Abstract
Purpose:
Patients with melanoma treated with ipilimumab and radiosurgery (stereotactic radiosurgery [SRS]) were reviewed for efficacy/safety.
Methods:
Patients who received ipilimumab and SRS for brain metastases were analyzed for control of SRS-treated metastasis and overall survival.
Results:
We identified 27 patients, 26 were assessable for outcomes. Median time-to-treated metastasis progression was 6.3 months (95% CI: 3.1–12.2). Overall survival was 23.4 months (95% CI: 5.7–not estimable) for SRS prior to/during ipilimumab (n = 14), and 10.4 months (95% CI: 1.9–not estimable) for SRS after ipilimumab (n = 12). Overall, no unexpected toxicities were seen: 11% of patients experienced grade 3 CNS toxicity and 7% developed radionecrosis.
Conclusion:
SRS for melanoma brain metastases with ipilimumab was well-tolerated. There may be improved survival for patients receiving SRS prior to/during ipilimumab.
KEYWORDS : brain metastases, ipilimumab, melanoma, radiosurgery
Summary points.
Purpose/objectives
Patients with metastatic melanoma treated with ipilimumab and stereotactic radiosurgery (SRS) were reviewed for efficacy and safety outcomes.
Methods
A retrospective analysis was performed of patients treated with ipilimumab at a single institution.
Patients who received SRS for brain metastases were analyzed for control of the metastasis treated with SRS (treated metastasis control), distant intracranial control, extracranial progression and overall survival.
Toxicity was scored using the CTCAE v4.0.
Results
A total of 26 patients were assessable for outcomes with a median follow-up after SRS of 7.1 months (interquartile range: 3.1–13.1).
In total, 14 patients received SRS before or during ipilimumab and 12 patients received SRS post-ipilimumab.
Median time to treated metastasis progression was 6.3 months (95% CI: 3.1–12.2).
Median extracranial progression-free survival was 3.0 months (95% CI: 1.6–4.6).
Median overall survival following initial SRS was 10.4 months (95% CI: 6.5–23.4) for all patients, 23.4 months (95% CI: 5.7–not estimable) for patients receiving SRS prior to or during ipilimumab and 10.4 months (95% CI: 1.9 – not estimable) for patients receiving SRS after ipilimumab.
Eleven percent of all patients experienced grade 3 CNS toxicity with no grade 4 or 5 toxicity reported.
Two patients (7%) had biopsy-proven radionecrosis.
Conclusion
Radiosurgery for melanoma brain metastases in patients treated with ipilimumab was well-tolerated with few side effects. The toxicity of radiosurgery in patients receiving ipilimumab was acceptable and similar to previously published series of patients treated with radiosurgery alone.
While treated metastasis control appears suboptimal, the favorable overall survival suggests that radiographic enlargement may not reflect true disease progression.
Extracranial effects of SRS were demonstrated with two cases of an abscopal effect.
There may be an association of improved survival and extracranial progression-free survival for patients receiving SRS prior to ipilimumab.
These findings are similar to other retrospective series, and prospective evaluation of the optimal combination of SRS and ipilimumab appears warranted.
Stereotactic radiosurgery (SRS) has become a standard treatment for patients with brain metastases [1]. Melanoma is the sixth most common malignancy diagnosed in the USA in 2015 [2]. The incidence of brain metastases in patients with metastatic melanoma can be as high as 66–75% in autopsy series [3]. SRS has been shown to have high rates of local control for melanoma brain metastases, while local control rates for whole-brain radiation therapy (WBRT) alone are poor relative to SRS [4,5]. Patients with melanoma comprise a small but consistent proportion in multiple randomized trials assessing the efficacy of the combination of SRS and WBRT [6–8]. The median survival for patients with brain metastases from melanoma ranges from 3.4 to 13.2 months depending on performance status and the number of brain metastases, highlighting the profound impact brain metastases have on prognosis [9].
Immune checkpoint inhibitors represent a paradigm shift in the treatment of metastatic melanoma. Ipilimumab, a monoclonal antibody that blocks CTLA-4, enhances T-cell activation and proliferation [10]. It was the first systemic therapy to demonstrate an overall survival (OS) benefit in the treatment of metastatic melanoma [11]. A Phase II study demonstrated modest activity of ipilimumab alone in the treatment of brain metastases, particularly those who were neurologically asymptomatic at diagnosis [12]. The interaction of immune modulators and radiation therapy are virtually unknown, particularly with high-dose-per-fraction radiation therapy.
Ipilimumab was first used as standard of care at our institution in 2011. We have an active radiosurgery program with a large institutional experience utilizing SRS for the management of melanoma brain metastases [13]. Therefore, we conducted a retrospective analysis of melanoma patients treated with radiosurgery and ipilimumab to determine the impact on patient toxicity and outcomes.
Methods
We queried our institutional retrospectively compiled database (including demographic-, pathologic- and treatment-related data) of consecutive melanoma patients treated with ipilimumab. Patients were included if they received at least one SRS course for melanoma brain metastases. Patients were excluded if they did not receive ipilimumab or if data regarding radiosurgery administration or follow-up were not available.
All patients received systemic therapy and radiosurgery per best clinical practice. Ipilimumab was administered at a dose of 3 mg/kg every 3 weeks for a total of four doses. Radiosurgery was delivered either as initial management of a limited number of brain metastases, in conjunction with WBRT as a boost, or as adjuvant treatment to a resection cavity following surgical management of brain metastases.
Our radiosurgery technique has been previously described in detail [13]. In general, patients underwent computed tomographic (CT) simulation for Radiation therapy planning in a thermoplastic facemask (BrainLAB, Munich, Germany). Fine-cut contrast-enhanced T1-weighted MRI, typically a 3D spoiled gradient series with 1 mm slice thickness, was obtained at the time of CT simulation or a diagnostic MRI of equal quality was fused if it was performed <2 weeks prior to CT simulation. Target definition was conducted in the iPlan treatment planning software (BrainLAB) environment. The gross tumor volume was defined as the contrast-enhancing lesion on the fine-cut T1-weighted MRI. Individual metastases were contoured and expanded 1 mm for setup and imaging uncertainty. In general, planning target volumes (PTVs) <2, 2–2.9 or 3–3.9 cm in greatest dimension received 20, 18 or 15 Gy in a single fraction, respectively [14,15]. These doses were marginally reduced for PTVs in close proximity to eloquent structures (e.g., a metastasis in the motor strip). PTVs >4 cm in greatest dimension received a total of 25 Gy in 5 Gy fractions. SRS treatment plans consisted of three to five dynamic conformal arcs or volumetric-modulated arc therapy. The plans were selected in such a manner that the minimum PTV coverage was at least 99.5% and the conformality index was minimized (ideally <2.0). A linear accelerator-based radiosurgery system equipped with a micromultileaf collimator (Novalis Tx, Varian Medical Systems, CA, USA and BrainLAB) was used for all treatments [16]. Immediately before treatment, all patients underwent kV onboard imaging and cone-beam CT with appropriate adjustment of the isocenter and reimaging to ensure a translational position deviation <1 mm in any direction and minimal rotational deviation.
All patients were followed in a multidisciplinary setting in both melanoma medical oncology and radiation oncology clinics. At each visit, all patients underwent complete history and physical examination, and appropriate laboratory analysis with careful and thorough neurologic examination. Patients were imaged every 3 months with a multisequence gadolinium-enhanced brain MRI to assess treated metastasis response as well as for detection of new metastases. For patients in whom an MRI was contraindicated, a contrast-enhanced CT of the brain was obtained instead. Surveillance imaging, most commonly PET-CT, was obtained after four doses and then every 2–3 months after to assess extracranial disease status.
Treated metastasis progression was defined using the same criteria as in EORTC 22952-26001 [7]. Briefly, metastases were considered to have progressed when a treated metastasis demonstrated a >25% increase in cross-sectional diameter following SRS. If a biopsy was obtained and was negative for malignancy, metastases were not considered to have progressed. If post-SRS brain imaging was not available, treated metastases were considered not assessable. Acute toxicities were scored using the CTCAE v4.0. Distant intracranial progression was defined as the appearance of any new brain metastases outside the treatment field after the first SRS course. Treatments for distant intracranial progression were based on the number, location and size of brain metastases as well as the extracranial disease burden and performance status of the patient.
Distant intracranial progression-free survival (PFS), extracranial PFS and time to first treated metastasis progression were defined as the time from the start date of the first SRS course to the date of distant intracranial progression, date of extracranial progression and date of first treated metastasis progression, respectively. Time to treated metastasis progression on the lesion level was defined as the time from the start date of the SRS course where the given metastasis was treated to the date of progression of that metastasis. The term ‘treated metastasis control’ specifically refers to the lack of progression of a brain metastasis that received SRS. OS was defined as the time from the start date of the first course of SRS to death. The Kaplan–Meier method was used to estimate PFS and OS. PFS and OS rates were also estimated with 95% CIs. PFS and OS analyses included only patients who had data available for the indicated PFS or OS outcome. The melanoma-specific graded prognostic assessment score was calculated for each patient based on Karnofsky performance status and the number of brain metastases (BMs). This is a validated prognostic score of 0–4 (best) that predicts the survival of patients with melanoma BMs [9]. Follow-up for progression ended on 1 September 2014.
A multilevel mixed model was utilized to estimate the association of SRS type (SRS alone or SRS + WBRT), SRS timing in relation to ipilimumab (SRS before/during ipilimumab or SRS after ipilimumab) and metastasis number on the probability of achieving treated metastasis control at the lesion level. This model was used because it allows for the inclusion of random effects that account for the fact that metastases are nested within an SRS course and that SRS courses are nested within a patient, and therefore have correlated outcomes. Point estimates are reported with 95% CIs where appropriate. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, NC, USA). This study was institutional review board (IRB) approved with a waiver of informed consent.
Results
• Patient & metastasis characteristics
From 2010 to 2014, 27 patients were identified. Patient and tumor-related characteristics are summarized in Table 1. One patient was excluded from the SRS treatment characteristics analysis due to missing metastasis information but was included in the toxicity analyses. The median time from SRS to ipilimumab administration was 6 weeks (range: 1–89 weeks). Seven patients received other systemic therapy prior to ipilimumab administration including five IL-2 courses, two vemurafenib courses and one course each of dacarbazine and temozolomide. For patients receiving ipilimumab before SRS, three underwent SRS during the ipilimumab course. Subsequent systemic therapy courses included one course each of nivolumab, pembroluzimab, IL-2, temozolomide, dacarbazine, dabrafenib/trametinib and vemurafenib. Median follow-up was 7.1 months (interquartile range [IQR]: 3.1–13.1).
Table 1. . Patient and tumor characteristics.
| Characteristic | n (range/%) |
|---|---|
| All patients | 27 (100) |
| Age (years), median (IQR) | 63 (55–68) |
| Gender: – Female – Male |
13 (48) 14 (52) |
| Race: – Hispanic – White/Caucasian |
1 (4) 26 (96) |
| ECOG performance status: – 0 – 1 – 2 |
17 (63) 9 (33) 1 (4) |
| Primary site: – Head/neck – Lower extremity – Trunk – Upper extremity – Unknown |
7 (26) 4 (15) 8 (30) 4 (15) 4 (15) |
|
BRAF: – Mutant – Nonmutant – Not tested – Unknown |
8 (30) 16 (59) 2 (7) 1 (4) |
| Non-ipilimumab systemic therapies prior to SRS (n): – 0 – 1 or more |
18 (67) 9 (33) |
| Largest brain metastasis diameter, median (IQR); mm | 8.1 (5.1–14.0) |
| m-GPA: – 1 – 2 – 3 – 4 |
2 (7) 7 (26) 11 (41) 7 (26) |
ECOG: Eastern Cooperative Oncology Group; IQR: Interquartile range; mGPA: Melanoma-specific graded prognostic assessment; SRS: Stereotactic radiosurgery.
• Radiosurgery treatment
A total of 67 metastases were irradiated, with 58 metastases assessable for response. A median of one (range: 1–2) SRS treatment course was delivered per patient with a median of two (range: 1–3) metastases treated per course. For single-fraction SRS, the median dose per fraction was 20 Gy (IQR: 18–24). One treatment was delivered as a total of 25 Gy in five fractions. In total, 20 received SRS as their initial intracranial treatment modality. WBRT was used in addition to SRS for six patients; five (19%) as a planned combination of WBRT (median dose: 35 Gy) and one (4%) as initial therapy with SRS delivered as a salvage therapy.
• Acute toxicity
Acute toxicity following SRS was generally mild, with three (11%) patients experiencing grade 3 CNS toxicity. No patient experienced grade 4 or higher toxicity. Ten (37%) patients required either a new prescription or an increase in oral dexamethasone within 3 months of initial SRS. Of the patients increasing oral dexamethasone, two (20%) were found to have progressive intracranial disease.
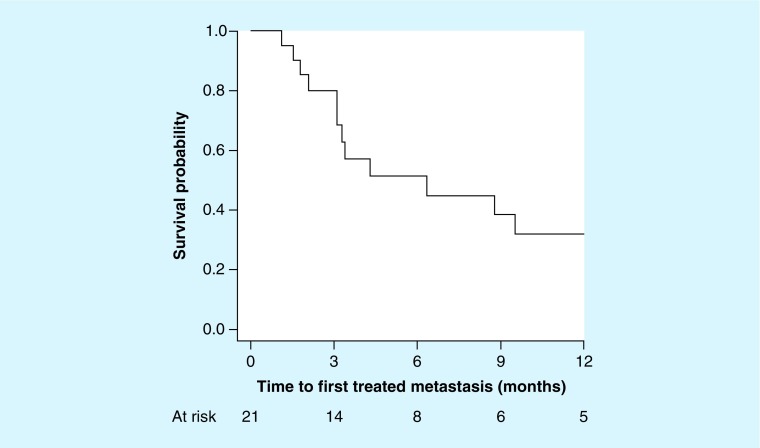
• Intracranial-treated metastasis control
Cumulative-treated metastasis control was 56% with a median time to progression of 8.8 months (3.2, nonestimable) (Figure 1). 6- and 12-month time to treated metastasis progression was 49% (95% CI: 33–62) and 44% (95% CI: 27–59), respectively. 6-month-treated metastasis control for SRS alone and SRS + WBRT were 67 and 43%, respectively. Two of the metastases meeting imaging criteria for progression were biopsied. Both were negative for malignancy. The first was located in the left frontal lobe, measured 22 mm in largest diameter, and received a single fraction of 16 Gy after receiving 35 Gy of WBRT in 2.5 Gy fractions. It was irradiated 2 months after completing four cycles of ipilimumab. The second was located in the left frontal lobe, measured 9 mm in largest diameter, and received a single fraction of 22 Gy. It was irradiated 2 weeks prior to initiating ipilimumab.
Figure 1. . Time to first treated metastasis progression for assessable patients (n = 21).
• Distant intracranial progression after SRS
Distant intracranial progression was common. At 6 months, the distant intracranial PFS was only 44% (95% CI: 22–64), and at 12 months 26% (95% CI: 9–47). Consistent with many previous studies, the addition of WBRT prolonged distant intracranial PFS at median 7.5 months (0.8–18.7 months) compared with SRS alone at 3.3 months (2.8–not estimable). Similarly, WBRT was associated with improved distant intracranial PFS at 6 months (67%; 95% CI: 19–90) compared with SRS alone (34%; 95% CI: 12–59). Treatment for distant intracranial progression included additional eight courses of SRS to nine brain metastases.
• Impact of ipilimumab timing
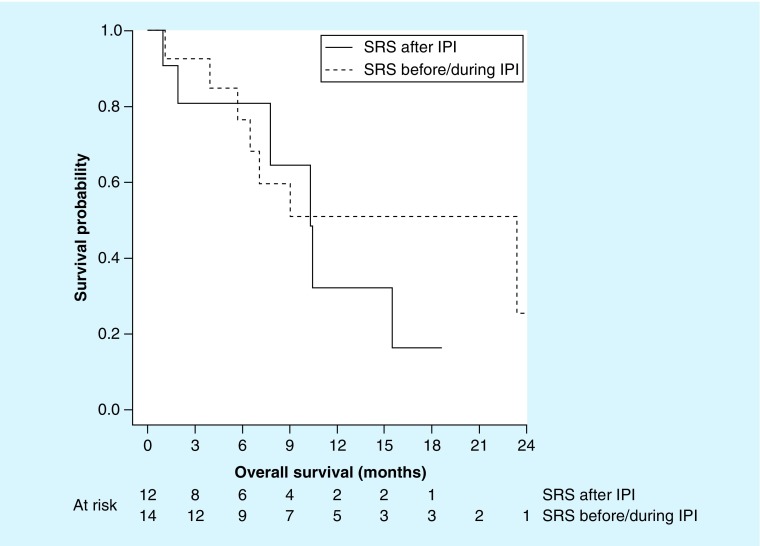
Given the potential for radiotherapy to sensitize tumors for an increased immune response [17] and the ability for ipilimumab to independently control intracranial metastases [12], we investigated the timing of ipilimumab on SRS outcomes. In total, 33 metastases (57%) were irradiated prior to or during ipilimumab and 25 (43%) were irradiated after ipilimumab. Table 2 describes the differences in treated metastasis control, distant intracranial progression, extracranial progression and OS for patients who received SRS prior to and during ipilimumab and those who received SRS after ipilimumab. Interestingly, we found that in patients who received SRS before/during ipilimumab median OS was 23.4 months compared with 10.4 months in those receiving SRS after ipilimumab as shown in Table 2 & Figure 2. Cause of death was generally unavailable in the patient records.
Table 2. . Treatment outcomes for all patients, patients who received radiosurgery prior to or during ipilimumab and patients who received radiosurgery after ipilimumab.
| Outcome | Time to first treated metastasis progression (months); (n = 21) | Distant intracranial progression (months); (n = 21) | Extracranial progression (months); (n = 26) | Overall survival (months); (n = 26) |
|---|---|---|---|---|
| All patients | 6.3 (3.1–12.2) | 4.7 (3.1–10.8) | 3.0 (1.6–4.6) | 10.4 (6.5–23.4) |
| SRS before/during ipilimumab | 3.4 (1.5–NE) | 4.0 (1.8–18.7) | 4.0 (1.5–11.1) | 23.4 (5.7–NE) |
| SRS after ipilimumab | 6.3 (3.1–9.5) | 5.2 (1.9–NE) | 2.0 (0.6–4.4) | 10.4 (1.9–NE) |
Number of patients assessable for each outcome are labeled. All values are reported as median (95% CI).
NE: Not estimable; SRS: Stereotactic radiosurgery.
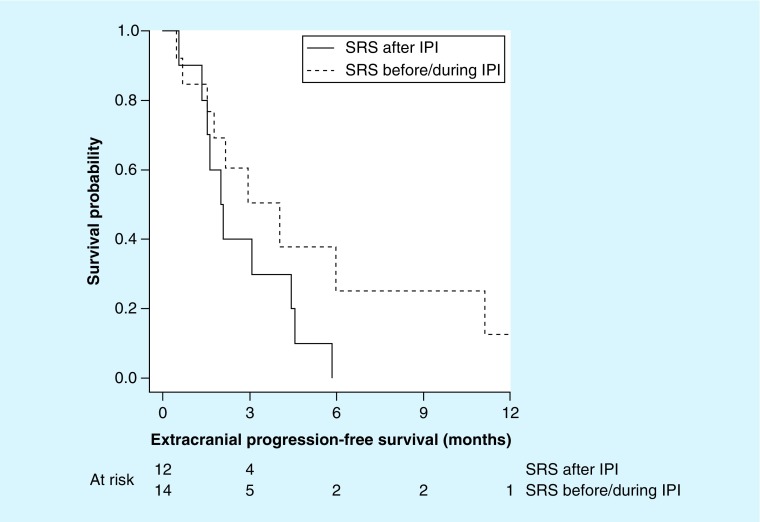
Figure 2. . Extracranial progression-free survival for patients receiving radiosurgery prior to or during ipilimumab (dashed line) or after ipilimumab (solid line) for assessable patients (n = 26).
IPI: Ipilimumab; SRS: Stereotactic radiosurgery.
Given the potential for radiation to cause an enhanced systemic response to immunotherapy [17], we investigated the impact, if any, SRS had on extracranial disease progression. Interestingly, we noted two patients who had an abscopal effect, and we also noted that the median time to extracranial progression was doubled in patients receiving SRS before/during ipilimumab (4 months) compared with those who received SRS after ipilimumab (2 months) as shown in Table 2 & Figure 3.
Figure 3. . Overall survival in assessable patients (n = 26) receiving radiosurgery prior to or during ipilimumab (dashed line) or after ipilimumab (solid line).
Extracranial progression-free survival for patients receiving radiosurgery prior to or during ipilimumab (dashed line) or after ipilimumab (solid line) for assessable patients (n = 26).
IPI: Ipilimumab; SRS: Stereotactic radiosurgery.
Discussion
In this analysis, we found that stereotactic radiosurgery delivered to patients who have received ipilimumab was well-tolerated and efficacious. Our findings are similar to other single-institution series published within the last several years (Table 3). Given the known adverse effect of brain metastases on prognosis, the 23.4-month median OS in our patients treated with SRS prior to or during ipilimumab is particularly striking. This is nearly double the expected median survival of patients with the best combination of factors derived from the diagnosis-specific graded prognostic assessment (Karnofsky performance status, number of brain metastases) and nearly eight-times as long as the median survival in one of the largest analyses of this patient population [9,18].
Table 3. . Summary of retrospective studies of ipilimumab and stereotactic radiosurgery for brain metastases from melanoma.
| Institution | Patients receiving SRS and CTLA-4 inhibition (n) | Median follow-up (months) | Survival | Toxicity | Radionecrosis†, n (%) | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Hofstra | 27 | 12.2 | OS (median): 21.3 months | NR | 3 (11) | No difference in OS if SRS given pre- or post-IPI | [19] |
| New York University | 25 | NR | LC: 65 vs 63%, FFBM: 35 vs 47%, OS: 56 vs 46% IPI + SRS vs SRS alone (NSSD) | Intracranial hemorrhage 7 vs 10% (NSSD) | NR | [20] | |
| Memorial Sloan-Kettering | 46 | 22 | OS (1 year): 56% pre-IPI OS (1 year): 65% during IPI OS (1 year): 40% post-IPI (p = 0.008) |
15% CNS grade 3–4 toxicity | 5 (11) | 50% rate of tumor enlargement >150% for SRS pre- or during IPI vs 13% SRS post-IPI | [21] |
| University of Michigan | 17 | NR | OS (median): 19.9 months | No unexpected toxicities | 0 (0) | [22] | |
| Emory | 20 | 7.3 | OS (1 year): 37.1% | No unexpected toxicities | 6 (30) | [23] | |
| Duke University (present study) | 27 | 7.1 | OS (median): 10.4 months | 11% grade 3 toxicity | 2 (7) | ||
†Biopsy-proven unless noted otherwise.
FFBM: Freedom from new brain metastases; GK: Gamma Knife; IPI: Ipilimumab; LC: Local control; NR: Not reported; NSSD: No statistically significant difference; OS: Overall survival; SRS: Stereotactic radiosurgery.
The generally favorable survival of this group of patients appears difficult to reconcile with the fact that the cumulative incidence of treated metastasis progression was unexpectedly high at 39%. Another recent series reported a 1-year local recurrence rate of 0, 13 and 11% for SRS delivered during ipilimumab, before and after ipilimumab, respectively [21]. There are key methodological differences in our series that may explain the apparent lower treated metastasis control rate in this study. First, we reported cumulative incidence of treated metastasis progression rather than censor our findings at the first evidence of progression, similar to EORTC 22952-26001. Second, we defined progression following SRS using an objective imaging-based definition of a 25% increase in diameter, which may be overly strict in this patient population. This is the same definition used in a contemporary Phase III randomized trial that enrolled patients with melanoma brain metastases who were treated with SRS alone [7]. There was a high rate of tumor enlargement seen in the aforementioned series, particularly for patients who received SRS during ipilimumab [21]. This phenomenon would result in an apparent local control rate much lower than the ‘true’ lack of disease progression [13].
There is no universally accepted definition of treated metastasis progression following radiosurgery and it is even more difficult to determine if a treated metastasis has progressed in patients who have also received immunotherapy. Indeed, a recent publication from the RANO-BM working group highlighted the difficulty in assessing targeted metastases in patients who have received radiosurgery and immunotherapy [24]. In our series, two patients who met the definition of radiographic progression had biopsies that were negative for malignancy, highlighting the phenomenon of ‘pseudoprogression’ that renders surveillance of these patients in a challenging clinical dilemma. Case series have reported symptomatic radionecrosis in a total of seven patients who received both SRS and ipilimumab [25,26]. For SRS, the most important predictive variables for radionecrosis include dose, irradiated volume and location of the targeted lesion. The rate of brain necrosis in our series is similar to other series, with the appropriate caveats noted for cross-trial comparisons. The RANO-BM group notes that standard MRI alone is insufficient to detect true progression versus treatment effect. A combination of more sensitive imaging techniques and greater clinical experience could yield a set of immune-related SRS response criteria to more confidently surveil these patients. Generating such a set of criteria is beyond the scope of this manuscript.
Similar to other series, we noted a possible association with improved outcomes for SRS delivered prior to or during the administration of ipilimumab compared with SRS after ipilimumab, although our series was too small to calculate inferential statistics. The hypothesis that high-dose-per-fraction radiation therapy can synergize with CTLA-4 blockade to achieve tumor rejection is intriguing and forms the basis of many ongoing clinical and translational studies. The impact of radiation on the tumor microenvironment is complex, but a recent article by Victor et al. provides some evidence for the immunologic interaction of high-dose-per-fraction radiation therapy with CTLA-4 blockade [27]. This was a clinical trial of 22 patients with metastatic melanoma who received hypofractionated radiation therapy to a single index metastasis, either two or three fractions of 8 Gy to lung or bone metastases or 6 Gy to liver or subcutaneous metastases, followed by four cycles of CTLA-4 blockade systemic therapy. Treatment was well-tolerated overall and, using response evaluation criteria in solid tumors (RECIST) criteria, evaluation of the unirradiated metastases showed that 36% of patients had either a partial response or stable disease. Radiation therapy enhanced the diversity of T-cell receptor repertoire of intratumoral T cells and was needed to achieve high complete response rates. Other studies have noted the potential importance of both dose-per-fraction and the interval between fractions as important modifiers of radiation’s effect on priming antitumor T cells, with higher dose per fraction (15 Gy in one study, 20 Gy in another) demonstrating improved priming of the antitumor response compared with radiation schedules with lower doses per fraction [28,29]. These data may help explain our findings of a possible association of increased survival for patients receiving SRS before or during ipilimumab compared with SRS after ipilimumab (23.4 vs 10.4 months).
Our results compare favorably the studies of SRS for melanoma brain metastases in the preimmunotherapy era [4,30]. In the largest of these series, local progression was scored using identical criteria as our study [30]. Of the 61 patients treated with SRS alone, the crude treated metastasis progression rate at the treated metastasis level was 34% and 1-year treated metastasis progression rates for SRS alone were 60.4%. Distant intracranial metastasis rate was 18% and the 1-year OS rate was 25%. The superior OS in our analysis likely results from the availability and use of more active systemic therapies. Other possible factors include routine use of PET-CT for staging of extracranial disease.
Our study has several limitations, chiefly its retrospective nature and the inherent weaknesses of such a study design. One other consideration would be the potential for lead time bias in selecting the date of SRS as the start date for progression and survival, though this methodology is similar to other series. Our study is relatively small, but comparable to other published series of ipilimumab and SRS. Prospective trials are needed to rigorously analyze the safety and efficacy of radiosurgery and ipilimumab, with several underway (NCT01950195, NCT02107755, NCT01703507).
Conclusion
This study demonstrates that SRS for brain metastases from melanoma for patients who have received ipilimumab is efficacious and well-tolerated. There may be an association of improved survival in patients who receive SRS prior to or during ipilimumab. These findings, coupled with results of other similar published series, warrant prospective evaluation of the optimal sequence and timing of SRS and ipilimumab.
Footnotes
Financial & competing interests disclosure
AKS Salama receives research funding and is a consultant for Bristol-Myers Squibb. She also receives research funding from Genentech, Merck, Reata and Celldex. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract. Radiat. Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat. Rev. 2004;30(6):515–520. doi: 10.1016/j.ctrv.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Liew DN, Kano H, Kondziolka D, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases. Clinical article. J. Neurosurg. 2011;114(3):769–779. doi: 10.3171/2010.5.JNS1014. [DOI] [PubMed] [Google Scholar]
- 5.Rades D, Heisterkamp C, Huttenlocher S, et al. Dose escalation of whole-brain radiotherapy for brain metastases from melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2010;77(2):537–541. doi: 10.1016/j.ijrobp.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Sahgal A, Ma L, Chang E, et al. Advances in technology for intracranial stereotactic radiosurgery. Technol. Cancer Res. Treat. 2009;8(4):271–280. doi: 10.1177/153303460900800404. [DOI] [PubMed] [Google Scholar]
- 7.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J. Clin. Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The Phase III randomized controlled trial comparing surgery or radiosurgery with or without adjuvant whole-brain radiotherapy. It demonstrated equivalent functional dependence and overall survival between the two arms, although intracranial relapse and neurologic deaths were reduced with the addition of whole-brain radiotherapy.
- 8.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 9.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin. Cancer Res. 2011;17(14):4622–4628. doi: 10.1158/1078-0432.CCR-10-2232. [DOI] [PubMed] [Google Scholar]
- 11.Hodi FS, O’Day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this Phase III randomized controlled trial, patients who received ipilimumab with or without a gp100 vaccine were compared with gp100 alone in metastatic melanoma. This was the first trial to demonstrate an overall survival benefit for ipilimumab in this patient population.
- 12.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, Phase 2 trial. Lancet Oncol. 2012;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]; •• The Phase II trial enrolled patients with melanoma brain metastases to receive ipilimumab alone. Ipilimumab demonstrated modest activity with no unexpected toxic effects.
- 13.Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2015;91(1):100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int. J. Radiat. Oncol. Biol. Phys. 2000;47(2):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 15.Shehata MK, Young B, Reid B, et al. Stereotatic radiosurgery of 468 brain metastases ≤2 cm: implications for SRS dose and whole brain radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(1):87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Wu QJ, Wang Z, Kirkpatrick JP, et al. Impact of collimator leaf width and treatment technique on stereotactic radiosurgery and radiotherapy plans for intra- and extracranial lesions. Radiat. Oncol. 2009 doi: 10.1186/1748-717X-4-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: from concept to the Clinic. Cancer. 2016 doi: 10.1002/cncr.29889. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 19.Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012;117(2):227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23(3):191–195. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 21.Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015;92(2):368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This single institution retrospective series compared outcomes for radiosurgery and ipilimumab based on timing of radiosurgery (before, during or after ipilimumab). It showed an association with favorable locoregional control and survival with concurrent ipilimumab and radiosurgery.
- 22.Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am. J. Clin. Oncol. 2015 doi: 10.1097/COC.0000000000000199. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]; • The RANO-BM working group summarizes the current controversies surrounding response assessment following radiotherapy. They emphasize the difficulty in judging response of brain metastases to radiosurgery in patients who have also received immunotherapy.
- 25.Du Four S, Hong A, Chan M, et al. Symptomatic histologically proven necrosis of brain following stereotactic radiation and ipilimumab in six lesions in four melanoma patients. Case Rep. Oncol. Med. 2014 doi: 10.1155/2014/417913. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Four S, Wilgenhof S, Duerinck J, et al. Radiation necrosis of the brain in melanoma patients successfully treated with ipilimumab, three case studies. Eur. J. Cancer. 2012;48(16):3045–3051. doi: 10.1016/j.ejca.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Using a variety of in vivo and in vitro experiments, the complex interplay between radiotherapy, CTLA-4 inhibition and PD-1 blockade is further characterized.
- 28.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local Radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selek U, Chang EL, Hassenbusch SJ, 3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(4):1097–1106. doi: 10.1016/j.ijrobp.2003.12.037. [DOI] [PubMed] [Google Scholar]