Abstract
Immunotherapy has radically transformed the management of metastatic malignant melanoma. Ipilimumab, a CTLA-4-targeted monoclonal antibody, was the first immunotherapeutic drug to reach a survival benefit compared with traditional chemotherapy. PD-1 targeted therapies, pembrolizumab and nivolumab, have demonstrated, in recent clinical trials, to be even more effective and safer. PD-1 and CTLA-4 blockade combination appears to improve the outcomes achieved so far, although increasing toxicity. However, many questions concerning the optimal timing of administration or the most adequate sequence of treatment are yet to be answered.
KEYWORDS : CTLA-4, immunotherapy, melanoma, oncology, PD-1, PD-L1, review
Practice points.
CTLA-4-directed therapies
Ipilimumab, a CTLA-4-specific monoclonal antibody, blocks the binding of CTLA-4 with CD-80 and CD-86, triggering an immune response against the neoplasm. The Phase III trial CA194–023 demonstrated that this drug is superior to chemotherapy in terms of overall survival (OS) in advanced malignant melanoma patients. As adjuvant therapy in stage III resected melanoma, ipilimumab improved recurrence-free survival compared with placebo in the essay EORTC 18071, although IFN-α-2b is considered the standard of care in this context.
PD-1/PD-L1 blockade
PD-1/PD-L1 binding elicits downstream signaling that inhibits T-cell proliferation and activation. Two drugs targeting PD-1 and thus disrupting this interaction have demonstrated clinical activity in metastatic malignant melanoma: nivolumab and pembrolizumab. Both achieved an improvement in OS compared with chemotherapy in melanoma patients previously treated with ipilimumab and BRAF-directed therapies, when indicated. The trial KEYNOTE-006 with pembrolizumab demonstrated a benefit in OS compared with ipilimumab in untreated advanced melanoma.
Immunotherapy combinations
Recent trials evaluating immunotherapy combinations suggest that this strategy could overcome the results achieved in melanoma so far, even though the severe toxicity and the elevated costs may limit its use. The Phase III CheckMate 067 revealed that the simultaneous administration of nivolumab and ipilimumab is more effective than either single treatment in terms of overall response ratio and progression-free survival, although more than 50% of the patients experienced grade 3–4 toxicity. The results are premature and the data of OS have still not been published, so this information should be interpreted attentively.
Background
Even though malignant melanoma represents less than 5% of all malignancies, its relevance has progressively increased in the last decades [1]. Over the past 50 years its incidence has grown worldwide, especially in regions with a high proportion of fair-skinned population [2]. According to the estimations by GLOBOCAN 2012, 323,130 new cases will be diagnosed every year [1].
Diagnosis is commonly made at early stages, when surgical excision can be curative. Therefore, most cases have a favorable prognosis where 5-year overall survival (OS) overcomes 80%. However, 1-year OS drops to 62% when considering only advanced melanoma with skin metastases, to 53% when located in the lungs and to 33% if spread to other visceral sites [3].
For more than 30 years chemotherapy had been the main therapeutic strategy for patients with advanced malignant melanoma, with dacarbazine as the standard of treatment since 1975; however, the outcomes were poor with an overall response rate (ORR) of around 20% and a median duration of 4–6 months [4]. Different chemotherapy agents such as temozolomide or fotemustine, among others, failed to demonstrate superiority [5–7].
Fortunately, a better understanding of cancer biology promoted the development of new therapeutic approaches including targeted therapies and immunotherapy.
The MAPK pathway is upregulated in many malignant melanomas with 40–60% of cases associated to the BRAF V600 mutation [8]. Two BRAF-targeted tyrosine-kinase inhibitors, vemurafenib and dabrafenib, have been approved for BRAF V600E/K mutated melanoma with a benefit in OS [9,10]. Fast responses with an ORR of about 50% were observed, although the median duration did not exceed 5–7 months [11,12].
Drugs targeting MEK, a kinase downstream BRAF, have also attracted interest with trametinib as the only one accepted to date [13]. Last, simultaneous administration of BRAF and MEK inhibitors could delay the acquired resistance to these treatments and improve the short duration of response – one of their main weaknesses [14–16].
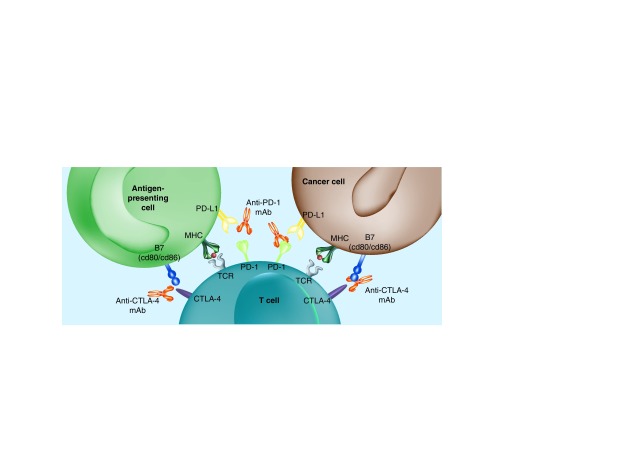
Finally, before the incursion of the most recent immunotherapy drugs, IL-2 was used by its ability to activate T cells causing proliferation and differentiation into memory and T-helper subsets [17]. However, its severe toxicity limited its application to carefully selected patients. In 2009, the first data on the activity of the CTLA-4-blocking human IgG1 monoclonal antibody ipilimumab were published. During the following years drugs targeting additional immune checkpoints were incorporated to the therapeutic arsenal, with PD-L1–PD-1-blocking antibodies occupying the center of attention (Figure 1).
Figure 1. . When naive T cells are stimulated through the T-cell receptor, CTLA-4 is upregulated early and its expression dampens T cells by outcompeting CD28 in binding ligands B7.1 (CD80) and B7.2 (CD86) on antigen presenting cells or cancer cells determining the suppression of T-cell activity.
The activity of PD-1 is related to its interaction with its ligand PD-L1. Upon antigen recognition, activated T cells express PD-1 on their surface and produce interferons that lead to the expression of PD-L1 in multiple tissues, including cancer, and consequently to the inactivation of T cells, mediated by the recruitment of phosphatases to the immune synapsis that dephosphorylate molecules related to T-cell receptor signaling.
mAb: Monoclonal antibody; TCR: T-cell receptor.
CTLA-4-directed therapies
CTLA-4, which was first described by Brunet et al. in 1987, is a type I transmembrane glycoprotein member of the CD28 family of receptors expressed on the surface of T cells [18]. Upon CD80/CD86 binding on antigen presenting cells, it delivers a strong inactivating signal to T cells. CTLA-4 is essential for preventing severe autoimmune processes [19], but malignancies profit from this as an immune escape mechanism. CTLA-4 blockade persistently activates T cells, triggering an immune reaction against the neoplasm. The clinical application of CTLA-4 blocking antibodies caused a radical change of the strategy in the treatment of melanoma, as thenceforth cancer cells were no longer the targets.
Malignant melanoma has been the main neoplasm in which the efficacy of CTLA-4 directed drugs was evaluated. Three monoclonal antibodies were initially used in clinical trials: CP-642,570 (withdrawn due to thrombocytopenia); tremelimumab (CP-642,206); and MDX-010 (rebranded as ipilimumab).
Ipilimumab is a CTLA-4-specific human IgG1 monoclonal antibody which was positioned as the standard of care for metastatic malignant melanoma over dacarbazine. The Phase II trials showed disease control rates (DCR) of about 25–30%, and in approximately 20% of the patients’ long-term survival for up to 4 years [20–22]. These trials uncovered the necessity of new methods for evaluating responses to immunotherapy, as increase of pre-existing lesion size or the appearance of new ones could be observed during the first months of treatments without necessarily being associated with treatment refractoriness [23].
A few months later the results of the Phase III trial MDX010–20 were published, in which pretreated melanoma patients received four cycles of 3 mg/kg ipilimumab every 21 days along with a melanoma vaccine containing gp100, ipilimumab alone, or only gp100 as a control arm. This was the first assay to demonstrate a benefit in OS in advanced melanoma, with median OS around 10 months when the patients received ipilimumab and 6 and 4 months in the control arm. A subset of about 20% of the patients who received ipilimumab lived longer than 3 years. ORR and DCR in the ipilimumab-alone group were significantly higher, although there were no differences in progression-free survival (PFS). The most common adverse events (AEs) were immune-related and mainly affected the skin and gastrointestinal tract. The rate of grade 3–4 toxicity was 10–15% with ipilimumab and 3% with gp100 alone [24].
The Phase III trial CA194–023 consolidated ipilimumab as first-line of treatment for advanced melanoma. This trial confronted the combination of ipilimumab and dacarbazine followed by maintenance with ipilimumab with eight cycles of dacarbazine. It demonstrated a small, but statistically significant, benefit in median OS (11.2 vs 9.1 months) at the expense of a much worse tolerance. Grade 3–4 toxicity reached 56.3 and 36% leading to treatment discontinuation. It should be emphasized that the dose of ipilimumab used was 10 mg/kg, three-times greater than in the MDX010–20 trial, with the current approved dose being 3 mg/kg. No differences in DCR or ORR were observed [25]. These results led to the approval of ipilimumab by the US FDA and the EMA.
Ipilimumab has also been tested as an adjuvant therapy. In the EORTC 18071 trial, 951 patients with a resected stage III melanoma were randomized to four cycles of ipilimumab 10 mg/kg every 3 weeks followed by this same dose every 3 months for 3 years versus a placebo treatment. Median recurrence-free survival was 26.1 versus 17.1 months in the placebo group; hazard ratio (HR): 0.75; p = 0.0013. Data on OS are still unknown. However, some concerns on the toxicity of the experimental regime should be raised with 52% of the patients with interrupted treatments due to drug-related AEs [26]. In addition, the choice of placebo as a control arm was not appropriate, as IFN-α-2b was considered the standard of care according to the benefits in recurrence-free survival and OS reported in the E-1684 Phase III trial [27].
Regarding tremelimumab, it was remarkable that two of the six patients who received a dose of 15 mg/kg showed response at the stage of Phase I clinical trial [28].
The Phase I/II trial published in 2009 showed encouraging outcomes, although toxicity was significant. In the Phase II regimen the proportion of grade 3–4 AEs was 40%, and ten patients out of 89 had to discontinue treatment [29].
Unfortunately the Phase III trial comparing tremelimumab to chemotherapy in nonresectable stage IIIC/IV melanoma failed to demonstrate a survival benefit [30]. When this study was published the standard of care had changed following the approval of ipilimumab and vemurafenib. Thus, clinical use of tremelimumab came to a halt.
PD-1/PD-L1 blockade
PD-1 is an inhibitory receptor of the extended CD28 family of T-cell regulators. It is expressed mainly on antigen-stimulated T cells, as well as in B cells, monocytes, Tregs and NK cells. PD-1 regulates immunity at multiple phases of the immune response, inducing downstream signaling that inhibits T-cell proliferation, cytokine release and cytotoxicity [31–33]. PD-1 engagement by PD-L1 inactivates T cells mainly through two mechanisms. First, PD-1 recruits Src homology region 2 domain-containing phosphatase (SHP) which dephosphorylate components of the TCR signalosome [34]. Second, PD-1 engagement leads to TCR downmodulation which prevents direct antigen recognition by T cells [35]. When activated T cells infiltrate tumors that are frequently inactivated through PD-L1–PD-1 interactions, as PD-L1 is usually expressed by cancer cells. When this interaction is blocked with monoclonal antibodies, T cells recover their cytotoxic activities leading to killing of tumor cells. Therefore, PD-1 functions as a checkpoint of the effector stage of the immune response, has a different role than CTLA-4 in limiting T-cell activation [36,37].
Malignant melanoma was the first neoplasm in which PD-L1–PD-1 blockade was tested. Up to date two monoclonal antibodies targeting PD-1, namely pembrolizumab and nivolumab, have shown clinical efficacy.
Pembrolizumab is a humanized IgG4 monoclonal antibody, highly selective for PD-1 and first evaluated in the Phase I study KEYNOTE-001 [38–40]. In a pooled analysis of 411 patients with advanced melanoma, ORR was 34% and it was maintained at 81% after a median follow-up of 18 months. The median OS was 25.9 months [39].
In the randomized Phase II trial KEYNOTE-002, pembrolizumab was superior to chemotherapy after progression to ipilimumab in terms of PFS, with HR of 0.57 for pembrolizumab 2 mg/kg and 0.50 for 10 mg/kg. A third of the pembrolizumab-treated patients treated were progression-free at 6 months, whereas only 16% assigned to chemotherapy had not progressed. ORR according to RECIST criteria were 21% in the pembrolizumab 2 mg/kg cohort and 25% in the 10 mg/kg group, compared with 4% with chemotherapy (p < 0.0001).
This drug was well tolerated. The incidence of grade 3–4 treatment-related AEs was higher with chemotherapy (26%) than with pembrolizumab (11% in the 2 mg/kg group; 14% in the 10 mg/kg group) [41].
A significant expectation was held to the results of the Phase III trial KEYNOTE 006, which evaluated the efficacy of pembrolizumab as first-line therapy. 834 patients with untreated advanced melanoma received pembrolizumab 10 mg/kg every 2 weeks, pembrolizumab 10 mg/kg every 3 weeks or ipilimumab. The proportion of melanoma with mutated BRAF V600 was 36.2%, and 80.5% of the tumors were PD-L1 positive. Pembrolizumab demonstrated superiority to ipilimumab, regardless the cadence of administration, in terms of 1-year OS (74.1, 68.4, 58.2%), 6-month PFS (47.3, 46.3, 26.5%) and ORR (33.7, 32.9, 11.9%). The benefit of pembrolizumab was evident in all subgroups with the exception of the group with PD-L1 negative tumors, even though the number of patients was low.
Pembrolizumab was better tolerated than ipilimumab with lower incidence of grade 3–5 AEs (13.3% for pembrolizumab 10 mg/kg every 2 weeks, 10.1% for pembrolizumab 10 mg/kg every 3 weeks and 19.9% for ipilimumab) and less treatment discontinuations [42].
Nivolumab it is a fully humanized IgG4 mAb that disrupts PD-1 interaction with its ligands PD-L1 and PD-L2 [43].
In a Phase I study, nivolumab had acceptable toxicity and achieved an ORR of 28% in heavily pretreated malignant melanoma patients [44,45]. These promising results led to two Phase III trials published in 2015, CheckMate-066 and CheckMate-037.
CheckMate-066 assigned 418 untreated melanoma patients to nivolumab 3 mg/kg every 2 weeks or dacarbazine treatments, and detected an improvement in OS, PFS and ORR. For nivolumab, 72.9% of the patients were alive at 1 year with only 42.1% for dacarbazine treated patients (HR: 0.42; p < 0.001). Interestingly, this benefit was independent of the PD-L1 tumor status. Median PFS was 5.1 months for nivolumab and 2.2 months for dacarbazine (HR: 0.43; p < 0.001), and ORR was 40 and 13.9%, respectively (OR: 4.06; p < 0.001).
The incidence of grade 3–4 AEs was lower with nivolumab (17.6 vs 11.7%) with lower treatment discontinuations (6.8 vs 11.7%).
It should be pointed out that this trial confronted the nivolumab treatment with chemotherapy (no longer than standard of treatment) instead of ipilimumab. In addition, BRAF-mutated melanomas were excluded even though they represent 40–60% of melanoma tumors. Therefore, conclusions on these patients ought to be taken carefully [46].
Last, preliminary data on the outcomes of the Phase III trial CheckMate-037 have been released. 405 patients with advanced malignant melanoma received nivolumab 3 mg/kg every 3 weeks or chemotherapy. These patients had been previously treated with ipilimumab, or a BRAF inhibitor when indicated. A significant advantage in ORR was reported (31.7 vs 10.6%), along with a manageable safety profile, with only 5% of drug-related serious AEs [47].
With respect to PD-L1 inhibitors, only the results from premature studies have been published so far. In a Phase I trial, objective responses were observed in nine out of 52 patients treated with BMS-936559, while another 14 patients had stable disease for at least 24 weeks [48]. MPDL3280A achieved an ORR of 28% in 45 melanoma patients from a Phase I study [49]. Durvalumab (MEDI4736) has also been tested in a cohort of melanoma patients, with evidence of activity [50]. The toxicity profile of these drugs appears to be manageable.
Immunotherapy combinations
The combined blockade of PD-1 and CTLA-4 achieved more pronounced antitumor activities than blockade of either pathway alone in preclinical models. However, in human patients it is associated with more severe toxicities (Tables 1 & 2).
Table 1. . Overview of the results of the main recent Phase III trials in advanced malignant melanoma.
| Study (year) | Therapy | Patients, n | OS (months) | PFS (months) |
|---|---|---|---|---|
| CTLA-4 targeted | ||||
| MDX010–20 | Ipilimumab + gp100 vs Ipilimumab vs gp100 | 676 | 10 vs 10.1 vs 6.4 (p < 0.001) | 2.76 vs 2.86 vs 2.76 (p > 0.05) |
| CA194–023 | Ipilimumab + dacarbazine vs dacarbazine | 502 | 11.2 vs 9.1 (p < 0.01) | 24% reduction (p = 0.006) |
| BRAF targeted | ||||
| BRIM-3 | Vemurafenib vs dacarbazine | 675 | 13.6 vs 9.7 (p = 0.0085) | 6.9 vs 1.6 (p = 0.0001) |
| BREAK-3 | Dabrafenib vs dacarbazine | 250 | 20.0 vs 15.6 | 6.9 vs 2.7 (p < 0.0001) |
| MEK targeted | ||||
| METRIC | Trametinib vs chemotherapy | 322 | 81 vs 67% (1-year OS; p = 0.01) | 4.8 vs 1.5 (p < 0.001) |
| BRAF + MEK targeted | ||||
| Long et al. (2014) | Dabrafenib + trametinib vs dabrafenib | 423 | 93 vs 83% (6-month OS; p = 0.02) | 9.3 vs 8.8 (p = 0.03) |
| Robert et al. (2015) | Dabrafenib + trametinib vs vemurafenib | 704 | 72 vs 65% (1-year OS; p = 0.005) | 11.4 vs 7.3 (p < 0.001) |
| PD-1 targeted | ||||
| KEYNOTE-006 | Pembrolizumab every 3 weeks vs pembrolizumab every 2 weeks vs ipilimumab | 834 | 74.1 vs 68.4 vs 58.2% (1-year OS; p < 0.05) | 46.4 vs 47.3 vs 26.5% (6-month PFS; p < 0.001) |
| CheckMate 066 | Nivolumab vs dacarbazine | 418 | NR vs 10.8 (p < 0.001) | 5.1 vs 2.2 (p < 0.001) |
| PD-1 + CTLA-4 targeted | ||||
| CheckMate 067 | Nivolumab + ipilimumab vs nivolumab vs ipilimumab | 945 | Pending | 11.5 vs 6.9 vs 2.9 (p < 0.001) |
| Postow et al. (2015) | Nivolumab + ipilimumab vs nivolumab | 142 | Pending | NR vs 4.4 (p < 0.001) |
NR: Not reached; OS: Overall survival; PFS: Progression-free survival.
Table 2. . Grade 3–4 treatment-related adverse events reported with different immunotherapy regimes.
| Study (year) | Therapy | Patients, n | Proportion of grade 3–4 AEs (%) | Most common grade 3–4 AEs |
|---|---|---|---|---|
| Ipilimumab | ||||
| MDX010–20 | Ipilimumab 3 mg/kg (+ gp100) | 676 | 45.5–45.8 | Fatigue (5–6.9%), diarrhea (4.5–5.3%), dyspnea (3.7–3.9%), dermatologic (1.5–2.4%) |
| CA184–023 | Ipilimumab 10 mg/kg + dacarbazine | 502 | 56.3 | ALT increase (21.9%), AST increase (18.2%), fatigue (10.9%), diarrhea (4%), dyspnea (3.2%), vomiting (3.2%), dermatologic (3.2%) |
| Pembrolizumab | ||||
| KEYNOTE-002 | Pembrolizumab 2–10 mg/kg | 540 | 11–14 | Fatigue (1%), vomiting (1%), asthenia (1%), diarrhea (0–1%) |
| KEYNOTE-006 | Pembrolizumab 10 mg/kg | 834 | 10.1–13.3 | Diarrhea (1.1–2.5%), colitis (1.4–1.8%), hypertension (0.4–0.7%) |
| Nivolumab | ||||
| CheckMate 066 | Nivolumab 3 mg/kg | 418 | 11.7 | Diarrhea (1%), rash (0.5%), pruritus (0.5%), vomiting (0.5%), increased AST (0.5%), hypophysitis (0.5%) |
| CheckMate 037 | Nivolumab 3 mg/kg | 405 | 9 | Fatigue (1%), anemia (1%), increased ALT (1%), increased lipase (1%) |
| Nivolumab and ipilimumab | ||||
| Postow et al. (2015) | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg | 142 | 54 | Colitis (17%), diarrhea (10.6%), ALT increase (10.6%), AST increase (7.4%), dermatologic (9.6%), hepatitis (3.2%), hypophysitis (2.1%), pneumonitis (2.1%) |
| CheckMate 067 | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg | 945 | 55 | Diarrhea (9.3%), colitis (7.7%), ALT increase (8.3%), AST increase (6.1%), rash (4.8%), fatigue (4.2%), vomiting (2.6%), nausea (2.2%) |
AE: Adverse event; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
In a Phase I dose-escalation study, 53 patients with advanced melanoma were treated with a concurrent regimen of nivolumab and ipilimumab and 33 with a sequential administration of these two drugs. The combination treatment achieved an ORR of 40% and evidence of clinical activity in 65%. In the sequential regimen ORR was 20%, even though the proportion of treatment-naive patients was lower. Responses were observed regardless of BRAF mutation status, absolute lymphocyte count or baseline PD-L1 tumor expression, with durable responses in the majority of patients. Unprecedented OS were achieved, reaching 85% at 1 year and 79% at 2 years.
Grade 3 or 4 AEs were observed in 72% of the patients treated with the simultaneous administration compared with 18% in the sequential scheme [51].
A Phase III study included 142 patients with untreated stage III/IV melanoma, randomly assigned to receive both nivolumab and ipilimumab or ipilimumab alone. Randomization was stratified according to BRAF mutation status, and melanoma was considered PD-L1 positive with a 5% of PD-L1 surface expression.
In patients with wild-type BRAF tumors, ORR with the combination was higher than with ipilimumab alone, 61 versus 11%, and 22% achieved complete responses with the combination. PFS was also significantly prolonged.
BRAF V600 mutated melanoma had a greater ORR with ipilimumab plus nivolumab, suggesting that the benefit is independent of BRAF mutation. Another point of interest was that no differences in ORR were observed according to PD-L1 expression, which may indicate that PD-L1 tumor expression is not a good predictive biomarker.
Grade 3–4 treatment-related AEs occurred more frequently with combination therapy than in the ipilimumab cohort (54 vs 24%), with a toxicity profile comparable to the reported in the Phase I trial [52].
The subsequent Phase III study CheckMate 067 was organized to confirm these results. This trial compared nivolumab–ipilimumab combination with either single treatment. 945 treatment-naive stage III/IV malignant melanoma patients were included and stratified according to PD-L1 expression, BRAF mutation and stage of disease.
The interim results were presented at the 2015 Annual ASCO Meeting. Median PFS was 11.5 months for the combination, 6.9 months for nivolumab and 2.9 months for ipilimumab, being these differences statistically significant between the three groups. These results were consistent in all the predefined subgroups. ORR was 57.6% for the combination, 43.7% with nivolumab and 19% with ipilimumab.
Considering patients with PD-L1 positive tumors with 5% expression as a cut-off, both nivolumab plus ipilimumab, or nivolumab alone, reached a PFS of 14 months, compared with 3.9 months in the ipilimumab group. In the PD-L1-negative subgroup, the combination was superior to both monotherapies with a PFS of 11.2 versus 5.3 months in the nivolumab cohort and 2.8 months in the ipilimumab cohort.
AEs were higher in the nivolumab-plus-ipilimumab group. Fifty-five percent of the patients treated with the combination presented grade 3–4 AEs, 27.3% in the ipilimumab group and 16.3% with nivolumab alone. Again the most common toxicities affected the gastrointestinal tract and skin, resulting in treatment discontinuation in 36.4, 14.8 and 7.7% of the patients, respectively [53].
Due to the severe toxicity exhibited by the concurrent administration of nivolumab and ipilimumab, alternative schemes are now under investigation.
The randomized Phase II trial CheckMate-064 is exploring the efficacy and safety of the sequential administration of nivolumab and ipilimumab. One cohort of patients received nivolumab followed by ipilimumab and the second cohort received the same treatments but in reverse sequence. Preliminary data were presented in the European Cancer Congress 2015, showing a toxicity profile comparable with the simultaneous administration, while the efficacy seemed to be inferior to the reported in CheckMate-067 [54].
In the Phase I/II trial KEYNOTE-029, patients with advanced melanoma and renal cancer received pembrolizumab with a lower dose of ipilimumab (1 mg/kg) or IFN-α-2b. Preliminary data reported so far disclosed antitumor activity and acceptable toxicity [55].
Last, a Phase I trial evaluated the combination of immunotherapy with MAPK-targeting drugs. Dabrafenib plus ipilimumab were active and well tolerated, although the enrollment for dabrafenib plus trametinib plus ipilimumab had to be interrupted due to grade 3 colitis and perforation [56]. The ongoing Phase I/II KEYNOTE-022 with pembrolizumab plus dabrafenib plus trametinib will probably bring light to this matter.
Conclusion & future perspective
The incorporation of immunotherapy has radically transformed the treatment of metastatic malignant melanoma. Systemic administration of monoclonal antibodies blocking CTLA-4/B7 and PD-1/PD-L1 interactions are more effective and safer than traditional chemotherapy. The simultaneous administration of drugs targeting different immune checkpoints could even overcome the advances achieved so far, albeit significantly increasing toxicity.
In ASCO 2016 Annual Meeting, updates from the most recent clinical trials confirming unprecedented survival rates were reported [57–61]. Likewise, promising outcomes from preliminary essays evaluating the combination of immunotherapy with IL-2 [62], HF-10 (oncolytic virus) [63] or MAPK-targeting drugs [64–66] were presented. During the forthcoming years, more essays with different combinations and sequences of immunotherapies and molecularly targeted drugs are expected. The molecular pathways associated with treatment resistance are being progressively detailed, and this knowledge may hopefully lead to relevant improvements in the prognosis of this malignancy [67,68].
The main clinical trials showed that only a relatively small percentage of the patients achieve long survival intervals. The search for biomarkers that allow the identification of the individuals more likely to benefit from CTLA-4 and PD-1 targeting therapies will surely be a main subject of investigation during the subsequent years.
Last, the optimal duration of each treatment has still not been clearly established, and neither has the most beneficial sequence of treatments, as the majority of the new therapies have not been directly compared. Studies evaluating these aspects will probably be arranged, therefore enhancing the clinical practice of malignant melanoma.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64(1):9. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Guy GP, Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections – United States, 1982–2030. MMWR Morb. Mortal Wkly Rep. 2015;64:591. [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrone L, Zeuli M, Sega FM, et al. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J. Exp. Clin. Cancer Res. 2000;19(1):21–34. [PubMed] [Google Scholar]
- 5.Middleton MR, Grob JJ, Aaronson N, et al. Randomized Phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 6.Hersh E, Del Vecchio M, Brown MP, et al. Final overall survival from a Phase III trial of nab-paclitaxel vs dacarbazine in chemotherapy-naive patients with metastatic melanoma. J. Clin. Oncol. 2014;32(Suppl. 5) doi: 10.1093/annonc/mdv324. Abstract 9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a Phase III study. J. Clin. Oncol. 2004;22(6):1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 8.Ott PA, Bhardwaj N. Impact of MAPK pathway activation in BRAF V600 melanoma on T cell and dendritic cell function. Front Immunol. 2013;4:346. doi: 10.3389/fimmu.2013.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, Phase III randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 11.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kefford RA, Arkenaua H, Brown MP. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J. Clin. Oncol. 2010;28(Suppl. 15) Abstract 8503. [Google Scholar]
- 13.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 14.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 15.Larkin JM, Yan Y, McArthur GA, et al. Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: Phase III study of cobimetinib (cobi) plus vemurafenib (vem) in advanced BRAF-mutated melanoma. J. Clin. Oncol. 2015;33(Suppl. 15) Abstract 9006. [Google Scholar]
- 16.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A comprehensive review of the development of the first successful immunotherapeutic approach against cancer.
- 18.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily – CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 19.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl Acad. Sci. USA. 2010;107(4):1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, Phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 21.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, Phase II, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 22.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with previously treated, advanced melanoma: a multicenter, single-arm Phase II study. Ann. Oncol. 2010;21(8):1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 23.Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med. 2015;4(5):661–672. doi: 10.1002/cam4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]; • After decades without significant advances in metastatic malignant melanoma, MDX010–20 was the first study to achieve an improvement in overall survival in this context.
- 25.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]; • This Phase III trial established immunotherapy as the standard of treatment for metastatic malignant melanoma.
- 26.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, Phase III trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 27.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon α-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 28.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a Phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 2005;23(35):8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 29.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 2009;27(7):1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 30.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 32.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gato-Cañas M, Arasanz H, Blanco-Luquin I, et al. Novel immunotherapies for the treatment of melanoma. Immunotherapy. 2016;8(5):613–632. doi: 10.2217/imt-2015-0024. [DOI] [PubMed] [Google Scholar]
- 35.Karwacz K, Bricogne C, MacDonald D, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol. Med. 2011;3(10):581–592. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribas A. Tumor immunotherapy directed at PD-1. N. Engl. J. Med. 2012;366:2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 38.Hamid O, Robert C, Daud A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribas A, Wolchok JD, Robert C, et al. P0116 Updated clinical efficacy of the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in 411 patients with melanoma. Eur. J. Cancer. 2015;51:e24. [Google Scholar]
- 40.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a Phase I trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 41.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, Phase II trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]; • KEYNOTE-006 demonstrated that PD-1 targeting antibodies were more effective than CTLA-4-directed therapies for the treatment of advanced melanoma.
- 43.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014;2(9):846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 44.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First evidence of the activity of PD-1/PD-L1 targeting immunotherapy.
- 45.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 47.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, Phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 48.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodi F, Kluger H, Sullivan RJ, et al. Clinical activity of the PD-L1 inhibitor MPDL3280A in patients with metastatic melanoma: updated Phase I data. Pigment Cell Melanoma Res. 2014;27:1169. [Google Scholar]
- 50.Lutzky J, Antonia S, Blake-Haskins A, et al. Proceedings of the 2014 ASCO Annual Meeting. Chicago, IL, USA: 29 May–2 June 2014. A Phase 1 study of MEDI4736, an anti-PD-L1 antibody, in patients with advanced solid tumors. [Google Scholar]
- 51.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Even though only preliminary results were presented in ASCO Annual Meeting 2015, and overall survival data have still not been published, the outcomes from CheckMate-067 have no precedent in malignant melanoma and suggest that combination immunotherapy could be a very effective strategy to improve the prognosis of this disease.
- 54.Hodi FS, Gibney GT, Sullivan R, et al. An open-label, randomized, Phase II study of nivolumab given sequentially with ipilimumab in patients with advanced melanoma (Checkmate 064) ECCO-ESMO. 25–29 September 2015 Abstract 23L. [Google Scholar]
- 55.Atkins MB, Choueiri TK, Hodi FS, et al. Pembrolizumab (MK-3475) plus low-dose ipilimumab (IPI) in patients (pts) with advanced melanoma (MEL) or renal cell carcinoma (RCC): data from the KEYNOTE-029 Phase I study. J. Clin. Oncol. 2015;33 Abstract 3009. [Google Scholar]
- 56.Puzanov I. Combining targeted and immunotherapy: BRAF inhibitor dabrafenib (D) ± the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation-positive unresectable or metastatic melanoma (MM) J. Transl. Med. 2015;13(Suppl. 1):K8. [Google Scholar]
- 57.Robert C, Ribas A, Hamid O, et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J. Clin. Oncol. 2016;34 Abstract 9503. [Google Scholar]
- 58.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival analysis of KEYNOTE-006. J. Clin. Oncol. 2016;34 Abstract 9504. [Google Scholar]
- 59.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Updated results from a Phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067) J. Clin. Oncol. 2016;34 Abstract 9505. [Google Scholar]
- 60.Long GV, Atkinson V, Cebon JS, et al. Pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma: results of the KEYNOTE-029 expansion cohort. J. Clin. Oncol. 2016;34 Abstract 9506. [Google Scholar]
- 61.Daud A, Blank CU, Robert C, et al. KEYNOTE-006 study of pembrolizumab (pembro) versus ipilimumab (ipi) for advanced melanoma: efficacy by PD-L1 expression and line of therapy. J. Clin. Oncol. 2016;34 Abstract 9513. [Google Scholar]
- 62.Patel SP, Milton D, Milhem MM, et al. Sequential administration of high-dose interleukin-2 and ipilimumab in patients with metastatic melanoma. J. Clin. Oncol. 2016;34 Abstract e21041. [Google Scholar]
- 63.Andtbacka RHI, Ross MI, Agarwala SS, et al. Preliminary results from Phase II study of combination treatment with HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab in patients with stage IIIb, IIIc, or IV unresectable or metastatic melanoma. J. Clin. Oncol. 2016;34 Abstract 9543. [Google Scholar]
- 64.Long GV, Hamid O, Hodi FS, et al. Phase II study of the safety and efficacy of pembrolizumab (pembro) in combination with dabrafenib (D) and trametinib (T) for advanced melanoma (KEYNOTE-022) J. Clin. Oncol. 2016;34 Abstract TPS9596. [Google Scholar]
- 65.Barcena E, Trinh V, McIntyre SE, et al. Responses in patients with BRAF V600-mutant metastatic melanoma receiving anti-PD1/PDL1 therapy alone or combined with BRAF inhibitors. J. Clin. Oncol. 2016;34 doi: 10.1200/JCO.2015.62.9345. Abstract 9546. [DOI] [PubMed] [Google Scholar]
- 66.Rohlfs M, Bassett RL, Lacey C, et al. BRAF with or without MEK inhibition plus PD-1 checkpoint blockade for the treatment of metastatic melanoma. J. Clin. Oncol. 2016;34 Abstract 9548. [Google Scholar]
- 67.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]