Abstract
Background
Recent biotechnological advancements have allowed for the adoption of Lactococcus lactis, a typical component of starter cultures used in food industry, as the host for the production of food-grade recombinant targets. Among several advantages, L. lactis has the important feature of growing on lactose, the main carbohydrate in milk and a majoritarian component of dairy wastes, such as cheese whey.
Results
We have used recombinant L. lactis NZ9000 carrying the nisin inducible pNZ8148 vector to produce MNEI, a small sweet protein derived from monellin, with potential for food industry applications as a high intensity sweetener. We have been able to sustain this production using a medium based on the cheese whey from the production of ricotta cheese, with minimal pre-treatment of the waste. As a proof of concept, we have also tested these conditions for the production of MMP-9, a protein that had been previously successfully obtained from L. lactis cultures in standard growth conditions.
Conclusions
Other than presenting a new system for the recombinant production of MNEI, more compliant with its potential applications in food industry, our results introduce a strategy to valorize dairy effluents through the synthesis of high added value recombinant proteins. Interestingly, the possibility of using this whey-derived medium relied greatly on the choice of the appropriate codon usage for the target gene. In fact, when a gene optimized for L. lactis was used, the production of MNEI proceeded with good yields. On the other hand, when an E. coli optimized gene was employed, protein synthesis was greatly reduced, to the point of being completely abated in the cheese whey-based medium. The production of MMP-9 was comparable to what observed in the reference conditions.
Electronic supplementary material
The online version of this article (10.1186/s12934-018-0974-z) contains supplementary material, which is available to authorized users.
Keywords: Lactococcus lactis, MNEI, Nisin controlled expression system, Cheese whey, GRAS, Bioconversions, Recombinant proteins
Background
Lactic acid bacteria (LAB) are traditional components of starter preparations and have been used for centuries in the manufacturing of fermented food [1]. More recently, they have attracted much attention for their biotechnological potential, finding use in a variety of applications. Lactococcus lactis (L. lactis), a prototypical member of this family, is a gram-positive, non-sporulating, facultative anaerobic bacterium and a common gut colonizer, which has been used for centuries by the cheese-making industry, thus receiving the Generally Recognized As Safe (GRAS) status [2, 3]. Outside food industry, L. lactis has been successfully exploited for its metabolic machinery, to produce and accumulate high value chemicals, such as ethanol, l-lactate, diacetyl, acetaldehyde, but also l-alanine, mannitol and other sweeteners and group B vitamins [3, 4]. Much of the biotechnological advances that have taken place for L. lactis own to the achievement of complete genome sequencing of a few strains [5, 6], which has allowed for metabolic engineering manipulations, aimed at favoring the production of specific metabolites of industrial interest [4, 7–10]. In this respect, it is noteworthy the production of bacteriocins, antimicrobial peptides with a number of applications, ranging from food-preservation to anti-biofilm activity in clinical set-ups [11–13].
Together with genome sequencing, the discovery and development of both constitutive and inducible expression systems for L. lactis has favored its development as a cell factory for the production of recombinant proteins [2, 14, 15]. Being a food-grade microorganism, devoid of endotoxins, L. lactis is very convenient for the heterologous production, both intracellular and secreted, of a number of therapeutics and vaccines, recently reviewed by Song et al. in [3].
The greatest boost in the use of L. lactis for the production of heterologous protein has been the development of the nisin controlled gene expression (NICE) system [16], which has been used in combination with the nisin-negative NZ9000 strain and its derivatives for the production of numerous recombinant protein targets [17].
In parallel with their use as molecular biology tools, L. lactis and other LAB have found space in the field of bioconversions, where their ability to digest the components of industrial effluents has been coupled to the production of added value chemicals [18–25]. In the case of LAB, their ability to digest lactose has been functional to the possibility of using them for the valorization of dairy wastes. Cheese whey (CW), the serum portion of milk that survives the process of cheese making, is in fact one of the most polluting by-products of dairy industry and its disposal imposes a considerable economic burden on cheese producers, due to its high biological and chemical oxygen demand [26]. The composition of CW varies according to its origin, but, in general, it is a nutritious mixture, containing roughly half the solids of raw milk. In particular, most of the lactose (that survives protein agglutination), roughly 20% of the original milk proteins, some vitamins and minerals are still present in CW, which is in fact often used as feeding for livestock [27–29]. In addition to its use “as is”, strategies for the valorization of CW have been proposed, often employing microbes to convert it in biomass or added value compounds, like ethanol, organic acids or bacteriocins [18, 20, 28, 30–32]. Nonetheless, to our knowledge, this material has never been used to sustain the production of high value recombinant proteins.
We here describe the use of the CW resulting from the making of mozzarella and ricotta cheese, to sustain the growth of L. lactis NZ9000 and the heterologous production of the small (~ 12 kDa), globular protein MNEI. MNEI is a single chain derivative of the plant (Dioscoreophyllum cumminsii) protein monellin [33, 34] and has received much attention for its high intensity sweetness. Due to this potential industrial interest, several biotechnological strategies have been devised to produce it, exploiting a variety of host systems [35, 36]. MNEI is one of the best characterized members of the sweet proteins family and it has been object of protein engineering to improve its taste profile and physicochemical characteristics, trying to meet the needs of the food and beverage industry [37–43]. In light of its potential applications in food preparations, the optimization of a food-grade production system becomes particularly important. The possibility of coupling such system to the valorization of a largely available industrial by-product makes our strategy particularly appealing. Moreover, we demonstrate that these growth conditions can be successfully used to produce other recombinant proteins in L. lactis strains. In particular, we show that the expression levels of the previously characterized protein MMP-9 in L. lactis [44, 45] are comparable in the CW-based medium and in the reference conditions in rich medium.
Results
Expression of MNEI in L. lactis NZ9000
The synthetic genes coding for MNEI with optimized codon usage for E. coli (MNEI-ec) and L. lactis (MNEI-ll) were cloned into the pNZ8148 vector. This was motivated by the fact that previous studies aimed at the production of brazzein, another sweet protein, in L. lactis had shown the counterintuitive result of greater production yields when employing an E. coli optimized gene [46–48]. In our case, the two synthetic genes differed for their GC content (49% and 37% for MNEI-ec and MNEI-ll, respectively) and their sequence alignment is reported in Additional file 1: Figure S1. Unlike the previous results for brazzein, when L. lactis NZ9000 was transformed with the vector containing the MNEI-ec gene, negligible protein production was observed (Additional file 2: Figure S2). Conversely, the system carrying the pNZ8148-MNEI-ll vector proved to be quite efficient: Fig. 1 shows the Coomassie stained SDS-PAGE and the western blot of 10 μg of total protein extracts from recombinant L. lactis producing MNEI. Densitometric quantification of the blot provided an estimate of the yield ~ 140 ng MNEI in 10 μg of total protein extract, corresponding to ~ 0.40 mg of protein per liter of culture medium, which remains quite stable throughout the fermentation, based on the intensity of the bands corresponding to 2, 4 and 16 h post-induction in comparison to purified protein samples.
Fig. 1.
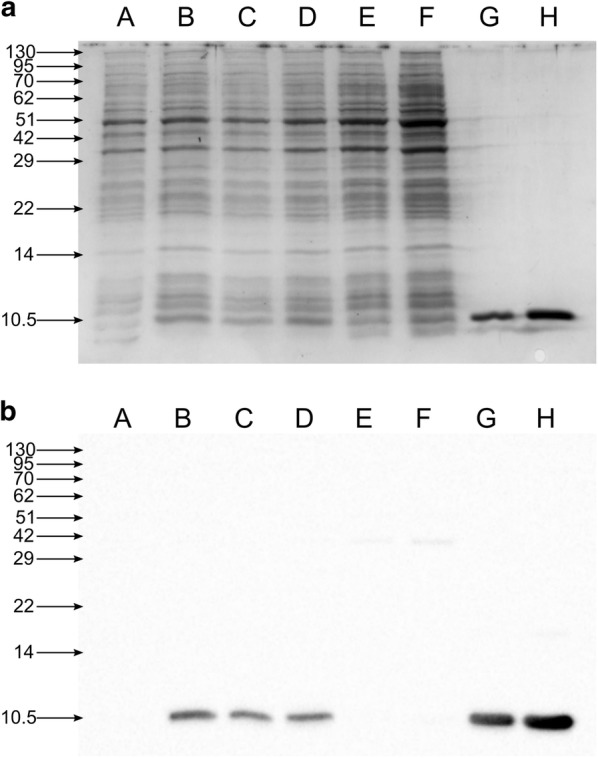
Production of recombinant MNEI in G-M17 medium. Coomassie-stained SDS-PAGE (a) and western blot (b) of the total protein extract (10 μg) from L. lactis NZ9000 carrying the pNZ8148-MNEI-ll vector (A–D) or the empty pNZ8148 vector (E–F). A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction; E: no induction; F: 4 h post induction; G: MNEI standard, 200 ng; H: MNEI standard, 500 ng
Growth of L. lactis NZ9000 on cheese whey based media
The above experiments were all carried out in the conventional growth conditions for L. lactis, namely static cultures in M17 rich medium supplemented with 0.5% glucose (G-M17) at 30 °C. We then moved to comparing the microbial growth on CW. We used the CW obtained as by-product of the manufacturing of ricotta, a typical fresh cheese from Campania, Italy. Ricotta is produced by heating at 90 °C the whey resulting from the production of “Mozzarella di Bufala Campana” and collecting the flocculating suspension. The residual watery portion constitutes the CW employed in this study. Upon delivery to our lab, all CW samples contained variable amounts of particulate in suspension, formed during the cooling down of the exhausted whey. Several treatments have been proposed as preliminary workups when CW is used as a raw material for biotechnological applications, and these range from total deproteinization of CW, to treatment with proteolytic enzymes, to supplementation with other nutrients such as peptone and yeast extract [20, 21]. Since we were trying to set up a scalable protocol, ideally suitable to work on the large amounts of CW produced by a dairy industry, we decided to limit the steps prior to inoculation of L. lactis to pH adjustment to 6.8, sterilization by autoclave and clarification by centrifugation of CW (ac-CW). The average nutrients content of CW and ac-CW before bacterial culture is provided in Table 1. The counterintuitive increase in carbohydrate content upon sterilization might result from partial hydrolysis of lactose, the main sugar of milk, to glucose and galactose. This would provide a higher response to the phenol assay used to quantify reducing carbohydrates. Despite pH adjustment had been performed, the lack of buffering capacity of untreated CW resulted in a drop in pH after sterilization, to ~ 6.2. Nonetheless, given the tolerance of LAB for acidic pHs, no further adjustment was performed. The medium was also supplemented with a catalytic amount (0.05%) of yeast extract as a source of vitamins and cofactors, to improve the utilization of the nutrients, still abundant in ac-CW.
Table 1.
Mean composition and standard deviation of the cheese whey (CW) at reception and after sterilization/clarification (ac-CW)
| CW | ac-CW | |
|---|---|---|
| Carbohydrates (mg/mL) | 27 ± 2 | 40 ± 5 |
| Proteins (mg/mL) | 2.4 ± 0.3 | 2.3 ± 0.2 |
| Lipids (mg/mL) | 12 ± 5 | 7 ± 3 |
| pH | 6.1 ± 0.1 | 6.17 ± 0.04 |
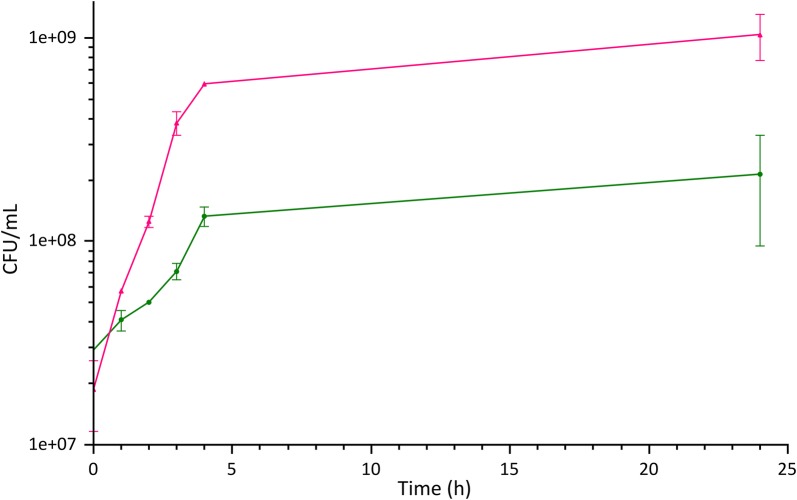
Explorative experiments showed that the growth on the CW-based medium was accompanied by a marked decrease in pH, due to the production of organic acids, paralleling what observed during the growth in G-M17 medium (Additional file 3: Figure S3). Therefore, since the proteins in CW had not been hydrolyzed, the growth in this medium was accompanied by additional protein precipitation, making it impossible to monitor biomass accumulation with turbidimetric methods (i.e. by measuring the OD600). Thus, all the growth curves were constructed measuring the CFUs/mL at different time points. Figure 2 shows the comparison of the growth curves, while Table 2 summarizes the growth parameters, for L. lactis NZ9000 in the standardized G-M17 medium and in the ac-CW-based medium. The marked difference in biomass at the end of the growth (2.14 × 108 vs 1.04 × 109 CFU/mL in ac-CW medium and G-M17, respectively) is likely the result of the significant protein deficiency of the ac-CW-based medium compared to G-M17 (2.3 vs 15.0 g/L, Additional file 4: Table S1) and of an unbalanced carbohydrates:proteins ratio (40:2.3 vs 10:15). Nonetheless, despite these suboptimal conditions, L. lactis NZ9000 could thrive in the CW-derived medium.
Fig. 2.
Growth curves of L. lactis NZ9000 in different media. The figure shows a comparison of the growth curve of L. lactis NZ9000 in the reference rich medium G-M17 (red curve) and on ac-CW supplemented with 0.05% yeast extract as a source of cofactors and vitamins (green curve). The final biomass in the two conditions differs by one order of magnitude
Table 2.
Comparison of the growth parameters for L. lactis NZ9000 in G-M17 medium and ac-CW + 0.05% yeast extract
| Growth rate µmax (h−1) |
Doubling time td (h) |
|
|---|---|---|
| G-M17 | 1.1 ± 0.2 | 0.6 ± 0.1 |
| Ac-CW + 0.05% yeast extract | 0.6 ± 0.1 | 1.2 ± 0.1 |
Expression of recombinant MNEI on CW-based medium
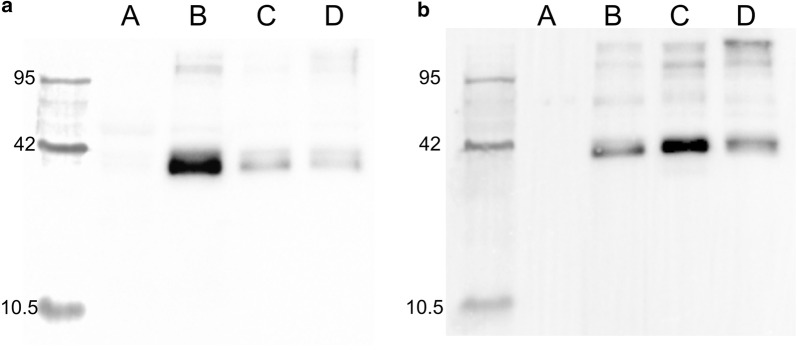
Once we had ascertained the capacity of L. lactis to grow on the ac-CW-based medium, we checked if these conditions could also sustain the expression of MNEI in the recombinant strain carrying the pNZ8148-MNEI-ll or pNZ8148-MNEI-ec vector. Both strains showed comparable growth profiles to the wild type strain (not shown); therefore, in all subsequent experiments, protein synthesis was induced after 2 h, corresponding to the mid-exponential phase, with 10 ng/mL nisin and checked at different time points post-induction by Western Blot. Figure 3 shows the Coomassie-stained SDS-PAGE (A) and Western blot (B) of the total protein extracts at different times post induction. Despite the discussed reduction in biomass accumulation compared to the standard G-M17 rich medium, the yield of recombinant MNEI on the ac-CW-based medium seems higher than in the reference condition. Densitometric quantification of MNEI by western blot, in comparison with pure protein samples, allowed us to estimate a yield of 270 ng MNEI/10 μg total proteins 2 h after induction, i.e. almost twice the observed yield in G-M17 medium. The yield of protein per liter of culture medium was nonetheless comparable to that in G-M17, i.e. ~ 0.49 mg, because of the lower biomass reached in ac-CW-based growth conditions. Interestingly, when the recombinant strain carrying the pNZ8148-MNEI-ec vector was grown on the ac-CW-based medium, no protein production could be detected (Additional file 5: Figure S4).
Fig. 3.
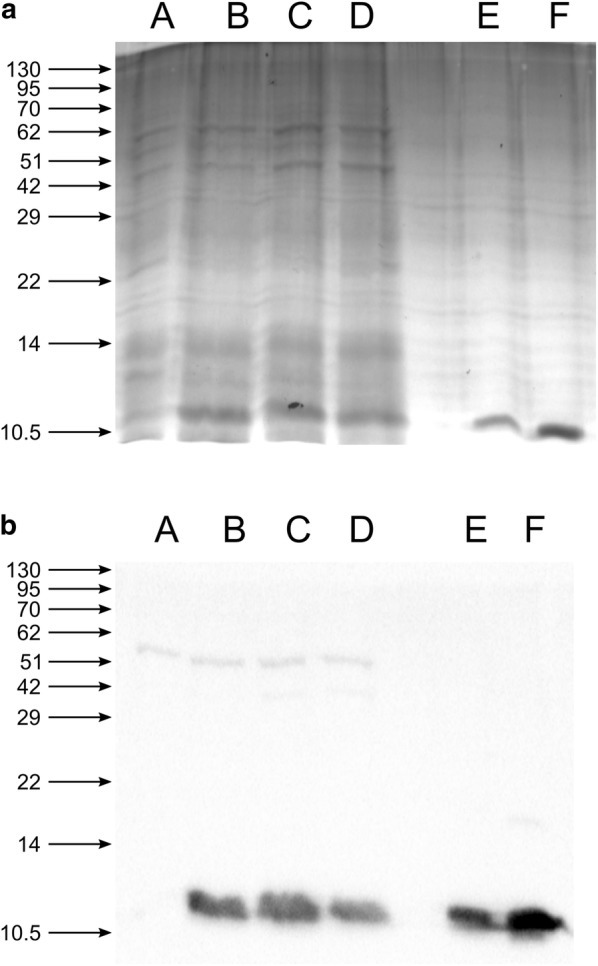
Production of recombinant MNEI in the ac-CW based medium. Coomassie-stained SDS-PAGE (a) and western blot (b) of the total protein extract (10 μg) from L. lactis NZ9000 carrying the pNZ8148-MNEI-ll and growing on ac-CW + 0.05% yeast extract. A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction; E: MNEI standard, 200 ng; F: MNEI standard, 500 ng
Expression of MMP-9 on CW-based medium
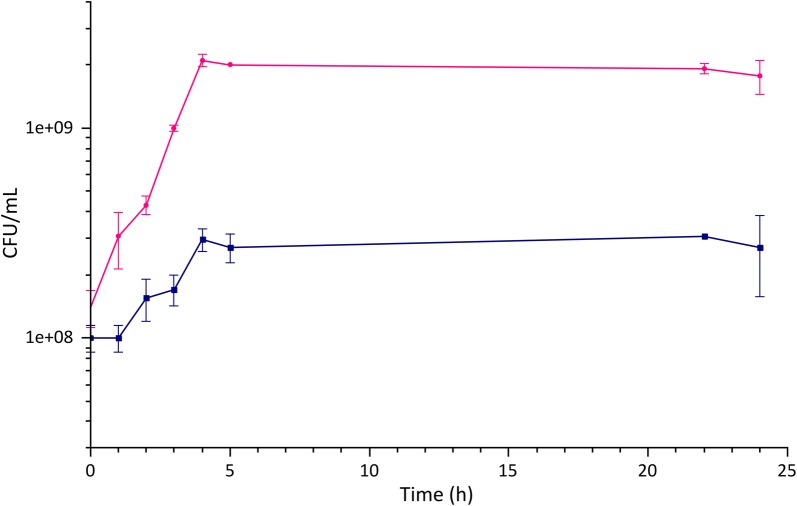
As a proof of concept, to demonstrate the suitability of the ac-CW-based medium to produce recombinant proteins in L. lactis, we tested its efficiency on a previously characterized system, namely L. lactis NZ9000 clpP− htrA− carrying the pNZ8148-MMP-9 vector coding for the catalytic domain (Phe107-Pro449, ~ 40 KDa) of metalloproteinase 9 (MMP-9) [44, 45]. Figure 4 shows the comparison between the growth curves in G-M17 and in ac-CW with 0.05% yeast extract. The comparison of the growth parameters for the two conditions is provided in Table 3. Compared to the reference condition in rich medium, we observe again the substantial reduction of the final biomass accumulation. Nonetheless, when MMP-9 expression was induced, with 10 ng/mL nisin after 2 h, comparable production of recombinant protein was obtained, as visible in Fig. 5, which shows the western blots of the total protein lysates obtained at different times post-induction in the two conditions examined. Maximal accumulation of the recombinant target seems only slightly delayed compared to the reference condition.
Fig. 4.
Comparison of the growth of L. lactis NZ9000 clpP− htrA− in different media. The figure shows a comparison of the growth curve of L. lactis NZ9000 clpP− htrA− carrying the pNZ8148-MMP-9 vector in the reference rich medium (pink curve), with the growth on ac-CW supplemented with 0.05% yeast extract (blue curve)
Table 3.
Comparison of the growth parameters for L. lactis NZ9000 clpP− htrA− (pNZ8148-MMP-9) in G-M17 medium and ac-CW + 0.05% yeast extract
| Growth rate µmax (h−1) |
Doubling time td (h) |
|
|---|---|---|
| G-M17 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Ac-CW + 0.05% yeast extract | 0.6 ± 0.2 | 1.2 ± 0.2 |
Fig. 5.
Production of recombinant MMP9 in the G-M17 and ac-CW based medium. The figure shows a comparison of the western blots of 10 μg of total protein extract of cells of L. lactis NZ9000 clpP− htrA− transformed with the pNZ8148-MMP9 vector after culture in rich medium (a) or in ac-CW supplemented with 0.05% Yeast extract (b). A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction. While protein expression levels seem comparable in the two cases, maximum production appears slightly delayed in the case of the CW derived medium
Discussion
The possibility of reintroducing waste materials in the productive cycle is a central issue of bioconversions and of the development of circular production strategies. The problem of handling the by-products of dairy industry has been long known: cheese whey (CW), the main by-product of cheese manufacturing, is a highly polluting waste, due to its high biological and chemical oxygen demand, which originates mostly from the residual lactose, but also from appreciable proteins and lipids content (Table 1) [29, 31]. Several strategies have been proposed to exploit these nutrients, reconverting them in added value compounds, often making use of LAB and of their ability of constitutively thrive on lactose. The growth of LAB on CW-based or CW-containing media, has allowed for the obtainment of various industrially relevant compounds, such as bacteriocins, ethanol, organic acids, often with the aid of metabolic engineering [18, 20, 21, 28, 30]. The utilization of CW as a suitable growth substrate, though, often requires preliminary treatments, which can range from complete deproteinization [20], to the supplementation of nutrients, with the addition of substantial amounts of peptone and yeast extract [21], to treatment with proteases or proteolytic microorganisms [32], and can greatly affect the sustainability and viability of the process. In this paper, we have described for the first time the possibility of using a CW-based medium also for the production of recombinant proteins in L. lactis. In the attempt of defining an industrially viable process, we have tried to keep the number of preliminary steps on CW prior to the cultures to a minimum, since pH adjustment and supplementation of catalytic amounts, i.e. 0.05%, of yeast extract have proven enough to produce acceptable biomass accumulation of L. lactis NZ9000. Using such a medium, it has been possible to also sustain recombinant protein production with the nisin-inducible NICE system. We have used the pNZ4184 vector carrying the gene coding for the sweet protein MNEI. This protein has been selected for its potential industrial application as a sugar replacer, which has motivated several efforts in the past to develop strategies for recombinant production [35]. Its production in L. lactis, a GRAS host, could facilitate the use of the protein as a food additive. Our results clearly indicate that the choice of the appropriate codon usage is a fundamental requisite for the correct expression of the protein, contradicting previous reports on the production of brazzein [46], for which E. coli optimized gene corresponded to higher production yields. In the case of MNEI, when the E. coli optimized gene was used, protein production was completely abated in the CW-based medium. This result could be related to the specific algorithm used for the E. coli codon usage optimization. The coding sequence was optimized to always use the same triplet for each codon, i.e. the most frequently occurring in the E. coli coding sequences. In almost 18% of the MNEI coding sequence, the E. coli preference is just the opposite of the L. lactis one, thus resulting in a coding sequence in which 18% of the codons are classified as “rare” for Lactococcus. Interestingly, L. lactis is one of the few microbial species for which a complete tRNAome determination was carried out, although in a growth condition investigated quite different from the two tested in the present work.
The general applicability of the CW-based medium has been demonstrated by producing the recombinant catalytic domain of MMP-9, using the previously described system L. lactis NZ9000 clpP− htrA− carrying the pNZ8148 vector coding for the catalytic domain of MMP-9 [44, 45]. Also in this case, recombinant protein production was comparable to the expression in standard rich medium. All the experiments described in this paper have been conducted in a laboratory set up, applying minimal control on the growth parameters, therefore with sub-optimal efficiency. The cultures on ac-CW-based medium always produced lower biomass than the corresponding control cultures in the standard medium. Even so, the yield of recombinant MNEI per liter of culture medium was comparable in the two conditions, due to the observation of higher titers of the target protein in the total protein extract. We can forecast that the introduction of controlled oxygenation and pH alone will compensate for the decrease in biomass accumulation. The production of organic acids throughout the fermentation, coupled to the lack of buffering capability of CW, causes in fact a quick drop in the culture pH to lethal values, which halts biomass accumulation. Moreover, ac-CW medium, as proposed in this paper, contains an unbalanced carbohydrates to proteins ratio that could be reduced by slight modification of the medium formulation. Optimization of these parameters and the use of fermenters will allow greater recoveries both in biomass and recombinant proteins.
Conclusions
We have presented a new strategy for the production of two recombinant proteins in two strains of L. lactis on a growth medium based solely on dairy by-products. Through minimal manipulation and the addition of minimal quantities of yeast extract, it has been possible to sustain microbial growth and to induce protein production levels comparable, if not superior, to the production in rich medium. The simplicity of the process makes it suitable for the application on the industrial scale and prone to wide margins of improvement through the control of the growth parameters.
Methods
Bacterial strains and plasmids
Lactococcus lactis NZ9000 (pepN::nisRnisK) (NIZO) and the double mutant L. lactis subsp. cremoris NZ9000 clpP− htrA− [44, 45, 49] used in this study were maintained as frozen glycerol stocks at − 80 °C. The CmR pNZ8148 plasmid (NIZO), under nisA promoter control, was used in this work. MNEI was expressed in L. lactis NZ9000, while the catalytic domain of metalloproteinase 9 (MMP-9), from Bos taurus, was produced, with a 6×His-tag, in L. lactis NZ9000 clpP− htrA− (clpP-htrA; erythromycin resistant (EmR)) (kindly provided by INRA, Jouy-en-Josas, France; patent nº EP1141337B1) [45, 49]. The gene sequences for MNEI with optimized codon usage for L. lactis (MNEI-ll) and E. coli (MNEI-ec) were purchased from Eurofins Genomics and received within commercial vectors. Both synthetic genes contained the NotI restriction enzyme site for the screening of the recombinant clones and were cloned into the pNZ8148 expression vector between the NcoI and HindIII restriction sites (Additional file 1: Figure S1). Plasmid isolation from L. lactis cells was achieved with the PureYield kit (Promega) after incubation of the cells with 5 mg/mL Lysozyme, 2 h, 37 °C.
Compositional analysis of CW
Cheese whey (CW) from the production of ricotta was obtained from “Caseificio Le Terre di Don Peppe Diana” and stored at − 20 °C until used. Ac-CW was obtained by adjusting the pH of CW to 6.8, sterilization by autoclave and centrifugation (14,000×g, 30′, 4 °C) to remove the precipitate. Protein determination was performed by Bradford assay (Bio-rad). Carbohydrates determination was obtained by the Phenol/Sulfuric Acid assay [50]. Lipid determination was obtained by the Bligh & Dyer extraction, as reported [51].
Cell cultures
Lactococcus lactis was grown in M17 medium supplemented with 0.5% d-glucose (G-M17), in ac-CW and in ac-CW supplemented with 0.05% Yeast extract. Culture were kept static at 30 °C. Typically, 30 mL cultures were performed in 50 mL tubes. For recombinant strains, the growth medium was supplemented with 5 µg/mL chloramphenicol. In general, overnight cultures of L. lactis in G-M17 were diluted into 30 mL of culture medium to an OD600 of 0.1 Bacterial growth rates in G-M17 and in ricotta cheese whey were measured by plating appropriate dilutions of the culture suspension on G-M17 agar plates and counting the CFU after incubation at 30 °C for 24 h.
Production of MNEI and MMP-9 in L. lactis
L. lactis NZ9000 competent cells were transformed by electroporation with either the pNZ8148-MNEI-ll or pNZ8148-MNEI-ec vector [52]. Electroporation was performed with a Gene Pulser from Bio-rad fitted with 2500 V, 200 X and 25 µF in a pre-cooled 2 cm electroporation cuvette. Samples were then incubated for 2 h at 30 °C in 900 µL restorative medium (G-M17 with 20 mM Mg2Cl2 and 2 mM Ca2Cl2). The electroporation mix was centrifuged for 10 min at 10,000×g at 4 °C and the pellet was resuspended in 100 µL of G-M17 media and plated. Recombinant cells were grown as described in the previous section. Expression of the MNEI gene was induced by administration of 10 ng/mL nisin in the mid-exponential phase, i.e. 2 h into the growth. At 2, 4 or 16 h post induction, 10 mL from the culture were harvested and the cells were pelleted by centrifugation (10,000×g, 4 °C, 30′), washed twice with cold PBS, resuspended in 1 mL of 50 mM sodium acetate buffer, pH 5.5, and disrupted by sonication. The MMP-9 recombinant production was carried out in L. lactis NZ9000 clpP− htrA− recombinant with pNZ8148-MMP-9 [44], following the same conditions previously described for MNEI production.
SDS-PAGE and Western blot
Total protein content of the sonicated fractions was estimated by Bradford assay using BSA as standard. The purified recombinant MNEI from E. coli (MW ~ 11 kDa), obtained as described in [53], was used as positive control. Samples of 10 μg total protein extract were loaded on 12% SDS-PAGE and then blotted onto Immobilon-P transfer membrane (EMD, Millipore Corporation, USA). Membranes were incubated with an rabbit anti-Y65R-MNEI antibody [36] (1:200, Primmbiotech, courtesy of Dr. Nunzia Scotti) and subsequently with a HRP-conjugated anti-rabbit antibody (1:50,000, BioFX Laboratories). Detection of the His-tagged MMP-9 expression in L. lactis clpP− htrA− was also performed by western blot. The membranes were incubated with anti-poly-His Peroxidase conjugate (1:2000). In both cases the signal was revealed by enhanced chemiluminescence (ECL) kit (Biorad, Clarity™ western ECL Substrate) and recorded on a ChemiDoc™ MP Imaging System (Biorad).
Additional files
Additional file 1: Figure S1. Alignment of the MNEI-ec and MNEI-ll gene sequences. Restriction sites used for cloning are indicated in blue (NcoI and Hind III). A NotI site (red) was included for plasmid screening.
Additional file 2: Figure S2. Effect of the codon usage on recombinant protein production. Western blot (B) of the total protein extract (10 μg) from L.lactis NZ9000 carrying the pNZ8148-MNEI-ec vector. A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction; E: MNEI, 50 ng; F: MNEI, 200 ng; G: MNEI, 500 ng.
Additional file 3: Figure S3. Evolution of the pH during cell culture. Plot of the pH vs time during the growth of L. lactis NZ9000 in G-M17 medium (blue curve) and in ac-CW + 0.05% yeast extract (red curve).
Additional file 4: Table S1. Composition of the G-M17 medium.
Additional file 5: Figure S4. Effect of the codon usage on recombinant protein production in CW-based medium. Western blot (B) of the total protein extract (10 μg) from L.lactis NZ9000 carrying the pNZ8148-MNEI-ec vector growth on ac-CW + 0.05% yeast extract. A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction; E: MNEI, 50 ng; F: MNEI, 200 ng; G: MNEI, 500 ng.
Authors’ contributions
MB carried out the experiments, performed the data analysis and wrote the manuscript; AC and AP performed experiments and data analysis, SL designed the experiments, performed the data analysis and wrote the manuscript; EGF, EP, AA and MMC contributed to experimental design, data analysis and revised the manuscript; DP and MLT supervised the study, participated in its design and data analysis and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Francesco Rega for the precious technical help. SL would like to thank Mario Minieri for the precious scientific collaboration.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated during this study are included in this article, and all material is available upon request.
Consent for publication
All authors approved publication.
Ethics approval and consent to participate
Not applicable.
Funding
This work has been funded by the Fondazione con il Sud (Grant 2011-PDR-19). MB was supported by a fellowship from Fondazione con Il Sud, Rome—Italy and a scholarship from the Italian government—the Minister for Foreign Affairs and International Cooperation. Rome—Italy. EGF received a post-doctoral fellowship from INIA (DOC-INIA, MINECO). EGF is also indebted to CERCA Programme (Generalitat de Catalunya) and European Social Fund for supporting our research.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CW
cheese whey
- ac-CW
autoclaved/centrifuged cheese whey
- NICE
nisin controlled gene expression system
Contributor Information
Mohamed Boumaiza, Email: m.boumaiza@yahoo.fr.
Andrea Colarusso, Email: andrea.colarusso@unina.it.
Ermenegilda Parrilli, Email: erparril@unina.it.
Elena Garcia-Fruitós, Email: elena.garcia@irta.cat.
Angela Casillo, Email: angela.casillo@unina.it.
Anna Arís, Email: anna.aris@irta.cat.
Maria Michela Corsaro, Email: mariamichela.corsaro@unina.it.
Delia Picone, Email: delia.picone@unina.it.
Serena Leone, Email: serena.leone@unina.it.
Maria Luisa Tutino, Email: tutino@unina.it.
References
- 1.Leroy F, De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol. 2004;15:67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- 2.Morello E, Bermúdez-Humarán LG, Llull D, Solé V, Miraglio N, Langella P, et al. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J Mol Microbiol Biotechnol. 2007;14:48–58. doi: 10.1159/000106082. [DOI] [PubMed] [Google Scholar]
- 3.Song AA-L, In LLA, Lim SHE, Rahim RA. A review on Lactococcus lactis: from food to factory. Microb Cell Factories. 2017;16:55. doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papagianni M. Metabolic engineering of lactic acid bacteria for the production of industrially important compounds. Comput Struct Biotechnol J. 2012;3:e201210003. doi: 10.5936/csbj.201210003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11:731–753. doi: 10.1101/gr.GR-1697R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linares DM, Kok J, Poolman B. Genome sequences of Lactococcus lactis MG1363 (revised) and NZ9000 and comparative physiological studies. J Bacteriol. 2010;192:5806–5812. doi: 10.1128/JB.00533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Quintáns N, Repizo G, Martín M, Magni C, López P. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl Environ Microbiol. 2008;74:1988–1996. doi: 10.1128/AEM.01851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Felipe FL, Kleerebezem M, de Vos WM, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen HW, Pedersen MB, Hammer K, Jensen PR. Lactate dehydrogenase has no control on lactate production but has a strong negative control on formate production in Lactococcus lactis. Eur J Biochem. 2001;268:6379–6389. doi: 10.1046/j.0014-2956.2001.02599.x. [DOI] [PubMed] [Google Scholar]
- 10.Sybesma W, Burgess C, Starrenburg M, van Sinderen D, Hugenholtz J. Multivitamin production in Lactococcus lactis using metabolic engineering. Metab Eng. 2004;6:109–115. doi: 10.1016/j.ymben.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.McAuliffe O, Ross RP, Hill C. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev. 2001;25:285–308. doi: 10.1111/j.1574-6976.2001.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 12.Alkhatib Z, Abts A, Mavaro A, Schmitt L, Smits SHJ. Lantibiotics: how do producers become self-protected? J Biotechnol. 2012;159:145–154. doi: 10.1016/j.jbiotec.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Cirkovic I, Bozic DD, Draganic V, Lozo J, Beric T, Kojic M, et al. Licheniocin 50.2 and bacteriocins from Lactococcus lactis subsp. lactis biovar. diacetylactis BGBU1-4 inhibit biofilms of coagulase negative Staphylococci and Listeria monocytogenes clinical isolates. PLoS ONE. 2016;11:e0167995. doi: 10.1371/journal.pone.0167995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Fruitós E. Lactic acid bacteria: a promising alternative for recombinant protein production. Microb Cell Factories. 2012;11:157. doi: 10.1186/1475-2859-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer-Miralles N, Villaverde A. Bacterial cell factories for recombinant protein production; expanding the catalogue. Microb Cell Factories. 2013;12:113. doi: 10.1186/1475-2859-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuipers OP, de Ruyter PGGA, Kleerebezem M, de Vos WM. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64:15–21. doi: 10.1016/S0168-1656(98)00100-X. [DOI] [Google Scholar]
- 17.Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 18.de Arauz LJ, Jozala AF, Pinheiro GS, Mazzola PG, Júnior AP, Vessoni Penna TC. Nisin expression production from Lactococcus lactis in milk whey medium. J Chem Technol Biotechnol. 2008;83:325–328. doi: 10.1002/jctb.1813. [DOI] [Google Scholar]
- 19.Lee N-K, Paik H-D. Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. J Microbiol Biotechnol. 2017;27:869–877. doi: 10.4014/jmb.1612.12005. [DOI] [PubMed] [Google Scholar]
- 20.Roukas T, Kotzekidou P. Lactic acid production from deproteinized whey by mixed cultures of free and coimmobilized Lactobacillus casei and Lactococcus lactis cells using fedbatch culture. Enzyme Microb Technol. 1998;22:199–204. doi: 10.1016/S0141-0229(97)00167-1. [DOI] [Google Scholar]
- 21.Rodrigues LR, Teixeira JA, Oliveira R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem Eng J. 2006;32:135–142. doi: 10.1016/j.bej.2006.09.012. [DOI] [Google Scholar]
- 22.Yadav AK, Chaudhari AB, Kothari RM. Bioconversion of renewable resources into lactic acid: an industrial view. Crit Rev Biotechnol. 2011;31:1–19. doi: 10.3109/07388550903420970. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Dantoft SH, Würtz A, Jensen PR, Solem C. A novel cell factory for efficient production of ethanol from dairy waste. Biotechnol Biofuels. 2016;9:33. doi: 10.1186/s13068-016-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez N, Torrado A, Cortés S, Domínguez JM. Use of waste materials for Lactococcus lactis development. J Sci Food Agric. 2010;90:1726–1734. doi: 10.1002/jsfa.4008. [DOI] [PubMed] [Google Scholar]
- 25.Bhanwar S, Singh A, Ganguli A. Effective conversion of industrial starch waste to l-lactic acid by Lactococcus lactis in a dialysis sac bioreactor. Ann Microbiol. 2014;64:1447–1452. doi: 10.1007/s13213-013-0754-2. [DOI] [Google Scholar]
- 26.Robinson RK, Wilbey RA. Cheesemaking Practice. Boston, MA: Springer; 1998. Cheese whey and its uses. [Google Scholar]
- 27.Mollea C, Marmo L, Bosco F. Valorisation of cheese whey, a by-product from the dairy industry, food industry innocenzo muzzalupo. IntechOpen. 2013 [Google Scholar]
- 28.Panesar PS, Kennedy JF, Gandhi DN, Bunko K. Bioutilisation of whey for lactic acid production. Food Chem. 2007;105:1–14. doi: 10.1016/j.foodchem.2007.03.035. [DOI] [Google Scholar]
- 29.Prazeres AR, Carvalho F, Rivas J. Cheese whey management: a review. J Environ Manag. 2012;110:48–68. doi: 10.1016/j.jenvman.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 30.González-Toledo SY, Domínguez-Domínguez J, García-Almendárez BE, Prado-Barragán LA, Regalado-González C. Optimization of Nisin Production by Lactococcus lactis UQ2 using supplemented whey as alternative culture medium. J Food Sci. 2010;75:347–353. doi: 10.1111/j.1750-3841.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 31.Mawson AJ. Bioconversions for whey utilization and waste abatement. Bioresour Technol. 1994;47:195–203. doi: 10.1016/0960-8524(94)90180-5. [DOI] [Google Scholar]
- 32.Vasala A, Panula J, Neubauer P. Efficient lactic acid production from high salt containing dairy by-products by Lactobacillus salivarius ssp. salicinius with pre-treatment by proteolytic microorganisms. J Biotechnol. 2005;117:421–431. doi: 10.1016/j.jbiotec.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Morris JA, Cagan RH. Purification of monellin, the sweet principle of Dioscoreophyllum cumminsii. Biochim Biophys Acta BBA Gen Subj. 1972;261:114–122. doi: 10.1016/0304-4165(72)90320-0. [DOI] [PubMed] [Google Scholar]
- 34.Tancredi T, Iijima H, Saviano G, Amodeo P, Temussi PA. Structural determination of the active site of a sweet protein A 1H NMR investigation of pMNEI. FEBS Lett. 1992;310:27–30. doi: 10.1016/0014-5793(92)81138-C. [DOI] [PubMed] [Google Scholar]
- 35.Masuda T, Kitabatake N. Developments in biotechnological production of sweet proteins. J Biosci Bioeng. 2006;102:375–389. doi: 10.1263/jbb.102.375. [DOI] [PubMed] [Google Scholar]
- 36.Castiglia D, Leone S, Tamburino R, Sannino L, Fonderico J, Melchiorre C, et al. High-level production of single chain monellin mutants with enhanced sweetness and stability in tobacco chloroplasts. Planta. 2018;248:465. doi: 10.1007/s00425-018-2920-z. [DOI] [PubMed] [Google Scholar]
- 37.Leone S, Picone D. Molecular dynamics driven design of pH-stabilized mutants of MNEI, a sweet protein. PLoS ONE. 2016;11:e0158372. doi: 10.1371/journal.pone.0158372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leone S, Pica A, Merlino A, Sannino F, Temussi PA, Picone D. Sweeter and stronger: enhancing sweetness and stability of the single chain monellin MNEI through molecular design. Sci Rep. 2016;6:34045. doi: 10.1038/srep34045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Li L, Yang L, Liu T, Cai C, Liu B. Modification of the sweetness and stability of sweet-tasting protein monellin by gene mutation and protein engineering. Biomed Res Int. 2016;2016:e3647173. doi: 10.1155/2016/3647173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai C, Li L, Lu N, Zheng W, Yang L, Liu B. Expression of a high sweetness and heat-resistant mutant of sweet-tasting protein, monellin, in Pichia pastoris with a constitutive GAPDH promoter and modified N-terminus. Biotechnol Lett. 2016;38:1941–1946. doi: 10.1007/s10529-016-2182-4. [DOI] [PubMed] [Google Scholar]
- 41.Zheng W, Yang L, Cai C, Ni J, Liu B. Expression, purification and characterization of a novel double-sites mutant of the single-chain sweet-tasting protein monellin (MNEI) with both improved sweetness and stability. Protein Expr Purif. 2018;143:52–56. doi: 10.1016/j.pep.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Spadaccini R, Leone S, Rega MF, Richter C, Picone D. Influence of pH on the structure and stability of the sweet protein MNEI. FEBS Lett. 2016;590:3681–3689. doi: 10.1002/1873-3468.12437. [DOI] [PubMed] [Google Scholar]
- 43.Pica A, Leone S, Di G, Donnarumma F, Emendato A, Rega MF, et al. pH driven fibrillar aggregation of the super-sweet protein Y65R-MNEI: a step-by-step structural analysis. Biochim Biophys Acta Gen Subj. 2018;1862:808–815. doi: 10.1016/j.bbagen.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Cano-Garrido O, Sánchez-Chardi A, Parés S, Giró I, Tatkiewicz WI, Ferrer-Miralles N, et al. Functional protein-based nanomaterial produced in microorganisms recognized as safe: a new platform for biotechnology. Acta Biomater. 2016;43:230–239. doi: 10.1016/j.actbio.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 45.Cortes-Perez NG, Poquet I, Oliveira M, Gratadoux JJ, Madsen SM, Miyoshi A, et al. Construction and characterization of a Lactococcus lactis strain deficient in intracellular ClpP and extracellular HtrA proteases. Microbiology. 2006;152:2611–2618. doi: 10.1099/mic.0.28698-0. [DOI] [PubMed] [Google Scholar]
- 46.Berlec A, Jevnikar Z, Majhenič AČ, Rogelj I, Štrukelj B. Expression of the sweet-tasting plant protein brazzein in Escherichia coli and Lactococcus lactis: a path toward sweet lactic acid bacteria. Appl Microbiol Biotechnol. 2006;73:158–165. doi: 10.1007/s00253-006-0438-y. [DOI] [PubMed] [Google Scholar]
- 47.Berlec A, Tompa G, Slapar N, Fonović UP, Rogelj I, Štrukelj B. Optimization of fermentation conditions for the expression of sweet-tasting protein brazzein in Lactococcus lactis. Lett Appl Microbiol. 2008;46:227–231. doi: 10.1111/j.1472-765X.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 48.Berlec A, Štrukelj B. Large increase in brazzein expression achieved by changing the plasmid /strain combination of the NICE system in Lactococcus lactis. Lett Appl Microbiol. 2009;48:750–755. doi: 10.1111/j.1472-765X.2009.02608.x. [DOI] [PubMed] [Google Scholar]
- 49.Poquet I, Saint V, Seznec E, Simoes N, Bolotin A, Gruss A. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol. 2000;35:1042–1051. doi: 10.1046/j.1365-2958.2000.01757.x. [DOI] [PubMed] [Google Scholar]
- 50.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 51.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 52.Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leone S, Sannino F, Tutino ML, Parrilli E, Picone D. Acetate: friend or foe? Efficient production of a sweet protein in Escherichia coli BL21 using acetate as a carbon source. Microb Cell Factories. 2015;14:106. doi: 10.1186/s12934-015-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Alignment of the MNEI-ec and MNEI-ll gene sequences. Restriction sites used for cloning are indicated in blue (NcoI and Hind III). A NotI site (red) was included for plasmid screening.
Additional file 2: Figure S2. Effect of the codon usage on recombinant protein production. Western blot (B) of the total protein extract (10 μg) from L.lactis NZ9000 carrying the pNZ8148-MNEI-ec vector. A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction; E: MNEI, 50 ng; F: MNEI, 200 ng; G: MNEI, 500 ng.
Additional file 3: Figure S3. Evolution of the pH during cell culture. Plot of the pH vs time during the growth of L. lactis NZ9000 in G-M17 medium (blue curve) and in ac-CW + 0.05% yeast extract (red curve).
Additional file 4: Table S1. Composition of the G-M17 medium.
Additional file 5: Figure S4. Effect of the codon usage on recombinant protein production in CW-based medium. Western blot (B) of the total protein extract (10 μg) from L.lactis NZ9000 carrying the pNZ8148-MNEI-ec vector growth on ac-CW + 0.05% yeast extract. A: no induction; B: 2 h post-induction; C: 4 h post induction; D: 16 h post induction; E: MNEI, 50 ng; F: MNEI, 200 ng; G: MNEI, 500 ng.
Data Availability Statement
All data generated during this study are included in this article, and all material is available upon request.