Abstract
Melanocytic lesions of acral sites are common, with an estimated prevalence of 28–36% in the USA. While the majority of these lesions are benign, differentiation from acral melanoma (AM) is often challenging. AM is a unique subtype of melanoma, with distinct molecular characteristics that are thought to contribute to its high rate of locoregional recurrence and worse prognosis. The advent of dermoscopy has since improved the diagnostic accuracy of AM, resulting in earlier detection and arguably improved survival. Additionally, the identification of unique genomic amplifications in AM invites the potential for future AM-specific targeted therapies. Herein, we discuss the importance of dermoscopy in the diagnosis of acral melanocytic lesions and review the treatment strategies for AM.
KEYWORDS : acral, acral melanoma, dermoscopy, glabrous skin, imatinib, ipilimumab, melanocytic lesions, sunitinib
Practice points.
Acral melanocytic lesions often pose a diagnostic & therapeutic challenge for many clinicians
Unlike acral melanoma (AM), which is the least common subtype of cutaneous melanoma, benign acral melanocytic lesions are prevalent in the USA.
Acral nevi often share similar clinical characteristics as AM, causing many clinicians to perform biopsies on otherwise benign acral lesions.
Dermoscopy provides an effective noninvasive modality to differentiate benign acral melanocytic nevi from AM
On dermoscopy, acral lesions that demonstrate a parallel-ridge pattern or do not show typical benign patterns (i.e., parallel-furrow pattern, lattice-like pattern, fibrillar pattern) may require histopathologic evaluation.
In addition to the parallel-furrow pattern, congenital nevi of glabrous skin may demonstrate the crista dotted (peas-in-a-pod) pattern.
Nail apparatus melanoma may present with melanonychia that is characterized by the dermoscopic appearance of brown/black bands with irregular thickness and spacing.
Measure the maximum diameter of acral lesions that do not show typical benign dermoscopic patterns to determine management
Short-term sequential digital dermoscopy of 3 months may be used to follow acral melanocytic lesions <7 mm in maximum diameter.
Acral lesions >7 mm in maximum diameter should be biopsied for histopathological evaluation.
AM is associated with a high rate of locoregional recurrence & worse prognosis
Melanocytes with amplifications in the CCND1 gene have been identified beyond the histopathological margin of AM, potentially contributing to the high rate of locoregional recurrence.
While the surgical treatment guidelines are currently uniform for all melanoma subtypes, widening the surgical margins when excising AM may improve locoregional control and AM-specific survival.
The unique genetic aberrations of AM, specifically the high frequency of KIT mutations, may signify the potential for systemic therapies in the treatment of advanced disease.
Acral melanoma (AM) is a subtype of melanoma that involves the palms, soles or nail apparatus [1,2]. AM accounts for 3–15% of all melanoma, making it the least common subtype of cutaneous malignant melanoma (CMM) [2–4]. Unlike AM, acral melanocytic nevi are relatively common, with an estimated prevalence of 28–36% in the USA [1,5]. The ability to clinically differentiate AM and acral melanocytic nevi is often challenging – albeit imperative – as AM is associated with a worse prognosis compared with other CMM subtypes [1–3,6]. At present, the surgical treatment of primary melanoma is based on tumor thickness, regardless of melanoma subtype. However, the unique clinical and biological behavior of AM may signify the need for AM-specific treatment guidelines [6–10].
Epidemiologic trends of acral melanocytic lesions
The incidence of AM in the USA has remained relatively stable over the past few decades, a finding that stands in opposition to the overall incidence of CMM [2]. While the incidence of AM is similar across racial and ethnic groups, AM disproportionately affects darker-skinned patients and is considered the most common subtype of melanoma in blacks [1,2,4,5]. AM accounts for 60–75% of all CMM in blacks and 5–7% of CMM in whites [2,11]. Additionally, studies have revealed that approximately half of all melanomas in Japanese patients are AM [12].
AM is suggested to have a worse prognosis than CMM. The 5-and 10-year melanoma-specific survival rates for AM (80.3 and 67.5%, respectively) are significantly less than those of CMM (91.3 and 87.5%, respectively) [2]. In fact, in the largest population-based evaluation of AM, Bradford et al. demonstrated that when adjusted for thickness or tumor stage, AM survival rates were still significantly lower than those for CMM overall [2]. At present, the absolute cause of the aggressive character of AM is unclear. Prior studies have suggested that delay in diagnosis may explain the worse prognosis [4,13–14]. In fact, numerous reports unfortunately describe the misdiagnosis of AM for common disorders, such as fungal infections [15], warts [16], diabetic foot ulcers [17] and traumatic ulcers [4]. However, the inherent molecular and biological differences in AM may also contribute to the poor outcomes [6–10].
In contrast to AM, benign acral melanocytic lesions are relatively common in the general population [1,6]. Acral pigmented lesions are more common in skin-of-color patients compared with non-Hispanic whites [1,18]. In fact, the prevalence of palmoplantar nevi is directly correlated with degree of skin pigmentation [1,18–20]. Interestingly, acral nevi in non-Hispanic whites are associated with younger age and are positively correlated with arm nevi density [1,18,21]. While pigmented lesions on glabrous sites are common, patients often lack awareness of their presence [1]. Thus, thorough skin examination that includes evaluation of both palmar and plantar surfaces is necessary to detect and monitor acral pigmented lesions that may otherwise be unknown to the patient.
Challenges in diagnosis & treatment
Melanocytic lesions of acral sites often pose a diagnostic and therapeutic challenge for many clinicians [1,13–14]. Dermatoglyphs of the palms and soles are responsible for the unusual clinical and histologic features of acral melanocytic lesions [22]. As a result, the conventional diagnostic criteria for melanoma are not applicable for acral sites [1]. Additionally, the biopsy process is often complicated, as the procedure may be distressing to patients in terms of pain and limitation afterwards. For these reasons, a potential delay in the diagnosis and treatment of AM may occur.
The importance of dermoscopy in diagnosis
Prognosis of CMM, irrespective of site, is directly dependent on the depth of invasion at the time of diagnosis. At present, early detection is recognized as the only effective strategy to reduce melanoma-related mortality. However, benign acral nevi and early AM may share similar clinical features, making the potential misdiagnosis of a benign acral lesion in the setting of AM an unnerving consequence. Dermoscopy offers an effective noninvasive approach for the differentiation of benign acral nevi from AM. Additionally, dermoscopy allows for the identification of microscopic patterns of early melanoma before macroscopic characteristics of advanced AM develop [23,24]. The dermoscopic patterns of acral surfaces reflect the distribution of melanocytes and melanin granules with regard to the rete ridges [25,26]. The different pigment distributions of benign acral lesions and AM reflect the independent development from each other, with each lesion arising from different portions of the epidermis [26,27]. Thus, by supplementing clinical examination with dermoscopy, the unique dermoscopic patterns exhibited by benign and malignant acral lesions can adequately be identified, further increasing the diagnostic accuracy for AM [23,27–29]. Herein, we describe the dermoscopic features of benign and malignant melanocytic lesions of acral sites.
Dermoscopic features of AM
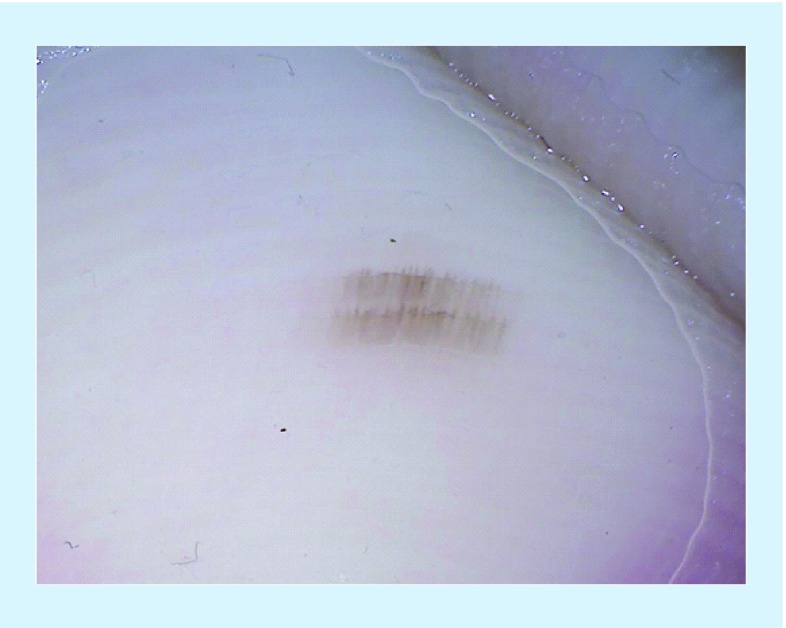
The parallel ridge pattern (PRP) is the hallmark dermoscopic feature for the diagnosis of AM, both invasive and in situ. In a study of 712 melanocytic acral lesions, the PRP demonstrated an extremely high specificity (99.0%) and negative predictive value (97.7%) for AM [27]. This pattern reveals band-like pigmentation on the ridges of skin markings (Figures 1 & 2) and reflects the proliferation of melanocytes in the crista profunda intermedia during early radial growth [27–28,30]. As AM becomes more advanced, the PRP may become disorganized and focally distributed. Importantly, while PRP is considered the hallmark for AM, approximately a third of AM do not display a PRP on dermoscopy [24].
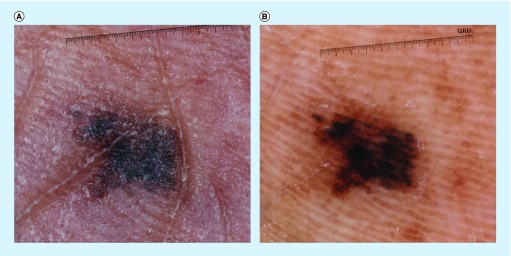
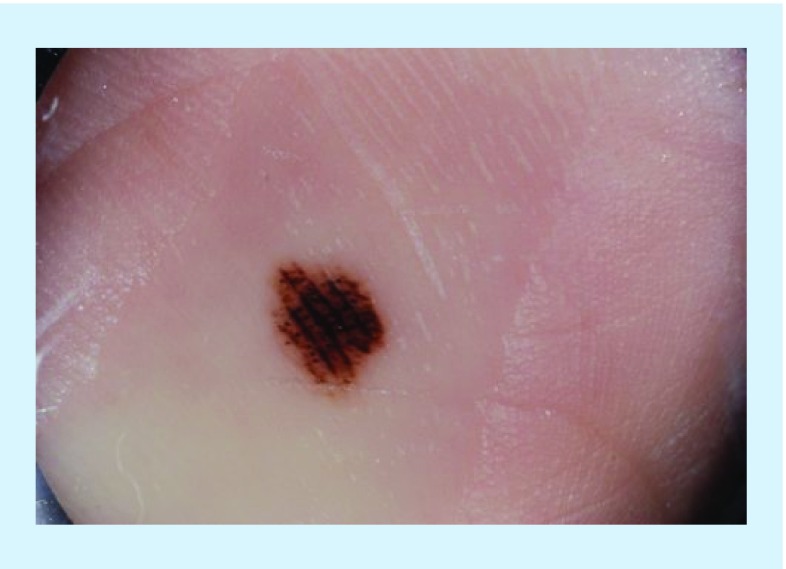
Figure 1. . Clinical and dermoscopic images of acral melanoma.
(A) Clinical image of an asymmetric, irregularly bordered pigmented lesion on the plantar surface of an African American female. (B) Dermoscopy of the above lesion displayed the parallel ridge pattern, as defined by the presence of pigment in the ridges of the skin markings.
Reproduced with permission from © Jennifer A Stein.
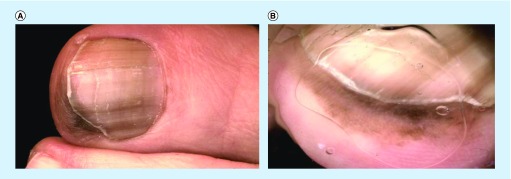
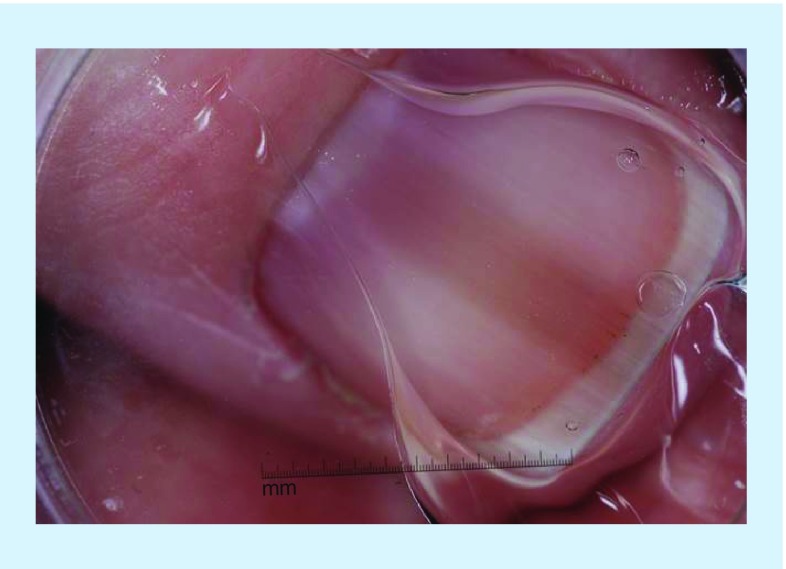
Figure 2. . Clinical and dermoscopic images of acral melanoma.
(A) Clinical image of melanonychia with pigment extending onto the hyponychium of the great toe. (B) Dermoscopic evaluation of pigment extending to the hyponychium revealed a parallel ridge pattern. This lesion was found to be an acral melanoma on histopathologic analysis.
Reproduced with permission from © Jennifer A Stein.
Interestingly, the presence of PRP may precede the presence of atypia on histopathologic evaluation, causing some to suggest that dermoscopy may be superior to histopathologic analysis for the detection of early AM. AM in situ can be divided into three histopathologic phases based on number of melanocytes, degree of atypia and distribution within the epidermis [31]. However, the degree of atypia for the diagnosis of early AM is sometimes subtle, as is often the case for atypical melanosis of the foot [32–34]. Atypical melanosis of the foot represent lesions with clinical and dermoscopic findings suggestive of AM or AM in situ, but without any histologic evidence of melanoma [32–34]. These plantar pigmented lesions present as large macules with irregular borders and variegated colors, and usually show PRP on dermoscopy [30,33–34]. It has been suggested that these lesions possess CCND1 amplifications, a genetic marker of AM and may represent the early or slowly-evolving AM in situ, although additional research is necessary to confirm this finding [25,30,32–35].
In addition to PRP, irregular diffuse pigmentation is another highly characteristic pattern of AM, with a specificity of 96.6% and a negative predictive value of 97.5% [27]. Irregular diffuse pigmentation is characterized by structureless tan-to-black pigmented areas. Compared with PRP, irregular diffuse pigmentation is considered a dermoscopic feature of more advanced AM, as it represents the invasion of neoplastic melanocytes across the entire epidermis and/or upper dermis [27–28,36].
Advanced AM may also demonstrate non-site specific dermoscopic features, such as a blue white veil, regression structures, irregular dots/globules, irregular streaks and polymorphous vessels [28]. Additionally, benign dermoscopic patterns may also be detected in advanced AM [37]. However, unlike benign lesions, these patterns are usually found focally within melanoma on acral volar skin. Lesions that display any of the above patterns or those that cannot be confidently classified as benign after dermoscopic evaluation, should always be biopsied for histopathologic evaluation.
Dermoscopic patterns of benign pigmented lesions
• Major dermoscopic patterns
Approximately two-thirds of acquired acral nevi exhibit at least one of three major dermoscopic patterns: the parallel-furrow pattern (Figure 3); the lattice-like pattern (Figure 4); and the fibrillar pattern (Figure 5) [28]. Approximately 50% of acquired acral nevi exhibit the parallel-furrow pattern, which can be defined as linear pigmentation along the sulci of the skin. Subtle variations of this pattern include the single dotted-line variant, double dotted-line variant, the double-lined variant and a variant with a fine reticulated background [28]. Additionally, the parallel-furrow pattern may present in an organized distribution with the other two major dermoscopic patterns [38,39].
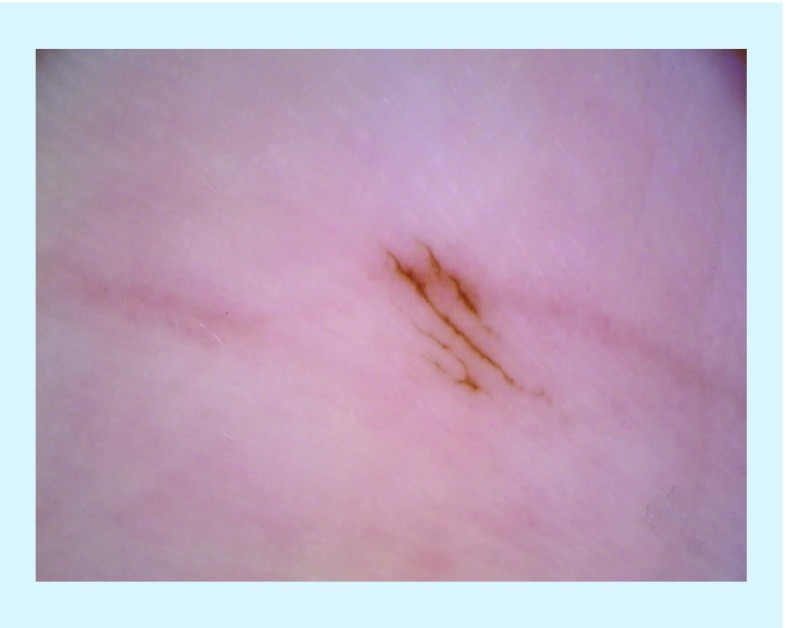
Figure 3. . Dermoscopic photograph of the parallel-furrow pattern, which reveals linear pigmentation along the sulci of the skin.
Reproduced with permission from © Jennifer A Stein.
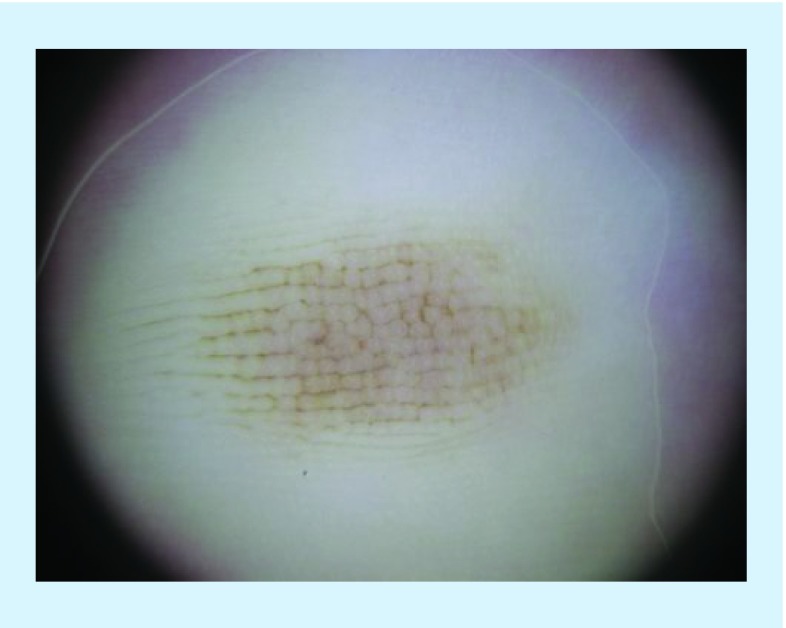
Figure 4. . Dermoscopic image of an acral melanocytic lesion displaying lattice-like pattern, as defined as linear pigmentation along and across the furrows of the skin.
Reproduced with permission from © Jennifer A Stein.
Figure 5. . Acral melanocytic lesion displaying fibrillar pattern on dermoscopy. The fibrillar pattern is a benign dermoscopic pattern that exhibits fine fibrillar or filamentous pigmented lines that run obliquely to the skin markings.
Reproduced with permission from © Jennifer A Stein.
The lattice-like pattern (Figure 4) is observed in 15% of acral lesions and is often considered a variant of the parallel-furrow pattern. The lattice-like pattern is defined by linear pigmentation along and across the furrows of the skin [39]. The majority of melanocytic nevi exhibiting the lattice-like pattern are located on the plantar arch [40]. It has been speculated that this may reflect the change in the skin markings, as the arch often loses the typical parallel pattern [38,40].
The fibrillar pattern (Figure 5) is defined by fine fibrillar or filamentous pigmented lines that run perpendicularly or obliquely to the skin markings. This pattern is detected in 10–20% of plantar nevi and is detected at a lower rate on the palms [38–40]. Acral nevi displaying the fibrillar pattern are most commonly found in pressure-bearing areas, as it is speculated that the fibrillar pattern results from the oblique arrangement of the plantar skin secondary to mechanical pressure [38–40]. For this reason, the fibrillar pattern is often recognized as a modified parallel-furrow pattern. In some instances, the lens of a contact dermatoscope may be used to push the cornified layer horizontally, modifying the fibrillar pattern to that of a parallel-furrow [41].
When assessing a lesion for benign dermoscopic patterns, it is important to confirm that the benign patterns are symmetrically and evenly distributed throughout the lesion. While the parallel-furrow pattern is considered the most potent predictor of a benign acral melanocytic lesion, an estimated 10% of AM may exhibit focally oriented parallel-furrow pattern [37,42]. Thus, the detection of benign dermoscopic patterns should not exclude the diagnosis of AM when other melanoma-specific criteria are present.
• Minor dermoscopic patterns
In addition to the three major dermoscopic patterns, minor patterns may also be detected in approximately a third of acquired acral lesions [23,29,39]. The homogenous pattern is the most prevalent minor pattern and includes diffuse regular light brown pigmentation that is not associated with any other features. The homogenous pattern is frequently observed in darker-skinned patients. It is often characterized clinically as mottled hyperpigmentation of plantar surfaces and may represent acral lentigines [1,19]. Additional minor patterns include the globular pattern, the globulo-streak-like pattern, the transition pattern and the acral reticular pattern.
• Dermoscopic patterns of congenital acral nevi
A congenital melanocytic nevus (CMN) is a melanocytic lesion that is present at birth or presents shortly after [43]. CMN of acral sites are usually larger, more asymmetric and have a greater degree of color variegation than acquired acral melanocytic lesions [44]. Additionally, acral CMN may evolve dramatically within the first few months, with clinical changes such as increase in size, thickness and darkness [43,44]. Importantly, cases of AM in the pediatric population are extremely rare [44,45]. Of 493 acral nevi excised in a cohort of pediatric patients, only three were melanoma, occurring in patients aged 15, 16 and 19 years [45]. Fortunately, dermoscopic features of acral CMN have been identified, thus supporting the use of dermoscopy in the diagnosis and management of volar CMN.
The parallel-furrow pattern is one of the major dermoscopic patterns in both congenital and acquired acral nevi [44]. The crista dotted pattern is most prevalent in acral nevi of the pediatric population. This pattern consists of dots and/or globules of pigment distributed on the ridges of surface skin and represents the adnexocentric distribution of nevus cells, a histopathologic feature of congenital nevi. The combination of the crista dotted with the parallel-furrow pattern is termed peas-in-a-pod (Figure 6). In addition to the three main dermoscopic patterns of acral CMN (i.e., peas-in-a-pod, parallel-furrow, crista dotted), acral CMN may also present with centrally enlarged pink ridges or central blue-gray structures [38,43–44,46]. The benign feature of central blue-gray structures emulates the presence of nevus cells in the mid- or deep-dermis [44].
Figure 6. . Dermoscopic photograph of the peas-in-a-pod pattern, a classic pattern of congenital acral nevi. The peas-in-a-pod pattern is a combination of the parallel-furrow and crista dotted pattern.
Reproduced with permission from © Jennifer A Stein.
Age-related dermoscopic changes of acral CMN are common, with >60% of acral CMN displaying an age-related morphological variation [47]. Several studies have suggested that the prevalence of specific dermoscopic patterns in acral CMN vary with age, with the peas-in-a-pod pattern more prevalent in persons younger than 20 years [43,47–48]. The most common age-related dermoscopic variation is a decrease of local criteria, such as pigmentation and/or globules. At present, the biological mechanism of fading CMN is unknown, although it is a phenomenon that is observed in both acral and non-acral CMN [47]. However, even after consideration of clinical and dermoscopic features, equivocal lesions should always be evaluated histologically.
• Dermoscopic patterns of the nail
There are various causes of melanonychia (Figure 7), a brown or black longitudinal pigmentation on the nail, of which can be grouped into nonmelanocytic and melanocytic origin. Melanonychia caused by melanin deposits may be due to activation or proliferation of nail matrix melanocytes, each with their own set of differential diagnoses [49]. The majority of melanonychia are benign, as the most common causes include subungual hemorrhage, nail matrix nevus, trauma-induced pigmentation, nail apparatus lentigo or ethnic-type nail pigmentation [50,51]. However, approximately two-thirds of nail apparatus melanoma, a disease that comprises approximately 0.7–3.5% of melanoma cases in Western countries, present with melanonychia [49–50,52–53]. Thus, the ability to differentiate the etiology of nail pigmentation is imperative. Dermoscopy of the nail offers a noninvasive approach to the diagnosis of nail pigmentation without the need for a nail matrix biopsy, a difficult procedure with a high potential for permanent nail deformity [54–56].
Figure 7. . Dermoscopic photograph of melanonychia, which exhibits brown background color with symmetric thickness, spacing and color of bands.
Reproduced with permission from © Jennifer A Stein.
• Dermoscopic features of nail apparatus melanoma
When using dermoscopy to evaluate nail pigmentation, it is important to assess background color, arrangement of lines, homogeneity of pigmentation and the presence of dots, globules or blood spots. Evaluation of the band color should always be the initial step. Bands with gray background are often due to melanocyte activation and usually do not require further evaluation. On the other hand, bands with brown/black background occur secondarily to melanocytic proliferation and include nail apparatus melanoma as a differential diagnosis [54,55]. Benign causes of melanocytic proliferation, such as a nail matrix nevus or lentigo, are characterized by melanonychia with regular thickness, spacing and color. Nail apparatus melanoma, on the other hand, usually exhibits melanonychia of irregular thickness, spacing and color [51,54,57–59]. Additionally, nail apparatus melanoma may be characterized by homogenous black pigmentation, with no visible lines present.
Dermoscopy can also be used to visualize the free edge of the nail plate, the proximal nail fold and the hyponychium, allowing further differentiation of benign from malignant nail lesions. Dermoscopy of the nail plate free edge allows for the localization of pigment within the nail plate. Since most bands originate from the distal matrix, nail pigment is most frequently observed in the ventral part of the nail plate [60]. Additionally, dermoscopy can be used to evaluate the proximal nail fold for a micro-Hutchinson sign, a specific, although uncommon, feature for early nail melanoma [54,57]. Interestingly, it is most prevalent in light skin individuals with early melanoma. Hutchinson's sign, pigmentation of the periungual skin, is a clinical feature that can be identified when evaluating the proximal nail fold. It represents the radial growth phase and is highly suggestive of nail apparatus melanoma [61]. Pigment that extends to the hyponychium should be evaluated dermoscopically, as lesions that display a PRP are highly suggestive of AM (Figure 2).
Surveillance of acral melanocytic lesions
Acral melanocytic lesions that display an organized combination of typical benign dermoscopic patterns do not require further dermoscopic follow-up [24,62]. The decision to not follow-up benign lesion is based on the premise that the risk of AM developing in a pre-existing nevus is extremely low [63,64]. In fact, previous studies have suggested that AM arises de novo [63,64]. At present, a change from a benign to a malignant pattern has not been reported in digital follow-up studies of acral melanocytic nevi [39,62]. However, changes within benign patterns have been reported in 20–70% of cases of acquired acral melanocytic nevi [65]. Regardless, any uncertainty in the classification of a dermoscopic pattern warrants biopsy or monitoring with clinical and dermoscopic surveillance.
When to follow-up: acral lesions that require surveillance
Acral lesions that do not show typical benign patterns may require surveillance. These lesions should be measured for their maximum diameter, as a diameter >7 mm is an independent predictor of AM, irrespective of clinical or dermoscopic appearance [37,66–67]. Based on this finding, it has been suggested that lesions >7 mm be excised or biopsied, whereas lesions <7 mm may be monitored clinically and dermoscopically at 3–6-month intervals [62].
The 3–6-month interval that we propose is based on the literature for the use of short-term sequential digital dermoscopy in the monitoring of non-AM. At present, there is no evidence regarding short-term dermoscopy specific to acral melanocytic lesions. However, short-term sequential digital dermoscopy has played a crucial role in the early diagnosis of melanoma [62]. Through this technique, dermoscopic images of melanocytic lesions are captured and assessed over a follow-up period of 3–4.5 months, with any morphologic change (i.e., size, shape, pattern, color) warranting excision [68,69]. The diagnostic accuracy of short-term dermoscopy for the diagnosis of melanoma is 83% [68]. Monitoring specific acral lesions for subtle dermoscopic changes offers an alternative to performing unnecessary excisions on acral sites, areas that are often complicated to biopsy due to hemostatic and mobility concerns. Although short-term dermoscopy provides an effective method for the monitoring of melanocytic lesions, it is important to acknowledge that the existing literature solely involves melanocytic lesions of nonglabrous skin.
Treatment of AM
In 1967, Clark and colleagues suggested a histogenetic classification that subdivided melanoma into four distinct subtypes based on histopathological features [8,38]. However, use of Clark's classification has been controversial, as a significant number of melanomas cannot be classified using this system [8,38]. Recently, Bastian et al. proposed a new classification of melanoma, defining AM as melanoma affecting the palms, soles or nail apparatus and possessing unique molecular and genetic characteristics distinct from other melanoma subtypes [8]. Interestingly, Clark's classification of acral lentiginous melanoma is not identical to Bastian's AM. According to Bastian's original study, five of the 36 AMs were diagnosed as superficial spreading melanoma according to Clark's criteria, thus demonstrating the inconsistency of the two systems in classifying AM [8].
Importantly, as suggested by Bastian et al., the unique genetic aberrations of AM may contribute to its distinct clinical and biologic behavior [8,38,70]. Compared with other CMM subtypes, AM is associated with a worse prognosis and a higher rate of locoregional recurrence after surgery. In fact, the locoregional recurrence rate of AM after surgical excision is estimated to range from 40 to 80% [4,71]. Despite these differences, AM is treated using the same surgical guidelines as those for other CMM subtypes. Additionally, the need for AM-specific treatment options is further highlighted by the recent identification of genomic amplifications exclusive to AM. Thus, revision of the current treatment recommendations for AM may be warranted based on its distinctive clinical and molecular characteristics.
• Genomic amplifications in AM
Compared with other CMM subtypes, AM possesses a unique set of genetic alterations and thus, biological behavior. Mutations in KIT are most prevalent in AM, with a frequency of 10–25% [7–8,72]. KIT mutations are rarely found in non-AM, of which more commonly consist of mutations in BRAF (∼50%) or NRAS (∼20%) [7,8]. In AM, the estimated rate of BRAF mutations varies between 15 and 20%. Interestingly, BRAF mutated AM may be associated with early age at diagnosis, female gender and a plantar presentation [7,73]. Furthermore, NRAS mutations are estimated to occur in approximately 10–15% of AM [7].
In addition to KIT mutations, AM also harbors a significantly greater frequency of genomic amplifications compared with other melanoma subtypes, most notably in the CCND1 or CDK4 genes [10,64]. A hypothetical genetic model of AM has been suggested, with amplification of the oncogene CCND1 recognized as the earliest genetic alteration identified [64]. The CCND1 gene is amplified in approximately 45% of AM and have been detected in both the radial growth phase of primary AMs as well as in melanocytes of early AM in situ [10,30,74–75].
Interestingly, amplifications in the CCND1 gene have also been identified in isolated melanocytes beyond the histopathologic margin of AM. These ‘field cells’ may extend to 10 mm beyond the histopathologic margin and represent the latent phase prior to epidermal melanocytic proliferation [10,64,76]. Thus, the high rate of locoregional recurrence after surgical excision of AM may be related to the retained presence of these isolated ‘field cells’ [4,71,76–77].
At present, the National Comprehensive Cancer Network recommends surgical treatment of primary melanoma, regardless of subtype, based on tumor thickness. Compared with CMM overall, AM has a unique genetic profile along with an aggressive biologic behavior and high rate of locoregional recurrence. Interestingly, Gumaste et al. demonstrated that AM tumors less than 2 mm in thickness had a higher recurrence rate compared with their nonacral counterparts [6]. Thus, modification of the current melanoma National Comprehensive Cancer Network guidelines may be necessary to account for the unique biologic behavior of AM [10]. In fact, some have speculated that widening the surgical margin for AM may improve locoregional control and thus, AM-specific survival [4,78]. Importantly, surgery on acral sites is associated with increased morbidity and it is often difficult to achieve even the recommended margins in acral sites. Thus, further widening the surgical margins may be practically and technically difficult, potentially further increasing morbidity [6]. Therefore, it is imperative that additional research on the utility of widening surgical margins for AM be completed prior to changing the current treatment recommendations.
• Systemic therapies
About a third of AM patients with disseminated disease may benefit from targeted therapies with BRAF or KIT inhibitors, as these oncogenes are mutated in approximately 17 and 15% of AM tumors respectively [7]. While there is a vast array of literature on the use of targeted therapies for CMM overall, the literature specific to AM is much less limited. Imatinib is a tyrosine-kinase inhibitor with c-kit as one of its biologic targets. Clinical trials have investigated the use of imatinib for the metastatic AM. The results of one clinical trial revealed that imatinib was associated with a positive response in patients only with KIT mutations. AM patients with KIT amplifications or wild-type did not demonstrate a significant response after imatinib use [79–81]. Interestingly, the presence of NRAS mutations and/or KIT copy number gain were hypothesized as potential mechanisms of therapeutic resistance to imatinib [79]. Additionally, a Phase II trial of sunitinib, a multi-targeted receptor tyrosine kinase inhibitor, was performed in patients with stage III or IV metastatic AM. Although the medication was poorly tolerated, sunitinib showed short-term activity in the treatment of AM that was not dependent on the presence of KIT mutations [82].
The use of immunotherapy in the treatment of AM has also recently been questioned. Ipilimumab has been successful in improving overall survival for patients with advanced CMM [83]. The results of a retrospective review of 35 patients with AM suggested clinical efficacy of ipilimumab in the treatment of patients with advanced AM. Compared with unselected melanomas, patients with advanced AM had comparable objective response (11%) and stable disease rates (23%) [83]. Although additional investigation regarding the immune biology of AM is necessary, the use of other emerging immunotherapies (i.e., anti-PD-1/PD-L1) for the treatment of AM may be on the horizon.
Conclusion
Melanocytic lesions of acral sites are common among the US population, with the majority of these lesions being benign. However, clinical differentiation of benign acral melanocytic lesions from AM is challenging and the potential for a missed diagnosis of AM is fatal. However, the use of dermoscopy has improved the diagnostic accuracy of AM, as the identification of unique dermoscopic features of AM has resulted in earlier detection and arguably improved survival. Yet, histopathology remains the gold standard for diagnosis and should be performed when dermoscopic diagnosis remains unclear.
Future perspective
As evident by its unique clinical and biologic behavior, AM may represent a distinct entity from other CMM subtypes. Thus, while melanoma treatment guidelines are currently uniform regardless of histo-subtype, AM-specific treatment guidelines may be developed and utilized in future clinical practice. Furthermore, additional research regarding the efficacy of AM-specific targeted therapies and immunotherapies for the treatment of advanced AM is needed.
Footnotes
Financial & competing interests disclosure
JA Stein was supported by the Irwin I Lubowe Fellowship in Dermatology. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J. Am. Acad. Dermatol. 2016;74(4):724–730. doi: 10.1016/j.jaad.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral melanoma: incidence and survival patterns in the United States, 1986–2005. Arch. Dermatol. 2009;145(4):427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durbec F, Martin L, Derancourt C, Grange F. Melanoma of the hand and foot: epidemiological, prognostic and genetic features. A systematic review. Br. J. Dermatol. 2012;166(4):727–739. doi: 10.1111/j.1365-2133.2011.10772.x. [DOI] [PubMed] [Google Scholar]
- 4.Gumaste P, Penn L, Cohen N, Berman R, Pavlick A, Polsky D. Acral melanoma of the foot misdiagnosed as a traumatic ulcer. A cautionary case. J. Am. Podiatr. Med. Assoc. 2015;105(2):189–194. doi: 10.7547/0003-0538-105.2.189. [DOI] [PubMed] [Google Scholar]
- 5.Tuma B, Yamada S, Atallah ÁN, Araujo FM, Hirata SH. Dermoscopy of black skin: a cross-sectional study of clinical and dermoscopic features of melanocytic lesions in individuals with type V/VI skin compared with those with type I/II skin. J. Am. Acad. Dermatol. 2015;73(1):114–119. doi: 10.1016/j.jaad.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 6.Gumaste PV, Fleming NH, Silva I, et al. Analysis of recurrence patterns in acral versus nonacral melanoma: should histologic subtype influence treatment guidelines? J. Natl Compr. Canc. Netw. 2014;12(12):1706–1712. doi: 10.6004/jnccn.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral melanoma. J. Dermatol. Sci. 2013;72(3):284–289. doi: 10.1016/j.jdermsci.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]; •• Highlights the distinct genetic pathways involved in the development of different melanoma subtypes.
- 9.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 10.Bastian BC, Kashani-Sabet M, Hamm H, et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000;60(7):1968–1973. [PubMed] [Google Scholar]; •• Reveals that acral melanoma (AM) is a distinct type of melanoma, as it is uniquely characterized by early gene amplifications in tumorigenesis. Additionally, this paper describes the presence of ‘field cells’ beyond the histopathological border, suggesting a potential mechanism for the high rate of locoregional recurrence in AM.
- 11.Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br. J. Dermatol. 2000;143(2):275–280. doi: 10.1046/j.1365-2133.2000.03651.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara K, Saida T, Yamamoto A. Japanese Skin Cancer Society Prognosis and Statistical Investigation Committee. Updated statistical data for malignant melanoma in Japan. Int. J. Clin. Oncol. 2001;6(3):109–116. doi: 10.1007/pl00012091. [DOI] [PubMed] [Google Scholar]
- 13.Albreski D, Sloan SB. Melanoma of the feet: misdiagnosed and misunderstood. Clin. Dermatol. 2009;27(6):556–563. doi: 10.1016/j.clindermatol.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Franke W, Neumann NJ, Ruzicka T, Schulte KW. Plantar malignant melanoma – a challenge for early recognition. Melanoma Res. 2000;10(6):571–576. doi: 10.1097/00008390-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Serarslan G, Akçalý C, Atik E. Acral melanoma misdiagnosed as tinea pedis: a case report. Int. J. Dermatol. 2004;43(1):37–38. doi: 10.1111/j.1365-4632.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosen T. Acral melanoma misdiagnosed as verruca plantaris: a case report. Dermatol. Online J. 2006;12(4):3. [PubMed] [Google Scholar]
- 17.Hussin P, Loke SC, Noor FM, Mawardi M, Singh VA. Malignant melanoma of the foot in patients with diabetes mellitus – a trap for the unwary. Med. J. Malaysia. 2012;67(4):422–423. [PubMed] [Google Scholar]
- 18.Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch. Dermatol. 2010;146(10):1085–1094. doi: 10.1001/archdermatol.2010.299. [DOI] [PubMed] [Google Scholar]
- 19.Coleman WP, 3rd, Gately LE, 3rd, Krementz AB, Reed RJ, Krementz ET. Nevi, lentigines, and melanomas in blacks. Arch. Dermatol. 1980;116(5):548–551. [PubMed] [Google Scholar]
- 20.Jaramillo-Ayerbe F, Vallejo-Contreras J. Frequency and clinical and dermatoscopic features of volar and ungual pigmented melanocytic lesions: a study in schoolchildren of Manizales, Colombia. Pediatr. Dermatol. 2004;21(3):218–222. doi: 10.1111/j.0736-8046.2004.21305.x. [DOI] [PubMed] [Google Scholar]
- 21.Green A, McCredie M, MacKie R, et al. A case-control study of melanomas of the soles and pAMs (Australia and Scotland) Cancer Causes Control. 1999;10(1):21–25. doi: 10.1023/a:1008872014889. [DOI] [PubMed] [Google Scholar]
- 22.Thomas L, Phan A, Pralong P, Poulalhon N, Debarbieux S, Dalle S. Special locations dermoscopy: facial, acral, and nail. Dermatol. Clin. 2013;31(4):615–624. doi: 10.1016/j.det.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Malvehy J, Puig S. Dermoscopic patterns of benign volar melanocytic lesions in patients with atypical mole syndrome. Arch. Dermatol. 2004;140(5):538–544. doi: 10.1001/archderm.140.5.538. [DOI] [PubMed] [Google Scholar]
- 24.Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br. J. Dermatol. 2015;173(4):1041–1049. doi: 10.1111/bjd.14045. [DOI] [PubMed] [Google Scholar]; • The BRAAFF checklist improves the diagnostic accuracy of dermoscopy for the diagnosis of AM, as it incorporates the finding that approximately a third of AMs do not demonstrate a parallel ridge pattern.
- 25.Ishihara Y, Saida T, Miyazaki A, Koga H, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am. J. Dermatopathol. 2006;28(1):21–27. doi: 10.1097/01.dad.0000187931.05030.a0. [DOI] [PubMed] [Google Scholar]
- 26.Saida T, Koga H, Goto Y, Uhara H. Characteristic distribution of melanin columns in the cornified layer of acquired acral nevus: an important clue for histopathologic differentiation from early acral melanoma. Am. J. Dermatopathol. 2011;33(5):468–473. doi: 10.1097/DAD.0b013e318201ac8f. [DOI] [PubMed] [Google Scholar]
- 27.Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar sites: results of a multicenter study in Japan. Arch. Dermatol. 2004;140(10):1233–1238. doi: 10.1001/archderm.140.10.1233. [DOI] [PubMed] [Google Scholar]; •• The diagnostic variables (i.e., sensitivity, specificity, positive predictive value, negative predictive value, diagnostic accuracy) of the major dermoscopic patterns observed in melanocytic lesions on acral volar skin were determined. The parallel ridge pattern aids in detecting early acral melanomas.
- 28.Saida T, Oguchi S, Miyazaki A. Dermoscopy for acral pigmented skin lesions. Clin. Dermatol. 2002;20(3):279–285. doi: 10.1016/s0738-081x(02)00219-5. [DOI] [PubMed] [Google Scholar]
- 29.Altamura D, Altobelli E, Micantonio T, Piccolo D, Fargnoli MC, Peris K. Dermoscopic patterns of acral melanocytic nevi and melanomas in a white population in central Italy. Arch. Dermatol. 2006;142(9):1123–1128. doi: 10.1001/archderm.142.9.1123. [DOI] [PubMed] [Google Scholar]
- 30.Yamaura M, Takata M, Miyazaki A, Saida T. Specific dermoscopy patterns and amplifications of the cyclin D1 gene to define histopathologically unrecognizable early lesions of acral melanoma in situ . Arch. Dermatol. 2005;141(11):1413–1418. doi: 10.1001/archderm.141.11.1413. [DOI] [PubMed] [Google Scholar]
- 31.Saida T. Malignant melanoma in situ on the sole of the foot. Its clinical and histopathologic characteristics. Am. J. Dermatopathol. 1989;11(2):124–130. doi: 10.1097/00000372-198911020-00003. [DOI] [PubMed] [Google Scholar]
- 32.Oh TS, Bae EJ, Ro KW, Seo SH, Son SW, Kim IH. Acral melanoma developing during long-standing atypical melanosis: usefulness of dermoscopy for detection of early acral melanoma. Ann. Dermatol. 2011;23(3):400–404. doi: 10.5021/ad.2011.23.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilinc Karaarslan I, Akalin T, Unal I, Ozdemir F. Atypical melanosis of the foot showing a dermoscopic feature of the parallel ridge pattern. J. Dermatol. 2007;34(1):56–59. doi: 10.1111/j.1346-8138.2007.00217.x. [DOI] [PubMed] [Google Scholar]
- 34.Chiu HH, Hu SC, Ke CL, Cheng ST. Dermoscopy identifies histopathologically indiscernible malignant lesion of atypical melanosis of the foot, an early lesion of acral melanoma in situ . Dermatol. Surg. 2008;34(7):979–983. doi: 10.1111/j.1524-4725.2008.34192.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Choi M, Jo SJ, Min HS, Cho KH. Acral melanoma: indolent subtype with long radial growth phase. Am. J. Dermatopathol. 2014;36(2):142–147. doi: 10.1097/DAD.0b013e31829bea8b. [DOI] [PubMed] [Google Scholar]
- 36.Kawabata Y, Tamaki K. Distinctive dermoscopic features of acral melanoma in situ from plantar melanocytic nevi and their histopathologic correlation. J. Cutan. Med. Surg. 1998;2(4):199–204. doi: 10.1177/120347549800200404. [DOI] [PubMed] [Google Scholar]
- 37.Braun RP, Thomas L, Dusza SW, et al. Dermoscopy of acral melanoma: a multicenter study on behalf of the international dermoscopy society. Dermatology. 2013;227(4):373–380. doi: 10.1159/000356178. [DOI] [PubMed] [Google Scholar]; • Evaluates dermoscopic patterns of acral lesions in a predominantly Caucasian population, as most studies on dermoscopy of acral lesions have been conducted in Asian populations. Additionally, this paper also confirmed the importance of maximum diameter in determining management of acral lesions.
- 38.Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J. Dermatol. 2011;38(1):25–34. doi: 10.1111/j.1346-8138.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 39.Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch. Dermatol. 2007;143(11):1423–1426. doi: 10.1001/archderm.143.11.1423. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki A, Saida T, Koga H, Oguchi S, Suzuki T, Tsuchida T. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J. Am. Acad. Dermatol. 2005;53(2):230–236. doi: 10.1016/j.jaad.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 41.Maumi Y, Kimoto M, Kobayashi K, Ito N, Saida T, Tanaka M. Oblique view dermoscopy changes regular fibrillar pattern into parallel furrow pattern. Dermatology. 2009;218(4):385–386. doi: 10.1159/000202986. [DOI] [PubMed] [Google Scholar]
- 42.Braun RP, Rabinovitz HS, Oliviero M, Kopf AW, Saurat JH. Dermoscopy of pigmented skin lesions. J. Am. Acad. Dermatol. 2005;52(1):109–121. doi: 10.1016/j.jaad.2001.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch. Dermatol. 2011;147(7):809–813. doi: 10.1001/archdermatol.2011.150. [DOI] [PubMed] [Google Scholar]
- 44.Chuah SY, Tsilika K, Chiaverini C, et al. Dermoscopic features of congenital acral melanocytic naevi in children: a prospective comparative and follow-up study. Br. J. Dermatol. 2015;172(1):88–93. doi: 10.1111/bjd.13187. [DOI] [PubMed] [Google Scholar]
- 45.Moscarella E, Zalaudek I, Cerroni L, et al. Excised melanocytic lesions in children and adolescents – a 10-year survey. Br. J. Dermatol. 2012;167(2):368–373. doi: 10.1111/j.1365-2133.2012.10952.x. [DOI] [PubMed] [Google Scholar]
- 46.Garrido-Ríos AA, Carrera C, Puig S, Aguilera P, Salerni G, Malvehy J. Homogenous blue pattern in an acral congenital melanocytic nevus. Dermatology. 2008;217(4):315–317. doi: 10.1159/000151442. [DOI] [PubMed] [Google Scholar]
- 47.Fargnoli MC, Suppa M, Micantonio T, Antonini A, Tambone S, Peris K. Dermoscopic features and follow-up changes of acral melanocytic naevi in childhood and adolescence. Br. J. Dermatol. 2014;170(2):374–381. doi: 10.1111/bjd.12667. [DOI] [PubMed] [Google Scholar]
- 48.Suzaki R, Ishizaki S, Iyatomi H, Tanaka M. Age-related prevalence of dermatoscopic patterns of acral melanocytic nevi. Dermatol. Pract. Concept. 2014;4(1):53–57. doi: 10.5826/dpc.0401a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin. Cutan. Med. Surg. 2009;28(1):49–54. doi: 10.1016/j.sder.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J. Am. Acad. Dermatol. 2016;46:1121–1127. doi: 10.1016/j.jaad.2015.12.039. [DOI] [PubMed] [Google Scholar]; • The most recent diagnostic algorithm for melanonychia is outlined in this paper.
- 51.Tosti A, Argenziano G. Dermoscopy allows better management of nail pigmentation. Arch. Dermatol. 2002;138(10):1369–1370. doi: 10.1001/archderm.138.10.1369. [DOI] [PubMed] [Google Scholar]
- 52.Cohen T, Busam KJ, Patel A, Brady MS. Subungual melanoma: management considerations. Am. J. Surg. 2008;195(2):244–248. doi: 10.1016/j.amjsurg.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am. J. Surg. Pathol. 2007;31(12):1902–1912. doi: 10.1097/PAS.0b013e318073c600. [DOI] [PubMed] [Google Scholar]
- 54.Ronger S, Touzet S, Ligeron C, et al. Dermoscopic examination of nail pigmentation. Arch. Dermatol. 2002;138(10):1327–1333. doi: 10.1001/archderm.138.10.1327. [DOI] [PubMed] [Google Scholar]
- 55.Lencastre A, Lamas A, Sá D, Tosti A. Onychoscopy. Clin. Dermatol. 2013;31(5):587–593. doi: 10.1016/j.clindermatol.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 56.Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol. Clin. 2015;33(2):185–195. doi: 10.1016/j.det.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Phan A, Dalle S, Touzet S, Ronger-Savlé S, BAMe B, Thomas L. Dermoscopic features of acral melanoma in a large series of 110 cases in a white population. Br. J. Dermatol. 2010;162(4):765–771. doi: 10.1111/j.1365-2133.2009.09594.x. [DOI] [PubMed] [Google Scholar]
- 58.Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J. Am. Acad. Dermatol. 2007;56(5):835–847. doi: 10.1016/j.jaad.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 59.Thomas L, Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol. Ther. 2007;20(1):3–10. doi: 10.1111/j.1529-8019.2007.00106.x. [DOI] [PubMed] [Google Scholar]
- 60.Braun RP, Baran R, Saurat JH, Thomas L. Surgical Pearl: dermoscopy of the free edge of the nail to determine the level of nail plate pigmentation and the location of its probable origin in the proximal or distal nail matrix. J. Am. Acad. Dermatol. 2006;55(3):512–513. doi: 10.1016/j.jaad.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 61.Baran R, Kechijian P. Hutchinson's sign: a reappraisal. J. Am. Acad. Dermatol. 1996;34(1):87–90. doi: 10.1016/s0190-9622(96)90839-7. [DOI] [PubMed] [Google Scholar]
- 62.Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch. Dermatol. 2011;147(6):741–743. doi: 10.1001/archdermatol.2011.136. [DOI] [PubMed] [Google Scholar]
- 63.Saida T. Lessons learned from studies of the development of early melanoma. Int. J. Clin. Oncol. 2005;10(6):371–374. doi: 10.1007/s10147-005-0539-0. [DOI] [PubMed] [Google Scholar]
- 64.Takata M, Murata H, Saida T. Molecular pathogenesis of malignant melanoma: a different perspective from the studies of melanocytic nevus and acral melanoma. Pigment Cell Melanoma Res. 2010;23(1):64–71. doi: 10.1111/j.1755-148X.2009.00645.x. [DOI] [PubMed] [Google Scholar]
- 65.Altamura D, Zalaudek I, Sera F, et al. Dermoscopic changes in acral melanocytic nevi during digital follow-up. Arch. Dermatol. 2007;143(11):1372–1376. doi: 10.1001/archderm.143.11.1372. [DOI] [PubMed] [Google Scholar]
- 66.Saida T, Yoshida N, Ikegawa S, Ishihara K, Nakajima T. Clinical guidelines for the early detection of plantar malignant melanoma. J. Am. Acad. Dermatol. 1990;23(1):37–40. doi: 10.1016/0190-9622(90)70182-h. [DOI] [PubMed] [Google Scholar]
- 67.Saida T, Ishihara Y, Tokuda Y. Effective detection of plantar malignant melanoma. Int. J. Dermatol. 1993;32(10):722–725. doi: 10.1111/j.1365-4362.1993.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 68.Altamura D, Avramidis M, Menzies SW. Assessment of the optimal interval for and sensitivity of short-term sequential digital dermoscopy monitoring for the diagnosis of melanoma. Arch. Dermatol. 2008;144(4):502–506. doi: 10.1001/archderm.144.4.502. [DOI] [PubMed] [Google Scholar]
- 69.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch. Dermatol. 2001;137(12):1583–1589. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 70.Yun J, Lee J, Jang J, et al. KIT amplifications and gene mutations in acral/mucosal melanoma in Korea. APMIS. 2011;119(6):330–335. doi: 10.1111/j.1600-0463.2011.02737.x. [DOI] [PubMed] [Google Scholar]
- 71.Phan A, Touzet S, Dalle S, Ronger-Savlé S, BAMe B, Thomas L. Acral melanoma: a clinicoprognostic study of 126 cases. Br. J. Dermatol. 2006;155(3):561–569. doi: 10.1111/j.1365-2133.2006.07368.x. [DOI] [PubMed] [Google Scholar]
- 72.Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008;14(21):6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 73.Ashida A, Uhara H, Kiniwa Y, et al. Assessment of BRAF and KIT mutations in Japanese melanoma patients. J. Dermatol. Sci. 2012;66(3):240–242. doi: 10.1016/j.jdermsci.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Sauter ER, Yeo UC, von Stemm A, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62(11):3200–3206. [PubMed] [Google Scholar]
- 75.Takata M, Goto Y, Ichii N, et al. Constitutive activation of the mitogen-activated protein kinase signaling pathway in acral melanomas. J. Invest. Dermatol. 2005;125(2):318–322. doi: 10.1111/j.0022-202X.2005.23812.x. [DOI] [PubMed] [Google Scholar]
- 76.Bastian BC. Understanding the progression of melanocytic neoplasia using genomic analysis: from fields to cancer. Oncogene. 2003;22(20):3081–3086. doi: 10.1038/sj.onc.1206463. [DOI] [PubMed] [Google Scholar]; •• Highlights the importance of genomic analysis in understanding the progression of melanocytic neoplasms. It also offers insight on the potential clinical applications of genomic analysis for the classification of melanoma and the detection of minimal residual disease.
- 77.Egger ME, McMasters KM, Callender GG, et al. Unique prognostic factors in acral melanoma. Am. J. Surg. 2012;204(6):874–879. doi: 10.1016/j.amjsurg.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Uehara J, Ito Y, Takahashi I, et al. Sequential acral melanomas of the foot. Case Rep. Dermatol. 2010;2(3):201–206. doi: 10.1159/000323467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. 2013;31(26):3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang X, Zhou J, Yuen NK, et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin. Cancer Res. 2008;14(23):7726–7732. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 81.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buchbinder EI, Sosman JA, Lawrence DP, et al. Phase II study of sunitinib in patients with metastatic mucosal or acral melanoma. Cancer. 2015;121(22):4007–4015. doi: 10.1002/cncr.29622. [DOI] [PubMed] [Google Scholar]
- 83.Johnson DB, Peng C, Abramson RG, et al. Clinical activity of ipilimumab in acral melanoma: a retrospective review. Oncologist. 2015;20(6):648–652. doi: 10.1634/theoncologist.2014-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]