SUMMARY
Use of dermoscopy has been proven to increase diagnostic accuracy for melanoma. It is frequently used by dermatologists and other healthcare providers during skin cancer screening and in the evaluation of concerning skin lesions. Studies have shown that it is useful in the diagnosis of many nononcologic cutaneous diseases as well as in the monitoring of disease progression and treatment response. Furthermore, dermoscopy has the potential to aid in pathology specimen sectioning, translational research and medical technology development. Its broad applications and ease of use will make it an increasingly influential tool in healthcare. In this article, we review the established uses of dermoscopy by different healthcare providers and its potential future applications.
KEYWORDS : dermoscopy, melanoma detection, mole monitoring, noninvasive imaging, pigmented lesions, skin cancer diagnosis, skin cancer screening, skin self-examination, teledermoscopy
Practice points.
Dermoscopy use in the diagnosis of skin cancer
- By dermatologists:
- – Dermoscopy use by trained dermatologists leads to improvements in diagnostic accuracy of melanoma and has led to new diagnostic modalities such as sequential digital dermoscopic monitoring and teledermoscopy.
- By primary care providers:
- – Dermoscopy use by primary care providers can increase the sensitivity for skin cancer detection while simultaneously decreasing the number of unnecessary biopsies and specialty referrals.
- By other medical specialties:
- – Dermoscopy can be of use to specialties such as gynecology, urology, ear nose and throat, plastic surgery, ophthalmology, dentistry and podiatry, who often encounter patients with concerning skin lesions.
- By medical students:
- – Training medical students in dermoscopy has shown to increase their awareness of skin cancer screening and diagnostic accuracy of pigmented skin lesions.
- By patients and their families:
- – Dermoscopy can assist patients and their families during skin self-examinations and mobile dermatoscopes allow patients to capture and transmit images of concerning lesions to their physicians.
- By nonmedical professionals:
- – Laypersons in the personal care service industry such as hair stylists, barbers, aestheticians and massage therapists may benefit from dermoscopy training as they could help provide skin cancer screening to the general population.
Dermoscopy beyond skin cancer diagnosis
Dermoscopy has the potential to help the diagnosis of other cutaneous diseases and to lead inspiring advancements in translational research and medical technologies.
- Diagnosis of other cutaneous conditions:
- – Dermoscopy use can aid in the diagnosis of inflammatory, infectious, autoimmune and connective tissue skin disorders.
- Longitudinal monitoring of skin conditions and treatment response:
- – Dermoscopy provides a noninvasive approach to follow various cutaneous diseases for progression, severity and response, as well as adverse effects to treatment.
- Ex vivo dermoscopy use in pathology:
- – Ex vivo dermoscopy has been shown to be helpful in guiding pathologists to identify the optimal area for cutaneous tissue sectioning.
- Dermoscopy use in translational research:
- – Mutational studies that characterized nevi and melanomas based on dermoscopic pattern have shed light on the molecular underpinnings of nevi and melanomas.
- Dermoscopy use in medical technologies:
- – Dermoscopy has facilitated the ongoing development and improvement of confocal laser microscopy, mobile teledermoscopy and videodermoscopy.
Future perspective
Similar to the impact of the otoscope, ophthalmoscope and stethoscope in improving the bedside diagnosis of ear, eye and heart conditions, the dermatoscope will likely become a routinely used handheld tool for the examination of skin lesions and rashes.
Dermoscopy is a noninvasive skin imaging technique that aids in the diagnosis of skin lesions. The dermatoscope is a handheld device that permits the visualization of subsurface colors, structures and patterns in skin lesions not visible to the naked eye. Dermoscopy works principally through modifying the cutaneous air-tissue optical interface and providing magnification (typically 10×). Two forms of dermoscopy can be used: nonpolarized and polarized dermoscopy. Nonpolarized dermoscopy requires use of a contact glass plate and liquid interface that leads to a significant reduction in surface reflected light. In contrast, polarized dermoscopy can be performed without skin contact and uses a detector that preferentially accepts light remitted from deeper skin layers, eliminating the visualization of surface reflected light [1].
Dermoscopy helps link the underlying microscopic histopathology to macroscopic clinical dermatology [2] and has been shown to improve diagnostic accuracy for primary cutaneous melanoma with appropriate training [3–8]. In addition to its ability to enhance visualization of pigmented structures, dermoscopy is also useful in identifying subtle structures such as hemorrhagic areas and vascular structures [2,9], therefore expanding its scope of application to evaluation of nonpigmented skin disorders including inflammatory diseases, infections/infestations and amelanotic neoplasms [10]. Furthermore, dermoscopy holds great potential in clinical and translational research [5]. Because of its multifunctionality and ease of use, dermoscopy is becoming increasingly popular among healthcare providers and researchers. Although articles have been published on the application of dermoscopy in specific fields, a current overview summarizing its use across medical disciplines is lacking. In this article, we review the established uses of dermoscopy by different healthcare providers and its potential future applications for professionals and nonprofessionals alike.
Dermoscopy use in the diagnosis of skin cancer
Dermoscopy is most frequently used for the diagnosis of skin cancer. The incidence of both melanoma and nonmelanoma skin cancer (NMSC) has been steadily rising worldwide over the past few decades [11]. Detection at early stages is essential for efficacy of treatment and disease outcome. For both dermatologists and nondermatologists, accurate diagnosis of skin cancers is crucial. Specific dermoscopic morphologic criteria have been defined for many types of skin cancers [2,12,13]. Table 1 lists the most common skin cancers and their associated dermoscopic features.
Table 1. . Common skin cancers and their associated dermoscopic structures.
| Skin cancer type | Associated dermoscopic structures |
|---|---|
Basal cell carcinoma 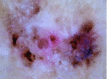
|
This basal cell carcinoma reveals multiple erosions, leaf-like areas, concentric structures and shiny white structures |
Squamous cell carcinoma 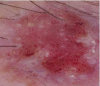
|
This squamous cell carcinoma reveals glomerular vessels and keratin pearls |
Melanoma 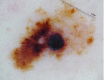
|
This melanoma reveals blue–white veil, off-centered blotch and peripheral tan structureless area |
• By dermatologists
While skin cancers are diagnosed by numerous healthcare providers from various disciplines, dermatologists were the first to adopt the routine use of dermoscopy in the evaluation of cutaneous malignancies. Systematic reviews have found that use of dermoscopy by trained dermatologists leads to significant improvements in diagnostic accuracy for primary cutaneous melanoma [6–8]. For instance, in a meta-analysis of 14 paired studies comparing melanoma diagnostic accuracy with and without dermoscopy, Kittler et al. found an increase of 49% (p = 0.001) when dermoscopy was used, primarily attributed to increase in sensitivity [7]. The improvement in clinical diagnostic accuracy also translates into a lower benign to malignant biopsy ratio for pigmented lesions, preventing many unnecessary biopsies and reducing costs [15,16]. In a randomized controlled trial of 913 consecutive subjects for pigmented lesion evaluation in a pigmented lesion clinic, Carli et al. observed a reduction in biopsy rate from 15.6 to 9.0% (p = 0.13) when clinicians used dermoscopy [15]. In addition to melanoma, dermoscopy use in the diagnosis of NMSC has also shown promising results of high sensitivity and specificity [17].
Dermoscopy has also led to new approaches to skin cancer diagnosis. For instance, it has made possible sequential digital dermoscopic monitoring of clinically equivocal melanocytic lesions. Sequential monitoring allows for images captured at different time points to be compared side by side to detect subtle changes that are not clinically apparent. Since most nevi in adults are in senescence, they will not change significantly over time. In contrast, melanomas can reveal changes detectable via dermoscopy within weeks to a few months. Nonetheless, some nevi that have not entered senescence are dynamic and undergo changes, limiting the specificity of this diagnostic modality [18]. While certain changes, such as symmetric enlargement and global pigment alterations, are frequently observed in common nevi, other changes have been specifically associated with melanoma, including focal enlargement and appearance of melanoma-associated dermoscopic structures [18,19]. Use of this technique prevents unnecessary biopsies of biologically indolent/nonchanging lesions and concurrently allows identification of clinically subtle melanomas lacking the ABCD features [19,20]. The sequential monitoring approach is particularly beneficial for patients with elevated total body nevus counts or dysplastic nevi, who are often subjected to numerous unnecessary biopsies of clinically atypical but histologically benign nevi.
Teledermoscopy introduced the possibility of skin cancer diagnosis conducted remotely. Teledermatology is gaining popularity because it saves time and increases access to specialty care. While limitations exist in teledermatology, especially in diagnosing ambiguous skin lesions, teledermoscopy renders more detailed information about skin lesions, which in turn translates into improved diagnostic accuracy and confidence in the teleconsultation [13]. Studies of teledermoscopy have reported high diagnostic concordance between teleconsultations and in-office visits [21]. Piccolo et al. compared the diagnoses rendered on 66 pigmented lesions by in-office visits to teledermoscopy consults and found the diagnostic concordance to be 91% [22]. All lesions were subsequently excised and evaluated on histopathology. The investigators found no statistically significant difference between the numbers of correct diagnoses made via teledermoscopy versus standard office visits [22]. Teledermoscopy can also improve efficiency by facilitating the triage of obviously benign or malignant lesions, leading to faster, more convenient and more cost-effective physician services. A prospective study in New Zealand that compared triage of pigmented lesions by a virtual teledermoscopy clinic and a standard dermatology clinic found a two thirds reduction in wait time for initial appointment and cost savings of 14% when using the virtual teledermoscopy clinic arm [23].
The prevalence of dermoscopy use among dermatologists is rising globally. Surveys in different countries show that in western Europe and Australia, dermoscopy is used by over 90% of dermatologists [24,25]. In the US, dermoscopy use is more limited at an estimated rate of 48% among dermatologists, but has been steadily increasing and is used by over 90% of dermatology chief residents [26,27]. With its numerous advantages, dermoscopy is anticipated to become an essential bedside tool for all dermatologists.
• By primary care providers
Primary care providers (PCPs) such as family doctors, pediatricians, nurse practitioners and physician's assistants may also benefit from dermoscopy use in skin cancer diagnosis and screening because they are often the first healthcare provider to encounter patients with skin cancers. Use of dermoscopy in the primary care setting has been found to reduce the number of unnecessary biopsies and specialty consult referrals [3]. In a study by Argenziano et al., 73 PCPs in Spain and Italy were given a 1-day training course in skin cancer detection with dermoscopic evaluation and were then randomly assigned to skin cancer screening groups with or without dermoscopy. After evaluation of 2522 lesions over a 16-month period, the referral sensitivity and negative predictive values of the dermoscopy group were significantly higher at 79.2 and 98.1%, respectively, versus 54.1 and 95.8% in the nondermoscopy group [28]. On histopathologic examination of equivocal lesions, PCPs without dermoscopy missed 23 malignant skin tumors compared with six missed by the dermoscopy group [28]. In another study, Grimaldi et al. found a 76.5% reduction in the number of unnecessary surgical procedures performed by PCPs trained in the use of dermoscopy during the assessment of 235 lesions in 197 consecutive patients screened over a 5-month period [3]. A systematic review of studies on the effect of dermoscopy use by family physicians showed that dermoscopy significantly increased the sensitivity and positive predictive value without compromising specificity in the diagnosis of melanoma [29]. Similarly, nurse practitioners trained to use dermoscopy in skin cancer screening were able to more accurately triage suspicious lesions and make fewer unnecessary specialty referrals [30]. The average wait time for a new dermatology appointment in the US is estimated to exceed 30 days; improved skin cancer screening and assessment of skin lesions in the primary care setting can free up valuable appointments and conserve healthcare resources [31]. In addition, the incorporation of dermoscopy in the primary care setting can boost the confidence of PCPs and increase their likelihood of performing skin cancer screening during routine physical examinations [28,29].
• By other medical specialties
Skin cancer diagnosis is not only relevant to dermatologists and PCPs. Other specialties such as Gynecology, Urology, Ear Nose and Throat (ENT), Plastic surgery, Ophthalmology, Dentistry and Podiatry often encounter patients who present for skin lesions. Pigmented lesions on the genitalia can be evaluated with dermoscopy during a pelvic or urological exam [32]. A study by Mannone et al. used dermoscopy in evaluating mucosal pigmentation in 170 consecutive patients seen in a vulva clinic in Italy over a period of 2 months. They found vulvar pigmented lesions to be present in 19% of the patients and that dermoscopy was helpful in differentiating benign melanosis from malignancy [32]. In addition, melanocytic lesions are often located in the pubic region. Melanocytic nevi located in the genitalia are referred to as ‘nevi of special sites’ and they can be difficult to distinguish from melanoma both clinically and histopathologically [33]. In evaluating melanocytic lesions in the pubic region, dermoscopy can be useful in differentiating nevi from melanoma. Some studies have reported dermoscopic criteria that can assist in characterization of these ‘special site’ nevi [32,34]. Use of dermoscopy by physicians that routinely examine the genital area can facilitate the early detection of skin cancer while at the same time reduce the number of benign nevus biopsies. Similarly, ENT, Ophthalmology, Dentistry and Podiatry focus on areas of the body where skin cancers frequently arise. Periocular and periorbital skin malignancies are often clinically subtle in early stages; visualization with dermoscopy increases diagnostic sensitivity and allows for prompt oculoplastic management [35]. A prospective study by Tosi et al. evaluated conjunctival pigmented lesions in 49 consecutive patients with dermoscopy and found that conjunctival melanoma showed significant dermoscopic differences in geometry, color, color distribution and texture compared with benign lesions [36]. Dermoscopy use simplified the evaluation process and allowed for sequential follow-up using reproducible high-quality digital images [36].
Another specialty in which dermoscopy is gaining popularity is plastic surgery, which manages a substantial proportion of cutaneous lesion excisions, particularly on cosmetically sensitive anatomic sites. In a study by Townley et al., 30 plastic surgeons who attended a 1-day training course in dermoscopy demonstrated improved ability to differentiate malignant from benign skin tumors, with a diagnostic accuracy increase of 44 to 56% (p < 0.05) when comparing their pre- and post-course tests consisting of 30 skin lesions [37]. This observed benefit has led to the incorporation of dermoscopy courses offered during plastic surgery and ENT facial plastics national conferences.
• By medical students
Dermoscopy use in the detection of skin cancer can be incorporated into medical school education, improving the confidence and diagnostic accuracy of skin cancer diagnosis among medical students. A survey study showed that when medical school students received a single 15-minute dermoscopy training session in their preclinical years, they paid more attention to skin inspection during physical examinations in subsequent clerkships and had significantly improved diagnostic accuracy of malignant skin lesions compared with students who did not receive dermoscopy training [38]. This improved diagnostic accuracy was retained 1 year later when the students were asked to distinguish malignant from benign lesions. A majority of medical students in the US report having insufficient dermatologic education while in school and as a result, low confidence during skin lesion assessments [38]. Approximately 80% of students exposed to dermoscopy training have reported positive attitudes toward learning dermoscopy [38]. More widespread dermoscopy training during medical school might enhance the ability and confidence of physicians to perform skin cancer screenings. This in turn may have a positive impact on future skin cancer related morbidity and mortality.
• By patients & their families
Patients have been found to detect the majority of melanomas [39]. As melanomas detected by patients are diagnosed at a more advanced stage than physician-detected melanomas, patients at high risk for skin cancer are recommended to perform monthly skin self-examinations (SSEs) [40–42]. As dermoscopy is simple, quick and noninvasive, motivated patients could potentially incorporate dermoscopy into their SSEs to improve their evaluation and triage of new or changing lesions. Dermoscopy use by patients has not been widely explored, but case reports and preliminary studies have illustrated its potential use as an aid during SSEs. Goulart et al. reported 2 cases where patients used dermatoscopes to identify a melanoma and a dysplastic nevus, respectively [39].
The introduction of mobile dermatoscopes, assembled by adding a compact low-cost dermatoscope attachment to a mobile device equipped with a camera, has facilitated the possibility of patient-initiated teledermoscopy. Patients have the ability to save and transmit dermoscopic images of concerning lesions to their physicians. The advantages of patient-acquired teledermoscopy include faster and potentially more efficient care, as well as patient convenience. Janda et al. examined the feasibility of using teledermoscopy during SSEs in ten patients at high-risk for melanoma and reported that patients had little difficulty in the teledermoscopy consult process but had relatively low specificity in selecting suspicious lesions [43]. With more education or dermatologist guidance in selecting lesions for patients to self-monitor, patient-driven teledermoscopy may be practical and cost-efficient for patients at high-risk for skin cancer [43]. More studies are needed to explore the feasibility of this emerging role of dermoscopy.
• By nonmedical professionals
Laypersons in the personal care service industry whose professional duties often involve cutaneous sites, such as hair stylists, barbers, aestheticians and massage therapists, may represent a potential public health resource that could aid in providing skin cancer screening to the general population. There are multiple case reports of these nonmedical professionals identifying melanomas [44]. Hairdressers and barbers, for example, could be educated on the use of dermoscopy to identify skin cancers common to the head and neck [45,46]. Studies are needed to assess the feasibility of utilizing this group in skin cancer screening.
Dermoscopy use beyond skin cancer diagnosis
• Diagnosis of other cutaneous conditions
In addition to skin malignancies, use of dermoscopy has been studied in various inflammatory, infectious, autoimmune and connective tissue skin disorders [2]. For instance, dermoscopy can be used to improve the visualization and identification of medically important infestations, such as pediculosis, scabies, ticks, tungiasis and cutaneous larva migrans [10]. Dermoscopic patterns have also been described for viral cutaneous infections, including warts and molluscum contagiosum [10]. Delays in the diagnosis of these conditions can lead to significant morbidity, disease spread and epidemiological burden. A systematic review on dermoscopy use in the diagnosis of skin infestations and infections has shown comparably high diagnostic accuracy with ex vivo clinical diagnosis via skin scrapings under light microscopy, which can be significantly more time consuming and unpleasant for patients [10].
Dermoscopy has also been used in the diagnosis of hair disorders, such as alopecia. Features such as hair diameter diversity and perihilar signs are associated with androgenic alopecia whereas yellow dots and dystrophic hair shaft features indicate alopecia areata [47]. The diagnosis of inflammatory disorders such as psoriasis and lichen planus can also benefit from incorporation of dermoscopy. Visualization of dilated, elongated and convoluted capillaries showing a typical ‘glomerular’ or ‘bushy’ quality can aid the diagnosis of psoriasis. Similarly, visualization of Wickham striae along with other deeper vascular structures invisible to the naked eye can facilitate the diagnosis of lichen planus [47].
In addition to infectious and inflammatory disorders, dermoscopy can be helpful in the evaluation of connective tissue diseases through examination of cutaneous vascular patterns present in skin lesions and nailfolds. Pilot studies have documented high diagnostic accuracy when dermoscopy was incorporated into the diagnosis of conditions including Raynaud's phenomenon [48], dermatomyositis, scleroderma and systemic lupus erythematosus [49,50]. Dermoscopy has also been shown to be useful in differentiating common urticaria and urticarial vasculitis based on the presence of purpuric globules unique to urticarial vasculitis [51]. These applications of dermoscopy can assist dermatologists and rheumatologists in discriminating a spectrum of clinically ambiguous connective tissue diseases.
• Longitudinal monitoring of skin conditions & treatment response
Because of its noninvasive nature, dermoscopy is well-suited for longitudinal monitoring of chronic skin conditions, treatment response and/or side effects [2]. Dermoscopy has been used to monitor disease progression of conditions such as leg ulcers, psoriasis, alopecia and autoimmune disorders [52–54]. A prospective study by Caramaschi et al. evaluated the nailfold capillary pattern of 103 consecutive patients with scleroderma and found that specific dermoscopic nailfold capillary patterns were associated with different stages of disease severity, including systemic organ involvement [55]. Improved assessment of disease severity can lead to more precise disease staging and prognosis, as well as appropriate treatment [55]. For instance, certain patients with connective tissue disorders and conditions such as lymphedema and venous stasis can benefit from physiotherapy when incorporated at the appropriate disease stage. The use of dermoscopy can improve identification of subtle changes in disease staging. Le Forum et al. have shown that dermoscopy was useful in identifying changes in the nail unit in patients with secondary lymphedema and timely decompressive physiotherapy can prevent long term changes in nail growth [56]. Other studies have examined the use of dermoscopy in monitoring adverse events of topical therapies. For example, topical steroid overuse can result in clinically unapparent but dermoscopically identifiable ‘red lines’, indicating initial signs of skin atrophy [57].
• Ex vivo dermoscopy use in pathology
Sectioning the diagnostically important area of a cutaneous pathology specimen is crucial for pathologists to make accurate diagnoses. Ex vivo dermoscopy has proven to be helpful in guiding pathologists to identify the optimal area of sectioning [58]. Scope et al. tested the feasibility of this technique in six biopsied pigmented lesions. A good correlation of ex vivo dermoscopy images was found with those taken during in vivo diagnosis prior to biopsy, suggesting that dermoscopy can be used to guide fixed tissue sectioning of pigmented lesions [58]. The findings from Scope et al. were confirmed in a second study that retrospectively reviewed 517 skin biopsies and found that when correlated with ex vivo dermoscopy, more definitive histopathologic diagnoses could be made for 18/25 (72%) previously ambiguous lesions reviewed by histopathology alone [59]. Ex vivo dermoscopy has also been successfully used to guide incisional tissue sampling of 10 melanoma specimens for the purpose of molecular studies, while preserving the histologic integrity of the tumor in the gross specimens [60]. Although future studies are needed, dermoscopy has shown promise in bridging pathologists and dermatologists by delivering more accurate and effective histopathologic diagnoses.
• Dermoscopy use in translational research
Dermoscopy may be able to assist in translational research. Cytogenetic studies that explore nevogenesis and the underlying genetics related to increased melanoma risk have associated specific dermoscopic structures in nevi with certain genotypes. Pujana et al. found a t(9;12)(p21;q13) balanced translocation to be correlated with a phenotype of >150 dysplastic nevi in two generations within a family. Members carrying this genetic translocation had nevi that shared a similar atypical reticular dermoscopic pattern, which was not present in family members without the mutation [61]. Other investigators have used dermoscopy to improve the molecular characterization of melanocytic neoplasms. For example, two studies found a higher frequency of oncogenic BRAF mutations in globular nevi than in reticular nevi [62,63]. Melanomas with a dark homogeneous streak dermoscopic pattern were found to be associated with a specific and unique KIT mutation [64]. These studies suggest that stratifying melanocytic neoplasms by dermoscopic pattern may illuminate molecular characterization of nevi and melanomas, thereby facilitating translational researchers in studies of nevogenesis and melanomagenesis.
• Dermoscopy use in medical technologies
Dermoscopy can facilitate the development and incorporation of other emergent technologies in the diagnosis of cutaneous diseases. Reflectance confocal microscopy (RCM) is used to noninvasively visualize microscopic structures and cellular detail in the epidermis and superficial dermis. Its application in skin tumor diagnosis is currently being explored but has shown great promise [65]. Dermoscopy can facilitate the understanding of RCM via direct correlation of dermoscopic features to RCM findings. This is possible because both RCM and dermoscopy image the cutaneous surface in a horizontal plane, unlike the vertical sectioning used in histopathology [66]. This correlation is synergistic in that it validates features observed via RCM, which in turn allows for the study of tissue correlated to dermoscopic structures and the in vivo monitoring of cellular changes over time. Recent advancements in RCM show promising results in its ability to assist melanoma and NMSC diagnosis in clinically ambiguous cases and to provide better margin definition to reduce re-excision rates [67–69]. For melanocytic neoplasms, RCM is currently most frequently used as a second-level diagnostic test in combination with dermoscopy and has been shown to improve diagnostic accuracy for melanoma and to reduce unnecessary biopsies of ultimately benign melanocytic neoplasms [70,71]. Besides RCM, dermoscopy has also promoted the advancement of telemedicine technology use in cutaneous disease diagnosis and monitoring, as previously mentioned. The introduction of teledermoscopy and mobile teledermoscopy has the potential to improve collaboration and communication between patients and healthcare providers [72].
Furthermore, dermoscopy has been adapted to obtain images at much higher magnification (10–1000×) with real-time recordings in videodermoscopy, which combines a video camera equipped with optic fibers and lenses to transmit recorded video for projection and storage in a computer. The evolution of videodermoscopy has allowed for more detailed visualization of skin structures and has shown great promise in the diagnosis of a wide spectrum of cutaneous disorders [53]. The incorporation of these technologies is not only valuable for clinicians, but also opens up new opportunities for professionals across industries, spanning from biomedical and electronic engineers to software and mobile application developers.
Conclusion
Dermoscopy is a useful tool across many areas of dermatologic clinical practice. Its effectiveness in skin cancer diagnosis is well-documented but further studies are needed to investigate other emerging applications. In order to fully exploit the potential of dermoscopy, all healthcare providers, including medical students, should obtain a basic level of dermoscopy education. Although the accuracy and effectiveness of dermoscopy is dependent on the expertise of the user, studies have shown that even short educational courses can help nonexperts to more accurately and appropriately triage skin lesions [38,73,74]. We believe that dermoscopy will become more influential in the future as healthcare providers across more disciplines learn its applications and benefits.
Future perspective
We speculate that over the next 5–10 years, automated or semiautomated clinical decision support tools will be successfully developed to aid dermoscopy users in their assessment of skin lesions. These tools would greatly improve the ability of nonexperts to triage or diagnose skin lesions concerning for skin cancer. We also anticipate standardization, simplification and unification regarding dermoscopy nomenclature and diagnostic algorithms. Ultimately, given its efficacy, noninvasive nature and ease of use, dermoscopy will be recognized worldwide as a standard of care in the evaluation and diagnosis of melanoma and possibly other skin cancers.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Pan Y, Gareau DS, Scope A, Rajadhyaksha M, Mullani NA, Marghoob AA. Polarized and nonpolarized dermoscopy: the explanation for the observed differences. Arch. Dermatol. 2008;144(6):828–829. doi: 10.1001/archderm.144.6.828. [DOI] [PubMed] [Google Scholar]
- 2.Zalaudek I, Argenziano G, Di Stefani A, et al. Dermoscopy in general dermatology. Dermatology. 2006;212(1):7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- 3.Grimaldi L, Silvestri A, Brandi C, et al. Digital epiluminescence dermoscopy for pigmented cutaneous lesions, primary care physicians, and telediagnosis: a useful tool? J. Plast. Reconstr. Aesthet. Surg. 2009;62(8):1054–1058. doi: 10.1016/j.bjps.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br. J. Dermatol. 2000;143(5):1016–1020. doi: 10.1046/j.1365-2133.2000.03836.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun RP, Rabinovitz H, Tzu JE, Marghoob AA. Dermoscopy research‐‐an update. Semin. Cutan. Med. Surg. 2009;28(3):165–171. doi: 10.1016/j.sder.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch. Dermatol. 2001;137(10):1343–1350. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 7.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–165. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008;159(3):669–676. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]; •• This meta-analysis showed that dermoscopy has a relative diagnostic odds ratio of 15.6 (p = 0.016) for primary melanoma detection compared to naked-eye examination alone.
- 9.Kreusch JF. Vascular patterns in skin tumors. Clin. Dermatol. 2002;20(3):248–254. doi: 10.1016/s0738-081x(02)00227-4. [DOI] [PubMed] [Google Scholar]
- 10.Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216(1):14–23. doi: 10.1159/000109353. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Geer S, Siemerink M, Reijers HA, et al. The incidence of skin cancer in dermatology. Clin. Exp. Dermatol. 2013;38(7):724–729. doi: 10.1111/ced.12080. [DOI] [PubMed] [Google Scholar]
- 12.Marghoob AA, Braun R. Proposal for a revised 2-step algorithm for the classification of lesions of the skin using dermoscopy. Arch. Dermatol. 2010;146(4):426–428. doi: 10.1001/archdermatol.2010.41. [DOI] [PubMed] [Google Scholar]
- 13.Argenziano G, Soyer HP. Dermoscopy of pigmented skin lesions – a valuable tool for early diagnosis of melanoma. Lancet Oncol. 2001;2(7):443–449. doi: 10.1016/s1470-2045(00)00422-8. [DOI] [PubMed] [Google Scholar]
- 14.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J. Am. Acad. Dermatol. 2003;48(5):679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 15.Carli P, De Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J. Am. Acad. Dermatol. 2004;50(5):683–689. doi: 10.1016/j.jaad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Carli P, De Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997–2001. Br. J. Dermatol. 2004;150(4):687–692. doi: 10.1111/j.0007-0963.2004.05860.x. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen M, Jemec GB. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: a review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatol. Surg. 2007;33(10):1158–1174. doi: 10.1111/j.1524-4725.2007.33251.x. [DOI] [PubMed] [Google Scholar]
- 18.Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J. Am. Acad. Dermatol. 2000;43(3):467–476. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- 19.Menzies SW, Gutenev A, Avramidis M, Batrac A, Mccarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch. Dermatol. 2001;137(12):1583–1589. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 20.Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J. Invest. Dermatol. 2006;126(5):980–985. doi: 10.1038/sj.jid.5700119. [DOI] [PubMed] [Google Scholar]; • Using dermoscopy to take sequential digital dermoscopic images improves the management of patients with atypical nevi and facilitates the diagnosis of melanoma.
- 21.Massone C, Brunasso AM, Campbell TM, Soyer HP. Mobile teledermoscopy – melanoma diagnosis by one click? Semin. Cutan. Med. Surg. 2009;28(3):203–205. doi: 10.1016/j.sder.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Piccolo D, Smolle J, Wolf IH, et al. Face-to-face diagnosis vs telediagnosis of pigmented skin tumors – a teledermoscopic study. Arch. Dermatol. 1999;135(12):1467–1471. doi: 10.1001/archderm.135.12.1467. [DOI] [PubMed] [Google Scholar]
- 23.Lim D, Oakley AM, Rademaker M. Better, sooner, more convenient: a successful teledermoscopy service. Australas. J. Dermatol. 2012;53(1):22–25. doi: 10.1111/j.1440-0960.2011.00836.x. [DOI] [PubMed] [Google Scholar]; • By comparing diagnostic outcome, waiting time and cost between a virtual teledermoscopy clinic and conventional face-to-face clinic, this study suggests that community based teledermoscopy clinics may improve efficiency and save cost without compromising care.
- 24.Venugopal SS, Soyer HP, Menzies SW. Results of a nationwide dermoscopy survey investigating the prevalence, advantages and disadvantages of dermoscopy use among Australian dermatologists. Australas. J. Dermatol. 2011;52(1):14–18. doi: 10.1111/j.1440-0960.2010.00708.x. [DOI] [PubMed] [Google Scholar]
- 25.Moulin C, Poulalhon N, Duru G, Debarbieux S, Dalle S, Thomas L. Dermoscopy use by French private practice dermatologists: a nationwide survey. Br. J. Dermatol. 2013;168(1):74–79. doi: 10.1111/j.1365-2133.2012.11216.x. [DOI] [PubMed] [Google Scholar]
- 26.Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J. Am. Acad. Dermatol. 2010;63(3):412–419. doi: 10.1016/j.jaad.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 27.Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int. J. Dermatol. 2009;48(9):951–952. doi: 10.1111/j.1365-4632.2009.04095.x. [DOI] [PubMed] [Google Scholar]
- 28.Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J. Clin. Oncol. 2006;24(12):1877–1882. doi: 10.1200/JCO.2005.05.0864. [DOI] [PubMed] [Google Scholar]; •• This study shows that a short 1-day dermoscopy course can be remarkably effective in improving the ability of primary care physicians to triage skin lesions suggestive of skin cancer.
- 29.Herschorn A. Dermoscopy for melanoma detection in family practice. Can. Fam. Physician. 2012;58(7):740–745. e372–e748. [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveria SA, Nehal KS, Christos PJ, Sharma N, Tromberg JS, Halpern AG. Using nurse practitioners for skin cancer screening – a pilot study. Am. J. Prev. Med. 2001;21(3):214–217. doi: 10.1016/s0749-3797(01)00354-3. [DOI] [PubMed] [Google Scholar]
- 31.Rubin CB, Kovarik CL. Teledermatologic care, the Affordable Care Act, and 20 million new patients: picturing the future. JAMA Dermatol. 2014;150(3):243–244. doi: 10.1001/jamadermatol.2013.9603. [DOI] [PubMed] [Google Scholar]
- 32.Mannone F, De Giorgi V, Cattaneo A, Massi D, De Magnis A, Carli P. Dermoscopic features of mucosal melanosis. Dermatol. Surg. 2004;30(8):1118–1123. doi: 10.1111/j.1524-4725.2004.30337.x. [DOI] [PubMed] [Google Scholar]
- 33.Elder DE. Precursors to melanoma and their mimics: nevi of special sites. Mod. Pathol. 2006;19:S4–S20. doi: 10.1038/modpathol.3800515. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ – correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am. J. Dermatopathol. 2006;28(1):21–27. doi: 10.1097/01.dad.0000187931.05030.a0. [DOI] [PubMed] [Google Scholar]
- 35.Mannor GE, Chern PL, Barnette D. Eyelid and periorbital skin basal cell carcinoma: oculoplastic management and surgery. Int. Ophthalmol. Clin. 2009;49(4):1–16. doi: 10.1097/IIO.0b013e3181b7ebe8. [DOI] [PubMed] [Google Scholar]
- 36.Tosi GM, Rubegni P, Schuerfeld K, et al. Digital surface microscopy analysis of conjunctival pigmented lesions: a preliminary study. Melanoma Res. 2004;14(5):375–380. doi: 10.1097/00008390-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Townley WA, Cassell OC, Bowling J. Dermoscopy – time for plastic surgeons to embrace a new diagnostic tool? J. Plast. Reconstr. Aesthet. Surg. 2011;64(10):1386–1387. doi: 10.1016/j.bjps.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Chen LL, Liebman TN, Soriano RP, Dusza SW, Halpern AC, Marghoob AA. One-year follow-up of dermoscopy education on the ability of medical students to detect skin cancer. Dermatology. 2013;226(3):267–273. doi: 10.1159/000350571. [DOI] [PubMed] [Google Scholar]; • This study showed that a short dermoscopy course given to medical students was able to enhance their ability to distinguish benign and malignant skin lesions in their future clinical practice.
- 39.Goulart JM, Malvehy J, Puig S, Martin G, Marghoob AA. Dermoscopy in skin self-examination: a useful tool for select patients. Arch. Dermatol. 2011;147(1):53–58. doi: 10.1001/archdermatol.2010.387. [DOI] [PubMed] [Google Scholar]
- 40.Epstein DS, Lange JR, Gruber SB, Mofid M, Koch SE. Is physician detection associated with thinner melanomas? JAMA. 1999;281(7):640–643. doi: 10.1001/jama.281.7.640. [DOI] [PubMed] [Google Scholar]
- 41.Friedman LC, Webb JA, Bruce S, Weinberg AD, Cooper HP. Skin-cancer prevention and early detection intentions and behavior. Am. J. Prev. Med. 1995;11(1):59–65. [PubMed] [Google Scholar]
- 42.Robinson JK, Rigel DS, Amonette RA. What promotes skin self-examination? J. Am. Acad. Dermatol. 1998;38(5):752–757. doi: 10.1016/s0190-9622(98)70204-x. [DOI] [PubMed] [Google Scholar]
- 43.Janda M, Loescher LJ, Soyer HP. Enhanced skin self-examination: a novel approach to skin cancer monitoring and follow-up. JAMA Dermatol. 2013;149(2):231–236. doi: 10.1001/jamadermatol.2013.1218. [DOI] [PubMed] [Google Scholar]; • This study showed that mobile teledermoscopy can complement skin self-examinations in high risk patients for melanoma. Most participants were able to take quality images of their pigmented lesions and transmit them to their physicians.
- 44.Roosta N, Wong MK, Woodley DT. Utilizing hairdressers for early detection of head and neck melanoma: an untapped resource. J. Am. Acad. Dermatol. 2012;66(4):687–688. doi: 10.1016/j.jaad.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Martin R, Emanuel P. Combined cellular blue nevus and trichoepithelioma. J. Clin. Aesthet. Dermatol. 2013;6(8):35–38. [PMC free article] [PubMed] [Google Scholar]
- 46.Pillemer BB, Pugliano-Mauro MA, Ferris LK, Patton TJ. Three cases of scalp melanomas discovered by hairdressers. J. Clin. Aesthet. Dermatol. 2013;6(8):32–34. [PMC free article] [PubMed] [Google Scholar]
- 47.Micali G, Lacarrubba F, Massimino D, Schwartz RA. Dermatoscopy: alternative uses in daily clinical practice. J. Am. Acad. Dermatol. 2011;64(6):1135–1146. doi: 10.1016/j.jaad.2010.03.010. [DOI] [PubMed] [Google Scholar]; • This article provides a comprehensive review of the different uses of dermoscopy and videodermoscopy in the diagnosis and monitoring of a wide spectrum of skin conditions including infectious, inflammatory and autoimmune disorders.
- 48.Moore TL, Roberts C, Murray AK, Helbling I, Herrick AL. Reliability of dermoscopy in the assessment of patients with Raynaud's phenomenon. Rheumatology (Oxford) 2010;49(3):542–547. doi: 10.1093/rheumatology/kep408. [DOI] [PubMed] [Google Scholar]
- 49.Sontheimer RD. A portable digital microphotography unit for rapid documentation of periungual nailfold capillary changes in autoimmune connective tissue diseases. J. Rheumatol. 2004;31(3):539–544. [PubMed] [Google Scholar]
- 50.Giampetruzzi AR, Mondino C, Facchiano A, et al. Association of dermoscopic profiles of telangiectases with nailfold videocapillaroscopic patterns in patients with systemic sclerosis. J. Rheumatol. 2013;40(9):1630–1632. doi: 10.3899/jrheum.130171. [DOI] [PubMed] [Google Scholar]
- 51.Vazquez-Lopez F, Maldonado-Seral C, Soler-Sanchez T, Perez-Oliva N, Marghoob AA. Surface microscopy for discriminating between common urticaria and urticarial vasculitis. Rheumatology. 2003;42(9):1079–1082. doi: 10.1093/rheumatology/keg301. [DOI] [PubMed] [Google Scholar]
- 52.Binder B, Hofmann-Wellenhof R, Salmhofer W, Okcu A, Kerl H, Soyer HP. Teledermatological monitoring of leg ulcers in cooperation with home care nurses. Arch. Dermatol. 2007;143(12):1511–1514. doi: 10.1001/archderm.143.12.1511. [DOI] [PubMed] [Google Scholar]
- 53.Lacarrubba F, D'Amico V, Nasca MR, Dinotta F, Micali G. Use of dermatoscopy and videodermatoscopy in therapeutic follow-up: a review. Int. J. Dermatol. 2010;49(8):866–873. doi: 10.1111/j.1365-4632.2010.04581.x. [DOI] [PubMed] [Google Scholar]
- 54.Lallas A, Apalla Z, Lefaki I, et al. Dermoscopy of discoid lupus erythematosus. Br. J. Dermatol. 2013;168(2):284–288. doi: 10.1111/bjd.12044. [DOI] [PubMed] [Google Scholar]
- 55.Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology (Oxford) 2007;46(10):1566–1569. doi: 10.1093/rheumatology/kem190. [DOI] [PubMed] [Google Scholar]
- 56.Le Fourn E, Duhard E, Tauveron V, et al. Changes in the nail unit in patients with secondary lymphoedema identified using clinical, dermoscopic and ultrasound examination. Br. J. Dermatol. 2011;164(4):765–770. doi: 10.1111/j.1365-2133.2010.10179.x. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Lopez F, Marghoob AA. Dermoscopic assessment of long-term topical therapies with potent steroids in chronic psoriasis. J. Am. Acad. Dermatol. 2004;51(5):811–813. doi: 10.1016/j.jaad.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Scope A, Busam KJ, Malvehy J, et al. Ex vivo dermoscopy of melanocytic tumors: time for dermatopathologists to learn dermoscopy. Arch. Dermatol. 2007;143(12):1548–1552. doi: 10.1001/archderm.143.12.1548. [DOI] [PubMed] [Google Scholar]; • This pilot study showed that dermoscopy could be applied to fixed tissue to guide tissue sectioning in gross pathology by comparing in vivo and ex vivo dermoscopic findings.
- 59.Amin K, Fraga GR. Ex vivo dermoscopy of cutaneous biopsies for melanocytic neoplasms: a retrospective review of 517 cases with histopathologic correlation. Am. J. Dermatopathol. 2012;34(7):710–715. doi: 10.1097/DAD.0b013e3182476bba. [DOI] [PubMed] [Google Scholar]
- 60.Malvehy J, Aguilera P, Carrera C, et al. Ex vivo dermoscopy for biobank-oriented sampling of melanoma. JAMA Dermatol. 2013;149(9):1060–1067. doi: 10.1001/jamadermatol.2013.4724. [DOI] [PubMed] [Google Scholar]
- 61.Pujana MA, Ruiz A, Badenas C, et al. Molecular characterization of a t(9;12)(p21;q13) balanced chromosome translocation in combination with integrative genomics analysis identifies C9orf14 as a candidate tumor-suppressor. Genes Chromosomes Cancer. 2007;46(2):155–162. doi: 10.1002/gcc.20396. [DOI] [PubMed] [Google Scholar]
- 62.Zalaudek I, Catricala C, Moscarella E, Argenziano G. What dermoscopy tells us about nevogenesis. J. Dermatology. 2011;38(1):16–24. doi: 10.1111/j.1346-8138.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 63.Marchetti MA, Kiuru MH, Busam KJ, et al. Melanocytic naevi with globular and reticular dermoscopic patterns display distinct BRAF V600E expression profiles and histopathologic patterns. Br. J. Dermatol. 2014 doi: 10.1111/bjd.13260. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez MI, Rabinovitz HS, Oliviero MC, et al. Dark homogeneous streak dermoscopic pattern correlating with specific KIT mutations in melanoma. JAMA Dermatol. 2014;150(6):633–639. doi: 10.1001/jamadermatol.2013.8442. [DOI] [PubMed] [Google Scholar]
- 65.Rajadhyaksha M. Confocal microscopy of skin cancers: translational advances toward clinical utility. Conf. Proc. IEEE Eng. Med. Biol. Soc. 20092009:3231–3233. doi: 10.1109/IEMBS.2009.5333600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scope A, Benvenuto-Andrade C, Agero AL, Halpern AC, Gonzalez S, Marghoob AA. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch. Dermatol. 2007;143(2):176–185. doi: 10.1001/archderm.143.2.176. [DOI] [PubMed] [Google Scholar]
- 67.Peppelman M, Wolberink EA, Blokx WA, Van De Kerkhof PC, Van Erp PE, Gerritsen MJ. In vivo diagnosis of basal cell carcinoma subtype by reflectance confocal microscopy. Dermatology. 2013;227(3):255–262. doi: 10.1159/000354762. [DOI] [PubMed] [Google Scholar]
- 68.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J. Am. Acad. Dermatol. 2009;61(2):216–229. doi: 10.1016/j.jaad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Ulrich M, Lange-Asschenfeldt S, Gonzalez S. In vivo reflectance confocal microscopy for early diagnosis of nonmelanoma skin cancer. Actas Dermosifiliogr. 2012;103(9):784–789. doi: 10.1016/j.ad.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J. Invest. Dermatol. 2009;129(1):131–138. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]
- 71.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br. J. Dermatol. 2014;171(5):1044–1051. doi: 10.1111/bjd.13148. [DOI] [PubMed] [Google Scholar]
- 72.Kroemer S, Fruhauf J, Campbell TM, et al. Mobile teledermatology for skin tumour screening: diagnostic accuracy of clinical and dermoscopic image tele-evaluation using cellular phones. Br. J. Dermatol. 2011;164(5):973–979. doi: 10.1111/j.1365-2133.2011.10208.x. [DOI] [PubMed] [Google Scholar]
- 73.Liebman TN, Goulart JM, Soriano R, et al. Effect of dermoscopy education on the ability of medical students to detect skin cancer. Arch. Dermatol. 2012;148(9):1016–1022. doi: 10.1001/archdermatol.2012.509. [DOI] [PubMed] [Google Scholar]
- 74.Pagnanelli G, Soyer HP, Argenziano G, et al. Diagnosis of pigmented skin lesions by dermoscopy: web-based training improves diagnostic performance of non-experts. Br. J. Dermatol. 2003;148(4):698–702. doi: 10.1046/j.1365-2133.2003.05168.x. [DOI] [PubMed] [Google Scholar]


