Summary
The oral and maxillofacial region has a complicated anatomy with critical contiguous organs, including the brain, eyes, vital teeth, and complex networks of nerves and blood vessels. Therefore, advances in basic scientific research within the field of intraoperative oral and maxillofacial surgery have enabled the introduction of the features of these techniques into routine clinical practice to ensure safe and reliable surgery. A navigation system provides a useful guide for safer and more accurate complex in oral and maxillofacial surgery. The effectiveness of a navigation system for oral and maxillofacial surgery has been indicated by clinical applications in maxillofacial trauma surgery including complex midfacial fractures and orbital trauma reconstruction, foreign body removal, complex dentoalveolar surgery, skull base surgery including surgery of the temporomandibular joint (TMJ), and orthognathic surgery. However, some fundamental issues remain involving the mobility of the mandible and difficulty in updating images intraoperatively. This report presents an overview and feasible applications of available navigation systems with a focus on the clinical feasibility of the application of navigation systems in the field of oral and maxillofacial surgery and solutions to current problems.
Keywords: Navigation systems, Oral and maxillofacial surgery, Review
1. Introduction
The oral and maxillofacial region has a complicated anatomy with critical contiguous organs, including the brain, eyes, vital teeth, and complex networks of nerves and blood vessels. In many cases, it is necessary to perform imaging diagnosis with computed tomography (CT) and/or magnetic resonance imaging (MRI) prior to oral and maxillofacial surgery to clarify the surgical site and surrounding anatomical structures. The rapid development of imaging technology has made it possible to quickly process and visualize the large amount of data produced by various digital imaging modalities. Prerequisites for three-dimensional (3D) visualization and programs for computer-assisted 3D planning of surgical procedures have been established. These information sources are available in the operating room to assist the surgeon at the time of surgery.
Computer-assisted surgery uses image processing data and can be divided into two main categories: computer-assisted pre-surgical planning and navigation. Computer-assisted pre-surgical planning includes preoperative surgical simulation with 3D images or models. Preoperative surgical simulations with 3D images have been used to determine the appropriate positions and sizes of dental implants and to assess the degree of movement during orthognathic surgery. Preoperative surgical simulations with 3D models, such as stereolithographic models, are useful to evaluate treatment plans and to acquire precise representations of the underlying skeletal anatomy of the patient [1]. A system for navigation was developed as a next step forward in the sequence of “diagnosis-surgical planning-surgery,” allowing the surgeon to visualize the actual position of surgical instruments in real time on the monitor displaying the CT or MRI 3D data of the patient. Navigation systems involve the integration of imaging with the surgical field, which allows simultaneous visualization of different types of images to reveal structures that are normally visible only intraoperatively and permits navigation in areas of anatomical sensitivity. These systems have recently evolved to improve precision and simplify the surgical procedure by minimizing intraoperative invasiveness. The development of navigation assisted surgery has improved execution and predictability, allowing for greater precision during oral and maxillofacial surgery [2].
This review report presents an overview of available navigation systems and applications with a focus on clinical usefulness and solutions to current problems with navigation systems for use in the field of oral and maxillofacial surgery.
2. Surgical navigation
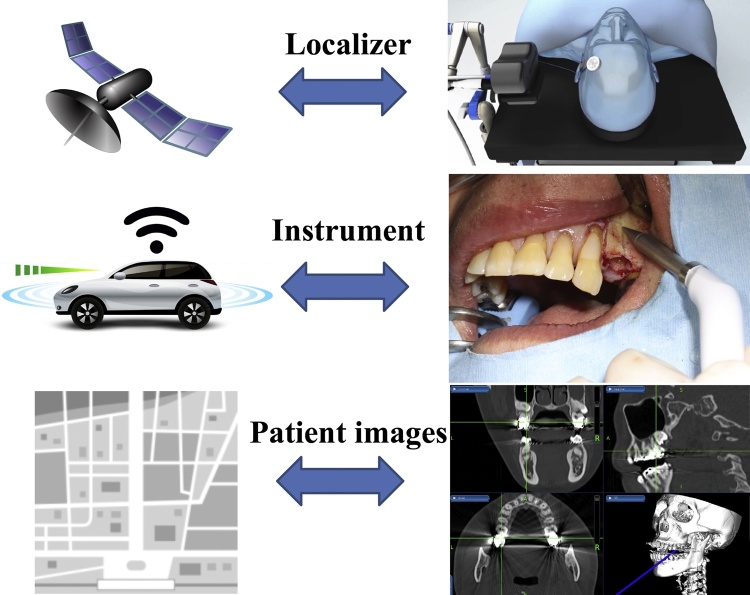
Surgical navigation is comparable to a global positioning system (GPS) commonly used in automobiles and is composed of three primary components: a localizer, which is analogous to a satellite in space; an instrument or surgical probe, which represents the track waves emitted by the GPS unit in the vehicle; and a CT scan data set, which is analogous to a road map (Figure 1, Figure 2). Navigation systems were initially developed for use in neurosurgery and are now commonly used in craniomaxillofacial surgery because of the reliability and an accuracy of less than 1–2 mm [3], [4], [5], [6], [7].
Figure 1.
Components of a surgical navigation system.
Navigation system is comparable to a global positioning system (GPS) commonly used in automobiles and is composed of three primary components: a localizer, which is analogous to a satellite in space; an instrument or surgical probe, which represents the track waves emitted by the GPS unit in the vehicle; and a CT scan data set, which is analogous to a road map.
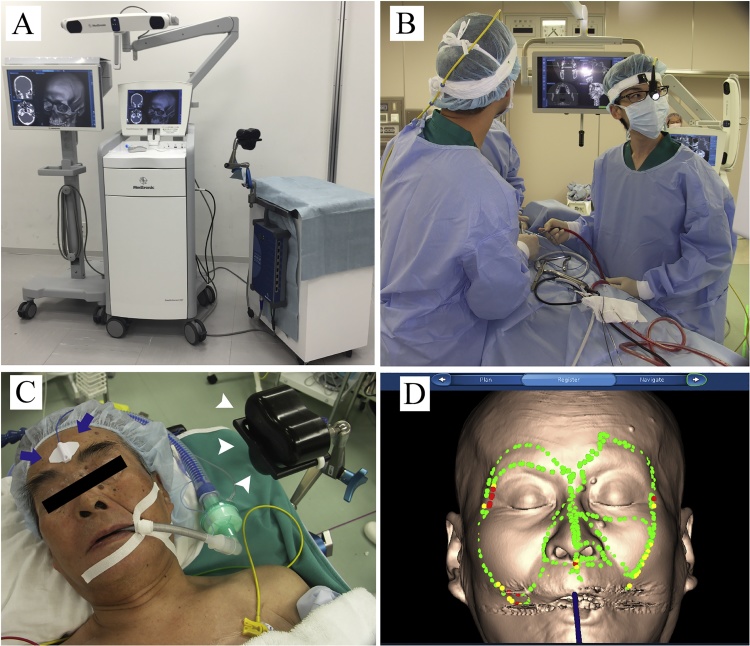
Figure 2.
(A) The StealthStation surgical navigation system.
This system has accurate patient registration software and advanced visualization to navigate the maxillofacial area.
(B) Intraoperative referencing and image-to-patient registration of the image-guided visualization display and the navigation unit.
(C) Preparation for registration using the reference points (arrows) and magnetic field generator (arrowheads) in place before final draping.
(D) Marker-free registration with the tracer probe.
2.1. Differences between the optical and electromagnetic tracking systems
There are two main types of navigation systems currently available: optical and electromagnetic. Both systems perform the same functions. However, the technology used to provide the information to the surgeon is very different. An optical system, or infrared system, as its name suggests, uses infrared sensors in combination with light-emitting structures or light reflectors that are fixed to the patient’s head and to a hand-held probe. Both the light-emitting structures and instrument must be detected or “seen” by the system camera or computer in order to track the position of the instruments within the surgical field [8]. Meanwhile, electromagnetic systems use electromagnetic fields and reference points on a device attached to the patient’s head and a wired instrument that the surgeon uses within the surgical field. Unlike an optical system, an electromagnetic system does not have to be “seen” by the computer, meaning that it does not matter if other devices or equipment in the operating theatre are placed between the computer and patient. However, too much metal within the electromagnetic field can cause inaccuracies [9].
2.2. Registration techniques
Registration techniques can be categorized into two main groups: marker-based [10], [11], [12] and marker-free [13], [14]. Marker-based registration requires markers that are apparent on pre-operative images and are easily detectable on the patient during the procedure, for example a referencing dental splint [15], skin adhesive reference markers [16], and bone-implanted screws [17]. As opposed to a marker-based method, marker-free registration relies on the patient’s craniofacial anatomy itself. One approach is to register defined bone protuberances to the corresponding structures apparent on a CT scan. Laser surface scanning is applied in the distinct marker-free registration technique, where random points on the facial skin surface are matched to the corresponding points on the soft tissues on CT/MRI images [14], [18]. More recently, a hybrid registration of a combination of these methods has also been proposed [19], [20]. When using different imaging modalities, for example CT and MRI, it is necessary to devise the use of set image data. Basically, registration uses one data and overlays another image data on that data. We are using CT which allows both registrations by hard tissue and soft tissue. By superimposing the MRI image data on it, it was possible to use the navigation exactly.
2.3. Application to the mandible
The use of a navigation system can improve preoperative planning and provide a high degree of intraoperative accuracy and precision. However, the navigational accuracy is limited by the system used, the method of obtaining the imaging data, and syncing the imaging data with the patient’s actual position during the procedure. In maxillary and midfacial surgery, the accuracy of currently available navigation systems is considered to be reliable because most were originally developed for neurosurgical purposes [21]. However, navigation systems are not approved for use in the mandible region because of the constant movement in this area. It is difficult to use a navigation system for surgery of the mandible because the mobile nature of the mandible complicates synchronization with the preoperative imaging data during surgery. However, if the mandible were to be held in an identical position during image acquisition and the surgical procedure, then all structures within the image could be fixed in an identical position, thereby ensuring the accuracy of the navigation system.
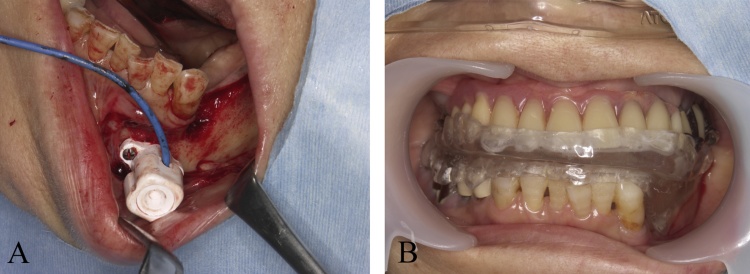
At present, there are other possible solutions for the application of navigation in the mandible. One approach is to mount a dynamic reference frame to the mandible that enables continuous tracking of mandibular movement during surgery [22] (Fig. 3A). This method enables direct tracking of the mandible via a sensor frame and tooth/mandible-supported fiducial markers useful for mandibular navigation [23]. With this approach, the mandible is allowed to freely move during the surgery. However, fixation of the reference points requires a special procedure and is, therefore, more time-consuming and complicated. As a second strategy, an immobile intercuspal position is maintained so that mandibular synchronization can be intraoperatively ensured [24]. However, most mandibular surgeries require the jaw to be open during transoral surgery. Therefore, a third strategy is to position the mandible in a reproducible posture or a defined position against the maxilla, using an occlusion splint (Fig. 3B). Artificial fixation of the mandible via a template appears to introduce no additional error. This strategy is sensitive to the relative movement of the mandible and, in turn, reduces the accuracy of the navigation system [5], [25], [26]. In the current system, navigation can be used in mandibular surgery by devising to position the mandible.
Figure 3.
The application of navigation in the mandible.
(A) The direct tracking of the mandible via a sensor frame and tooth/mandible-supported fiducial markers.
(B) The mandibular position in a reproducible posture or a defined position against the maxilla using an occlusion splint.
2.4. Navigation using intraoperative updated images
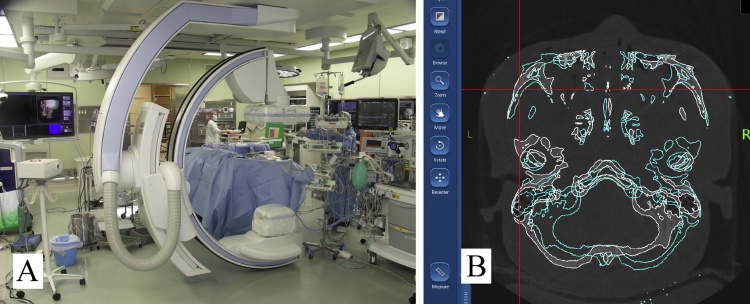
The use of intraoperative imaging is not yet standard in oral and maxillofacial surgery. However, many oral and maxillofacial experts believe that postoperative CT imaging with multiplanar reconstruction (MPR) images should be mandatory for repair of midfacial fractures with orbital wall involvement. These images are supported by the fact that the correct position of the bones, such as the orbital walls, zygoma, and zygomatic arch, and the precise placement of implant material after fracture reduction or movement of bone segments for orthognathic surgery can be accurately evaluated only on MPR images [27]. A clinical study investigating intraoperative CT imaging has already shown that high-contrast structures, such as bone, can be imaged at a quality comparable to that of conventional CT, even in close proximity to the implant material. In recent years, intraoperative CT imaging by cone-beam (CB) CT, C-arm, and O-arm systems has become possible [28], [29]. Furthermore, although not documented in the field of oral surgery, the effectiveness of a navigation system using intraoperative CT images was demonstrated in the orthopedic field for surgery involving complicated movements [30]. Therefore, once an intraoperative imaging CT system is available, navigation using an intraoperative updated image should prove to be effective for complex oral surgery (Fig. 4).
Figure 4.
(A) An intraoperative C-arm CT system. (B) Use of navigation system with updated CT images acquired intraoperatively.
3. Clinical significance
Numerous clinical applications of computer-assisted surgery are possible. With more than a decade of experience, most surgeons concluded that navigation systems are both efficient and useful for application in oral and maxillofacial surgery.
3.1. Clinical application in maxillofacial trauma surgery
The orbito-zygomatic complex is a cornerstone bone that strongly contributes to midfacial width, anteroposterior malar eminence, and ocular projections, thus playing a role of paramount importance in both facial esthetics and ocular function [31], [32], [33]. Orbito-zygomatic midfacial fractures are among the most commonly encountered fractures in oral and maxillofacial traumatology, and treatment of such injuries is largely dependent on the degree of bone stability and displacement. However, functional and esthetic correction of deformities resulting from inadequate treatment of midfacial fractures continues to be one of the most difficult and challenging procedures for oral-maxillofacial surgeons [31], [32], [33].
Repair of orbital or complex midfacial fractures involving orbital fracture by computer-assisted surgery has been performed using 3D repositioning with a mirroring technique of the bones in a virtual environment [34]. The advantage of this treatment method is that the surgeon has 3D visualization of where the virtual bone model should be reconstructed to achieve symmetry with the uninjured side. With this technique, preoperative preparation can easily be performed, resulting in accurate orbital reconstruction. In conventional orbital reconstruction, accurate reconstruction is created by constructing a model for custom-made preparation, such as a stereolithographic model, using 3D computer simulation data mirroring the uninjured side [35], [36]. These methods are very useful to achieve precise orbital reconstruction. However, application is limited because model creation and custom made-plate production in fabricating implant material are expensive and time-consuming, rendering these methods unsuitable for emergency surgery. With surgical navigation, it is possible to develop an accurately fitted plate for use in emergency surgery. Furthermore, it is possible to evaluate the status of orbital floor reconstruction and accurate plate positioning several times during the procedure. Although orbital reconstruction can be performed to such an extent that no functional disorder appears clinically in the conventional method, more accurate orbital reconstruction is possible with the use of navigation system [37]. Surgical navigation is a useful method for emergency maxillofacial surgery for repair of complex midfacial orbito-zygomatic fractures (Fig. 5).
Figure 5.
A navigation-assisted orbital floor reconstruction using the mirroring technique.
(A) The reconstructed site was confirmed to match the mirror image using a tip pointer with a navigation system.
(B) Screenshot of the navigation system showing a multiplane view of the position of the navigation probe in relation to the reconstructed orbit at the time of location. The white line shows the reconstructed orbital planning image created using the mirroring technique.
(C) Confirming the accuracy of the reconstructed orbital floor on the mirrored 3D image.
3.2. Clinical applications for removal of foreign bodies
The removal of foreign bodies from the craniomaxillofacial region can be risky due to the proximity to vital structures and access difficulties [34]. The location of deep aberrant foreign bodies with intense trauma, such as gun-shot and blast injuries, the normal anatomy may also be altered or damaged [39]. Therefore, it is difficult to remove the foreign body safely. Accurate identification of the precise location of the foreign object is essential for complication-free removal. Preoperative CT scanning and 3D image reconstruction can facilitate precise object location and offer a clear picture of the surrounding anatomy, including vital blood vessels. Even if the surgeon grasped the exact foreign body position preoperatively, it is difficult to accurately and quickly detect the foreign body intraoperatively. Therefore, several intraoperative techniques for localizing a foreign body have been described, such as the use of a stereotactic technique using two venipuncture needles. With this technique, two reference needles are positioned sequentially until both meet radiographically at the foreign body. Then, blunt dissection is conducted with one or the other venipuncture needle until the tip of the foreign body is encountered [39]. However, intraoperative plain radiographic films are often difficult to obtain in an emergency situation because of the time needed for imaging. Furthermore, it can be difficult to discriminate between small changes in position on plain radiographs. Nezafati reported the use of C-arm digital fluoroscopy to provide rapid radiography [40]. However, as with plain films, fluoroscopic images are two-dimensional, thus it is not possible to provide an accurate position of the foreign body in 3D space, as one must review images in different directions in order to precisely locate the needle in a 3D space. Intraoperative ultrasound imaging has been suggested to localize foreign bodies [40]. However, an ultrasound device does not provide precise positioning and its use in the small oral cavity is limited by the size of the ultrasound device.
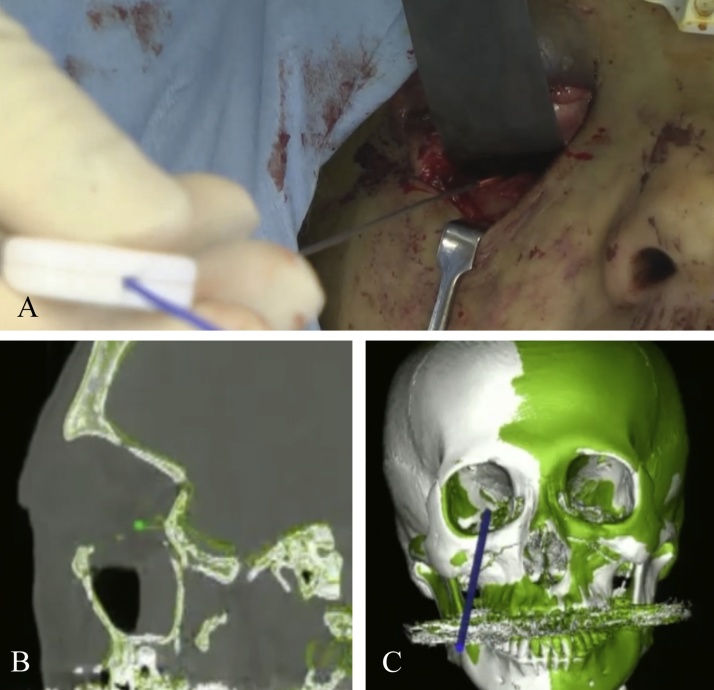
Navigation systems could provide an accurate means to locate a foreign body in a 3D space [5]. Therefore, such systems are very effective for removal of a foreign body during oral and maxillofacial surgery. Although, as an unfortunate limitation, a navigation system cannot account for the change in soft tissue architecture during the surgical procedure [41]. However, if only a minimal degree of soft tissue manipulation is required, such as finding a foreign body under a relatively small thickness of soft tissue, then this limitation would be minimal. In addition, careful and limited manipulation of the tissues when using the navigational probe will aid in mitigating this issue (Fig. 6).
Figure 6.
Removal of a foreign body (adapted and modified from Ref. [5]).
(A) Screenshot of the navigation system showing the position of the navigation probe in relation to the broken endodontic instrument fragment at the time of its location in the mandible.
(B) A broken endodontic instrument.
(C) The location of the broken instrument was accurately confirmed using a navigation system.
3.3. Clinical applications in dentoalveolar surgery
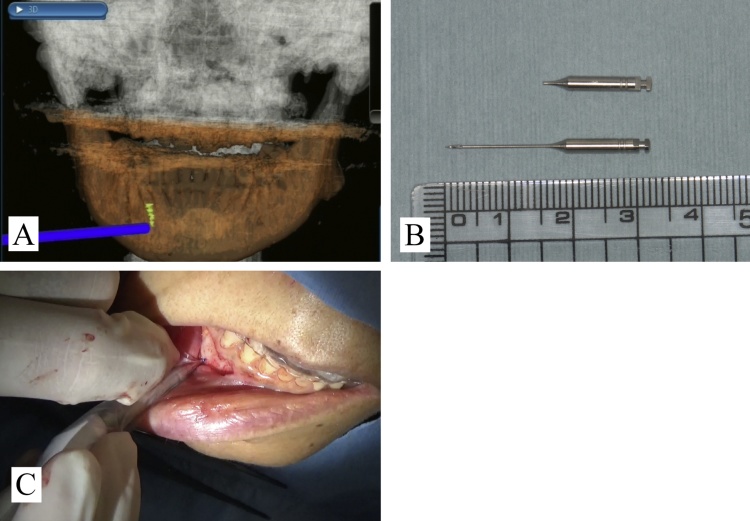
Extraction of supernumerary teeth or malposed teeth that are deeply impacted in the bone can be both complicated and challenging. Especially, determining the location of deeply impacted supernumerary teeth is a critical step during surgical extraction because it can prolong the surgical duration, negatively impact the surgeon’s confidence, and increase the incidence of trauma or complications, such as injury to an adjacent tooth germ or root [42]. In such cases, 3D images showing the exact location of the supernumerary teeth and their positional relation with adjacent structures are crucial before and during surgery. Therefore, a navigation system could help resolve these issues and promote the confidence of the surgeon during the procedure by displaying real-time corresponding relationships between an actual scenario and the sectional anatomy recorded using preoperative CT/CBCT imaging. Use of navigation systems for extraction of impacted teeth could be rather beneficial in complicated cases (Fig. 7).
Figure 7.
Dental alveolar surgery for bone lid surgery (adapted and modified from Ref. [26]).
(A) Screenshot of the intraoperative real-time navigation system showing a 3D view of the position of the navigation probe in relation to the mandibular lesion at the time of location.
(B) The location of the mandibular lesion was accurately confirmed using a navigation system.
(C) Accurate removal of the bone lid provided precise access to the lesion in the mandible.
(D) The bone lid was completely replaced in its former position.
Wang reported that navigation systems can provide the following advantages in complicated extractions of deeply impacted teeth [43]: (1) Identification of the location of deeply impacted teeth for accurate selection of an access point and to minimize bone loss and trauma. (2) To distinguish deeply impacted teeth from permanent tooth germs. (3) To ensure that the preoperative design is accurately transferred to the surgical procedure. (4) To mark safe margins to preserve adjacent structures, such as the incisive canal and apical papilla, to avoid complications.
In theory, a navigation system can be used for any displaced tooth, including the maxillary third molar. The retrieval of an accidentally displaced maxillary third molar is especially challenging when the molar is displaced into areas difficult to access, such as the infratemporal space or maxillary sinus [44], [45], [46]. There is no doubt that the use of a navigation system can benefit retrieval of displaced maxillary third molars [47].
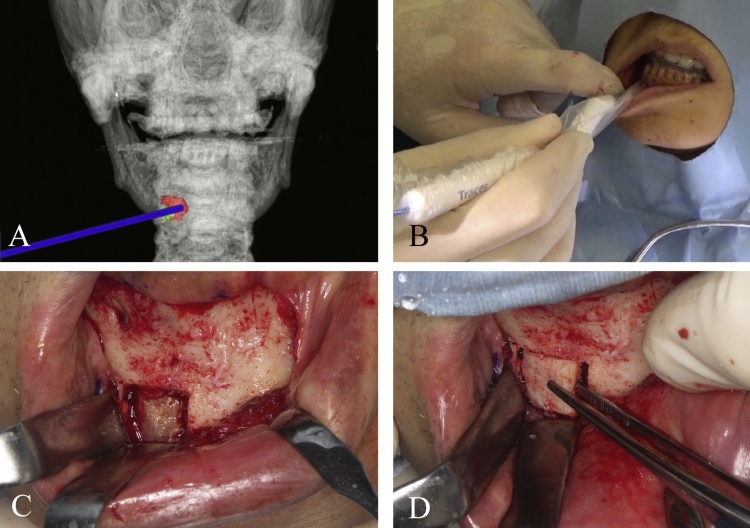
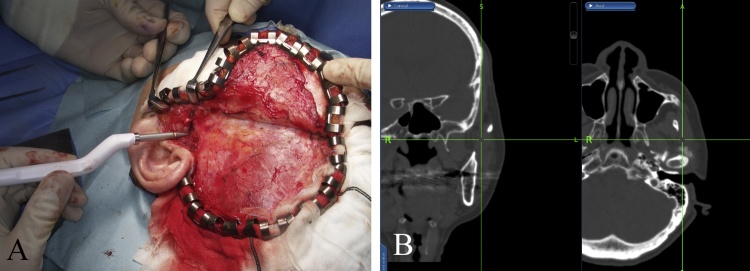
3.4. Clinical applications in skull base surgery
Management of tumors at the skull base or end-stage degenerative temporomandibular joint (TMJ) disorders require detailed anatomical knowledge of the region, precise 3D operative planning of the tumor extent, especially regarding the tumor resection margin, the attention to injury to structures within the middle cranial fossa, and preservation of vital structures in a confined space [48]. The location, invasion, and extent of the tumor are determining factors in the selection of the surgical approach. In the past, malignant tumors that had infiltrated inaccessible areas, such as the middle of the skull base or the infratemporal fossa, were considered inoperable due to the limited access to achieve tumor control or hemostasis in the event of massive hemorrhage [49].
However, the application of a navigation system can ensure safer and quicker drilling of the skull base by a dynamic actual display of the precise operating site and the extent of bone drilling on preoperative CT/MRI images. Thus, quality improvement and risk reduction can be anticipated [49]. Furthermore, outlining of the anatomical structures can be conducted before surgery, so that important landmarks, such as the foramen ovale and foramen rotundum, can be protected during reconstructive surgery [50]. In addition, the incorporation of other imaging modalities, such as 3D CT angiography and MRI, into navigation surgery facilitates the comprehension of surgical field anatomy of the skull base and internal carotid artery region [51]. As navigation at the skull base is not influenced by shifting of the brain or soft tissue, precision is greater than in other neurosurgical procedures [52], [53]. In tumor surgery, the use of navigation procedures reduces the need for resection of vital structures and provides reliable mapping of the positional relationship with lesions at the skull base [53], [54]. The use of a navigation system for resection of tumors at the skull base or TMJ lesions enhances the oral and maxillofacial surgeon’s confidence to embark on a radical approach and optimizes the surgical duration (Fig. 8).
Figure 8.
(A) Intraoperative photograph demonstrating the approach for surgical resection to include composite resection of the TMJ via the coronal approach. (B) Navigation used to assist in safe and accurate TMJ resection.
3.5. Clinical applications in orthognathic surgery
The advantages of using navigation for orthognathic surgery are the avoidance of critical structures and the opportunity to reflect on the surgical plan. Consideration of the pertinent anatomy influences the application of orthognathic surgery. One of the complicating factors of these procedures is that the pertinent anatomy is often hidden. Therefore, it is important to accurately understand the anatomical features and positional relationships of each patient. Furthermore, as the positional information of important organs is reflected during surgery, the procedure can be performed more safely. In fact, the course of the inferior alveolar nerve, as well as the thickness, marrow space, and length of the mandible differ widely among patients [55], [56].
With the use of an navigation system, the exact position of the tip of any surgical instrument can be evaluated as the instrument is being used, including assessing the tip of a pterygoid osteotome within the pterygomaxillary fissure and the location of an oscillating saw in relation to the lingual nerve and sigmoid notch during vertical ramus osteotomy [57]. Also, the inferior alveolar nerve can be avoided in blind inferior border osteotomy.
The use of 3D planning software allows overlay of Digital Imaging and Communications in Medicine planning files with navigated CT images. Preoperative computer-assisted surgical plans can be transferred to the system and navigated intraoperatively. Therefore, as compared with conventional surgical planning, computer-assisted surgical simulation is more useful to place the maxillary and mandibular segments in ideal positions. Navigation for distraction osteogenesis allows accurate localization of the osteotomy sites, placement of screw holes for the distractor, and orientation of the distractor [57]. Moreover, navigation surgery can be used to evaluate other anatomic points during orthognathic surgical procedures. With computer-assisted surgical simulation, surgeons receive information, such as a long list of specific anatomic points with the degree of surgical change, such as the anterior nasal spine, A/B points, and pogonion. Access to this information and verifying these positions with the use of navigation allows more accurate intraoperative verification of the desired bony changes [58], [59]. It is possible to improve the accuracy and safety of orthognathic surgery through verifying the surgical plan with navigation.
4. Conclusions and perspectives
There have been substantial improvements in the orientation and safety of accessing complex anatomical regions for the localization of pathological changes or foreign objects, as well as for the surgical correction of distinct malformations. In our clinical study [37], we reported the usefulness of the navigation system compared with conventional surgical methods. Particularly, not to mention the safety, we also recognized a significant difference in the accuracy of surgery. Therefore, it is possible for this system to perform useful and minimally invasive surgery. The use of a navigation system for osteotomy and resection in tumor surgery, particularly at the skull base, allows the procedure to be performed more quickly, safely, and precisely. The use of navigation for areas where surgical approaches are difficult and areas requiring anatomical attention provides confidence in the approach. In the near future, the application of computer-assisted surgery is expected to further reduce operative risks and time, accompanied with a considerable decrease in patient stress. Therefore, the use of a navigation system will also be effective and feasible in oral and maxillofacial surgery.
However, current navigation systems have some drawbacks as well. For example, although a navigation system is easy to use for the maxillary/midfacial area because of immovable parts, the mobility of the mandible makes it difficult to synchronize positions with pre-acquired images. For midfacial surgical treatment, the use of a navigation system for the mandibular region is feasible and could be beneficial for accurate location of a foreign object and provides the surgeon with intraoperative guidance, while ensuring both safety and reliability. Although preoperative preparation of the device to determine the position of the mandible requires extra effort, sufficient preparation will shorten the surgical duration and ensure the safety and reliability of the procedure. We expect the further development of simple and small tools as a dynamic reference frame method, which will enable direct tracking of the mandible via a sensor frame and mandible-supported fiducial markers useful for mandibular navigation.
In addition, it is difficult to renew intraoperative images, which was conventionally impossible. However, recent advances in technology has made it possible to edit and use images simultaneously with intraoperative CT imaging due to the popularization of hybrid operating rooms equipped with C-arm CT systems. Moreover, CT images and navigation during osseous surgery, including orthodontic surgery for repair of complex maxillofacial fractures accompanying bone segment movement, has made it possible to conduct intraoperative evaluations after segmental movement. Hence, navigation surgery will become more familiar with the continued spread of this technology.
Navigation systems are effective for delicate and accurate oral and maxillofacial surgery, neurosurgery, as well as otolaryngology and orthopedic surgery currently covered by the national health insurance program in Japan. We hope to further promote navigation system development in the future.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank our colleagues at the Division of Oral and Maxillofacial Surgery of Kagawa Prefectural Central Hospital (Takamatsu, Japan).
References
- 1.Hannen E.J.M. Recreating the original contour in tumor deformed mandibles for plate adapting. Int J Oral Maxillofac Surg. 2006;35:183–185. doi: 10.1016/j.ijom.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Azarmehr I., Stokbro K., Bell R.B., Thygesen T. Surgical navigation: a systematic review of indications, treatments, and outcomes in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2017;75:1987–2005. doi: 10.1016/j.joms.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Barnett G.H. Surgical management of convexity and falcine meningiomas using interactive image-guided surgery systems. Neurosurg Clin N Am. 1996;7:279–284. [PubMed] [Google Scholar]
- 4.Berger M., Nova I., Kallus S., Ristow O., Eisenmann U., Freudlsperger C. Electromagnetic navigated positioning of the maxilla after Le Fort I osteotomy in preclinical orthognathic surgery cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:298–304. doi: 10.1016/j.oooo.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Sukegawa S., Kanno T., Shibata A., Matsumoto K., Sukegawa-Takahashi Y., Sakaida K. Use of an intraoperative navigation system for retrieving a broken dental instrument in the mandible: a case report. J Med Case Rep. 2017;11:14. doi: 10.1186/s13256-016-1182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y., Huang T., Zhang Y., An J., He L. Application of a computer-assisted surgical navigation system in temporomandibular joint ankylosis surgery: a retrospective study. Int J Oral Maxillofac Surg. 2017;46:189–197. doi: 10.1016/j.ijom.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Luebbers H.T., Messmer P., Obwegeser J.A., Zwahlen R.A., Kikinis R., Graetz K.W. Comparison of different registration methods for surgical navigation in cranio-maxillofacial surgery. J Craniomaxillofac Surg. 2008;36:109–116. doi: 10.1016/j.jcms.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Yang J., Chu Y., Wu W., Xue J., Liang P. A novel augmented reality navigation system for endoscopic sinus and skull base surgery: a feasibility study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samarakkody Z.M., Abdullah B. The use of image guided navigational tracking systems for endoscopic sinus surgery and skull base surgery: a review. Egypt J Ear Nose Throat Allied Sci. 2016;17:133–137. [Google Scholar]
- 10.Altobelli D.E., Kikinis R., Mulliken J.B., Cline H., Lorensen W., Jolesz F. Computer-assisted three-dimensional planning in craniofacial surgery. Plast Reconstr Surg. 1993;92:576–585. [PubMed] [Google Scholar]
- 11.Hassfeld S., Mühling J., Zöller J. Intraoperative navigation in oral and maxillofacial surgery. Int J Oral Maxillofac Surg. 1995;24:111–119. doi: 10.1016/s0901-5027(05)80871-9. [DOI] [PubMed] [Google Scholar]
- 12.Howard M.A., 3rd, Dobbs M.B., Simonson T.M., LaVelle W.E., Granner M.A. A noninvasive, reattachable skull fiducial marker system. Technical note. J Neurosurg. 1995;83:372–376. doi: 10.3171/jns.1995.83.2.0372. [DOI] [PubMed] [Google Scholar]
- 13.Marmulla R., Lüth T., Mühling J., Hassfeld S. Markerless laser registration in image-guided oral and maxillofacial surgery. J Oral Maxillofac Surg. 2004;62:845–851. doi: 10.1016/j.joms.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann J., Westendorff C., Leitner C., Bartz D., Reinert S. Validation of 3D-laser surface registration for image-guided cranio-maxillofacial surgery. J Craniomaxillofac Surg. 2005;33:13–18. doi: 10.1016/j.jcms.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Bettschart C., Kruse A., Matthews F., Zemann W., Obwegeser J.A., Grätz K.W. Point-to-point registration with mandibulo-maxillary splint in open and closed jaw position. Evaluation of registration accuracy for computer-aided surgery of the mandible. J Craniomaxillofac Surg. 2012;40:592–598. doi: 10.1016/j.jcms.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Hardy S.M., Melroy C., White D.R., Dubin M., Senior B. A comparison of computer-aided surgery registration methods for endoscopic sinus surgery. Am J Rhinol. 2006;20:48–52. [PubMed] [Google Scholar]
- 17.Siniković B., Kramer F.J., Swennen G., Lübbers H.T., Dempf R. Reconstruction of orbital wall defects with calcium phosphate cement: clinical and histological findings in a sheep model. Int J Oral Maxillofac Surg. 2007;36:54–61. doi: 10.1016/j.ijom.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Marmulla R., Eggers G., Mühling J. Laser surface registration for lateral skull base surgery. Minim Invasive Neurosurg. 2005;48:181–185. doi: 10.1055/s-2005-870906. [DOI] [PubMed] [Google Scholar]
- 19.Jeon S., Park J., Chien J., Hong J. A hybrid method to improve target registration accuracy in surgical navigation. Minim Invasive Ther Allied Technol. 2015;24:356–363. doi: 10.3109/13645706.2015.1020555. [DOI] [PubMed] [Google Scholar]
- 20.Jaesung Hong J., Matsumoto N., Ouchida R., Komune S., Hashizume M. Medical navigation system for otologic surgery based on hybrid registration and virtual intraoperative computed tomography. IEEE Trans Biomed Eng. 2009;56:426–432. doi: 10.1109/TBME.2008.2008168. [DOI] [PubMed] [Google Scholar]
- 21.Gumprecht H.K., Widenka D.C., Lumenta C.B. BrainLab VectorVision neuronavigation system: technology and clinical experiences in 131 cases. Neurosurgery. 1999;44:97–105. doi: 10.1097/00006123-199901000-00056. [DOI] [PubMed] [Google Scholar]
- 22.Casap N., Wexler A., Eliashar R. Computerized navigation for surgery of the lower jaw: comparison of 2 navigation systems. J Oral Maxillofac Surg. 2008;66:1467–1475. doi: 10.1016/j.joms.2006.06.272. [DOI] [PubMed] [Google Scholar]
- 23.Wittwer G., Adeyemo W.L., Schicho K., Figl M., Enislidis G. Navigated flapless transmucosal implant placement in the mandible: a pilot study in 20 patients. Int J Oral Maxillofac Implants. 2007;22:801–807. [PubMed] [Google Scholar]
- 24.Li P., Li Z., Tian W., Tang W. A strategy for removal of foreign body in mandible with navigation system. Int J Oral Maxillofac Surg. 2015;44:885–888. doi: 10.1016/j.ijom.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Stein K.M. Use of intraoperative navigation for minimally invasive retrieval of a broken dental needle. J Oral Maxillofac Surg. 2015;73:1911–1916. doi: 10.1016/j.joms.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Sukegawa S., Kanno T., Shibata A., Matsumoto K., Sukegawa-Takahashi Y., Sakaida K. Intraoperative navigation-assisted accurate bone lid surgery to remove a mandibular lesion: a case report. Oral Maxillofac Surg Cases. 2017;3:15–19. [Google Scholar]
- 27.Wilde F., Lorenz K., Ebner A.K., Krauss O., Mascha F., Schramm A. Intraoperative imaging with a 3D C-arm system after zygomatico-orbital complex fracture reduction. J Oral Maxillofac Surg. 2013;71:894–910. doi: 10.1016/j.joms.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 28.van Hout W.M., Van Cann E.M., Muradin M.S., Frank M.H., Koole R. Intraoperative imaging for the repair of zygomaticomaxillary complex fractures: a comprehensive review of the literature. J Craniomaxillofac Surg. 2014;42:1918–1923. doi: 10.1016/j.jcms.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Wilde F., Schramm A. Intraoperative imaging in orbital and midface reconstruction. Facial Plast Surg. 2014;30:545–553. doi: 10.1055/s-0034-1393700. [DOI] [PubMed] [Google Scholar]
- 30.Xiao R., Miller J.A., Sabharwal N.C., Lubelski D., Alentado V.J., Healy A.T. Clinical outcomes following spinal fusion using an intraoperative computed tomographic 3D imaging system. J Neurosurg Spine. 2017;26:628–637. doi: 10.3171/2016.10.SPINE16373. [DOI] [PubMed] [Google Scholar]
- 31.Scolozzi P., Terzic A. Mirroring computational planning, navigation guidance system, and intraoperative mobile C-arm cone-beam computed tomography with flat-panel detector: a new rationale in primary and secondary treatment of midfacial fractures? J Oral Maxillofac Surg. 2011;69:1697–1707. doi: 10.1016/j.joms.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 32.Manson P.N., Clark N., Robertson B., Slezak S., Wheatly M., Vander Kolk C. Subunit principles in midface fractures: the importance of sagittal buttresses, soft-tissue reductions, and sequencing treatment of segmental fractures. Plast Reconstr Surg. 1999;103:1287–1306. [PubMed] [Google Scholar]
- 33.Sukegawa S., Kanno T., Shibata A., Matsumoto K., Sukegawa-Takahashi Y., Sakaida K. Treatment of orbital fractures with orbital-wall defects using anatomically preformed orbital wall reconstruction plate system. J Hard Tissue Biol. 2017;26:231–236. [Google Scholar]
- 34.Tarsitano A., Badiali G., Pizzigallo A., Marchetti C. Orbital reconstruction patient-specific orbital floor reconstruction using a mirroring technique and a customized titanium mesh. J Craniofac Surg. 2016;27:1822–1825. doi: 10.1097/SCS.0000000000002907. [DOI] [PubMed] [Google Scholar]
- 35.Mustafa S.F., Evans P.L., Bocca A., Patton D.W., Sugar A.W., Baxter P.W. Customized titanium reconstruction of post-traumatic orbital wall defects: a review of 22 cases. Int J Oral Maxillofac Surg. 2011;40:1357–1362. doi: 10.1016/j.ijom.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Sukegawa S., Kanno T., Shibata A., Takahashi Y., Furuki Y. Use of a titanium mesh plate with high three-dimensional flexibility to repair an orbital floor fracture: clinical note. Clin Surg. 2016;1:1–3. [Google Scholar]
- 37.Sukegawa S., Kanno T., Koyama Y., Matsumoto K., Sukegawa-Takahashi Y., Masui M. Precision of post-traumatic orbital reconstruction using unsintered hydroxyapatite particles/poly-l-lactide composite bioactive/resorbable mesh plate with and without navigation: a retrospective study. J Hard Tissue Biol. 2017;26:274–280. [Google Scholar]
- 39.Wei R., Xiang-Zhen L., Bing G., Da-Long S., Ze-Ming T. Removal of a foreign body from the skull base using a customized computer-designed guide bar. J Craniomaxillofac Surg. 2010;38:279–283. doi: 10.1016/j.jcms.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Nezafati S., Shahi S. Removal of broken dental needle using mobile digital C-arm. J Oral Sci. 2008;50:351–353. doi: 10.2334/josnusd.50.351. [DOI] [PubMed] [Google Scholar]
- 41.Lee T.Y., Zaid W.S. Broken dental needle retrieval using a surgical navigation system: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:e55–e59. doi: 10.1016/j.oooo.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Sukegawa S., Kanno T., Kawakami K., Shibata A., Takahashi Y., Furuki Y. Use of a piezosurgery technique to remove a deeply impacted supernumerary tooth in the anterior maxilla. Case Rep Dent. 2015 doi: 10.1155/2015/974169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Cui N.-H., Guo Y.-J., Zhang W. Navigation-guided extraction of impacted supernumerary teeth: a case report. J Oral Maxillofac Surg. 2017;75:1136. doi: 10.1016/j.joms.2017.02.001. e1–1136.e5. [DOI] [PubMed] [Google Scholar]
- 44.Iwai T., Matsui Y., Hirota M., Tohnai I. Endoscopic removal of a maxillary third molar displaced into the maxillary sinus via the socket. J Craniofac Surg. 2012;23:e295–e296. doi: 10.1097/SCS.0b013e318252f1cf. [DOI] [PubMed] [Google Scholar]
- 45.Kunkel M., Kleis W., Morbach T., Wagner W. Severe third molar complications including death-lessons from 100 cases requiring hospitalization. J Oral Maxillofac Surg. 2007;65:1700–1706. doi: 10.1016/j.joms.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Patel M., Down K. Accidental displacement of impacted maxillary third molars. Br Dent J. 1994;177:57–59. doi: 10.1038/sj.bdj.4808507. [DOI] [PubMed] [Google Scholar]
- 47.Campbell A., Costello B.J. Retrieval of a displaced third molar using navigation and active image guidance. J Oral Maxillofac Surg. 2010;68:480–485. doi: 10.1016/j.joms.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Schmelzeisen R., Gellrich N.-C., Schramm A., Schön R., Otten J.-E. Navigation-guided resection of temporomandibular joint ankylosis promotes safety in skull base surgery. J Oral Maxillofac Surg. 2002;60:1275–1283. doi: 10.1053/joms.2002.35724. [DOI] [PubMed] [Google Scholar]
- 49.Voss P.J., Leow A.M., Schulze D., Metzger M.C., Liebehenschel N., Schmelzeisen R. Navigation-guided resection with immediate functional reconstruction for high-grade malignant parotid tumour at skull base. Int J Oral Maxillofac Surg. 2009;38:886–890. doi: 10.1016/j.ijom.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Marin P.C., Love T., Carpenter R., Iliff N.T., Manson P.N. Complications of orbital reconstruction: misplacement of bone grafts within the intramuscular cone. Plast Reconstr Surg. 1998;101:1323–1327. [PubMed] [Google Scholar]
- 51.Leong J.-L., Batra P.S., Citardi M.J. Three-dimensional computed tomography angiography of the internal carotid artery for preoperative evaluation of sinonasal lesions and intraoperative surgical navigation. Laryngoscope. 2005;115:1618–1623. doi: 10.1097/01.mlg.0000173156.26930.15. [DOI] [PubMed] [Google Scholar]
- 52.Brinker T., Arango G., Kaminsky J., Samii A., Thorns U., Vorkapic P. An experimental approach to image guided skull base surgery employing a microscope-based neuronavigation system. Acta Neurochir (Wien) 1998;140:883–889. doi: 10.1007/s007010050189. [DOI] [PubMed] [Google Scholar]
- 53.Sure U., Alberti O., Petermeyer M., Becker R., Bertalanffy H. Advanced image-guided skull base surgery. Surg Neurol. 2000;53:563–572. doi: 10.1016/s0090-3019(00)00243-3. discussion 572. [DOI] [PubMed] [Google Scholar]
- 54.Sukegawa S., Kanno T., Yoshimoto A., Matsumoto K., Sukegawa-Takahashi Y., Masui M. Use of an intraoperative navigation system and piezoelectric surgery for styloidectomy in a patient with Eagle’s syndrome: a case report. J Med Case Rep. 2017;15(11):322. doi: 10.1186/s13256-017-1464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hallikainen D., Iizuka T., Lindqvist C. Cross-sectional tomography in evaluation of patients undergoing sagittal split osteotomy. J Oral Maxillofac Surg. 1992;50:1269–1273. doi: 10.1016/0278-2391(92)90225-o. [DOI] [PubMed] [Google Scholar]
- 56.Noleto J.W., Marchiori E., Da Silveira H.M. Evaluation of mandibular ramus morphology using computed tomography in patients with mandibular prognathism and retrognathia: relevance to the sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2010;68:1788–1794. doi: 10.1016/j.joms.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 57.Kang S.-H., Kim M.-K., Choi Y.-S., Park W., Lee S.-H. Navigation-assisted intraoral vertical ramus osteotomy. J Oral Maxillofac Surg. 2011;69:931–934. doi: 10.1016/j.joms.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Mazzoni S., Badiali G., Lancellotti L., Babbi L., Bianchi A., Marchetti C. Simulation-guided navigation: a new approach to improve intraoperative three-dimensional reproducibility during orthognathic surgery. J Craniofac Surg. 2010;21:1698–1705. doi: 10.1097/SCS.0b013e3181f3c6a8. [DOI] [PubMed] [Google Scholar]
- 59.Zinser M.J., Mischkowski R.A., Dreiseidler T., Thamm O.C., Rothamel D., Zöller J.E. Computer-assisted orthognathic surgery: waferless maxillary positioning, versatility, and accuracy of an image-guided visualisation display. Br J Oral Maxillofac Surg. 2013;51:827–833. doi: 10.1016/j.bjoms.2013.06.014. [DOI] [PubMed] [Google Scholar]