Abstract
Advances in immune therapy have changed the landscape of advanced melanoma treatment. Intralesional therapy is an important type of immune therapy due to its efficacy and safety, especially in the setting of locoregional metastases. These therapies induce frequent responses in injected lesions as well as distant nontreated lesions through a ‘bystander’ effect of priming an antitumor immune response. The culmination of nearly a century of innovation has led to the approval of the first US FDA approved intralesional therapy for melanoma in talimogene laherparepvec. Numerous efforts to combine intralesional therapies with systemic immune checkpoint inhibitors are ongoing, whereby a synergistic effect may continue to improve outcomes for patients.
KEYWORDS : intralesional therapy, melanoma, talimogene laherparepvec
Practice points.
The management of in-transit metastases is a unique challenge in melanoma.
The first intralesional therapy, heat-killed bacteria known as Coley’s toxins, was the earliest form of immunotherapy.
Recent development of intralesional therapies has provided additional options for clinicians to tackle this challenge.
Talimogene laherparepvec, a herpes-based oncolytic viral injectable therapy, is the first US FDA approved intralesional therapy for advanced melanoma.
Other intralesional therapies including PV-10 (Rose Bengal), IL-12 plasmid electroporation and Coxsackievirus A21 have shown promising clinical results.
There has been some evidence of a bystander effect where there are responses in noninjected tumor lesions; however, there are few responses in visceral lesions.
The combination of intralesional and systemic therapy is being explored to enhance the immune response in melanoma.
As the deadliest form of skin cancer, melanoma will account for over 10,000 deaths annually in the USA alone [1]. Despite the historical poor prognosis for metastatic disease, there are now promising systemic immune therapy options such as immune checkpoint therapy. These innovative treatments have improved the overall survival (OS) in advanced melanoma from a 5-year OS of less than 10% in 2008 to over 30% for patients treated with anti-PD-1 [2]. In some cases, however, advanced melanoma blurs the lines between locoregional and disseminated disease, posing challenging clinical dilemmas. In these situations, locoregional metastases develop between the primary melanoma and the draining lymph-node basin, known as in-transit metastasis. These types of metastasis have been reported to occur in 4% of melanoma and up to 8% in patients with primary melanoma ≥1 mm [3]. In many cases, these in-transit lesions are technically resectable, but operations would cause substantial morbidity with minimal chance of cure. Historic options for these lesions included limb perfusion, systemic therapies or surgery, which were all associated with suboptimal safety and efficacy. However, the recent development of effective intralesional therapy appears to particularly benefit patients with extensive locoregional disease with minimal toxicity. This approach has led to the development of talimogene laherparepvec (T-VEC), the first US FDA approved intralesional therapy for melanoma. In this review, we will discuss the history of the development of intralesional therapies, current effective therapies and the future direction of these injectable therapies (see Table 1 for summary of current agents).
Table 1. . Summary of intralesional therapy in melanoma.
| Agent | Type or mechanism | Phase | n | ORR in injected lesions (%) | ORR in bystander lesions (%) | CR rate (%) | PFS (months) | OS (months) | Grade 3–4 AE (%) | Trial information |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasmid IL-12 | Plasmid DNA | 2 | 29 | 33 | 62 | 11 | NR | NR | 0 | NCT01502293 |
| PV-10 (Rose Bengal) | Xanthene dye | 2 | 80 | 51 | 33 | 26 | 8.2 | NR | 15 | NCT00521053 |
| Coxsackievirus 21 | Oncolytic virus | 2 | 57 | 28 | NR | 14 | 4.2 | 27 | 0 | NCT01227551 |
| Talimogene laherparepvec | Oncolytic virus | 3 | 436 | 26 | 15 | 11 | 8.2 (TTF) | 23 | 11 | NCT00769704 |
AE: Adverse event; CR: Complete response; NR: Not reported; ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival; TTF: Time-to-treatment failure.
Historic intralesional agents (Coley’s toxins, Bacillus Calmette–Guérin, IL-2, granulocyte macrophage colony-stimulating factor)
The advent of cancer immunotherapy and intralesional therapy began with observations of spontaneous regressions after infections in the 19th century. WB Coley attempted to leverage this observation by using heat-killed cultures of Streptococcus pyogenes and Serratia maracescens, better known as Coley’s toxins, for intratumoral injection in sarcoma patients in 1891 [4]. Although Coley’s toxins had some success, the underlying mechanism was unknown at the time and met with skepticism by his contemporaries [5,6]. The use of Coley’s toxins eventually became unfavorable as it was considered illegal to use as a ‘new drug’ by the FDA in 1963 and was added to the ‘unproven methods’ list by the American Cancer Society in 1965 [7].
Bacillus Calmette–Guérin (BCG) is a live strain of Myobacterium bovis that was first administered in humans in 1921 as a vaccine against tuberculosis. Using the principle that BCG could trigger a nonspecific immune response to tumors in murine models, BCG was used as a form of intralesional therapy in melanoma beginning in the 1970s [8,9]. Early success was seen in locally injected lesions and distant metastases in stage II–III melanoma patients with some trials reporting regression of 90% of injected lesions and 17% of noninjected lesions [10,11]. In a Phase III trial (ECOG1673), over 700 patients with stage I–III melanoma were randomized to receive adjuvant BCG injections in the regional lymph node drainage area alone or in combination with intravenous dacarbazine [12]. With 30 years of follow-up, the mature results of this study were conclusively negative with no improvement in OS or disease-free survival with the addition of BCG. Toxicity was generally mild, with the exception of frequent punctate abscesses and occasional cases of anaphylaxis. This study thus concluded that intralesional BCG does not play a role in adjuvant therapy for melanoma.
GM-CSF plays a critical role in the expansion of antigen-presenting cells, such as dendritic cells (DCs), in the tumor microenvironment which then promotes antitumor cytotoxic T-cell responses [13,14]. Several early studies have evaluated direct injection of GM-CSF into melanoma lesions with modest response and tolerable side effect profile [15,16]. Currently, GM-CSF is being used in distinct ways as adjunctive therapy incorporated into other treatment regimens (discussed below).
IL-2 has been a mainstay of systemic melanoma therapy for many years, becoming the first FDA-approved immunotherapy for metastatic melanoma in 1998. Recombinant IL-2 was shown to enhance immune responses against cancer cells in melanoma mouse models [17]. Systemic IL-2 therapy, while showing durable responses (approximately 6–8%) lasting over 10 years in some patients, is restricted to patients with excellent performance status with preserved organ function [18]. As an intralesional therapy, IL-2 has shown to be well tolerated with only low-grade flu-like symptoms and local injection-side adverse events (AEs) [19]. Response rates for injected lesions have generally exceeded 80%, but did not induce a significant bystander effect in noninjected lesions [19,20]. Randomized trials have not evaluated injectable IL-2, and it is not commonly used currently.
Velimogene aliplasmid
Velimogene aliplasmid or Allovectin is an investigational plasmid DNA-based immunotherapy. It was designed with a bicistronic plasmid (VCL-1005) encoding two transgene proteins, an MHC class I heavy chain (HLA-B7), and the light chain common to class I molecules β2-microglobulin and formulated in a cationic lipid suspension [21]. Based on this design, intralesional administration of velimogene aliplasmid was thought to stimulate both innate and adaptive immune responses [21].
A Phase II dose-escalation study was conducted to evaluate the safety and efficacy of this intralesional therapy in patients with stage IIIB/C and IV M1a/b melanoma [22]. Of the 127 patients evaluated for efficacy, 11.8% had an objective response with a median duration of response of 13.8 months in those with stage IV melanoma. Responses in noninjected target lesions were seen in 21% of the patients.
The subsequent Phase III Allovectin Immunotherapy for Metastatic Melanoma trial included 390 patients with stage IIIB–IV M1a/b melanoma randomized 2:1 to velimogene aliplasmid or chemotherapy in the form of dacarbazine or temozolomide [23]. The trial failed to meet its primary end point of response rate at 24 weeks with velimogene aliplasmid (4.6%) as compared with the chemotherapy arm (12.3%, p = 0.01). Duration of response among velimogene aliplasmid responders was marginally longer but this did not reach statistical significance (p = 0.066), and median OS appeared shorter (18.8 vs 24.1 m, p = 0.49). Given these results, further development of velimogene aliplasmid was halted.
Plasmid IL-12 electroporation
IL-12 plays an important role in antitumor response by expanding the T-helper-1 response and subsequent production of IFN-γ, leading to the proliferation of cytotoxic natural killer and T cells in preclinical models [24,25]. Furthermore, it was shown that IL-12 could improve antitumor immune responses in human melanoma cells in vitro by enhancing the levels of HLA class I/II and ICAM-1 expression [26]. However, early trials were largely unsuccessful due to the severe toxicity and narrow therapeutic window involved with systemic therapy [27,28]. It was not until the advancements in intratumoral electroporation with gene transfer of plasmid IL-12 that this cytokine could be deployed in melanoma [29]. Intratumoral electroporation involves the delivery of an electrical field by an electrode directly to a lesion, increasing the permeability of the cell membrane transiently to the cytotoxic agent [30].
A Phase II clinical trial in patients with stage IIIB–IV M1a melanoma revealed an objective response rate (ORR) of 33% in 27 evaluable patients with a 11% complete response (CR) rate [31]. There was a notable bystander effect with regression of noninjected lesions in 62% of the patients. The treatment was well tolerated with no grade 3–4 drug-related AEs and transient pain (57%) and inflammation (17%) at the treatment site being the most common side effect. An expansion protocol has been planned to evaluate treatment frequency, and a combination study with pembrolizumab is also ongoing.
PV-10 (Rose Bengal disodium 10%)
PV-10 is a sterile, nonpyrogenic solution derived from Rose Bengal disodium, a red-dark xanthene-based dye developed for use in liver and ophthalmological diagnostic studies [32,33]. Mouse models have shown that PV-10 has direct cytotoxic effects on cancer cells, by lysozyme-induced apoptosis and necrosis [34,35]. Additionally, it appears that the necrosis does not cause the denaturation of tumor antigens [36]. In 11 patients with metastatic melanoma, a Phase I trial demonstrated that intralesional injection of PV-10 can induce regression in both injected (OR: 48%) and noninjected lesions (OR: 27%) [37]. The single-arm Phase II trial, involving 80 patients with stage III/IV disease, demonstrated an ORR of 51% (CR in 26%) with 8% of patients having no evidence of disease after 1 year [38]. An OR in bystander lesions was seen in 33% of patients. In terms of safety, the treatment was well tolerated with predominately mild-to-moderate injection site-related AEs. Approximately 15% of patients had at least one grade 3 AEs, which were largely injection site related such as pain. A Phase III clinical trial comparing PV-10 with either systemic chemotherapy (dacarbazine, temozolomide) or T-VEC in stage IIIB/C melanoma patients is currently ongoing (NCT02288897). Additionally, a Phase Ib/II trial evaluating the combination therapy of intralesional PV-10 with pembrolizumab in patients with metastatic melanoma with at least one injectable lesion (NCT02557321).
Based on the clinical data, there seems to be an immunologic basis for these responses given the responses in nontreated bystander lesions. In patients with regression of injected lesions, there appeared to be increased circulating CD3+ T cells (p = 0.03) in peripheral blood as compared with pretreatment samples [39]. T cells from these samples also produced increased levels of IFN-γ in response to autologous tumor after treatment, which implies antigen-specific T-cell activation and proliferation. In addition, murine studies revealed that PV-10 treatment led to elevated serum levels of HMGB1 protein, a damage-associated molecular pattern molecule, crucial in the activation of DCs [40]. The authors demonstrated increased infiltration of DCs in the lymph nodes and proliferation of tumor-specific CD8+ T cells in mice after intralesional PV-10 treatment, which suggests that PV-10 plays a critical role in activation of DCs and induction of a systemic antitumor immune response. Elevated serum HMGB1 levels in patients 7–14 days after intralesional PV-10 also suggest an analogous mechanism in humans.
Coxsackievirus A21 (CAVATAK)
The coxsackievirus A21 (CVA21) is a naturally occurring ‘common cold’ enterovirus, which has been shown to have potent oncolytic activity in both in vitro and in vivo models of cancer [41]. This activity appears related to specific viral capsid interactions with surface expressed virus receptors such as ICAM-1 and decay-accelerating factor [41]. These receptors are upregulated on the surface of melanoma cells compared with normal tissue, causing CVA21 to preferentially infect these cancer cells and cause oncolysis. CAVATAK is a proprietary formulation of genetically unmodified CVA21 that can be given intralesionally in patients.
A Phase I trial (NCT00438009) demonstrated that even two intralesional injections of CVA21 were sufficient to cause regression or stabilization of injected melanoma lesions. A subsequent Phase II trial evaluated 57 patients with unresectable stage IIIC–IV M1c melanoma [42]. Patients were treated with up to ten series of multi-intralesional CVA21 injections. The primary end point of the trial was met with 38.6% of patients experiencing immune-related progression-free survival (irPFS) at 6 months with a median irPFS of 4.2 months. The overall response rate by irRECIST was 28.1% with a ≥6-month durable response rate (DRR) of 19%. Median OS was 26 months with a 1-year survival of 75.4%. In terms of safety, the most common AEs were grade 1 fatigue, fevers, chills and local injection site reactions. No grade 3 or 4 drug-related AEs were observed. A subsequent study was performed to evaluate the nature of the systemic responses by collection of sequential tumor biopsies of both injected and noninjected lesions in 13 patients [43]. The samples were assessed for levels of viral replication and evidence of viral-induced immune activation within the tumor microenvironment as well as serum levels of viral loads, anti-CVA21 neutralizing antibody and levels of immune-inflammatory cytokines. In five of the six cases, there were increases in CD8+ infiltrates within the tumor microenvironment with elevated expression of PD-L1+ cells. Four patients were also noted to have increased immune cell infiltrates after failing treatment with single or double immune checkpoint blockade. The authors concluded that the findings provided a strong rationale for investigation of intralesional CVA21 administration in sequential or concurrent treatment with checkpoint inhibitors. Currently, there are several Phase I clinical trials exploring the use of intralesional CVA21 in combination with ipilimumab (NCT02307149) or pembrolizumab (NCT02565992).
Talimogene laherparepvec
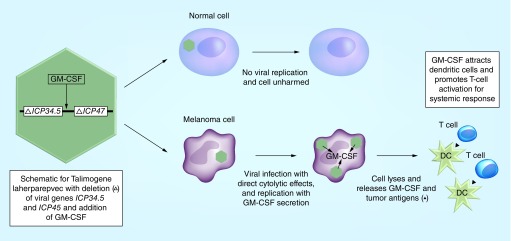
T-VEC is another oncolytic virus based on a genetically modified herpes simplex virus type 1 (HSV-1). The modifications include the deletion of viral genes ICP34.5, which enhances the oncolytic properties by providing tumor-selective replication and reduces neurotropism, and ICP47, which increases the level of antigen presentation in HSV-infected cells [44,45]. Instead, a coding sequence for GM-CSF was inserted, promoting antigen-presenting cell recruitment and enhancing the tumor-specific T-cell response (Figure 1) [13].
Figure 1. . Proposed mechanism for Talimogene laherparepvec.
DC: Dendritic cell.
The Phase I trial proved that T-VEC was generally well tolerated with the only AEs being local inflammation, erythema and fever [46]. These effects were more noticeable in patients that were HSV seronegative. Post-treatment biopsies also revealed areas of tumor necrosis or apoptosis that stained strongly for HSV and expression of GM-CSF. A multidose regimen every 2–3 weeks was instituted in the subsequent trials to account for the side effects in the seronegative patient, which allowed for eventual seroconversion and less toxicity.
The single-arm Phase II trial evaluated the efficacy of T-VEC every 3 weeks in 50 patients with unresectable or metastatic melanoma [47]. The ORR was 26% with 92% with durable responses maintained for 7–31 months. Regressions were seen in both injected and noninjected lesions including visceral lesions. At 1- and 2-year OS was 58 and 52%, respectively.
The subsequent Phase III trial, OPTiM, randomly assigned 436 patients in a 2:1 ratio to either intralesional T-VEC or subcutaneous GM-CSF in patients with stage IIIB–IV melanoma [48]. The DRR, the primary end point defined as the proportion of patients with a response lasting 6 months or more, was significantly higher in the T-VEC arm as compared with the patients who received GM-CSF (16.3 vs 2.1%, p <001). Key secondary end points such as ORR (26.4 vs 5.7%) were significantly higher in the T-VEC arm with a CR rate of 10.8%, but median OS had a nearly statistically significant trend toward benefit with T-VEC (23.3 vs 18.9 months, p = 0.051). Among patients that responded in the T-VEC arm, the median time to response was 4.1 months, but more than half had pseudoprogression (≥25% increase in the size of lesions or appearance of new lesions) before achieving a response, a similar phenomenon seen in other immune therapies [49,50]. Further exploratory analyses revealed that the differences in DRR between T-VEC and GM-CSF were more pronounced in stage IIIB/C (33 vs 0%) and IV M1a (16 vs 2%) as compared with stage IV M1b (3 vs 4%) and IV M1c (7 vs 3%). These key differences favoring in patients with more locoregional disease (stage IIIB/C and IV M1a) in the T-VEC group were also noted in OS (HR: 0.57; 95% CI: 0.4–0.8). Patients who were previously untreated also had a survival benefit (HR: 0.50; 95% CI: 0.35–0.73).
Based on these studies, monotherapy with T-VEC has shown to be an effective intralesional agent in advanced melanoma, especially in stage III or stage IV M1a disease. It is the first FDA-approved oncolytic viral therapy for the treatment of melanoma.
Future perspective
A major issue surrounding the development of intralesional therapies is the difficulty in determining response rates and PFS. As these agents modulate the immune response, immune-related pseudoprogression may frequently be observed. Furthermore, injected and noninjected lesions may have discordant growth in many cases. Another major concern surrounding intralesional therapy surrounds distant, including visceral metastases. While noninjected lesions may respond, patients with stage IV M1c disease (nonlung visceral metastases) had extremely low response rates to T-VEC and other intralesional injections. Thus, combination therapies or novel methods of delivery are major priorities.
Based on the underlying mechanism, efficacy and tolerable side effect profile, intralesional therapy combined with a systemic immune therapy seems to be an intriguing strategy supported by preclinical studies [51,52]. Several trials combining intralesional therapy with immune checkpoint inhibitors have been previously mentioned. Of note, a Phase Ib/II trial of T-VEC combined with ipilimumab revealed an ORR in 10 out of 18 patients with stage IIIB–IV M1c melanoma, a 33% CR rate and a median PFS of 10.8 months without any dose-limiting toxicities (DLTs) [53]. Results from a randomized study of ipilimumab + T-VEC versus ipilimumab alone are pending (NCT01740297). Another randomized study, the Phase I/III MASTERKEY-265 (NCT02263508), compares the combination of T-VEC with pembrolizumab with pembrolizumab alone in stage IIIB–IV M1c melanoma patients. Results from the Phase Ib trial revealed no DLTs with confirmed ORR of 10 out of 21 (56%) evaluable patients and a 14% CR rate [54]. The Phase III portion is now enrolling patients.
In addition to combination T-VEC therapy, trials with combinations of other intralesional agents have been initiated. Interim data presented at AACR 2016 of the Phase Ib trial of CVA21 and ipilimumab (NCT02307149) revealed no DLTs and only one grade 3 AE with the best ORR of four out of six evaluable patients with stage IIIC/IV melanoma [55]. A Phase Ib, multicenter trial evaluating the safety and tolerability of combination CVA21 and pembrolizumab is ongoing (NCT02565992). Similarly, a Phase Ib/II trial of combination PV-10 and pembrolizumab dosed the first patient in January 2016 (NCT02557321). Finally, a single-center Phase II clinical trial evaluating the efficacy of intratumoral plasmid IL-12 and pembrolizumab is also underway (NCT02493361).
Besides intralesional agents for melanoma in the metastatic or unresectable setting, investigators have been exploring the potential for these agents in the neoadjuvant setting. Given the high risk of recurrence and death with locoregional melanoma, there is strong rationale to see if the use of neoadjuvant therapy will outweigh the risks in delaying surgery. A Phase II trial comparing neoadjuvant T-VEC of resectable IIIB–IV M1a melanoma with surgery alone will help to determine the efficacy of this treatment [56].
Last, the role of intralesional therapy has expanded outside the realm of advanced melanoma. As seen from the benefits of systemic immunotherapy in other solid tumor types, multiple trials have been initiated. These include liver-directed therapy with T-VEC in hepatocellular carcinoma (NCT02509507), PV-10 in hepatocellular carcinoma (NCT00986661) and neuroendocrine tumors metastatic to the liver (NCT02693067) as well as CVA21 and pembrolizumab in non-small-cell lung cancer and bladder cancer (NCT02043665).
Conclusion
The advancement of injectable therapy for melanoma has evolved over the past several decades from the early days of Coley’s toxins in the 1890s. As the basis for immunotherapy has been elucidated over the past several years, the role of intralesional therapy may expand in both monotherapy and combinatorial strategies. Given its particular efficacy in advanced stage III and stage IV M1a melanoma, intralesional therapy currently plays an important role in local management where surgery or regional chemotherapy may not be amenable. However, as seen by the bystander effect on noninjected and systemic disease, intralesional therapy may be useful in augmenting current systemic immunotherapy in advanced melanoma.
Footnotes
Financial & competing interests disclosure
DB Johnson is on the advisory board for Genoptix and Bristol-Myers Squibb and has received research funding from Incyte. DY Wang has no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Read RL, Haydu L, Saw RP, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann. Surg. Oncol. 2015;22(2):475–481. doi: 10.1245/s10434-014-4100-0. [DOI] [PubMed] [Google Scholar]
- 4.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 1991;(262):3–11. [PubMed] [Google Scholar]
- 5.The failure of the erysipelas toxins. J. Am. Med. Assoc. 1894;XXIII(24):919. [Google Scholar]
- 6.Johnston BJ, Novales ET. Clinical effect of Coley’s toxin. II. A seven-year study. Cancer Chemother. Rep. 1962;(21):43–68. [PubMed] [Google Scholar]
- 7.Grabstald H. Unproven methods of cancer treatment Coley’s mixed toxins. CA Cancer J. Clin. 1965;15(3):139–140. doi: 10.3322/canjclin.15.3.139. [DOI] [PubMed] [Google Scholar]
- 8.Morton D, Eilber FR, Malmgren RA, Wood WC. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970;68(1):158–163. discussion 163–154. [PubMed] [Google Scholar]
- 9.Old LJ, Clarke DA, Benacerraf B. Effect of bacillus Calmette–guerin infection on transplanted tumours in the mouse. Nature. 1959;184(Suppl. 5):291–292. doi: 10.1038/184291a0. [DOI] [PubMed] [Google Scholar]
- 10.Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann. Surg. 1974;180(4):635–643. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karakousis CP, Douglass HO, Jr, Yeracaris PM, Holyoke ED. BCG immunotherapy in patients with malignant melanoma. Arch. Surg. 1976;111(6):716–718. doi: 10.1001/archsurg.1976.01360240096018. [DOI] [PubMed] [Google Scholar]
- 12.Agarwala SS, Neuberg D, Park Y, Kirkwood JM. Mature results of a Phase III randomized trial of bacillus Calmette–guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I–III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer. 2004;100(8):1692–1698. doi: 10.1002/cncr.20166. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman HL, Ruby CE, Hughes T, Slingluff CL., Jr Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer. 2014;(2):11. doi: 10.1186/2051-1426-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA. 1993;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Si Z, Hersey P, Coates AS. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996;6(3):247–255. doi: 10.1097/00008390-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Nasi ML, Lieberman P, Busam KJ, et al. Intradermal injection of granulocyte–macrophage colony-stimulating factor (GM-CSF) in patients with metastatic melanoma recruits dendritic cells. Cytokines Cell. Mol. Ther. 1999;5(3):139–144. [PubMed] [Google Scholar]
- 17.Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J. Exp. Med. 1985;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 19.Boyd KU, Wehrli BM, Temple CL. Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J. Surg. Oncol. 2011;104(7):711–717. doi: 10.1002/jso.21968. [DOI] [PubMed] [Google Scholar]
- 20.Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br. J. Cancer. 2003;89(9):1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doukas J, Rolland A. Mechanisms of action underlying the immunotherapeutic activity of Allovectin in advanced melanoma. Cancer Gene Ther. 2012;19(12):811–817. doi: 10.1038/cgt.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedikian AY, Richards J, Kharkevitch D, Atkins MB, Whitman E, Gonzalez R. A Phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. 2010;20(3):218–226. doi: 10.1097/CMR.0b013e3283390711. [DOI] [PubMed] [Google Scholar]
- 23.Agarwala SS. Intralesional therapy for advanced melanoma: promise and limitation. Curr. Opin. Oncol. 2015;27(2):151–156. doi: 10.1097/CCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunda MJ, Luistro L, Rumennik L, et al. Antitumor activity of interleukin 12 in preclinical models. Cancer Chemother. Pharmacol. 1996;38(Suppl.):S16, S21. doi: 10.1007/s002800051031. [DOI] [PubMed] [Google Scholar]
- 25.Cavallo F, Signorelli P, Giovarelli M, et al. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J. Natl Cancer Inst. 1997;89(14):1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 26.Yue FY, Geertsen R, Hemmi S, et al. IL-12 directly up-regulates the expression of HLA class I, HLA class II and ICAM-1 on human melanoma cells: a mechanism for its antitumor activity? Eur. J. Immunol. 1999;29(6):1762–1773. doi: 10.1002/(SICI)1521-4141(199906)29:06<1762::AID-IMMU1762>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 27.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 1997;3(3):409–417. [PubMed] [Google Scholar]
- 28.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- 29.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr. Gene Ther. 2010;10(4):312–317. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 30.Cha E, Daud A. Plasmid IL-12 electroporation in melanoma. Hum. Vaccin. Immunother. 2012;8(11):1734–1738. doi: 10.4161/hv.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daud A, Algazi A, Ashworth M, Fong L, Lewis J. Systemic antitumor effect and clinical response in a Phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. J. Clin. Oncol. 2014;32, 5s(Suppl.) Abstract 9025. [Google Scholar]
- 32.Norn MS. Rose bengal vital staining. Staining of cornea and conjunctiva by 10 percent rose bengal, compared with 1 percent. Acta Ophthalmol. (Copenh.) 1970;48(3):546–559. doi: 10.1111/j.1755-3768.1970.tb03756.x. [DOI] [PubMed] [Google Scholar]
- 33.Epstein NN, Delprat GD, Kerr WJ. The rose bengal test for liver function: further studies. J. Am. Med. Assoc. 1927;88(21):1619–1623. [Google Scholar]
- 34.Toomey P, Kodumudi K, Weber A, et al. Intralesional injection of rose bengal induces a systemic tumor-specific immune response in murine models of melanoma and breast cancer. PLoS ONE. 2013;8(7):e68561. doi: 10.1371/journal.pone.0068561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wachter EA, Dees C, Harkins J, Fisher WG, Scott T. International Symposium on Biomedical Optics. San Jose, CA, USA: 28 May 2002. Imaging photosensitizer distribution and pharmacology using multiphoton microscopy. Presented at. [Google Scholar]
- 36.Maker AV, Prabhakar B, Pardiwala K. The potential of intralesional rose bengal to stimulate T-cell mediated anti-tumor responses. J. Clin. Cell. Immunol. 2015;6(4):343. doi: 10.4172/2155-9899.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18(6):405–411. doi: 10.1097/CMR.0b013e32831328c7. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann. Surg. Oncol. 2015;22(7):2135–2142. doi: 10.1245/s10434-014-4169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Kodumudi K, Weber A, Sarnaik AA, Pilon-Thomas S. Abstract 630: Induction of anti-melanoma immunity after intralesional ablative therapy. Cancer Res. 2014;74(Suppl. 19):630. [Google Scholar]
- 40.Liu H, Innamarato PP, Kodumudi K, et al. Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1. Oncotarget. 2016;7(25):37893–37905. doi: 10.18632/oncotarget.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafren DR, Au GG, Nguyen T, et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin. Cancer Res. 2004;10(1 Pt 1):53–60. doi: 10.1158/1078-0432.ccr-0690-3. [DOI] [PubMed] [Google Scholar]
- 42.Andtbacka RH, Curti BD, Kaufman HL, et al. Final data from CALM: A Phase II study of Coxsackievirus A21 (CVA21) oncolytic virus immunotherapy in patients with advanced melanoma. J. Clin. Oncol. 2015;33(Suppl.) Abstract 9030. [Google Scholar]
- 43.Andtbacka RHI, Curti BD, Hallmeyer S, et al. Phase II calm extension study: Coxsackievirus A21 delivered intratumorally to patients with advanced melanoma induces immune-cell infiltration in the tumor microenvironment. J. Immunother. Cancer. 2015;3(Suppl. 2):343–343. [Google Scholar]
- 44.Hill A, Jugovic P, York I, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375(6530):411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 45.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 46.Hu JC, Coffin RS, Davis CJ, et al. A Phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 47.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 48.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]; •• Phase III clinical trial that led to the approval of talimogene laherparepvec (T-VEC) by the US FDA.
- 49.Hodi FS, O’day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 51.Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6(226):226ra232. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Preclinical data that provide strong support for the use of intralesional therapy combined with systemic immunotherapy.
- 52.Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014;22(11):1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puzanov I, Milhem M, Andtbacka R, et al. Survival, safety, and response patterns in a Phase Ib multicenter trial of talimogene laherparepvec (T-VEC) and ipilimumab (Ipi) in previously untreated, unresected stage IIIB–IV melanoma. J. Clin. Oncol. 2015;33(Suppl.) Abstract 9063. [Google Scholar]; • Phase Ib data on the combination therapy of T-VEC with ipilimumab.
- 54.Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 Phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB–IV melanoma. J. Clin. Oncol. 2016;34(Suppl.) Abstract 9568. [Google Scholar]; • Phase Ib data on combination of T-VEC and pembrolizumab in advanced melanoma.
- 55.Curti B, Richards J, Faries M, Grose M, Karpathy R. 107th Annual Meeting of the American Association for Cancer Research. LA, USA: April 2016. Phase Ib study of a novel immunotherapy combination therapy of intralesional coxsackievirus A21 and systemic ipilimumab in patients with advanced melanoma; p. Abstract CT021. Proceedings of: 16–20. [Google Scholar]; • Phase Ib data on combination of CVA21 and ipilimumab in advanced melanoma.
- 56.Andtbacka R, Chastain M, Li A, Shilkrut M, Ross M. Phase 2, multicenter, randomized, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment (tx) plus surgery vs surgery for resectable stage IIIB/C and IVM1a melanoma (MEL) J. Clin. Oncol. 2015;33(Suppl.) Abstract TPS0904. [Google Scholar]