SUMMARY
Follow-up examinations in melanoma aim to detect recurrences or secondary melanomas in an early phase of development. Follow-up guidelines that have been developed in many European countries, the USA and Australia show varying recommendations and are controversial, especially in patients with melanomas of 1.0 mm tumor thickness or less. This group contains 50–70% of all melanoma patients and the majority is unlikely to develop recurrences. On the other hand, within this entity, subgroups at higher risk for recurrences can be defined who require a more intense follow-up. This article discusses recommendations for the frequency, duration and costs of follow-up in low-risk melanoma patients. Patient preferences are addressed and a risk-adapted follow-up scheme is proposed.
KEYWORDS : costs, follow-up, low risk, melanoma, risk groups
Practice points.
The worldwide increasing incidence of melanoma challenges health systems in terms of primary therapy, follow-up and the treatment of recurrences.
For follow-up, a minimum of two visits per year is recommended for the detection of recurrences, as longer periods may result in the development of already-advanced growth of recurrences. The probability for recurrences is highest in the first 3 years after the primary diagnosis and declines rapidly thereafter. Therefore, an intense follow-up schedule up to four-times yearly is recommended in the first 3 years in stage IB patients and can be reduced to twice yearly afterwards.
In stage IA patients, follow-up examinations twice yearly can be performed in order to reassure patients and introduce them to self-examinations, and this can be reduced to once yearly examinations after 2 years. The patient's needs should be taken into account.
Surveillance over a total duration of 10 years seems to be appropriate for the detection of recurrences. Depending on the risk for secondary melanomas, lifelong examinations once a year, particularly in patients with dysplastic nevus syndrome, are recommended.
In stage IB patients, physical examination and lymph node sonography seems to be adequate and cost effective. In stage IA patients, only physical examinations are recommended.
The worldwide increasing incidence of cutaneous melanoma (CM) challenges health systems in terms of primary therapy, follow-up and the treatment of recurrences. Follow-up schedules have been proposed in order to achieve two major objectives: first, to detect recurrences early; and second, to detect subsequent primary melanomas. Therefore, guidelines have been developed in many European countries, the USA and Australia with varying recommendations, but they remain controversial, especially in patients with a tumor thickness of ≤1.0 mm. According to the literature, low-risk melanoma is defined as melanoma with a tumor thickness of 1.0 mm or less or stage I melanoma [1,2]. This group covers 50–70% of patients with CM [3], and the majority of these patients is unlikely to develop recurrences. Due to this reason, many guidelines recommend a less frequent and less intensive follow-up scheme in stage I patients, as this could by adequate and cost effective [4]. On the other hand, up to 11% of stage I patients develop recurrences during a 10-year follow-up [5]. Therefore, it is important to identify the subgroup of stage I patients who is at high risk for metastases. Mitotic rate and ulceration have proven to be independently significant prognostic factors in patients with thin melanomas [3,6]. The 10-year survival probability in patients with melanomas ≤1.0 mm (stage IA according to the American Joint Committee on Cancer [AJCC]) without mitotic activity or ulceration is 95%, whereas in melanoma patients with the same tumor thickness with ulceration or a positive mitotic rate (stage IB according to the AJCC), this drops to 88% [3]. In addition, patients, especially those with a tumor thickness of more than 0.75 mm, are considered to pose a higher risk for a positive sentinel node biopsy [7], whereas the impact of mitotic activity is controversial [8]. One further important issue in planning follow-up schedules is the impact on psychosocial aspects and patient needs, which could differ from current recommendations. A Dutch study showed that 80% of patients with a Breslow thickness of ≤1 mm received more frequent follow-up visits than the guideline recommends, and only 5% of the patients wanted to reduce their follow-up frequency [9].
The current article discusses the following questions: how long and how often should melanoma follow-up be performed in low-risk melanoma patients? Which individuals are at a higher risk for recurrence in the group of stage I patients? Which examination techniques seem to be reasonable with respect to their costs? What are patient preferences?
Detection of recurrences
When follow-up examinations aim at the early detection of recurrences, guidelines and publications recommend that the period between two follow-up examinations should not exceed 6 months, as a longer time period does not match with the aim of the early detection of recurrences [10,11]. Early detection of locoregional recurrences was described to be associated with a more favorable survival outcome, as is indicated by the current N-staging system [12,13]. Early detection of distant metastases allows for a greater percentage of surgical treatments with complete removal of all detectable metastases, and this also seems to be associated with a more favorable prognosis [14]. Early detection of recurrences also underlies length time bias, mainly due to tumor biology [15]. However, even if the percentage of recurrence diagnosis in the early phase of metastasis development may be influenced by the length time bias, the classification of the early and advanced phases of metastasis development remains valid for comparisons of the results of different surveillance schedules [15]. There is evidence from a prospective study that early detection of recurrences is the basis of a better melanoma-specific survival prognosis [2]. On the other hand, only one study described a survival benefit in patients with asymptomatic recurrences detected by scheduled follow-up examinations, while others did not [16,17]. Furthermore, a review by Francken and colleagues showed that none of the evaluated studies revealed any benefit in disease-free or overall survival associated with follow-up surveillance, although one study detected a survival benefit of doctor-detected (asymptomatic) recurrences [12]. It is certainyl true that a survival effect has not been convincingly shown. The reason for this is that the appropriate studies comparing follow-up with no follow-up in a randomized setting are not acceptable for our patients. The authors concluded that follow-up surveillance of localized melanoma does not necessarily result in a benefit for patients, as 62% of patients detected their first recurrence themselves [12]. Consequently, only infrequent follow-up visits (once or twice yearly) for patients with primary CM, with a stronger emphasis on patient self-examination, were suggested [18,19]. In addition, a further study of this group concluded that current guidelines on the frequency of follow-up after treatment for localized melanoma probably provide rather small gains (in terms of earlier diagnosis of recurrences or new primaries) at the expense of a large number of additional clinic visits, and so proposed a less frequent monitoring schedule [20].
However, all of these are retrospective studies and, for this reason, have to be interpreted with caution. It remains unclear in all of these studies as to what is the percentage of patients who accurately followed the proposed follow-up schedule. The more rarely the patients are seen in scheduled follow-up examinations, the more likely it becomes that the patient will detect a recurrence at first on their own [4]. In summary, a poor follow-up schedule increases the percentage of patients detecting their recurrences by themselves in an advanced phase. Therefore, patients should be thoroughly taught how to self-examine cutaneous and superficial nodes and what to do in case of doubt of a new lesion or a recurrence.
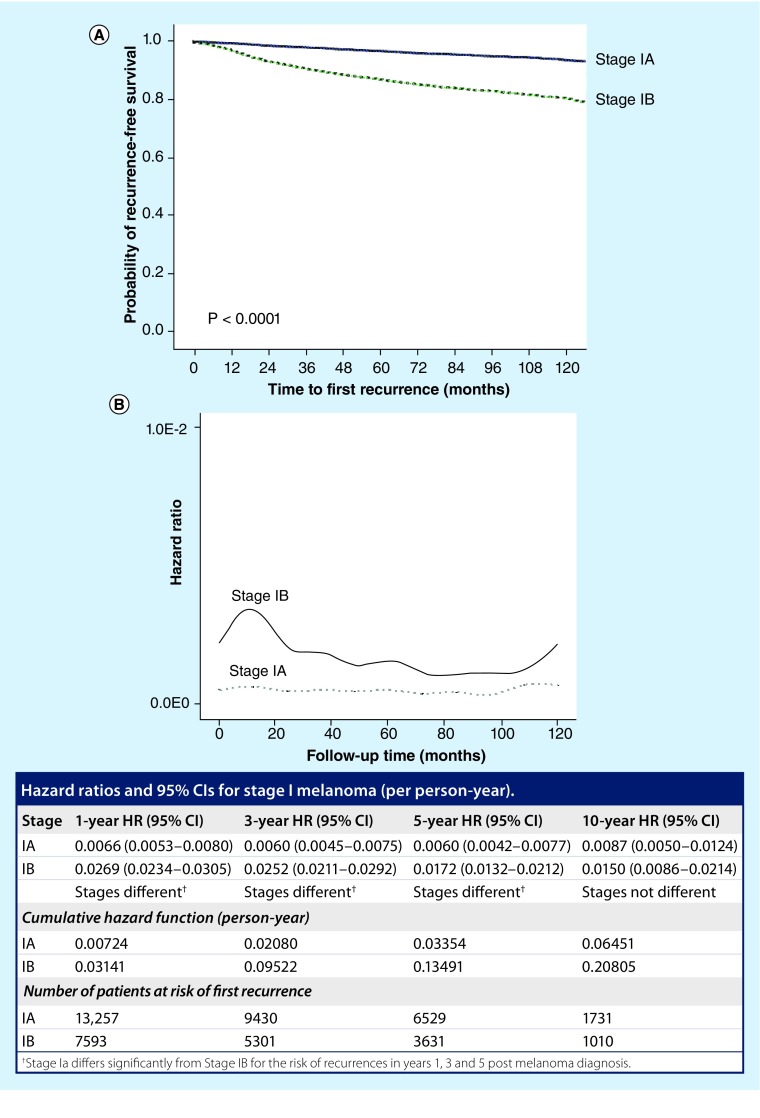
Concerning the intensity of follow-up examinations, an analysis of the specific risk of recurrence calculated by hazard ratios (HRs) in low-risk melanoma showed that HRs remained at a low level during a 10-year follow-up of stage I melanoma (Figure 1) [5]. While the HRs remained stable in stage IA patients (≤1:125; i.e. one case per 125 persons per year) for 10 years, increased HRs were observed in stage IB patients during the first 36 months (one case per 37 persons per year in year 1 to one case per 40 persons per year in year 3), with overlapping 95% CIs after 10 years [5]. Due to these results, an intensified follow-up seems reasonable in the first 3 years postdiagnosis in stage IB patients [5]. For HRs of less than one recurrence per 40 persons per year, less frequent and less intensive surveillance seems appropriate. In stage IA patients with HRs of less than one recurrence per 125 persons per year during the entire follow-up period, the need for surveillance is questionable and should mainly focus on the detection of secondary or multiple primary melanomas. This threshold has been set according to an article that was published in the Journal of the American Academy of Dermatology in 2011 [5].
Figure 1. . Recurrence-free survival and risk of recurrences in patients with melanoma of a tumor thickness of equal or less than 1.0 mm.
(A) Probability of recurrence-free survival after primary cutaneous melanoma diagnosis according to stages IA (bold line) and IB (dotted line). (B) HRs for first recurrences of 23,842 stage I patients recorded by the Central Malignant Melanoma Registry of the German Society of Dermatology between 1976 and 2007. The results are presented as one recurrence per number of person-years.
HR: Hazard ratio.
Reproduced with permission from [5].
Concerning the duration of follow-up schedules, most guidelines recommend a period of 10 years or longer, with some of them recommending life-long follow-up [21–26]. A few guidelines recommend follow-up for only 3–5 years after primary melanoma diagnosis [27], while others recommend a 10-year follow-up only for thick melanomas [28]. A life-long follow-up was recommended by the national conference of the NIH for stage IB melanoma and higher [23]. In Table 1, the follow-up recommendations of current guidelines in the USA, UK, Australia, France and Germany are listed [29–33].
Table 1. . Follow-up recommendations of current guidelines in stage I melanoma.
| Country | Study (year) | Stage | Frequency and duration; further recommendations | Ref. |
|---|---|---|---|---|
| US guidelines of the American Cancer Society | Coit et al. (2013) | Stage IA–IIA | 1–4× yearly (years 1–5), 1× yearly thereafter; no imaging or blood tests | [30] |
| UK guidelines | Marsden et al. (2010) | Stage IA | 2–4× yearly (year 1) then discharged | [31] |
| Stage IB | 4× yearly (years 1–3), 2× yearly (years 4–5); no routine imaging or blood tests | |||
| Australia and New Zealand clinical practice guidelines | Cancer Council Australia, Australian Cancer Network, Ministry of Health NZ (2008) | Stage I | 2× yearly (years 1–5), 1× yearly thereafter | [29] |
| German guidelines | Pflugfelder et al. (2013) | Stage IA | 2× yearly (years 1–3), 1× yearly thereafter; only clinical examinations | [33] |
| Stage IB | 4× yearly (years 1–3), 2× yearly (years 4–5), 1× yearly thereafter; lymph node sonography 2× yearly (years 1–3), then S100b 2× yearly (years 1–3) | |||
| French guidelines | Negrier et al. (2006) | Stage I | 2× yearly (years 1–5) | [32] |
Analyses of stage I–III melanoma showed that 50% of all recurrences arose during the first year postdiagnosis and 80% arose during the first 3 years [4,17,22,34–37]. Looking at melanoma patients in stages I–II, recurrences were found in 8.9–10.1%, and of these, 78% occurred by 18 months postdiagnosis [38]. In melanoma with a tumor thickness of <1.0 mm, some authors found that 10% of the recurrences occurred 10 or more years after diagnosis, although the majority of recurrences appeared in the first 3 years [17,39,40]. Others showed that the risk of recurrences after more than 10 years varied between 1 and 25% [22,41–44]. However, this assessment is biased because of the retrospective nature of these analyses and the frequencies therefore might be overestimated [41–43,45–47].
As melanoma can relapse after a long disease-free interval, a most effective benefit might be gained by educating patients to conduct skin self-examinations, because education can be potentially maintained and reinforced life-long [4,12]. Therefore, patient education and self-examination should be provided in the first years of follow-up.
• Risk groups in thin melanoma patients
Within the group of thin melanomas of ≤1.0 mm tumor thickness, a subset of patients develop recurrent disease and also have a melanoma-related mortality [3,48,49]. Approximately a decade ago, large series of patients with thin (tumor thickness ≤1.0 mm) incident primary invasive CM were analyzed [1,48,50]. In 12,728 patients, a multivariate Cox proportional hazard model found tumor thickness, sex, age, body site and histopathologic subtype to be significant prognostic factors of thin CM. Classification and regression trees analysis identified prognostic subgroups with highest significance in thin CM, and the classification by tumor thickness was improved by the introduction of age and sex. The 10-year survival rates varied between 91.8% for male patients with a tumor thickness of >0.75 mm and 98.1% for female patients with a tumor thickness of ≤0.75 mm [50]. A Surveillance, Epidemiology, and End Results (SEER)-based analysis of 26,291 CM patients (tumor thickness ≤1.0 mm) identified additional criteria to be Clark's level of invasion, tumor cell mitotic rate and sex for explaining survival heterogeneity among patients with thin, nonulcerated lesions. Here, the 10-year survival rates ranged from 89.1 to 99% [48]. A further analysis of 884 patients with thin, invasive melanomas again showed that growth phase, mitotic rate and sex were important prognostic factors for patients with thin melanomas [1].
In the following years, mitotic rate has been added as a predictor of survival in thin melanoma patients in the current staging system of the AJCC [3]. Therefore, it was recommended to upstage these patients to stage IB, which resulted in an intensified primary staging [3]. For patients with a positive mitotic rate and a tumor thickness of <1.0 mm, sentinel lymph node biopsy (SLNB) is recommended, although the utility of this method in thin melanoma is controversial. On the other hand, the rate of nodal metastases in this group is low and the benefit for patients is not established [51]. In a retrospective study of 271 patients with melanomas of ≤1.0 mm undergoing SLNB, 8.1% showed a positive sentinel lymph node (SLN). Of these, 5% were T1a melanomas and had a tumor thickness of >0.75 mm and 13% were T1b melanomas and had a tumor thickness of >0.75 mm. Logistic regression analysis showed that the mitotic rate (≥1 mitosis/mm2) and ulceration were significantly correlated with nodal disease [51]. A further analysis of 189 patients with a tumor thickness of ≤1.0 mm showed that 1.6% had a positive SLN. Of these, 3.2% of patients developed recurrences, associated with a mitotic rate >3 mitoses/mm2. A further 1.6% of the patients died of CM within 5 years, four out of six patients died although they had a negative SLN biopsy result [7].
Other studies in melanomas of ≤1.0 mm revealed that tumor thickness ≥0.75 mm, Clark level ≥IV, ulceration and mitotic index significantly predicted SLN disease in thin melanomas [52]. SLN metastases were found in 10.2% of melanomas of ≥0.75–1.0 mm compared with 2.3% in melanomas of <0.75 mm [52]. By using a 5% metastasis risk threshold, SLNB was recommended for melanomas of ≥0.75 mm, but further study is needed in order to define the indications for SLNB in melanomas of <0.75 mm [52].
According to the recommendation of the AJCC, melanoma with a positive mitotic rate and ulceration should be classified as stage IB melanomas, resulting in an intensified follow-up program and also conducting SLNB. This is particularly applicable to patients with a tumor thickness of ≥0.75 mm or ulceration, which leads to upstaging to stage IB, in whom SLNB should be recommended [3,33].
Detection of subsequent melanomas
If the aim of follow-up examinations consists exclusively or predominantly of the detection of secondary melanomas, screening examinations once a year would probably be sufficient [53–55]. This kind of surveillance, mainly consisting of nevus screening in the follow-up examination using digital dermoscopy, should be preferentially offered to melanoma patients with dysplastic nevus syndrome, and once-yearly examinations would be appropriate for long-term follow-up [54].
Patients with an elevated risk for secondary CMs or with dysplastic nevus syndrome should be recommended for a continuation of annual examinations beyond the 10-year time period, although the majority of secondary melanomas were found in the first 2 years postdiagnosis, and detection rates declined afterwards [5,17,56,57]. Lifelong surveillance is recommended by several authors in order to detect secondary melanomas, as secondary CMs have also been found more than 30 years post-primary diagnosis [56,58,59].
Tests & costs for follow-up in stage I patients
As 50–70% of newly diagnosed CM patients belong to stage I, recommendations for regular follow-up examinations must also bear costs in mind. Due to the low recurrences rates (8–10%) in stage I patients, the costs for the detection of one recurrence in a regular surveillance are much higher compared with stage II–III patients. In stage I patients, physical examination was regarded to be the most effective method in routine follow-up [18,19,60,61]. Costs calculated to detect melanoma recurrences in 1554 stage I patients according to the German reimbursement system (Gebührenordnung für Ärzte 2004) and the US reimbursement system showed that the detection of one recurrence in stage I was €4289/US$4391 for physical examination and €18,035/US$131,423 for lymph node sonography [61]. Imaging techniques and blood tests led to false-positive results in 3.1%, resulting in further imaging and accounted for a further €26,700/US$118,000. Similar results were reported by Basseres et al., who evaluated follow-up examinations of 528 stage I/II patients [18]. A total of 95% of surgically removable metastases were detected by physical examination and thus the clinical examination was deemed to be most the cost effective. Blood tests were described as inefficient methods for detecting subclinical metastatic disease. Physical examinations had the lowest costs associated with the detection of metastasis, whereas the costs were tenfold higher for chest x-ray and 20-fold higher for abdomen sonography. The authors recommended no use of imaging techniques in melanoma patients with a tumor thickness of less than 1.5 mm [18]. The high cost:benefit ratio of physical examinations as compared with technical examinations in stage I–II patients has also been reported in other studies [16,19,62,63]. Brown et al. showed that the routine use of surveillance CXR provided no clinically useful information in the follow-up of patients with melanoma, as it did not detect recurrence at levels that were sufficient to justify its routine use [64]. Hofmann et al. came to a similar conclusion and recommended only physical examinations once to twice yearly in stage I patients, and lymph node sonography once to twice yearly as adequate surveillance methods [19].
To date, there is no clear knowledge of the number of life-years gained by follow-up examinations and the early detection of recurrences in melanoma. Therefore, it seems difficult to calculate cost–effectiveness in relationship to life-years saved by the respective diagnostic or therapeutics strategies. Further technical examination methods have been reported to be cost ineffective and are not included in the follow-up recommendations. A risk-adapted follow-up schedule was proposed based on the premise of not lowering the quality of medical care while still leading to significant savings [2].
Few studies have been published examining the costs associated with a false-positive result or indeterminate findings. High numbers of false-positive results were found in follow-up examinations in stage I–II disease (4.1%), which led to further technical examinations [19]. Lymph node sonography had the highest false-positive rate for all stages, followed by physical examination and abdomen sonography [19]. However, lymph node sonography revealed the highest sensitivity (85.7%) in routine follow-up examinations, followed by physical examination (68.4%) [65]. Thus, many of the true-positive lymph node metastases were detected in an early stage of development [2], allowing for surgical intervention. At present, lymph node sonography is not recommended in routine follow-up schedules in the USA [29,30,66].
Blood tests (lactate dehydrogenase and alkalic phosphatase) revealed a high number of false-positive values (3.7%) and had low sensitivity (11.2%) [65]. A positive correlation between the serum tumor marker S100b and patient survival was shown in a meta-analysis in 2008 [70]; in addition, other studies showed that serum S100b gains prognostic information in multivariate analyses, mainly in advanced-stage melanoma [67]. As S100b proved to be highly sensitive (86–91%) and specific (76–91%) [68–70], some guidelines, as in those from Germany, recommend blood tests with S100b in stage IB melanoma. Table 2 shows possible recommendations for the follow-up of melanoma with a tumor thickness of ≤1.0 mm according to the German S3 guidelines. In other countries, such as Australia, the USA or the UK, blood tests using S100b are not recommended in routine follow-up schedules [29,30,66].
Table 2. . Possible recommendations for the follow-up of melanoma patients with a tumor thickness of ≤1.0 mm (intervals in months).
| Stage | Physical examination(s)/year | Lymph node ultrasound(s)/year | S100b blood test(s)/year | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–3 years | 4/5 years | 6–10 years | 1–3 years | 4/5 years | 6–10 years | 1–3 years | 4/5 years | 6–10 years | |
| IA | 2× | 1× | 1× | – | – | – | – | – | – |
| IB | 4× | 2× | 1–2× | None or up to 2׆ | – | – | None or up to 4× | – | – |
†Only in sentinel lymph node biopsy-staged patients.
A recently published study investigating 515 stage IA–IIC patients who had received preoperative imaging studies (computed tomography [CT] or PET scans, chest x-rays or MRIs) revealed 0% true-positive findings [71]. CT and PET/CT scans were reported to have a very low rate in terms of cross-selectional screening in melanoma, associated with higher costs. Even if patients were selected according to prognostic indicators, such as tumor thickness, ulceration or large tumor burden, the true-positive rate remained below the false-positive one. Therefore, it was recommended that these methods should not be performed in early-stage CM in the staging of node-negative patients. At the 5-year follow-up, CT or PET/CT at 6-month intervals detected surgically treatable regional or distant recurrence in 6.4% of patients with stage I disease; 12-month intervals decreased the rates to 3.0%. The high false-positive rates of CT (20%) and PET/CT (9%) resulted in overall low positive predictive values. However, both CT and PET/CT effectively predicted the absence of disease. Life-expectancy gains were minimal (≤2 months) for all groups [72].
According to recent publications, imaging techniques in primary tumor stages are reported to be dispensable, except for lymph node ultrasound, which represents a sensitive examination method and enables the early detection of locoregional recurrences before they become palpable. In primary melanomas and particularly in those with a tumor thickness of <1.0 mm, a reduction of technical examinations is widely recommended [11,31,32,60,61,66,73]. Compared with physical examination, the rates of detection of regional or distant recurrences by imaging were shown to be exceedingly low in stage I patients, independent of the distinct imaging technique or frequency, and were 2.6–5.2% for regional recurrences and 1.8–3.6% for distant recurrences [72]. Therefore, costs of follow-up examinations can be clearly reduced without an increased risk for patients.
Follow-up
• Patient needs & perspectives
Recent guidelines from various countries recommend follow-up visits twice yearly in patients with stage I melanomas of <1.0 mm tumor thickness, resulting in a reduced burden of follow-up [11,31,32,66]. A reduction of visits may impact other components of care, such as information and the reassurance of patients. Therefore, the impact on psychosocial outcomes should also be evaluated [74]. Particularly in stage I patients, an increased demand for follow-up visits has been shown [75]. In this study, patients with thin melanomas in particular have more intense follow-up periods than those proposed by the current guidelines [75]. Up to three-quarters of patients asked for and received additional examinations, such as blood tests of diagnostic imaging, and experienced an important effect of reassurance [9,74]. Reasons such as the patient's clinical risk profile, level of anxiety, education requirements, possibility of performing self-examinations and management in case of suspicious lesions influenced the individual's follow-up [76]. Follow-up has other important functions in terms of establishing a trusting patient–doctor relationships [74]. A trial in The Netherlands (MELFO) is currently being performed in order to analyze the psychological effects of the reduction of follow-up visits in stage I–III patients on patient wellbeing, quality of life, anxiety and satisfaction with the schedule [77]. The evidence consistently shows that most melanomas are discovered by nonmedical persons [78,79], but nonmedical person detection is not associated with shallower tumors. The reason for this may be that nonmedical persons are unable to identify melanomas at an early phase of growth or atypical tumors without any correlation with the ABCDE rule. Publications suggest that melanoma risk is reduced by skin self-examination combined with physician visits, which seems to be a sensible practice for melanoma prevention and early detection. Therefore, the patient's education and support should be emphasized in follow-up examinations. More data are needed in order to determine the efficacy of this [78–80].
• Follow-up adapted to the risk of recurrence
For stage IA patients, most guidelines recommend follow-up examinations twice to four-times yearly in year 1 up to year 3 or even year 5, which should be performed in order to reassure patients and introduce them to self-examinations [29–33,66]. Only physical examinations are recommended. Patient needs should be taken into account.
For stage IB patients with an increased risk of recurrences (mainly in the first 3 years [5]; Figure 1), some guidelines recommend an intensified follow-up with physical examinations up to four-times yearly (see Table 1 [31,33]) complemented by lymph node sonography or even blood tests. Table 2 shows possible recommendations for the follow-up of melanoma patients with a tumor thickness of ≤1.0 mm.
Conclusion
Whereas there is an internationally accepted consensus based on randomized prospective trials, the recommendations for surveillance strategies in melanoma are still controversial. Follow-up guidelines have been developed in many European countries, the USA and Australia [23,28,29,33,73], with varying recommendations. An international consensus has not yet been reached. So long as surveillance is predominantly aimed at the detection of recurrences, a minimum of two follow-up visits per year is recommended, because longer periods may result in the presentation of already-advanced growth of recurrences when first detected within regular follow-up examinations. In general, the probability for recurrences is highest in the first 3 years after the primary diagnosis, and the probability declines thereafter rapidly. Therefore, in many countries, an intensified follow-up schedule is recommended in the first 3 years, which can be reduced afterwards. Surveillance over a total duration of 10 years seems to be appropriate for the detection of recurrences. Depending on the risk for secondary melanomas, lifelong examinations once a year, particularly in patients with dysplastic nevus syndrome, are recommended. More than 80% of all recurrences are primarily detected during follow-up examinations when a structured, stage-adapted schedule is applied. In the majority of cases, recurrences are detected by patients themselves or physical examinations. Lymph node ultrasound enables the early detection of locoregional recurrences before they become palpable and represents a sensitive examination method. In primary melanomas, particularly those with a tumor thickness of <1 mm, a reduction of technical examinations could be recommended. The costs of follow-up examinations can be clearly reduced without increased risks for the patients.
Moreover, patient needs as the psychosocial aspects of the impact of follow-up on patient well-being play an important role in potential adherence to schedules and so could influence clinician practice. Therefore, psychosocial impacts on patients must be explicitly addressed when planning follow-up schedules.
Future perspective
The worldwide increasing incidence of melanoma will continue over the next 20 years, challenging health systems in terms of primary therapy, follow-up and the treatment of recurrences. Due to increased awareness and screening programs, more melanomas with a lower tumor thickness will be detected. Therefore, there is a need for a risk-adapted follow-up scheme that takes medical care an also costs into account.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gimotty PA, Guerry D, Ming ME, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J. Clin. Oncol. 2004;22(18):3668–3676. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Garbe C, Paul A, Kohler-Spath H, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. J. Clin. Oncol. 2003;21(3):520–529. doi: 10.1200/JCO.2003.01.091. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francken AB, Shaw HM, Accortt NA, Soong SJ, Hoekstra HJ, Thompson JF. Detection of first relapse in cutaneous melanoma patients: implications for the formulation of evidence-based follow-up guidelines. Ann. Surg. Oncol. 2007;14(6):1924–1933. doi: 10.1245/s10434-007-9347-2. [DOI] [PubMed] [Google Scholar]
- 5.Leiter U, Buettner PG, Eigentler TK, et al. Hazard rates for recurrent and secondary cutaneous melanoma: an analysis of 33,384 patients in the German Central Malignant Melanoma Registry. J. Am. Acad. Dermatol. 2012;66(1):37–45. doi: 10.1016/j.jaad.2010.09.772. [DOI] [PubMed] [Google Scholar]; •• Analyzes the rates of recurrences during follow-up in patients with primary melanoma, including those with a tumor thickness of ≤1.0 mm.
- 6.Van Der Esch EP, Cascinelli N, Preda F, Morabito A, Bufalino R. Stage I melanoma of the skin: evaluation of prognosis according to histologic characteristics. Cancer. 1981;48(7):1668–1673. doi: 10.1002/1097-0142(19811001)48:7<1668::aid-cncr2820480732>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, Wayne JD, Damstetter EM, et al. A 10-year, single-institution analysis of clinicpatlogic features and sentinel lymph node biopsy in thin melanomas. J. Am. Acad. Dermatol. 2013;69(5):693–699. doi: 10.1016/j.jaad.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Wong SL, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann. Surg. Oncol. 2006;13(3):302–309. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Holterhues C, van de Poll-Franse LV, de Vries E, Neumann HA, Nijsten TE. Melanoma patients receive more follow-up care than current guideline recommendations: a study of 546 patients from the general Dutch population. J. Eur. Acad. Dermatol. Venereol. 2012;26(11):1389–1395. doi: 10.1111/j.1468-3083.2011.04297.x. [DOI] [PubMed] [Google Scholar]
- 10.Francken AB, Accortt NA, Shaw HM, et al. Follow-up schedules after treatment for malignant melanoma. Br. J. Surg. 2008;95(11):1401–1407. doi: 10.1002/bjs.6347. [DOI] [PubMed] [Google Scholar]
- 11.Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline – update 2012. Eur. J. Cancer. 2012;48(15):2375–2390. doi: 10.1016/j.ejca.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Francken AB, Bastiaannet E, Hoekstra HJ. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol. 2005;6(8):608–621. doi: 10.1016/S1470-2045(05)70283-7. [DOI] [PubMed] [Google Scholar]
- 13.Kleeberg UR. Wishful thinking, unicentric empiricism and the everyday world of the medical melanomologist. Melanoma Res. 1997;7(Suppl. 2):S143–S149. [PubMed] [Google Scholar]
- 14.Essner R. Surgical treatment of malignant melanoma. Surg. Clin. North Am. 2003;83(1):109–156. doi: 10.1016/S0039-6109(02)00205-0. [DOI] [PubMed] [Google Scholar]
- 15.Zelen M. Forward and backward recurrence times and length biased sampling: age specific models. Lifetime Data Anal. 2004;10(4):325–334. doi: 10.1007/s10985-004-4770-1. [DOI] [PubMed] [Google Scholar]
- 16.Mooney MM, Mettlin C, Michalek AM, Petrelli NJ, Kraybill WG. Life-long screening of patients with intermediate-thickness cutaneous melanoma for asymptomatic pulmonary recurrences: a cost–effectiveness analysis. Cancer. 1997;80(6):1052–1064. doi: 10.1002/(sici)1097-0142(19970915)80:6<1052::aid-cncr7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Poo-Hwu WJ, Ariyan S, Lamb L, et al. Follow-up recommendations for patients with American Joint Committee on Cancer stages I–III malignant melanoma. Cancer. 1999;86(11):2252–2258. [PubMed] [Google Scholar]
- 18.Basseres N, Grob JJ, Richard MA, et al. Cost–effectiveness of surveillance of stage I melanoma. A retrospective appraisal based on a 10-year experience in a dermatology department in France. Dermatology. 1995;191(3):199–203. doi: 10.1159/000246546. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow-up in melanoma patients – monocenter evaluation of methods, costs and patient survival. Br. J. Cancer. 2002;87(2):151–157. doi: 10.1038/sj.bjc.6600428. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Analyzes the costs of follow-up examination in stage I–III melanoma and questions technical examinations in stage I patients.
- 20.Turner RM, Bell KJ, Morton RL, et al. Optimizing the frequency of follow-up visits for patients treated for localized primary cutaneous melanoma. J. Clin. Oncol. 2011;29(35):4641–4646. doi: 10.1200/JCO.2010.34.2956. [DOI] [PubMed] [Google Scholar]; •• Performed in order to develop more evidence-based guidelines for the frequency of patient follow-up after the treatment of localized (American Joint Committee on Cancer stage I or II) melanoma. By providing less intensive monitoring, more efficient follow-up strategies were possible. Fewer visits with a more focused approach may address the needs of patients and clinicians for detecting recurrent or new melanomas.
- 21.Baughan CA, Hall VL, Leppard BJ, Perkins PJ. Follow-up in stage I cutaneous malignant melanoma: an audit. Clin. Oncol. (R. Coll. Radiol.) 1993;5(3):174–180. doi: 10.1016/s0936-6555(05)80321-8. [DOI] [PubMed] [Google Scholar]
- 22.Dicker TJ, Kavanagh GM, Herd RM, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Scottish Melanoma Group. Br. J. Dermatol. 1999;140(2):249–254. doi: 10.1046/j.1365-2133.1999.02657.x. [DOI] [PubMed] [Google Scholar]
- 23.Houghton AN, Coit DG, Daud A, et al. Melanoma. J. Natl Compr. Canc. Netw. 2006;4(7):666–684. doi: 10.6004/jnccn.2006.0057. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson H, Nystrom L, Tornberg S, Lenner P. Service screening with mammography of women aged 50–69 years in Sweden: effects on mortality from breast cancer. J. Med. Screen. 2001;8(3):152–160. doi: 10.1136/jms.8.3.152. [DOI] [PubMed] [Google Scholar]
- 25.Moloney DM, Gordon DJ, Briggs JC, Rigby HS. Recurrence of thin melanoma: how effective is follow-up? Br. J. Plast. Surg. 1996;49(6):409–413. doi: 10.1016/s0007-1226(96)90012-0. [DOI] [PubMed] [Google Scholar]
- 26.Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance. Patient or physician based? Ann. Surg. 1995;221(5):566–569. doi: 10.1097/00000658-199505000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts DL, Anstey AV, Barlow RJ, et al. U.K. guidelines for the management of cutaneous melanoma. Br. J. Dermatol. 2002;146(1):7–17. doi: 10.1046/j.1365-2133.2001.04614.x. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health Consensus Development Conference Statement on Diagnosis and Treatment of Early Melanoma, January 27–29, 1992. Am. J. Dermatopathol. 1993;15(1):34–43. doi: 10.1097/00000372-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Council Australia Australian Cancer Network, Ministry of Health NZ. Clinical Practice Guidelines for the management of melanoma in Australia and New Zealand. 2008. pp. 1–217.www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp111.pdf
- 30.Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J. Natl Compr. Canc. Netw. 2013;11(4):395–407. doi: 10.6004/jnccn.2013.0055. [DOI] [PubMed] [Google Scholar]
- 31.Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J. Plast. Reconstr. Aesthet. Surg. 2010;63(9):1401–1419. doi: 10.1016/j.bjps.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Negrier S, Saiag P, Guillot B, et al. Clinical practice guideline: 2005 update of recommendations for the management of patients with cutaneous melanoma without distant metastases (summary report) Bull. Cancer. 2006;93(4):371–384. [PubMed] [Google Scholar]
- 33.Pflugfelder A, Kochs C, Blum A, et al. S3-guideline 'diagnosis, therapy and follow-up of melanoma' – short version. J. Dtsch. Dermatol. Ges. 2013;11(6):563–602. doi: 10.1111/ddg.12044. [DOI] [PubMed] [Google Scholar]
- 34.Fusi S, Ariyan S, Sternlicht A. Data on first recurrence after treatment for malignant melanoma in a large patient population. Plast. Reconstr. Surg. 1993;91(1):94–98. doi: 10.1097/00006534-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Kelly JW, Blois MS, Sagebiel RW. Frequency and duration of patient follow-up after treatment of a primary malignant melanoma. J. Am. Acad. Dermatol. 1985;13(5 Pt 1):756–760. doi: 10.1016/s0190-9622(85)70218-6. [DOI] [PubMed] [Google Scholar]
- 36.Martini L, Brandani P, Chiarugi C, Reali UM. First recurrence analysis of 840 cutaneous melanomas: a proposal for a follow-up schedule. Tumori. 1994;80(3):188–197. doi: 10.1177/030089169408000305. [DOI] [PubMed] [Google Scholar]
- 37.Hohnheiser AM, Gefeller O, Gohl J, Schuler G, Hohenberger W, Merkel S. Malignant melanoma of the skin: long-term follow-up and time to first recurrence. World J. Surg. 2011;35(3):580–589. doi: 10.1007/s00268-010-0859-8. [DOI] [PubMed] [Google Scholar]
- 38.Stucky CC, Gray RJ, Dueck AC, et al. Risk factors associated with local and in-transit recurrence of cutaneous melanoma. Am. J. Surg. 2010;200(6):770–774. doi: 10.1016/j.amjsurg.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RC, Fenn NJ, Horgan K, Mansel RE. Follow-up of patients with a thin melanoma. Br. J. Surg. 1999;86(5):619–621. doi: 10.1046/j.1365-2168.1999.01079.x. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy WH, Shaw HM, Thompson JF, Milton GW. Time and frequency of recurrence of cutaneous stage I malignant melanoma with guidelines for follow-up study. Surg. Gynecol. Obstet. 1988;166(6):497–502. [PubMed] [Google Scholar]
- 41.Callaway MP, Briggs JC. The incidence of late recurrence (greater than 10 years); an analysis of 536 consecutive cases of cutaneous melanoma. Br. J. Plast. Surg. 1989;42(1):46–49. doi: 10.1016/s0007-1226(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 42.Crowley NJ, Seigler HF. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann. Surg. 1990;212(2):173–177. doi: 10.1097/00000658-199008000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leman JA, MacKie RM. Late (> 10 years) recurrence of melanoma: the Scottish experience. Br. J. Dermatol. 2003;148(2):372–373. doi: 10.1046/j.1365-2133.2003.05097_8.x. [DOI] [PubMed] [Google Scholar]
- 44.Schmid-Wendtner MH, Baumert J, Schmidt M, et al. Late metastases of cutaneous melanoma: an analysis of 31 patients. J. Am. Acad. Dermatol. 2000;43(4):605–609. doi: 10.1067/mjd.2000.107234. [DOI] [PubMed] [Google Scholar]
- 45.Shaw HM, Beattie CW, McCarthy WH, Milton GW. Late relapse from cutaneous stage I malignant melanoma. Arch. Surg. 1985;120(10):1155–1159. doi: 10.1001/archsurg.1985.01390340053010. [DOI] [PubMed] [Google Scholar]
- 46.Briele HA, Beattie CW, Ronan SG, Chaudhuri PK, Das Gupta TK. Late recurrence of cutaneous melanoma. Arch. Surg. 1983;118(7):800–803. doi: 10.1001/archsurg.1983.01390070012003. [DOI] [PubMed] [Google Scholar]
- 47.Tahery DP, Moy RL. Lack of predictive factors in late recurrence of stage I melanoma. Int. J. Dermatol. 1992;31(9):629–631. doi: 10.1111/j.1365-4362.1992.tb03980.x. [DOI] [PubMed] [Google Scholar]
- 48.Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J. Clin. Oncol. 2007;25(9):1129–1134. doi: 10.1200/JCO.2006.08.1463. [DOI] [PubMed] [Google Scholar]
- 49.McKinnon JG, Yu XQ, McCarthy WH, Thompson JF. Prognosis for patients with thin cutaneous melanoma: long-term survival data from New South Wales Central Cancer Registry and the Sydney Melanoma Unit. Cancer. 2003;98(6):1223–1231. doi: 10.1002/cncr.11624. [DOI] [PubMed] [Google Scholar]
- 50.Leiter U, Buettner PG, Eigentler TK, Garbe C. Prognostic factors of thin cutaneous melanoma: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. J. Clin. Oncol. 2004;22(18):3660–3667. doi: 10.1200/JCO.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 51.Han D, Zager JS, Shyr Y, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J. Clin. Oncol. 2013;31(35):4387–4393. doi: 10.1200/JCO.2013.50.1114. [DOI] [PubMed] [Google Scholar]; • Presents a large, multi-institutional study for determining the predictive factors of sentinel lymph node metastasis in thin melanomas.
- 52.Ranieri JM, Wagner JD, Wenck S, Johnson CS, Coleman JJ., 3rd The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann. Surg. Oncol. 2006;13(7):927–932. doi: 10.1245/ASO.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow-up protocols. Br. J. Dermatol. 2008;159(2):331–336. doi: 10.1111/j.1365-2133.2008.08649.x. [DOI] [PubMed] [Google Scholar]
- 54.Bauer J, Blum A, Strohhacker U, Garbe C. Surveillance of patients at high risk for cutaneous malignant melanoma using digital dermoscopy. Br. J. Dermatol. 2005;152(1):87–92. doi: 10.1111/j.1365-2133.2005.06370.x. [DOI] [PubMed] [Google Scholar]
- 55.Haenssle HA, Vente C, Bertsch HP, et al. Results of a surveillance programme for patients at high risk of malignant melanoma using digital and conventional dermoscopy. Eur. J. Cancer Prev. 2004;13(2):133–138. doi: 10.1097/00008469-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Goggins WB, Tsao H. A population-based analysis of risk factors for a second primary cutaneous melanoma among melanoma survivors. Cancer. 2003;97(3):639–643. doi: 10.1002/cncr.11116. [DOI] [PubMed] [Google Scholar]
- 57.Johnson TM, Hamilton T, Lowe L. Multiple primary melanomas. J. Am. Acad. Dermatol. 1998;39(3):422–427. doi: 10.1016/s0190-9622(98)70318-4. [DOI] [PubMed] [Google Scholar]
- 58.Brobeil A, Rapaport D, Wells K, et al. Multiple primary melanomas: implications for screening and follow-up programs for melanoma. Ann. Surg. Oncol. 1997;4(1):19–23. doi: 10.1007/BF02316806. [DOI] [PubMed] [Google Scholar]
- 59.Kang S, Barnhill RL, Mihm MC, Jr, Sober AJ. Multiple primary cutaneous melanomas. Cancer. 1992;70(7):1911–1916. doi: 10.1002/1097-0142(19921001)70:7<1911::aid-cncr2820700718>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 60.Hengge UR, Wallerand A, Stutzki A, Kockel N. Cost–effectiveness of reduced follow-up in malignant melanoma. J. Dtsch. Dermatol. Ges. 2007;5(10):898–907. doi: 10.1111/j.1610-0387.2007.06454.x. [DOI] [PubMed] [Google Scholar]
- 61.Leiter U, Marghoob AA, Lasithiotakis K, et al. Costs of the detection of metastases and follow-up examinations in cutaneous melanoma. Melanoma Res. 2009;19(1):50–57. doi: 10.1097/CMR.0b013e32831bc41c. [DOI] [PubMed] [Google Scholar]
- 62.Ardizzoni A, Grimaldi A, Repetto L, Bruzzone M, Sertoli MR, Rosso R. Stage I–II melanoma: the value of metastatic work-up. Oncology. 1987;44(2):87–89. doi: 10.1159/000226451. [DOI] [PubMed] [Google Scholar]
- 63.Weiss M, Loprinzi CL, Creagan ET, Dalton RJ, Novotny P, O'Fallon JR. Utility of follow-up tests for detecting recurrent disease in patients with malignant melanomas. JAMA. 1995;274(21):1703–1705. [PubMed] [Google Scholar]
- 64.Brown RE, Stromberg AJ, Hagendoorn LJ, et al. Surveillance after surgical treatment of melanoma: futility of routine chest radiography. Surgery. 2010;148(4):711–716. doi: 10.1016/j.surg.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 65.Saiag P. Recommendations for an effective follow-up strategy in melanoma patients should be tailored to the investigations performed during initial staging. J. Clin. Oncol. 2003;21(19):3706–3707. doi: 10.1200/JCO.2003.99.065. [DOI] [PubMed] [Google Scholar]
- 66.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2011;69(6):1049–1050. doi: 10.1016/j.jaad.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Weide B, Elsasser M, Buttner P, et al. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br. J. Cancer. 2012;107(3):422–428. doi: 10.1038/bjc.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deichmann M, Benner A, Bock M, et al. S100-beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J. Clin. Oncol. 1999;17(6):1891–1896. doi: 10.1200/JCO.1999.17.6.1891. [DOI] [PubMed] [Google Scholar]
- 69.Garbe C, Leiter U, Ellwanger U, et al. Diagnostic value and prognostic significance of protein S-100beta, melanoma-inhibitory activity, and tyrosinase/MART-1 reverse transcription-polymerase chain reaction in the follow-up of high-risk melanoma patients. Cancer. 2003;97(7):1737–1745. doi: 10.1002/cncr.11250. [DOI] [PubMed] [Google Scholar]
- 70.Mocellin S, Zavagno G, Nitti D. The prognostic value of serum S100B in patients with cutaneous melanoma: a meta-analysis. Int. J. Cancer. 2008;123(10):2370–2376. doi: 10.1002/ijc.23794. [DOI] [PubMed] [Google Scholar]; • Analysis suggesting that serum S100B detection has a clinically valuable independent prognostic value in patients with melanoma, particularly with regards to stage I–III disease.
- 71.Haddad D, Garvey EM, Mihalik L, Pockaj BA, Gray RJ, Wasif N. Preoperative imaging for early-stage cutaneous melanoma: predictors, usage, and utility at a single institution. Am. J. Surg. 2013;206(6):979–985. doi: 10.1016/j.amjsurg.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Rueth NM, Xing Y, Chiang YJ, et al. Is surveillance imaging effective for detecting surgically treatable recurrences in patients with melanoma? A comparative analysis of stage-specific surveillance strategies. Ann. Surg. 2014;259(6):1215–1222. doi: 10.1097/SLA.0000000000000233. [DOI] [PubMed] [Google Scholar]; •• Investigates the effectiveness of routine surveillance imaging for detecting treatable melanoma recurrences and discusses the value of imaging techniques with respect to life expectancy.
- 73.[Consensus conference. Follow-up of patients surgically treated for stage I melanoma. Paris, France, 30 March 1995] Ann. Dermatol. Venereol. 1995;122(5):250–391. [PubMed] [Google Scholar]
- 74.Rychetnik L, McCaffery K, Morton R, Irwig L. Psychosocial aspects of post-treatment follow-up for stage I/II melanoma: a systematic review of the literature. Psychooncology. 2013;22(4):721–736. doi: 10.1002/pon.3060. [DOI] [PubMed] [Google Scholar]
- 75.Livingstone E, Windemuth-Kieselbach C, Eigentler TK, et al. A first prospective population-based analysis investigating the actual practice of melanoma diagnosis, treatment and follow-up. Eur. J. Cancer. 2011;47(13):1977–1989. doi: 10.1016/j.ejca.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 76.Rychetnik L, McCaffery K, Morton RL, Thompson JF, Menzies SW, Irwig L. Follow-up of early stage melanoma: specialist clinician perspectives on the functions of follow-up and implications for extending follow-up intervals. J. Surg. Oncol. 2013;107(5):463–468. doi: 10.1002/jso.23278. [DOI] [PubMed] [Google Scholar]; • Demonstrates that psychosocial aspects of follow-up influence patient well-being and potential adherence to schedules and may influence clinician practice. Planning follow-up schedules relating to the psychosocial impacts on patients should be explicitly addressed.
- 77.Francken AB, Hoekstra-Weebers JW, Hoekstra HJ. Is GP-led follow-up feasible? Br. J. Cancer. 2010;102(10):1445–1446. doi: 10.1038/sj.bjc.6605667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carli P, De Giorgi V, Palli D, et al. Dermatologist detection and skin self-examination are associated with thinner melanomas: results from a survey of the Italian Multidisciplinary Group on Melanoma. Arch. Dermatol. 2003;139(5):607–612. doi: 10.1001/archderm.139.5.607. [DOI] [PubMed] [Google Scholar]
- 79.Epstein DS, Lange JR, Gruber SB, Mofid M, Koch SE. Is physician detection associated with thinner melanomas? JAMA. 1999;281(7):640–643. doi: 10.1001/jama.281.7.640. [DOI] [PubMed] [Google Scholar]
- 80.Titus L. Skin self-examination and the ABCDE rule in the early diagnosis of melanoma: is the game over? Reply from author. Br. J. Dermatol. 2013;168(6):1371–1372. doi: 10.1111/bjd.12251. [DOI] [PubMed] [Google Scholar]