Abstract
Approximately 50% of melanomas have mutations in the gene encoding BRAF. In recent years, new targeted therapies have transformed the landscape of metastatic melanoma treatment. Dabrafenib, a potent kinase inhibitor of mutated BRAF, has been showed to have high response rates with a rapid onset of response, as well as improved overall and progression-free survival when compared with chemotherapy. Dabrafenib in combination with trametinib, a MEK inhibitor, has demonstrated higher responses and improved clinical efficacy compared with monotherapy. Toxicity is distinct compared with chemotherapy but manageable. This article summarizes the pharmacology, key clinical trial data as well as practical experience with dabrafenib in clinical practice, and future directions.
KEYWORDS : BRAF, dabrafenib, metastatic melanoma, V600
Practice points.
Dabrafenib is a potent inhibitor of mutated BRAF and is suitable for use in the first or subsequent line treatment of patients with unresectable or metastatic melanoma harboring a BRAF V600 mutation.
Half of patients experience an objective response to therapy, with the median time to response around 6 weeks, which can lead to rapid symptomatic benefit for patients.
Dabrafenib also has activity in patients with brain metastases even after failure of local therapy.
In combination with trametinib, an MEK inhibitor, superior efficacy is seen with objective responses in 70% of patients but increased toxicity, namely fever.
The most common toxicities of monotherapy are cutaneous adverse events, pyrexia, fatigue, headache, hypertension and arthralgia.
Toxicity, although common is mostly low grade and manageable for both single agent and combination therapy.
Unfortunately, for the majority resistance is inevitable with a median progression-free survival with monotherapy of approximately 6–8 months and combination therapy of 9–11 months.
Future research is directed toward better understanding resistance mechanisms and how this can be overcome as well as the role of BRAF inhibition in combination with immune therapies.
Melanoma occurs due to genetic changes in melanocytes, which result in uncontrolled proliferation and invasion [1,2]. It has been increasing in incidence over the last two decades due to a number of environmental and social factors as well as increased surveillance [2]. In 2014, the incidence of melanoma in the USA was 76,100 [3].
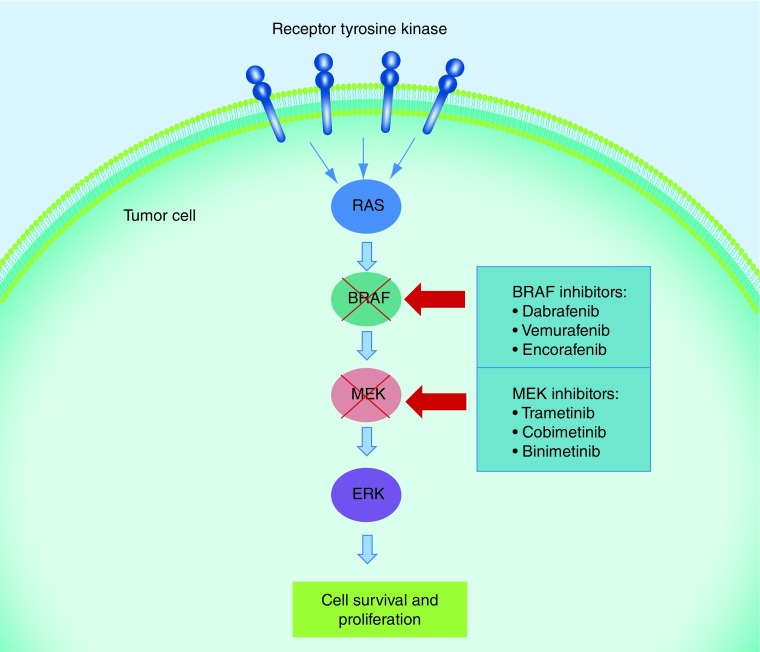
Activation of the MAPK pathway has been shown to be crucial to the progression of melanoma [4]. Approximately 50% of patients diagnosed with melanoma harbor a mutation in the proto-oncogene BRAF, which results in increased kinase activity and phosphorylation of MEK, which phosphorylates ERK [5,6]. ERK substrates include a number of kinases and transcription factors which result in increased cell proliferation and survival (Figure 1) [4]. Dabrafenib is a BRAF inhibitor resulting in disruption of the MAPK pathway and decreased ERK activity [7]. Here we review the mechanism of action of dabrafenib, its clinical efficacy as a single agent and in combination with trametinib, common toxicities and future strategies.
Figure 1. . Drug inhibition of the MAPK pathway.
Activation of the MAPK pathway due to mutations in BRAF leads to increased proliferation and survival of melanoma cells. This growth pathway can be disrupted by targeted agents such as BRAF and MEK inhibitors.
Clinical pharmacology
• Mechanism of action
Dabrafenib is a potent inhibitor of the RAF proteins BRAF and CRAF through ATP competitive binding of the active conformation of BRAF kinase [8,9]. This results in decreased MEK and ERK phosphorylation (see Figure 1), cell cycle arrest at G1 and activation of caspase-3/7 resulting in apoptosis [8]. Vemurafenib, another BRAF inhibitor, also acts by binding to the ATP binding site of mutated BRAF. Studies have shown that dabrafenib is a more selective inhibitor of BRAF with less potency for CRAF than vemurafenib [9]. Preclinical data in xenograft mouse models confirm dabrafenib inhibits the MAPK pathway in BRAF V600 mutated melanoma cell lines leading to decreased proliferation and regression [7].
Depending on the cellular context, ATP-competitive kinase inhibitors can have opposing functions as inhibitors or activators of signaling pathways [10]. Through a similar mechanism to that previously shown with vemurafenib [5], dabrafenib has been shown to cause RAS-dependent paradoxical activation of the MAPK pathway in BRAF wild-type cells [8]. Different mechanisms of paradoxical activation have been demonstrated but this results in increased cell survival and growth when exposed to a BRAF inhibitor. Clinically this is associated with hyperkeratosis, keratoacanthomas and squamous skin cancers in patients treated with BRAF inhibitors [5]. Combining dabrafenib with a MEK inhibitor prevents increased MAPK signaling and reduces these side effects [8].
• Pharmacokinetics
In cell proliferation assays, dabrafenib has been shown to inhibit BRAF V600E mutated cell lines with a drug concentration required to inhibit 50% of BRAF kinase activity (IC50) of 200 nM [8]. It is also able to inhibit cell lines with BRAF V600K, BRAF V600D and BRAF V600R mutations [8,11].
In vivo experiments showed inhibition of tumor growth in BRAF V600E (Colo 205) xenograft mouse models [8]. Immunohistochemical analysis of tumor markers 6 h post-final dose of dabrafenib demonstrated downregulation of ERK phosphorylation and Ki67 by 89 and 28%, respectively, as well as upregulation of the growth inhibition marker p27 by 54% [8].
• Pharmacodynamics & drug interactions
In vivo experiments with xenograft models (Colo 205) demonstrated rapid (within 2 h) inhibition of ERK. In addition, inhibition of ERK was sustained for up to 18 h postdose when the circulating concentration of dabrafenib was lower than that required for inhibition in the in vitro studies [8]. This was thought to be due to active circulating metabolites rather than accumulation of the drug within the tumor [8].
In a Phase I trial with a 3+3 design, maximum tolerated dose was not achieved and doses up to 300 mg twice daily were tolerated [12]. A dose of 150 mg twice daily was chosen for Phase II studies as maximal response in fluorodeoxyglucose – PET studies and tumor markers were seen at this dose and further increments resulted in toxicity [12].
At the 150 mg twice-daily dose, maximum plasma concentration was recorded 2 h after the dose was given and mean terminal half-life was 5.2 h [12]. Median inhibition of phosphorylated ERK was 83.9% compared with baseline in paired tumor biopsies in eight patients treated with 70–200 mg of dabrafenib [12].
Hepatic metabolism and biliary secretion are the main routes of elimination of dabrafenib and its metabolites. Although caution should be used in patients with moderate to severe hepatic impairment, in contrast to vemurafenib, dabrafenib has not been associated with hepatitis [9]. Dabrafenib is metabolized to hydroxyl-dabrafenib by CYP2C8 and CYP3A4 therefore drugs that are strong inhibitors or inducers of these enzymes should be avoided. Examples are provided in Table 1.
Table 1. . Examples of drugs that are strong inhibitors or inducers of CYP2C8 or CYP3A4.
| Class | Drug |
|---|---|
| Antibiotics | Clarithromycin, rifampacin class agents, telithromycin, troleandomycin |
| Anticancer therapies | Abiraterone acetate |
| Anticonvulsants | Phenobarbital, phenytoin, fosphenytoin, carbamazepine, stiripentol, primidone |
| Antidepressants | Nefazodone |
| Antifungals | Itraconazole, ketoconazole, posaconazole, voriconazole |
| Antilipaemic agents | Gemfibrozil |
| Antiplatelets | Clopidogrel |
| Antiretrovirals | Ritonavir |
| Miscillaneous | Amiodarone, bosentan, conivaptan, mibefranil |
Data taken from Lexicomp Online®.
Dabrafenib induces CYP3A4 and can induce other enzymes such as CYP2B6, CYP2C8, CYP2C9 and CYP2C19 therefore increased monitoring of drugs such as warfarin and digoxin should be performed. Dabrafenib was associated with a maximum increase in QTc interval of >60 ms from baseline ECG in 3% of subjects [13]. The concomitant use of drugs at high risk of prolonging QTc should be avoided.
Mild renal dysfunction does not appear to affect clearance of dabrafenib, however the drug has not been studied in patients with more severe impairment; therefore caution should be used.
Population pharmacodynamics analysis did not demonstrate age to have a significant effect on dabrafenib metabolism [13]. However, age greater than 75 years was predictive of 40% higher plasma concentrations of carboxy- and desmethyl-dabrafenib plasma compared with subjects <75 years [13].
Pyrexia was seen in 20% of patients in the Phase I study and was proportional to doses on day 1 but less so after repeat dosing [12]. The mechanism behind pyrexia remains unclear, however, it is thought may be due to the presence of hydroxy-dabrafenib; a metabolite of dabrafenib [14]. In Phase I/II studies, there was a trend toward increased pyrexia with higher circulating hydroxy-dabrafenib [14]. Despite the exact mechanism being unknown, it has been suggested that it may be immune-mediated and possibly related to cytokine release. In addition it has been proposed that trametinib, a MEK inhibitor, influences dabrafenib-driven pyrexia as the pyrexia is significantly more common in patients receiving the combination of these drugs [14].
Clinical efficacy
• Monotherapy
Dabrafenib has been approved by the US FDA and EMA for use in patients with unresectable stage III or stage IV BRAF V600E or BRAF V600K mutated melanoma [15]. The registration Phase III open-label study compared dabrafenib (150 mg twice daily) with dacarbazine (DTIC; 1000 mg/m2 every 3 weeks) as first-line therapy. Objective responses were reported in 50% of patients assigned to dabrafenib versus 6% for those treated with DTIC, with 3% of patients on dabrafenib experiencing a complete response. Median time to response was 6.2 weeks and median duration of response 5.5 months. Median progression-free survival (PFS) was 5.1 months for dabrafenib and 2.7 months for dacarbazine (HR: 0.30; 95% CI: 0.18–0.51; p < 0.0001) [7]. After a median follow-up of 10.2 months, PFS for dabrafenib improved to 6.9 months and was unchanged for dacarbazine (HR from progression or death: 0.37; 95% CI: 0.23–0.57) [16]. The most common adverse events reported with dabrafenib were skin-related effects, fever, fatigue, arthralgia and headache [7]. At median follow-up of 17.2 months, median overall survival (OS) was 20.1 months [4,7–24] in the dabrafenib arm versus 15.6 months [2,9–21] for DTIC. The 3-year survival was 31 and 28% for dabrafenib and DTIC, respectively [17]. This was not statistically significant; however, survival data are confounded by almost 60% of patients treated with DTIC crossing over to dabrafenib [18].
• Combination therapy
The FDA granted accelerated approval for the combination of dabrafenib and trametinib in January 2014 on the basis of results from Phase I/II studies [15,19]. The results of Phase III studies were consistent with the Phase II results and confirmed superior efficacy with combination therapy. 423 patients with previously untreated unresectable stage IIIc or stage IV melanoma were randomly assigned, in a 1:1 ratio, to receive either combination dabrafenib (150 mg twice daily) and trametinib (2 mg once daily) or dabrafenib (150 mg twice daily) and placebo [20]. Patients were stratified according to baseline lactate dehydrogenase and BRAF genotype. The primary end point of median PFS was significantly improved at 9.3 months in the combination group and 8.8 months in the monotherapy group (HR for progression or death: 0.75; 95% CI: 0.57–0.99; p = 0.03) [20]. PFS in the monotherapy group was longer than that seen in previous trials of BRAF inhibitors. This is thought due in part to censoring of more patients in the monotherapy arm who had clinical and not radiological progression, and went on to receive alternative effective systemic therapies. Objective responses were seen in 67% in the dabrafenib-trametinib group and 51% in the dabrafenib-placebo group (p = 0.002) [20]. Median OS was not reached at the time of reporting with an interim OS rate of 93% at 6 months in the combination group and 85% in the monotherapy group (HR for death: 0.63; 95% CI: 0.42–0.94; p = 0.02) [20]. As seen in the Phase II study, the addition of downstream blockade of MAPK signaling by trametinib resulted in a reduction in squamous cell carcinomas, including keratoacanthomas, (2 vs 9%) [20]. Pyrexia was a common feature with the combination (51 vs 28%) and was the commonest reason for dose interruptions or discontinuation of therapy. Hypertension, peripheral edema and diarrhea were also more common in the combination group [20].
Combination dabrafenib and trametinib has also been shown to be superior compared with single agent vemurafenib in an open label Phase III study of 704 patients [21]. Vemurafenib is another potent and specific inhibitor of BRAF, which has been shown to significantly improve response rates, PFS and OS when compared with chemotherapy [22]. Patients were randomized to receive dabrafenib (150 mg twice daily) and trametinib (2 mg daily) or vemurafenib (960 mg twice daily) as first-line therapy. The primary end point of OS on interim analysis at 12 months demonstrated an OS rate of 72% (95% CI: 67–77) in the combination group and 65% (95% CI: 59–70) in the vemurafenib monotherapy group (hazard ratio for death: 0.69; 95% CI: 0.53–0.89; p = 0.005). As a consequence the study was terminated early and patients in the vemurafenib group were allowed to cross over to combination therapy. Median PFS was 11.4 months for combination versus 7.3 months for vemurafenib (HR: 0.56; 95% CI: 0.46–0.69; p < 0.001) with an objective response rate of 64 versus 51% (p < 0.001). Pyrexia of any grade was more common with combination therapy occurring in 53 versus 21% of patients, and cutaneous squamous-cell carcinoma and keratoacanthoma less common (1 vs 18%) [21].
The combination of dabrafenib and trametinib versus placebo is currently being evaluated in a randomized Phase III double blind study in the adjuvant treatment of high risk BRAFV600 mutation positive melanoma after surgical resection (NCT01682083).
The combination of vemurafenib and cobimetinib, another BRAF/MEK combination has also shown superiority to single agent vemurafenib in the metastatic setting [23]. The combination of LGX818 plus MEK162 versus monotherapy BRAF inhibition is still currently under evaluation (NCT01909453) (Figure 1).
• Brain metastases
Brain metastases are common in melanoma and are associated with a poor prognosis due to limited effective therapies. Dabrafenib has been shown to have activity in asymptomatic brain metastases in an open label Phase II study (see Table 2) [24]. Responses were seen in those that had not received prior local therapy (cohort A) and in patients that had progressed following local therapy (cohort B). Response rates were highest in patients with a BRAF V600E mutation (A: 39% and B: 30%) compared with those with a BRAF V600K mutation (A: 6.7% and B: 22.2%) [24].
Table 2. . Results of clinical trials evaluating dabrafenib in metastatic melanoma.
| Outcome | BREAK 3 | BREAK MB | COMBI-D | COMBI-V |
|---|---|---|---|---|
| Objective response rate | 50% | Treatment naive and BRAFV600E 39% | Dabrafenib and BRAFV600E 53% | Vemurafenib and BRAFV600E 52% |
| Treatment naive and BRAFV600K 6% | Dabrafenib and BRAFV600K 40% | Vemurafenib and BRAFV600K 44% | ||
| Prior local therapy and BRAFV600E 31% | Dabrafenib/trametinib and BRAFV600E 68% | Dabrafenib/trametinib and BRAFV600E 64% | ||
| Prior local therapy and BRAFV600K 22% | Dabrafenib/trametinib and BRAFV600K 61% | Dabrafenib/trametinib and BRAFV600K 65% | ||
| Complete response | 3% | Treatment naive and BRAFV600E 3% | Dabrafenib and BRAFV600E 16% | Vemurafenib 8% |
| Treatment naive and BRAFV600K 0% | Dabrafenib and BRAFV600K 2% | Dabrafenib and trametinib 13% | ||
| Prior local therapy and BRAFV600E 0% | Dabrafenib/trametinib and BRAFV600E 19% | |||
| Prior local therapy and BRAFV600K 0% | Dabrafenib/trametinib and BRAFV600K 3% | |||
| Median progression-free survival | 6.9 months | Treatment naive and BRAFV600E 16 weeks | Dabrafenib 8.8 months | Vemurafenib 7.3 months |
| Treatment naive and BRAFV600K 8 weeks | Dabrafenib/trametinib 9.3 months | Dabrafenib/trametinib 11.4 months | ||
| Prior local therapy and BRAFV600E 17 weeks | ||||
| Prior local therapy and BRAFV600K 16 weeks | ||||
| OS | Median OS 20 months | Median OS: | OS rate at 6 months: | OS rate at 12 months: |
| Treatment naive and BRAFV600E 33 weeks | Dabrafenib 85% | Vemurafenib 65% | ||
| Treatment naive and BRAFV600K 16 weeks | Dabrafenib/trametinib 93% | Dabrafenib/trametinib 72% | ||
| Prior local therapy and BRAFV600E 31 weeks | ||||
| Prior local therapy and BRAFV600K 21 weeks | ||||
• Treatment beyond progression
There are concerns regarding the potential for rapid disease progression after the withdrawal of MAPK inhibitors. Data suggest some patients benefit from ongoing BRAF inhibition beyond progression, with prolonged OS even after adjusting for potential confounding prognostic factors at the time of progressive disease. However, currently there are conflicted preclinical data to support this. This approach can be considered in patients who lack alternative effective therapeutic options and continued assessment is imperative to ensure ongoing clinical benefit. Due to the heterogeneous nature of progression patients who progress rapidly though treatment are unlikely to benefit from this approach [25–27].
Safety & tolerability
Side effects are common with dabrafenib although usually manageable and low grade. The most common toxicities are cutaneous adverse events, pyrexia, fatigue, headache, hypertension and arthralgia [7,28]. Less frequent but severe toxicities that are increased when dabrafenib is combined with trametinib include pyrexia associated with hypotension and renal failure, and a decrease in ejection fraction [20]. In comparison to vemurafenib, dabrafenib is associated with decreased cutaneous toxicity (in particular less photosensitivity) and hepatotoxicity. Table 3 compares the toxicity experienced by patients treated with dabrafenib, the combination of dabrafenib and trametinib versus vemurafenib.
Table 3. . Toxicity profiles.
| Adverse event | BREAK 3 % (%G3/4), monotherapy (187 patients) | COMBI-D % (%G3/4), monotherapy (211 patients) | COMBI-D % (%G3/4), combination therapy (209 patients) | COMBI-V % (%G3/4), combination therapy (350 patients) | Vemurafenib % (%G3/4), monotherapy (3222 patients) |
|---|---|---|---|---|---|
| Pyrexia | 33 (5) | 28 (2) | 51 (6) | 53 (4) | 10 (<1) |
| Cutaneous: – Hyperkeratosis – Alopecia – SCC/KA – Phototoxicity – New primary melanomas |
41 29 10 3 1 |
32 26 9 NR 1 |
3 7 2 NR <1 |
4 6 1 4 1 |
19 (<1) 26 (<1) 12 30 (2) 1 |
| Arthralgias | 37 | 27 (0) | 24 (<1) | 24 (1) | 38 (3) |
| Fatigue | 5 | 35 (1) | 35 (2) | 29 | 32 (3) |
| Headache | 3 (1) | 29 (1) | 30 (<1) | 29 | 12 (<1) |
| Hypertension | NR | 14 (5) | 22 (4) | NR | 4 (2) |
| Decreased ejection fraction | NR | 2 (<1) | 4 (<1) | 8 (4) | NR |
| Noncutaneous malignancy | 2 | 3 (1) | 2 (<1) | 1 | 0.3 |
| Abnormal LFTs | NR | 3 (<1) | 11 (3) | NR | 11 (5) |
| Diarrhea | NR | 14 (1) | 24 (1) | 32 (1) | 15 (<1) |
| Nausea | 1 | 26 (1) | 30 (0) | 35 (<1) | 19 (1) |
• Cutaneous toxicity
Typical cutaneous adverse events due to paradoxical activation of the MAPK pathway include hyperkeratosis, papillomas, palmar-plantar erythrodysesthesia and less commonly cutaneous squamous cell carcinomas (cuSCC) and keratoacanthomas (KA) [28]. With single agent therapy, proliferative epidermal skin lesions are seen in less than 10% of patients, with cuSCC/KA observed in 6% of patients [7]. Typically cuSCCs appear between 6 and 24 weeks from commencement of therapy. Most are well differentiated and can occur on either sun-damaged or undamaged skin [30]. Combining dabrafenib with a MEK inhibitor leads to a reduction in squamoproliferative events with only 1–2% developing cuSCC or KA [20,21]. Treatment is with excision – no dose interruption or reduction is required [31]. Photo toxicity, which is common with vemurafenib, is rarely seen with dabrafenib. New primary melanomas are reported in approximately 1% of patient on monotherapy or combination therapy, available genotyping data demonstrate these lesions to have BRAF wildtype genotype [32].
• Pyrexia
Pyrexia is a frequent adverse event experienced with dabrafenib and can occur in up to 30% with monotherapy and 50% of patients when used in combination with trametinib [20,21]. The median time to onset of first episode is 4 weeks and the median duration 3 days. There are no known predictive correlates and it does not correspond with clinical outcome. Patients usually respond to an interruption in therapy but may require a dose reduction if symptoms recur. Recurrent pyrexia is common (79%) and low dose steroids have been successfully used as secondary prophylaxis to allow maintenance of dose intensity or dose re-escalation [14].
• Arthralgia
Arthralgia is a common side effect occurring in a third of patients receiving treatment with dabrafenib; however, severe debilitating arthralgia occurs in <1% [7,16]. The main stay of treatment is providing symptomatic relief with anti-inflammatories and analgesics. More severe arthralgia may require a short course of corticosteroids and an interruption in therapy until symptoms improve [33,34].
Monitoring
Patients should be under regular review for assessment of toxicity and response while on therapy. Grade 2 or higher adverse events were seen in greater than 50% of patient in Phase III studies and the incidence was greater than 80% in the brain metastases study [28]. On commencement of therapy patients should be seen regularly with blood tests (full blood count, renal and liver function tests) in the initial weeks to assess for early intolerance and need for dose reduction or interruption. Median time to response is 6 weeks allowing initial response assessment at this time point. Imaging with (18F) fluorodeoxyglucose-PET can be used as a marker of early biologic response to BRAF inhibitors with decreased tumor activity becoming evident before response on conventional cross-sectional imaging [35]. Patients should be informed of the risk of noninfectious pyrexia, particularly if receiving combination therapy. They should be advised to withhold dabrafenib and take a nonsteroidal antipyretic as well as seek consultation if their temperature is >38.5°C [31].
Patients should undergo regular skin evaluations and assessment for cuSCC. Consideration should also be given to the potential for other primary melanomas as well as noncutaneous squamous malignancy [31], particularly if patients are on therapy for a considerable length of time.
Dabrafenib should be used with caution in patients who are at risk of developing an arrhythmia and avoided in those with prolonged QTc at baseline [36]. Patients should have electrocardiogram monitoring at baseline, after 1 month on treatment and when dose modifications occur [31].
Uveitis, pancreatitis and renal failure are serious but infrequent toxicities (<1%) that should be monitored for at clinical review [31].
Tackling resistance
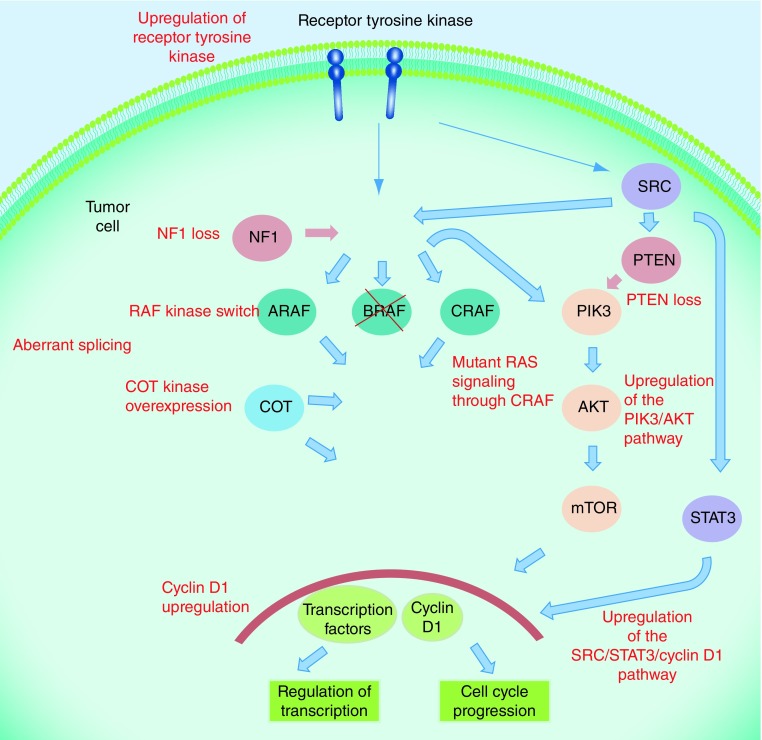
Dabrafenib produces significant clinical benefit in BRAF V600 mutation-positive metastatic melanoma and this is further increased in combination with trametinib. Unfortunately, response for the majority is measured in terms of months as complex drug resistance is acquired, commonly leading to reactivation of the MAPK pathway [37]. Resistance mechanisms to BRAF inhibitors include dimerization of aberrantly spliced BRAF, amplification of mutant BRAF, acquisition of mutations in RAS or MEK, upregulation of MAP3K8/COT, loss of NF1, upregulation of the EGF receptor-SRC family kinase-STAT3 signaling pathway and PI3K–PTEN–AKT pathway upregulating mutations (see Figure 2) [38–42]. The numbers of patients affected by these mechanisms of resistance remain uncertain and there are a large proportion of patients in whom a mechanism for resistance is unknown. Using the combination of dabrafenib and trametinib to block the MAPK pathway at two points has been shown to delay but not prevent resistance [20]. Resistance to combination therapy can also be mediated by development of other activating mutations of NRAS and MEK2 C125S [37].
Figure 2. . Mechanisms of resistance to BRAF inhibitors.
Multiple mechanisms of resistance to BRAF inhibitors exist including upregulation of alternative pathways, loss of tumor suppressors, aberrant splicing and upregulation of a variety of kinases.
Various strategies to delay and overcome resistance are currently being evaluated, including intermittent dosing and addition of novel agents. Mouse models evaluating tumors resistant to vemurafenib have demonstrated that these tumors can become drug dependent for their ongoing proliferation, with cessation of drug leading to tumor regression. In addition, intermittent dosing schedules can forestall the development of lethal drug resistance [26]. There are also reports of patients successfully being rechallenged with BRAF inhibition after a treatment-free interval [43]. Phase II clinical trials are planned to evaluate a variety of discontinuous dosing schedules to sustain the durability of response. A number of novel targets are currently being investigated in order to overcome resistance mechanisms. Preclinical data have shown that Pan-raf inhibitors have activity in BRAF/MEK inhibitor patient-derived xenografts and Phase I trial results are awaited [44]. Inhibition of targets further downstream of RAF and MEK such as ERK or cyclin-dependant kinase inhibitors are in early phase development [45]. Further combination strategies such as combined ERK and PI3K/mTOR inhibition may have more value [46].
Combining targeted therapy with immunotherapy
Immunotherapy is another evolving area that has dramatically improved outcomes in patients with advanced melanoma in recent years. Following progress in this field attention has been drawn to strategies for combining and sequencing targeted and immune therapy approaches. Vemurafenib has been shown to increase expression of antigens known to stimulate T cells and reduce immune inhibitory production of cytokines by tumor cells [47]. Unfortunately a Phase I trial combined vemurafenib and ipilimumab was closed due to grade 3 hepatotoxicity seen in six out of ten patients; however, as dabrafenib is not associated with hepatitis, combinations of dabrafenib, trametinib and ipilimumab are currently being investigated (NCT01767454) [48,49]. Initial results have not shown significant hepatotoxicity and the trial is being expanded [49]. In addition, clinical trials are currently being designed to investigate the optimal sequencing of targeted and immune therapy [50].
Conclusion
Dabrafenib has been shown to be comparable to other BRAF inhibitors in terms of efficacy in metastatic melanoma. It is well tolerated with main adverse events including pyrexia, arthralgia and cutaneous toxicity, all being manageable. In combination with trametinib, further improvements in efficacy have been seen. Future research will determine methods of overcoming resistance and if BRAF inhibition will be synergistic with immunotherapy.
Footnotes
Financial & competing interests disclosure
P Lorigan is a paid consultant to Merck, Bristol-Myers Squibb, Roche, Chugai, GlaxoSmithKline and Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365(9460):687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Marshall C. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell Elsevier. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 5.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siroy AE, Boland GM, Milton DR, et al. Beyond BRAF(V600): clinical mutation panel testing by next-generation sequencing in advanced melanoma. J. Invest. Dermatol. 2015;135(2):508–515. doi: 10.1038/jid.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, Phase III randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]; •• Pivotal first-line study of dabrafenib in patients with BRAF-mutated melanoma compared with the standard of care of the time, dacarbazine.
- 8.King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS ONE. 2013;8(7):e67583. doi: 10.1371/journal.pone.0067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzies AM, Long GV, Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des. Devel. Ther. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 11.Gentilcore G, Madonna G, Mozzillo N, et al. Effect of dabrafenib on melanoma cell lines harbouring the BRAF(V600D/R) mutations. BMC Cancer. 2013;13(1):17. doi: 10.1186/1471-2407-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a Phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Summary of product characteristics: Tafinlar. pp. 1–64.www.ema.europa.eu
- 14.Lee CI, Menzies AM, Haydu LE, et al. Features and management of pyrexia with combined dabrafenib and trametinib in metastatic melanoma. Melanoma Res. 2014;24(5):468–474. doi: 10.1097/CMR.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 15.Center for Drug Evaluation and Research. Approved Drugs – trametinib and dabrafenib. Center for Drug Evaluation and Research; 2014.
- 16.Hauschild A, Grob JJ, Demidov LV, et al. ASCO Annual Meeting 2013. Chicago, IL, USA: 2013. An update on BREAK3, a Phase III, randomized trial: dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E positive mutation metastatic melanoma (MM) Presented at. 31 May–4 June. [Google Scholar]
- 17.Grob JJ, Demidov L, Jouary T, Gutzmer R, Millward M. European Society for Medical Oncology (ESMO) 2014 Congress. Madrid, Spain: 2014. An update on overall survival (OS) and follow-on therapies in BREAK-3, a Phase III, randomized trial: dabrafenib (D) vs dacarbazine (DTIC) in patients (pts) with BRAF V600E mutation-positive metastatic melanoma. Society for Melanoma Research. Presented at. 26–30 September. [Google Scholar]
- 18.Hauschild A, Grob J-J, Demidov LV, et al. European Society for Medical Oncology (ESMO) 2014 Congress. Madrid, Spain: 2014. An update on overall survival (OS) and follow-on therapies in BREAK-3, a Phase III, randomized trial: dabrafenib (D) vs. dacarbazine (DTIC) in patient (pts) with BRAF V600E mutation-positive metastatic melanoma (MM) Presented at. 26–30 September. [Google Scholar]
- 19.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First-line study demonstrating superior progression-free survival of dabrafenib in combination with trametinib versus monotherapy in previously untreated patients harboring a BRAF mutation.
- 20.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014;29(371):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]; •• Combination therapy with dabrafenib and trametinib was demonstrated to have significantly improved survival compared with single agent vemurafenib with manageable toxicity in patients harboring a BRAF mutation.
- 21.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 22.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Open-label study demonstrating efficacy of dabrafenib in patients with brain metastases who were treatment naive and also those who have failed local therapy.
- 23.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 24.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, Phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]; • A prospective review of cutaneous manifestations identified in patients treated with dabrafenib.
- 25.Carlino MS, Gowrishankar K, Saunders CA, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol. Cancer Ther. 2013;12(7):1332–1342. doi: 10.1158/1535-7163.MCT-13-0011. [DOI] [PubMed] [Google Scholar]
- 26.Das Thakur M, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494(7436):251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120(20):3142–3153. doi: 10.1002/cncr.28851. [DOI] [PubMed] [Google Scholar]
- 28.Rutkowski P, Blank C. Dabrafenib for the treatment of BRAF V600-positive melanoma: a safety evaluation. Expert Opin. Drug Saf. 2014;13(9):1249–1258. doi: 10.1517/14740338.2014.939954. [DOI] [PubMed] [Google Scholar]
- 29.Larkin J, Del Vecchio M, Ascierto P, et al. Vemurafenib in patients with BRAFV600 mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15:436–444. doi: 10.1016/S1470-2045(14)70051-8. [DOI] [PubMed] [Google Scholar]
- 30.Anforth RM, Blumetti TCMP, Kefford RF, Sharma R, Scolyer RA, Kossard S. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br. J. Dermatol. 2012;167(5):1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 31.GlaskoSmithKline. Tafinlar: adverse reaction managment. https://hcp.gsk.co.uk
- 32.Gibney GT, Messina JL, Fedorenko IV, Sondak VK, Smalley KSM. Paradoxical oncogenesis and the long term consequences of BRAF inhibition in melanoma. Nat. Rev. Clin. Oncol. 2013;10(7):390–399. doi: 10.1038/nrclinonc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babacan T, Urkbeyler IHT, Balakan O, Pehlivan Y, Suner A, Kisacik B. A case of vemurafenib-induced polyarhritis in a patient with melanoma: how to manage it? Int. J. Rheum. Dis. 2014 doi: 10.1111/1756-185X.12396. doi:10.1111/1756-185X.12396. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Trinh VA, Davis JE, Anderson JE, Kim KB. Dabrafenib therapy for advanced melanoma. Ann. Pharmacother. 2014;48(4):519–529. doi: 10.1177/1060028013513009. [DOI] [PubMed] [Google Scholar]
- 35.McArthur GA, Puzanov I, Amaravadi R, et al. Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J. Clin. Oncol. 2012;30(14):1628–1634. doi: 10.1200/JCO.2011.39.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lexicomp Online®. Lexi-Interact™ Dabrafenib ; • Response of BRAF-resistant cell lines to intermittent dosing of vemurafenib as a method to delay development of resistance.
- 37.Long GV, Fung C, Menzies AM, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat. Commun. 2014;5:5694. doi: 10.1038/ncomms6694. [DOI] [PubMed] [Google Scholar]
- 38.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF V600E . Nature. 2011;480(7377):387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girotti MR, Marais R. Déjà vu: EGF receptors drive resistance to BRAF inhibitors. Cancer Discov. 2013;3(5):487–490. doi: 10.1158/2159-8290.CD-13-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Case series describing liver toxicity in a Phase I study combining BRAF inhibition with immunotherapy.
- 40.Girotti MR, Pedersen M, Sanchez-Laorden B, et al. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov. 2013;3(2):158–167. doi: 10.1158/2159-8290.CD-12-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi H, Hong A, Kong X, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov. 2014;4(1):69–79. doi: 10.1158/2159-8290.CD-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seghers AC, Wilgenhof S, Lebbé C, Neyns B. Successful rechallenge in two patients with BRAF-V600-mutant melanoma who experienced previous progression during treatment with a selective BRAF inhibitor. Melanoma Res. 2012;22:466–472. doi: 10.1097/CMR.0b013e3283541541. [DOI] [PubMed] [Google Scholar]
- 44.Girotti MR, Lopes F, Preece N, et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell. 2014;27(1):85–96. doi: 10.1016/j.ccell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samatar AA, Poulikakos PI. Targeting RAS–ERK signalling in cancer: promises and challenges. Nat. Rev. Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 46.Carlino MS, Todd JR, Gowrishankar K, et al. Differential activity of MEK and ERK inhibitors in BRAF inhibitor resistant melanoma. Mol. Oncol. 2014;8(3):544–554. doi: 10.1016/j.molonc.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 2013;368(14):1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 49.Puzanov I, Callahan MK, Linette GP, Patel SP. Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation-positive unresectable or metastatic melanoma (MM) J. Clin. Oncol. 2014;32(5s Suppl.) 2014 ASCO Annual Meeting Abstracts. Abstract 2511. [Google Scholar]
- 50.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J. Transl. Med. 2012;10:107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]