recommendations for dialysis treatment.1–5 A study investigating the incidence of various kidney-related AEs in patients newly diagnosed with CML-CP (N = 468) taking imatinib, dasatinib, or nilotinib found that 4% and 14% of patients developed acute kidney injury and chronic kidney disease, respectively, and that nilotinib had the most significant (p < 0.001) increase in glomerular filtration rate 3 months after treatment intiation.51 Unfortunately, this analysis did not include a control group, and comparisons to healthy individuals had to be made based on data from a previous study. Upon a rapid breakdown of cancerous cells, some patients taking dasatinib or nilotinib may experience tumor lysis syndrome (TLS), which can result in kidney failure.2,3 If patients initiate treatment in the presence of high white blood cell counts, allopurinol may be co-administered to help reduce TLS complications.51 Patients with chronic kidney disease and prescribed a BCR-ABL1 TKI should be closely monitored with blood tests analyzing kidney function to detect TLS and determine if dialysis treatment needs to be initiated.2,3
Pregnancy
All approved first- and second-line BCR-ABL1 TKIs have demonstrated embryo-fetal toxicity in experimental animals and have the potential to cause harm to the human fetus1–5; therefore, the treatment of patients of childbearing age and partners of patients with CML who want to become pregnant needs to be given special consideration. A recent study of dasatinib-treated patients who were either pregnant or male partners of pregnant women supported the current recommendation for women taking dasatinib to avoid becoming pregnant and not receive the medication if they become pregnant.2,52 Reported fetal risks included skeletal malformations and detrimental pharmacological effects.52 Fetal malformations and a slightly higher risk of spontaneous abortion have also been observed in imatinib-treated pregnant women.53 Dasatinib has not been shown to affect fertility; however, bosutinib and ponatinib may impair male or male and female fertility, respectively.4,5 While a larger dataset is required for definitive results, a review of the database cases did not identify any risks to offspring of dasatinib-treated male partners of pregnant women.52
Patients diagnosed with CML who are of reproductive age should be apprised of risks associated with fertility and pregnancy while on BCR-ABL1 TKIs and be advised to use effective contraceptive methods to avoid becoming pregnant.1–5 TKI therapy can be interrupted during deep response periods for planned pregnancies and hydroxyurea or interferon treatment used to control disease in place of interrupted TKIs for planned/unplanned pregnancies.54 Currently, clinical trials are investigating the ability of patients to maintain deep responses during treatment-free periods (discussed further below).55
Follow-up care of patients
Adherence and compliance
Patients who are not achieving molecular milestones at the recommended time points after initiation of therapy should be evaluated for adherence to their prescribed TKI.10 Table 5 lists several studies that have demonstrated 10%–98% adherence to approved TKIs and data to support that patients who are adherent to a medication plan have better early responses and/or long-term outcomes with BCR-ABL1 TKI therapy.56–62 Importantly, one factor to be mindful of adherence studies is that more complete data sets may be available for those patients with better adherence, inadvertently incorporating some bias into both prospective and retrospective analyses.56 Furthermore, methods for measuring adherence in patients and the selected cutoff values are not uniform across analyses and more prospective studies with a consistent methodology should be developed for future investigations.
Table 5.
| BCR-ABL1 TKI (patients with adherence assessed) | Adherent patients, n (%) | Adherence assessment method | Response |
|---|---|---|---|
| Imatinib (n = 165)56 | NR (67) | Survey | Imatinib not taken • CCyR: 9% • No CCyR: 26% |
| Imatinib (n = 87)57 | 64 (74) | MEMS (>90%) | 58% MMR (at 18 months) |
| Imatinib (n = 516)58 | 366 (71) | Drug interruptions | 5-year EFS • Adherent: 77% • Nonadherent: 60% |
| Imatinib (n = 87)59 | 69 (79) | MEMS (>85%) | 24-month EFS • Adherent: 91% • Nonadherent: 54% |
| Imatinib (n = 169)60 | 95 (56) | ≥90% actual vs recommended days of therapya | CCyR or MMR by 18 months • Adherent: 46% • Nonadherent: 43% |
| Dasatinib, nilotinib (n = 60) | 33 (55) | ||
| Imatinib (n = 68)61 | 16 (24) | Survey | MMR by adherence level • High: 27% • Medium: 41% • Low: 32% |
| Dasatinib (n = 9) | 1 (10) | ||
| Nilotinib (n = 9) | 3 (33) | ||
| Imatinib62 Year 1a (n = 53) Year 2 (n = 50) | NR (82) NR (72) | MPR ≥85% | Adherence by response in year 1 • CMR: 96% • MMR: 96% • Failure: 79% |
CCyR: confirmed cytogenetic response; CMR: complete molecular response; EFS: event-free survival; MEMS: medication event monitoring system; MMR: major molecular response; NR: not reported.
Adherence was measured based on patients being in the first or second year of treatment with imatinib.
To help preemptively address medication compliance, the adherence potential of a patient can be measured before initiating treatment that may have a more complex therapeutic regimen. The ASK-20 survey contains 47 items to assess the likelihood of a patient being adherent to a prescribed medicine.63 Analysis of responses from over 600 patients diagnosed with asthma, diabetes, or depression indicates that the ASK-20 survey could be a reliable resource to determine adherence barriers across a variety of chronic diseases.63 Similarly, the ASK-12 adherence survey was recently tested in a small group of patients (N = 42) with multiple myeloma or CML and reported that its use by healthcare providers improved patient adherence within an average time span of 5 months.64
Each BCR-ABL1 TKI has different administration requirements, with varying complexities and restrictions (Table 1).1–5 Studies examining patient compliance have reported that a therapeutic regimen that is straightforward and adaptable can produce higher adherence than therapies with twice-daily dosing and/or restrictive meal requirements.65 Recent studies analyzing patient information from medical records or insurance claims data indicate higher adherence rates among dasatinib-treated patients compared with patients treated with imatinib66 or nilotinib,66,67 respectively.
In addition to effects on the response to CML therapy, associations between nonadherence and healthcare costs have also been investigated. In one particular study, levels of adherence were calculated based on the mean possession ratio (MPR) of imatinib prescribed for CML patients (N = 592) and a significant association between lower adherence and higher healthcare costs was determined.68 The increased spending, analyzed over a 12-month period, was determined to be significantly greater in nonadherent versus adherent patients for inpatient hospital visits and non-TKI medication.68 A retrospective analysis of claims data from over 10,000 patients taking oral oncolytics, including imatinib, reported that 10% of patients abandoned a newly prescribed oral cancer therapy.69 In this study, a lower household income, a greater number of prescription claims, and higher patient cost-sharing were all associated with higher prescription abandonment rates.69 It was also observed that patients with Medicare have significantly higher cost-sharing than patients with commercial insurance.69
Educating patients about medication characteristics, administration guidelines, and AEs may help reduce dose-modifying AEs and improve adherence.70 Improvement in patient medication adherence was observed in a retrospective analysis of 56 patients taking oral TKIs and who were managed by oncology pharmacists.71 In this study, the adherence rate in patients in the intervention group was 89% compared with 66% in patients receiving the usual care regimen.71 Furthermore, a variety of pharmacist interventions were identified (e.g. monitoring/managing AEs and drug interactions, management of laboratory tests, adjustment of TKI dosage).71
Specialty pharmacies also have established systems to monitor adherence, including routine phone calls for follow-up and prescription reminders; however, the distance from this network compared with that from a local hospital, pharmacy, or clinic may pose a barrier to their successful monitoring of patient therapy.72 Also, while healthcare providers at a specialty pharmacy may potentially have more focused experience with oncolytics than a local pharmacist, telecommunication can provide a disadvantage for identification of AEs that would be more readily noticed during in-person interactions.72
AEs
The safety profiles of BCR-ABL1 TKIs are diverse, which is to be expected given that each agent has its own unique structure and mechanism of action.1–5 Some of the more common AEs that have been similarly reported in ≥10% of patients taking one of the approved first-line TKIs, imatinib, dasatinib, or nilotinib, include diarrhea, fatigue, headache, abdominal pain, and rash (Table 6).1–3
Table 6.
| Dasatinib2 100 mg QDa (n = 258) |
Imatinib1 400 mg QDb (n = 551) |
Nilotinib3 300 mg BIDc (n = 279) |
||||
|---|---|---|---|---|---|---|
| All grades | Grade 3/4 | All grades | Grade 3/4 | All grades | Grade 3/4 | |
| Nonhematologic AE | ||||||
| Abdominal pain | 11 | 0 | 37 | 4 | 15 | 2 |
| Constipation | NR | NR | 11 | 1 | 20 | <1 |
| Cough | NR | NR | 20 | <1 | 17 | 0 |
| Diarrhea | 22 | 1 | 45 | 3 | 19 | 1 |
| Dizziness | NR | NR | 19 | 1 | 12 | <1 |
| Dyspepsia | NR | NR | 19 | 0 | 10 | 0 |
| Fatigue | 11 | <1 | 39 | 2 | 23 | 1 |
| Fluid retention | 38 | 5 | 62 | 3 | NR | 4 |
| Headache | 14 | 0 | 37 | 1 | 32 | 3 |
| Influenza | NR | NR | 14 | <1 | 13 | 0 |
| Insomnia | NR | NR | 15 | 0 | 11 | 0 |
| Arthralgia/joint pain | 7 | 0 | 31 | 3 | 22 | <1 |
| Myalgia | 7 | 0 | 24 | 2 | 19 | <1 |
| Nasopharyngitis | NR | NR | 31 | 0 | 27 | 0 |
| Nausea | 10 | 0 | 50 | 1 | 22 | 2 |
| Pyrexia | NR | NR | 18 | 1 | 14 | <1 |
| Rash and related termsd | 14 | 0 | 40 | 3 | 38 | <1 |
| Upper respiratory tract infection | NR | NR | 21 | <1 | 17 | <1 |
| Vomiting | 5 | 0 | 23 | 2 | 15 | <1 |
| Hematologic AE | ||||||
| Neutropenia | 29 | 17 | 12 | |||
| Thrombocytopenia | 22 | 9 | 10 | |||
| Anemia | 13 | 4 | 4 | |||
AE: adverse event; BID: twice daily; NR: not reported in ≥10% of patients; QD: once daily; TKI: tyrosine kinase inhibitor.
Minimum of 60 months follow-up.2
Study versus interferon-α.1
60-month analysis.3
Includes erythema, erythema multiforme, rash, rash generalized, rash macular, rash papular, rash pustular, skin exfoliation, and rash vesicular.
Dasatinib is generally well tolerated, and clinical trials have demonstrated that AEs are typically reported within 2 years of starting treatment.24,31 In clinical trials, all AEs in dasatinib-treated patients, with the exception of pleural effusion, were reported less frequently or to a comparable degree as AEs in imatinib-treated patients and were mild to moderate in severity (Table 6).24,31 Fluid retention events are the most common category of AE reported in imatinib-treated (62%) or dasatinib-treated (38%) patients2; the majority of these events were due to pleural effusion (28% all grades, 3% grade 3/4) in dasatinib-treated patients and superficial localized edema (38% all grades, <1% grade 3/4) in imatinib-treated patients.2,31 The BCR-ABL1 TKIs have varying incidence of pleural effusion, although all frequencies are lower than what occurs with dasatinib (imatinib: ≤1%, all grades; first-line nilotinib: ≤2%, all grades; bosutinib: <2%, grade 3/4; ponatinib: 7%, all grades).1–5
Age (>55 years) was determined to be a statistically significant risk factor for pleural effusion in a retrospective analysis of imatinib-resistant/intolerant patients who received dasatinib second line in the phase III dose-optimization study.73 Risk factors in addition to age were identified in independent investigations of pleural effusion in dasatinib-treated patients and include advanced disease, a history of cardiac disease,74,75 hypertension, hypercholesterolemia, autoimmune disease, and skin rash while receiving imatinib.75 Dosage and schedule may also influence pleural effusion incidence. Both a retrospective analysis of the dasatinib dose-optimization study32,73 and the prospective OPTIM-DASA (Optimized TKIs Monotherapy) study76 analyzed AEs based on dasatinib dose measured by pharmacokinetics and reported that a higher dasatinib plasma concentration was associated with pleural effusion. Hypotheses of a mechanism for pleural effusion in dasatinib-treated patients include a potential immune-mediated hypersensitivity/response or a more potent inhibition of platelet-derived growth factor receptor-β (PDGFR-β).77
It is vital for healthcare professionals to identify symptoms of pleural effusion such as dry cough, tightness in the chest, and shortness of breath early in their development.77 Pleural effusions caused by BCR-ABL1 TKIs can be self-limiting or, if they are identified early, can be resolved with supportive care (e.g. diuretics, corticosteroids, therapeutic thoracentesis) or dose reductions/interruptions.2 In DASISION, 6% of dasatinib-treated patients discontinued dasatinib because of pleural effusion, and 41% and 62% were given a dose reduction or interruption, respectively.31 The median time to the initial grade 1/2 pleural effusion was 114 weeks (range 4–299 weeks), and the median time of dose interruption due to pleural effusion was 14 days (range 2–63 days).31 Radiography tests can confirm the presence of pleural effusion,2 which is most commonly managed with diuretics and short-course steroids. If these treatments do not correct the condition, dasatinib treatment should be interrupted and then returned with a reduced dosage once pleural effusion symptoms subside.2 Importantly, adjustments in dasatinib dose have not affected the ability of patients to respond to therapy, as 96% of patients with pleural effusion were able to achieve CCyR and 82% MMR by 5 years in DASISION.31
Myelosuppression, which includes thrombocytopenia, neutropenia, and anemia, can occur during treatment with any of the approved BCR-ABL1 TKIs1–5 and typically presents early after initiation of treatment.78 Neutropenia is the most common form of myelosuppression across all of the BCR-ABL1 TKIs approved for first-line therapy (Table 6).1–3 Blood tests, including complete blood counts, are performed at diagnosis to establish a baseline level of cells and blood tests are repeated weekly early on in treatment to detect cytopenias.34,40 The baseline number of blood cells can be compared with numbers at monitoring time points to determine the level of hematologic response to a BCR-ABL1 TKI.34 Blood cell counts should be monitored monthly after the first 3 months of therapy, but additional blood tests may be performed if resistance to the prescribed TKI is suspected or abnormal blood cell counts are detected during routine tests.78 If cytopenia is identified, dose adjustments or interruptions are recommended, depending on the severity of the event.1–5 If neutropenia, thrombocytopenia, or anemia recurs after repeated dose interruptions and/or reductions, switching therapies may be warranted. While a mechanism for myelosuppression in CML-CP patients is not known, it may be associated with the leukemic state of the bone marrow, since patients with cancers other than CML (e.g. gastrointestinal tumors) who take imatinib, report a lower incidence of cytopenias.79 For example, the 5-year follow-up of the IRIS trial reported grade 3/4 neutropenia in 17% of imatinib-treated CML-CP patients,19 whereas it occurred in only 7% of patients with gastrointestinal tumors treated with imatinib.79 Furthermore, 9% of CML-CP patients taking imatinib reported grade 3 thrombocytopenia, while no cases were observed in patients with gastrointestinal tumors.19,79
Other, more common, AEs of BCR-ABL1 TKIs include gastrointestinal disorders (e.g. diarrhea, nausea, vomiting) and rash (Table 6).1–5 These can typically be remedied with standard interventions, such as antidiarrheal/antiemetic medicines or topical corticosteroids/antihistamines, respectively.77 Taking medication with a meal may help lessen or eliminate nausea and vomiting in patients taking BCR-ABL1 TKIs that do not require fasting in relation to administration.78 Most dermatologic issues arising with BCR-ABL1 TKI use are mild to moderate and self-limiting, although severe reactions may require dose reductions/interruptions or an alternative TKI.78 For example, serious dermatologic reactions, including erythema multiforme and Stevens-Johnson syndrome, have been observed with use of dasatinib and imatinib and require immediate attention.1,2
Incidence of cardiovascular events has been reported across all of the BCR-ABL1 TKIs, but with varied frequency.1–5 Venous and arterial occlusive events are potentially severe complications that may occur during ponatinib use, and patients taking this medication should be actively monitored.15 In a phase II clinical trial of second-line ponatinib use (N = 449), 17% of patients reported arterial ischemic events (12% of patients reported serious arterial ischemic events).15 Cardiovascular events, which include arterial occlusive events, are reported to occur in 9%–15% of patients taking nilotinib,3 and cardiac ischemic events (including myocardial infarction, angina pectoris, coronary artery disease, and acute coronary syndrome) occurred in 4% of CML-CP patients treated with dasatinib in DASISION.31
Common AEs have been identified in clinical trials of BCR-ABL1 TKIs,14,15,31,32,48 which should be watched for and monitored closely during follow-up care through physical examination, discussion, and blood tests (e.g. complete blood count with differential) performed during regularly scheduled checkups.41 Pharmacists and other healthcare providers are all responsible and obligated to report any serious drug-related AEs.80
Mutational analysis
If a patient is confirmed as adherent and not achieving appropriate responses to their BCR-ABL1 TKI, it may indicate medication resistance and the patient should undergo mutational analysis.10 This includes patients who have not achieved the following: complete hematologic response at 3 months,33 partial cytogenetic response or BCR-ABL1 transcripts ≤10% by qPCR (IS) at 3 and 6 months, or CCyR or BCR-ABL1 transcripts ≤1%, but >0.1% by qPCR (IS) at 12 months.10 Additionally, any sign of loss of hematologic or cytogenetic response (defined as hematologic or cytogenetic relapse) or a 1-log increase in BCR-ABL1 transcripts and loss of MMR should also lead to mutational analysis.10,33
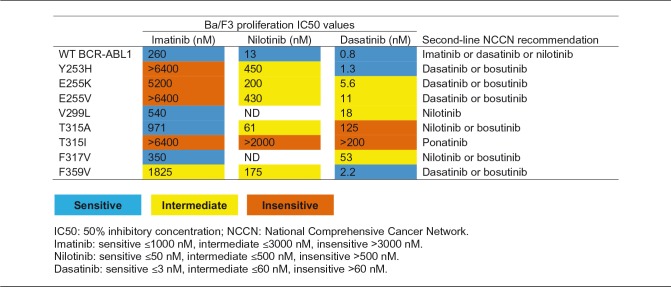
Each TKI has a unique sensitivity profile to BCR-ABL1 mutations,81 which can be attributed to its method of binding and inhibiting the BCR-ABL1 protein (Table 1)38 and may impact treatment decisions on a patient-by-patient basis (Table 7). Imatinib and nilotinib both bind the inactive conformation of BCR-ABL1 where dasatinib binds the active BCR-ABL1 protein and has dual inhibitory activity against BCR-ABL1 and the Src family of kinases (Table 1).38 The different binding sites of the TKIs results in their varying potencies and sensitivity to mutations in the target protein.38
Table 7.
Sensitivity of first-line BCR-ABL1 TKIs to various BCR-ABL1 point mutants.81,a
aSensitivity was determined by IC50 value for each TKI in respect to inhibition of cell proliferation in Ba/F3 cells expressing wild-type BCR-ABL1 or a BCR-ABL1 mutant.
Mutational analysis provides an opportunity for healthcare providers to optimize treatment and increase the potential for more positive short- and long-term outcomes.10 Occasionally, patients harboring specific TKI-insensitive mutations may still respond to the respective TKI, but at a lower rate compared with a nonresistant counterpart.81 Therefore, CML patients who are not achieving response milestones and are identified with resistant mutations should begin treatment with an agent with known activity in the presence of the mutation.10 In the case of a T315I mutation, specifically, ponatinib, omacetaxine, or enrollment in a clinical trial are recommended.10
Patient support
Assistance beyond a patient’s healthcare team is available for access to medication and managing treatment.82 For example, financial assistance for commercially insured patients is available through pharmaceutical industry co-pay cards.82 Several patient assistance foundations will help provide information about blood and bone marrow donation organizations, describe clinical recommendations for treatment, list available support networks, and provide information related to obtaining financial assistance for treatment including The National CML Society, CML Advocates Network, Patient Access Network Foundation, Patient Services, Inc, and Good Days.
Treatment-free remission (TFR)
For patients who have a deep, prolonged molecular response during TKI treatment, the concept of discontinuing CML therapy and maintaining a response is becoming a reality. Several TFR studies, either planned or already under way, have enrolled patients with CML-CP who are taking a BCR-ABL1 TKI (Table 8).83–99 To qualify for one of these discontinuation studies a patient must have maintained a deep molecular response (e.g. ≥MR4.0) for a certain period of time before the TKI is discontinued. Findings from the largest TFR study, the European Stop Kinase Inhibitor (EURO-SKI) trial (N = 750), have demonstrated successful TFR for approximately half of patients being treated with any of the approved first-line TKIs.83
Table 8.
| Study (ID) | Phase | n discontinued (Region) | TKI treatment at discontinuation | Study-defined DMR requirement for discontinuation | Definition of relapse | Relapse-free | Response after reinitiating TKI therapy |
|---|---|---|---|---|---|---|---|
| EURO-SKI (NCT01596114)83 | 3 | 750 (Europe) | Imatinib, dasatinib, or nilotinib | MR4.0 for ≥1 year | Any loss of MMR | MRFS 6 months: 62% 12 months: 56% 24 months: 52% | N/A |
| LAST (NCT02269267)84 | 2 | 173 (USA) | Imatinib, dasatinib, nilotinib, or bosutinib | MR4.0 for ≥2 years | Molecular recurrence | N/A | N/A |
| DESTINY (NCT01804985)85 | 2 | 174 (UK) | Imatinib, dasatinib, or nilotinib | MMR for 12 months on reduced (50%) TKI dose | Loss of MMR on 2 consecutive samples | N/A | N/A |
| STOP-2G-TKI86 | Observational | 60 | Dasatinib or nilotinib | MR4.5 for ≥2 years | Loss of MMR | 57% | 26/26 regained MMR and MR4.5 |
| DADI (UMIN000005130)87 | 2 | 63 (Japan) | Dasatinib | MR4.0 for 1 year | Loss of MR4.0 | 48% | 33/33 regained MMR by 3 months; 33/33 regained MR4.0 by 6 months |
| DASFREE (NCT01850004)88 | 2 | 30 (Global) | Dasatinib | MR4.5 ≥1 year | Loss of MMR | 63% | 10/10a regained MMR and MR4.5 |
| 1st-DADI (UMIN000011099)90 | 2 | Targeted enrollment: 100 (Japan) | Dasatinib | CMR ≥1 year | Loss of CMR | N/A | N/A |
| D-STOP (NCT01627132)91 | 2 | 54 (Japan) | Dasatinib | MR4.0 for 2 years | 2 consecutive >MR4.0 in 1 month | 63% | 20/20 |
| TRAD (NCT02268370)89 | 2 | 75 (Canada) | Imatinib | MR4.5 for ≥2 years | Loss of MR4.0 on 2 consecutive occasions or loss of MMR | 69% | 14/14b regained MMR and MR4.5 |
| STIM1 (NCT00478985)92 | Observational | 100 (France) | Imatinib | CMR for ≥2 years | 2 consecutive 1-log increases in BCR-ABL1 from baseline or single loss of MMR | Overall: 39% MMR: 83% | 55/57 |
| TWISTER(ACTRN 12606000118505)93 | 2 | 40 (Australia) | Imatinib | CMR ≥2 years | Loss of MMR for any sample or any 2 consecutive positive samples | 45% | 22/22 |
| ENESTFreedom (NCT01784068)94 | 2 | 190 (Global) | Nilotinib | All assessments ≥MR4.0, >MR4.0–<MR4.5 for ≤2 assessments, and MR4.5 at last assessment over 1 year | Loss of MMR | 52% | MMR: 85/85c MR4.5: 76/85 |
| ENESTGoal (NCT01744665)95 | 2 | 4 (USA) | Nilotinib | MR4.5 for 1–2 years | Confirmed loss of MMR | 25% | 3/3 |
| ENESTPath (NCT01743989)96 | 3 | 101 (Europe) | Nilotinib | Stable MR4.0 for ≥1 year | Loss of MR4.0 | N/A | N/A |
| ENESTop (NCT01698905)97 | 2 | 126 (Global) | Nilotinib | Confirmed MR4.5 for 1 year on nilotinib | Loss of MMR or confirmed loss of MR4.0 | 58% | ≥MMR: 50/51 ≥MR4.0: 48/51 MR4.5: 47/51 |
| Nilst (UMIN000007141)98 | 2 | 87 (Japan) | Nilotinib | MR4.5 for 2 years | Loss of MR4.5 | 59% | 32/34 |
| STAT2 (UMIN 000005904)99 | N/A | 73 (Japan) | Nilotinib | MR4.5 for 2 years | Confirmed loss of MR4.5 | 66% | N/A |
CMR: complete molecular response (undetectable BCR-ABL1 [IS]); DMR: deep molecular response; MMR: BCR-ABL1 <0.1% on the International Scale (IS); MR4.0: BCR-ABL1 <0.01 on the IS; MRFS: molecular recurrence-free survival; N/A: not available; TFR: treatment-free remission; TKI = tyrosine kinase inhibitor.
An 11th patient lost MMR, but discontinued the study, restarted therapy at another site, and was lost to follow-up.
Recovery of response occurred on dasatinib.
An 86th patient lost MMR, but discontinued the study.
Trial requirements and available TFR data from current studies can be viewed in Table 8. Several of these studies have reported similar outcomes as the EURO-SKI trial, in addition to finding that those patients who did relapse after TKI discontinuation were able to reestablish their molecular response soon after treatment was reinitiated (Table 8).83–99 ESMO notes the importance of including TFR as an endpoint in future clinical trials.33 NCCN Guidelines® discuss the concept of TFR, however they do not currently recommend discontinuing TKI therapy outside of a clinical trial.10
Summary
Healthcare providers need to maintain a comprehensive knowledge of current treatment options to ensure each patient is aligned with a therapy that is both effective and also provides the best quality of life. With CML treatment being life-long, patients require a supportive and diligent healthcare team that will appropriately monitor each patient throughout their therapy and take a patient’s changing medical and personal considerations into account. The patient and healthcare team need to discuss efficacy, safety, and affordability of a therapy plan to determine the best possible course of treatment. Pharmacists are a readily accessible and direct point of contact for patients and can help navigate this process, in addition to assisting patients in development of medication administration plans, identifying and answering questions about AEs, discussing changes in treatment, and receiving information on where to go for additional medical or financial support. The patient and healthcare team both benefit from an integrated approach to treatment, allowing for the consideration of each patient’s unique characteristics when tailoring and optimizing CML treatment with BCR-ABL1 TKIs.
Acknowledgments
The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoints and medical expertise. They also wish to acknowledge Kelly M. Fahrbach, PhD, of StemScientific, an Ashfield company, part of UDG Healthcare plc, funded by Bristol-Myers Squibb, for providing medical writing and editorial support.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Sandra Cuellar served on an advisory board or speakers’ bureau for Celgene and Merck. Michael Vozniak served on advisory boards for Eisai, Teva, and Sanofi. Jill Rhodes served on an advisory board or speakers’ bureau for Bristol-Myers Squibb. Nicholas Forcello served on a speakers’ bureau for Teva. Daniel Olszta served on an advisory board or speakers’ bureau for Bristol-Myers Squibb, Merck, and Incyte. Bristol-Myers Squibb did not influence the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Novartis. Gleevec® (imatinib mesylate) package insert, East Hanover, NJ: Novartis, 2016. [Google Scholar]
- 2.Bristol-Myers Squibb. Sprycel® (dasatinib) package insert, Princeton, NJ: Bristol-Myers Squibb, 2015. [Google Scholar]
- 3.Novartis. Tasigna® (nilotinib) package insert, East Hanover, NJ: Novartis, 2015. [Google Scholar]
- 4.Pfizer. Bosulif® (bosutinib) package insert, New York, NY: Pfizer, 2016. [Google Scholar]
- 5.ARIAD. Iclusig® (ponatinib) package insert, Cambridge, MA: ARIAD, 2016. [Google Scholar]
- 6.Di Gion P, Kanefendt F, Lindauer A, et al. Clinical pharmacokinetics of tyrosine kinase inhibitors focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet 2011; 50: 551–603. [DOI] [PubMed] [Google Scholar]
- 7.Eley J, Luo FR, Agrawal S, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharm 2009; 49: 700–709. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Roy A, Hochhaus A, et al. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure–response analysis of a Phase III study. Clin Pharmacol 2013; 5: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andolina JR, Neudorf SM, Corey SJ. How I treat childhood CML. Blood 2012; 119: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia (V.1.2017). © National Comprehensive Cancer Network, Inc 2015®. All rights reserved, see NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc (accessed 17 March 2017).
- 11.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270. [DOI] [PubMed] [Google Scholar]
- 13.Saglio G, Kim D-W, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259. [DOI] [PubMed] [Google Scholar]
- 14.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011; 118: 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med 2013; 369: 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neville K, Parise RA, Thompson P, et al. Plasma and cerebrospinal fluid pharmacokinetics of imatinib after administration to nonhuman primates. Clin Cancer Res 2004; 10: 2525–2529. [DOI] [PubMed] [Google Scholar]
- 17.Porkka K, Koskenvesa P, Lundan T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome–positive leukemia. Blood 2008; 112: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 18.Reinwald M, Schleyer E, Kiewe P, et al. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. BioMed Res Int 2014; 2014: 637059–637059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355: 2408–2417. [DOI] [PubMed] [Google Scholar]
- 20.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood 2009; 114 : abstract 1126. [Google Scholar]
- 21.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol 2008; 26: 3358–3363. [DOI] [PubMed] [Google Scholar]
- 22.Hanfstein B, Muller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012; 26: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood 2011; 118: 4541–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia 2003; 17: 2401–2409. [DOI] [PubMed] [Google Scholar]
- 26.Quintás-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood 2009; 113: 6315–6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol 2012; 30: 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood 2014; 123: 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006; 108: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003; 349: 1423–1432. [DOI] [PubMed] [Google Scholar]
- 31.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the DASatinib versus imatinib study in treatment-naive CML patients trial. J Clin Oncol 2016; 34: 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol 2016; 91: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baccarani M, Pileri S, Steegman J-L, et al. ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(suppl 7): vii72–vii77. [DOI] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network. Tests and treatment responses in chronic phase CML, http://education.nccn.org/sites/education.nccn.org/files/HEM-N-0333-0415%20CML%20Pt%20Vignette%20Tool.pdf (accessed 14 March 2016).
- 35.Leukemia and Lymphoma Society. The CML guide, information for patients and caregivers, chronic myeloid leukemia, https://www.lls.org/sites/default/files/file_assets/cmlguide.pdf (2014, accessed 30 March 2016).
- 36.Reddy EP, Aggarwal AK. The ins and outs of Bcr-Abl inhibition. Genes Cancer 2012; 3: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Erp NP, Gelderblom H, Guchelaar H-J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 2009; 35: 692–706. [DOI] [PubMed] [Google Scholar]
- 38.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 2005; 65: 4500–4505. [DOI] [PubMed] [Google Scholar]
- 39.Levinson NM, Boxer SG. Structural and spectroscopic analysis of the kinase inhibitor bosutinib and an isomer of bosutinib binding to the Abl tyrosine kinase domain. PLoS One 2012; 7: e29828–e29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novartis. Tasigna® (nilotinib) [European Medicines Agency product information]. East Hanover, NJ: Novartis.
- 41.American Cancer Society. Chronic myeloid leukemia, https://www.cancer.org/cancer/chronic-myeloid-leukemia.html (2017, accessed 17 May 2017).
- 42.Balducci L, Goetz-Parten D, Steinman MA. Polypharmacy and the management of the older cancer patient. Ann Oncol 2013; 23: vii36–vii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nightingale G, Hajjar E, Swartz K, et al. Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol 2014; 33: 1453–1459. [DOI] [PubMed] [Google Scholar]
- 44.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Older Adult Oncology (V.1.2017). © National Comprehensive Cancer Network, Inc 2015®. All rights reserved, see NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc (accessed 5 January 2017).
- 45.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Ann Rev Pharm Toxicol 1999; 39: 1–17. [DOI] [PubMed] [Google Scholar]
- 46.Haouala A, Widmer N, Duchosal MA, et al. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 2011; 117: e75–e87. [DOI] [PubMed] [Google Scholar]
- 47.Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf 2012; 3: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016; 30: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saussele S, Kraus M-P, Hehlmann R, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood 2015; 126: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jabbour E, Makenbaeva D, Lingohr-Smith M, et al. Use of real-world claim databases to assess prevalence of comorbid conditions relevant to the treatment of chronic myelogenous leukemia based on National Comprehensive Network Treatment Guidelines. Clin Lymphoma Myeloma Leuk 2015; 15: 797–802. [DOI] [PubMed] [Google Scholar]
- 51.Yilmaz M, Lahoti A, O’Brien S, et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer 2015; 121: 3894–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cortes JE, Abruzzese E, Chelysheva E, et al. The impact of dasatinib on pregnancy outcomes. Am J Hematol 2015; 90: 1111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abruzzese E, Trawinska MM, Perrotti AP, et al. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis 2014; 6: e2014028–e2014028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood 2012; 120: 1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US National Institutes of Health. ClinicalTrials.gov. Studies showing remission in CML, https://clinicaltrials.gov/ct2/results?term=remission&recr=&type=&rslt=&age_v=&gndr=&cond=CML&intr=&titles=&outc=&spons=&lead=&id=&state1=&cntry1=&state2=&cntry2=&state3=&cntry3=&locn=&rcv_s=&rcv_e=&lup_s=&lup_e= (accessed 18 October 2016).
- 56.Noens L, van Lierde M-A, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood 2009; 113: 5401–5411. [DOI] [PubMed] [Google Scholar]
- 57.Marin D, Bazeos A, Mahon F-X, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471–474. [DOI] [PubMed] [Google Scholar]
- 59.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 2011; 117: 3733–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Bella N, Bhowmik D, Bhor M, et al. The effectiveness of tyrosine kinase inhibitors and molecular monitoring patterns in newly diagnosed patients with chronic myeloid leukemia in the community setting. Clin Lymphoma Myeloma Leuk 2015; 15: 599–605. [DOI] [PubMed] [Google Scholar]
- 61.Kekale M, Talvensaari K, Koskenvesa P, et al. Chronic myeloid leukemia patients’ adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Pref Adher 2014; 8: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santoleri F, Lasala R, Ranucci E, et al. Medication adherence to tyrosine kinase inhibitors: 2-year analysis of medication adherence to imatinib treatment for chronic myeloid leukemia and correlation with the depth of molecular response. Acta Haematol 2016; 136: 45–51. [DOI] [PubMed] [Google Scholar]
- 63.Hahn SR, Park J, Skinner EP, et al. Development of the ASK-20 adherence barrier survey. Curr Med Res Opin 2008; 24: 2127–2138. [DOI] [PubMed] [Google Scholar]
- 64.Liles DK, Lea CS, Moore TN, et al. Barriers to adherence in hematologic malignancies in a rural regional hospital network. Presented at: American Society of Clinical Oncology Annual Meeting, Chicago, IL, 2–6 June 2016.
- 65.Hirji I, Gupta S, Goren A, et al. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspective. Health Qual Life Outcomes 2013; 11: 167–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Degli-Esposti L, Saragoni S, Buda S, et al. Medication adherence in patients with chronic myeloid leukemia using tyrosine-kinase inhibitors: a retrospective analysis. Presented at: American Society of Hematology Annual Meeting, San Francisco, CA, 6–9 December 2014.
- 67.Trivedi D, Landsman-Blumber P, Darkow T, et al. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J Manag Care Spec Pharm 2014; 20: 1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu EQ, Guerin A, Yu AP, et al. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin 2010; 26: 61–69. [DOI] [PubMed] [Google Scholar]
- 69.Streeter SB, Schwartzberg L, Husain N, et al. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract 2011; 7: 46S–51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Felton MA, van Londen GJ, Marcum ZA. Medication adherence to oral cancer therapy: the promising role of the pharmacist. J Oncol Pharm Pract 2016; 22: 378–381. [DOI] [PubMed] [Google Scholar]
- 71.Lam MSH, Cheung N. Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. J Oncol Pharm Pract 2016; 22: 741–748. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz RN, Eng KJ, Frieze DA, et al. NCCN task force report: Specialty pharmacy. J Natl Compr Canc Netw 2010; 8: S1–S11. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Roy A, Hochhaus A, et al. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure–response analysis of a Phase III study. Clin Pharmacol 2013; 5: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quintás-Cardama A, Kantarjian H, O’Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol 2007; 25: 3908–3914. [DOI] [PubMed] [Google Scholar]
- 75.de Lavallade H, Punnialingam S, Milojkovic D, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Hematol 2008; 141: 734–747. [DOI] [PubMed] [Google Scholar]
- 76.Rousselot P, Boucher S, Etienne G, et al. Pharmacokinetics of dasatinib as a first line therapy in newly diagnosed CML patients (OPTIM dasatinib trial): correlation with safety and response [abstract]. Blood 2010; 116: 3432–3432. [Google Scholar]
- 77.Khoury HJ, Guilhot F, Hughes TP, et al. Dasatinib treatment for Philadelphia chromosome-positive leukemias. Cancer 2009; 115: 1381–1394. [DOI] [PubMed] [Google Scholar]
- 78.Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol 2015; 94: S149–S158. [DOI] [PubMed] [Google Scholar]
- 79.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472–480. [DOI] [PubMed] [Google Scholar]
- 80.American Society of Hospital Pharmacy. ASHP guidelines on adverse drug reaction monitoring and reporting. Am J Health-Syst Pharm 1995; 52: 417–419. [DOI] [PubMed] [Google Scholar]
- 81.Kimura S, Ando T, Kojima K. BCR-ABL point mutations and TKI treatment in CML patients. J Hematol Transf 2014; 2: 1022–1022. [Google Scholar]
- 82.The National CML Society. Resources: assistance programs, http://www.nationalcmlsociety.org/resources/assistance-programs (July 2015, accessed 19 October 2016).
- 83.Mahon FX, Richter J, Guilhot J, et al. Cessation of tyrosine kinase inhibitors treatment in chronic myeloid leukemia patients with deep molecular response: Results of the Euro-Ski trial. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 84.US National Institutes of Health. ClinicalTrials.gov. The life after stopping tyrosine kinase inhibitors study (The LAST study), https://clinicaltrials.gov/ct2/show/NCT02269267?term=NCT02269267&rank=1 (accessed 18 January 2017).
- 85.Clark RE, Polydoros F, Apperley JF, et al. Chronic myeloid leukaemia patients with stable molecular responses (at least MR3) may safely decrease the dose of their tyrosine kinase inhibitor: Data from the British Destiny study. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 86.Rea D, Nicolini FE, Tulliez M, et al. France Intergroupe des Leucémies Myéloïdes Chroniques. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood 2017; 129: 846–854. [DOI] [PubMed] [Google Scholar]
- 87.Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicenter phase 2 trial. Lancet Haematol 2015; 2: e528–e535. [DOI] [PubMed] [Google Scholar]
- 88.Shah NP, Paquette R, Muller MC, et al. Treatment-free remission (TFR) in patients with chronic phase chronic myeloid leukemia (CML-CP) and in stable deep molecular response (DMR) to dasatinib – the Dasfree study. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 89.Kim DDH, Bence-Bruckler I, Forrest DL, et al. Treatment-free remission accomplished by dasatinib (TRAD): Preliminary results of the Pan-Canadian tyrosine kinase inhibitor discontinuation trial [abstract]. Blood 2016; 128: 1922–1922. [Google Scholar]
- 90.UMIN Clinical Trials Registry. UMIN.ac.jp. Dasatinib treatment discontinuation study implementation plan for initial chronic phase chronic myelogenous leukemia with 1 year complete molecular genetic effect, https://translate.google.com/translate?hl=en&sl=ja&u=https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi%3Ffunction%3Dbrows%26action%3Dbrows%26type%3Dsummary%26recptno%3DR000012999%26language%3DJ&prev=search (2013, accessed 22 March 2017).
- 91.Kumagai T, Nakaseko C, Nishiwaki K, et al. Discontinuation of dasatinib after deep molecular response for over 2 years in patients with chronic myelogenous leukemia and the unique profiles of lymphocyte subsets for successful discontinuation: A prospective, multicenter Japanese trial (D-STOP trial) [abstract]. Blood 2016; 128: 791–791. [Google Scholar]
- 92.Etienne G, Rea D, Guilhot F, et al. Long-term follow-up of the French 1 Stop Imatinib Study (STIM1) in chronic myeloid leukemia patients [abstract]. Blood 2015; 126: 345–345. [Google Scholar]
- 93.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013; 122: 515–522. [DOI] [PubMed] [Google Scholar]
- 94.Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study. Presented at: American Society of Clinical Oncology Annual Meeting, Chicago, IL, 3–7 June 2016.
- 95.Ritchie EK, Catchatourian R, Klisovic RB, et al. ENESTgoal treatment-free remission study: Updated preliminary results and digital polymerase chain reaction analysis in patients with chronic myeloid leukemia in chronic phase who switched from imatinib to nilotinib. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 96.Rea D, Rosti G, Cross NCP, et al. ENESTPath: A phase 3 study to assess the effect of nilotinib treatment duration on treatment-free remission (TFR) in patients with chronic myeloid leukemia in chronic phase (CML-CP) previously treated with imatinib: 24-month analysis of the first 300 patients in the induction/consolidation phase. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 97.Mahon FX, Boquimpani C, Takahashi N, et al. Patient-reported quality of life before and after stopping treatment in the ENESTop trial of treatment-free remission for patients with chronic myeloid leukemia in chronic phase. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 98.Kadowaki N, Kawaguchi T, Kuroda J, et al. Discontinuation of nilotinib in patients with chronic myeloid leukemia who have maintained deep molecular responses for at least 2 years: A multicenter phase 2 stop nilotinib (Nilst) trial. Presented at: American Society of Hematology Annual Meeting, San Diego, CA, 3–6 December 2016.
- 99.Takahashi N, Nakaseko C, Nishiwaki K, et al. Two-year consolidation by nilotinib is associated with successful treatment free remission in chronic myeloid leukemia with MR: Subgroup analysis from STAT2 trial in Japan. Presented at American society of hematology annual meeting, San Diego, CA, 3–6 December 2016.