Abstract
Background:
The Women’s Health Initiative (WHI) Dietary Modification (DM) trial did not show that reductions in dietary fat accompanied by increases in vegetable and fruit consumption decreases the incidence of colorectal cancer. Secondary analyses suggested that aspirin use may modify the intervention effects of DM on colorectal cancer development, although a recent re-analysis including the post-intervention period confirmed no main effect of the intervention on reducing colorectal cancer incidence
Methods:
We analyzed data from 48,834 postmenopausal women who were randomized into the low fat DM (N=19540) or comparison (N=29294) group for an average 8.1 years and followed for an additional 9.4 years through 8/31/2014. Exposure to specific class(es) or strength(s) of non-steroidal anti-inflammatory drugs (NSAIDs) was modeled at baseline and as time-dependent use through the 9-year clinic visit. A Cox proportional hazard model was employed to assess the association of the DM, medication use and their interaction with colorectal cancer events.
Results:
A total of 906 incident cases of colorectal cancer were identified during the intervention and post-intervention periods. By both exposure models, we found that colorectal cancer incidence was not different in the DM from the comparison group among any type of NSAID users. None of the interactions with any category of NSAID use was statistically significant; however there was most modest evidence for an interaction (P=0.07) with aspirin use at baseline (Hazard Ratio (HR) 0.81, 95% confidence interval (CI) 0.60–1.11 for users and HR 1.12, 95% CI 0.97–1.30 for non-users). Strength and duration of aspirin use at baseline did not alter the associations.
Conclusion:
Extended follow-up of women in the WHI DM trial did not confirm combined protective effects of aspirin and low fat diet on colorectal cancer risk among the post-menopausal women.
Keywords: Low-fat diet, clinical trial, aspirin, non-steroidal anti-inflammatory drugs, colorectal cancer
INTRODUCTION
The Women’s Health Initiative (WHI) Dietary Modification (DM) trial did not show that reductions in dietary fat accompanied by increases in vegetable and fruit consumption decreased the incidence of colorectal cancer (CRC). However, the initial results from this trial offered two interesting and potentially important findings [1]. First, the self-reported incidence of colorectal polyps was significantly lower in the DM group than in the comparison group, implying that the observation period may have been too short to see an effect on cancer incidence as progression from the formation of polyps to cancer may take decades, unless all polyps are removed by colonoscopic screening. Thus, if the DM suppresses the formation of adenoma, rather than progression from advanced adenoma to cancer, it may require a longer period than 8 years to see the effects of the DM. However, a recent analysis in which the post-intervention period was included did not reveal any protective effect of the diet on colorectal cancer incidence [2]. Second, among more than 20 variables examined for effect modification, aspirin use was the only one medication other than hormonal treatments that showed a significant interaction with the dietary intervention. Specifically, among women taking 325mg or more of aspirin per day at baseline, the risk of developing colorectal cancer in the DM group was 42% lower (0.11% per year) than in the comparison group (0.19% per year). This subgroup analysis did not consider other types of non-steroidal anti-inflammatory drugs (NSAIDs). Also, due to the number of comparisons, a chance finding of this interaction cannot be ignored. Further, the result was based on only 31 colorectal cases among aspirin users in the DM group. Extended follow-up of the study population provides a larger number of colorectal cancer cases, a longer induction period and the ability to more rigorously assess the potential interaction effect of the DM and aspirin on cancer.
In pharmaceutical clinical trials, exposures of interest usually cease at the time of closure. For example, participants in the WHI hormone trials were asked to stop taking HT at study closure due to the adverse effects of the drug, but the DM trial was different. The participants were not specifically instructed to go back to the original high fat or regular American diet. It was participants’ choice to maintain low fat dietary patterns or to change back. Women who had been well adapted to low fat diet for almost 8 years could have chosen to continue. Therefore, the distinction between the intervention and post-intervention periods are not as clear as in other trials.
There are substantial data to support the chemopreventive potential of aspirin and NSAIDs, particularly for colorectal cancer. Most evidence from epidemiologic cohorts were based on aspirin use [3, 4], but limited data from case-control studies analyzing non-aspirin NSAIDs separately were generally consistent with the results for aspirin [5–7]. Furthermore cardiovascular disease prevention trials using aspirin have confirmed a cancer preventive effect of aspirin [3, 4, 8]. Moreover, colorectal polyp prevention trials with various non-aspirin NSAIDs have provided evidence supporting their role in the early stages of colorectal tumor development [9–11]. Although the preventive effects of aspirin on cancer incidence and mortality are generally seen after a latent period of about 10 years [3], recent studies have highlighted their potential short term benefits on cancer incidence, death, survival and risk of metastasis [4, 8, 12, 13].
There is some evidence of an earlier or a larger reduction in risk associated with regular strength versus low dose aspirin from both clinical trials and observational studies [3, 4, 8], but the data are not conclusive. Aspirin and NSAIDs reduce the synthesis of prostaglandins and other oxidized lipid products from an n-6 fatty acid, arachidonic acid, via the inhibition of cyclooxygenase (COX 1 and 2) activities [9], resulting in inhibition in cell proliferation, angiogenesis, oxidative stress and promoting apoptosis, and leading to subsequent risk reduction in cancer development [9, 14]. Results from an epidemiologic cohort indicate higher intakes of fruits, vegetable and whole grains reduce circulating inflammation markers. In addition, diets with a high content of red and/or processed meats and fried foods as well as high trans fatty acid intake increase the activity of inflammatory markers such as Cox-2[15, 16]. Thus, there is a potential biological mechanism that may underlie the interaction between low-fat high vegetable/fruit intervention and use of aspirin and NSAIDs. The objectives of this study were to test whether the low-fat-high vegetable/fruit dietary modification is effective in reducing the incidence of colorectal cancer among women taking NSAIDS and to assess whether the effects of DM on colorectal cancer incidence are modified by use of specific class(es) or dose(s)/strength(s) of these medications. These aims are addressed in two ways, (1) using baseline exposure model with extended follow up to allow sufficient incubation time, and (2) based on time-dependent exposure model using multiple measurements made during the intervention period.
Materials and methods
Study design
Details of the WHI DM trial design have been published previously[1]. Recruitment of postmenopausal women aged 50 to 79 years was conducted at 40 clinical centers throughout the United States. Eligibility criteria for the DM trial included willingness to be randomized to the DM or comparison group (controls), and having a fat intake at baseline of 32% or more of total calories as evaluated by the WHI food frequency questionnaire. Between 1993 and 1998, 48,834 eligible women were randomly assigned to the DM or a comparison group in the ratio of 2:3 for cost-efficiency. The intervention was designed to promote dietary change with the goals of reducing total fat to 20% of energy intake, and increasing vegetables and fruits to at least 5 servings daily and grains to at least 6 servings daily through behavioral modification. Comparison group participants received a copy of the US Department of Health and Human Services’ Dietary Guidelines for Americans and other health-related materials but were not asked to make dietary changes. The trial was terminated on March 31, 2005, representing an average of 8.1 years of intervention.
Exposure Assessment
Medication use was obtained from direct examination of prescription and non-prescription pill bottles in use that participants brought to clinic visits. The duration of use and strength were recorded for those used at least twice a week. Medication generic/trade names were converted into National Drug Codes from the Master Drug DataBase (Medi-Span, Indianapolis, IN). Regular medication use was defined based on both the frequency and duration of medication use, i.e., at least twice per week for the prior 2 weeks as described previously [17, 18]. The medication inventory was collected at baseline and updated at year 1, 3, 6, and 9 clinic visits, regardless of assigned groups, until DM trial closure (3/31/2005). After trial closeout (extension study), a self-administered questionnaire was used to capture updated medication information in pre-defined categories. Accordingly, reconstruction of 2 times/week for 2 weeks using the extension study data was not possible, generating two vastly different sets of regular users, and thus the information from the extension study was not included in the time-dependent exposure model, censoring participants permanently based on the last medication inventory available during trial period. For the purpose of this study NSAIDs will include both aspirin and non-aspirin NSAIDs. However, except for aspirin and Ibuprofen, the frequencies of users of other individual NSAIDs (including selective COX-2 inhibitors) were very low, and therefore they were grouped together as “other NSAIDs” for the analyses.
Outcome ascertainment
Clinical outcomes were collected through annual clinic visits during the trial and semi-annual mailed questionnaires during the trial and follow-up period. Following the trial closure, remaining participants were invited to the follow-up extension study, to which 81% and 84% of the women from the DM and comparisons groups, respectively, consented to participate. Outcomes for cancer were verified initially by trained physician adjudicators at the local clinical centers by medical record and pathology review, followed by final central blinded adjudication[19]. The vital status of all participants was cross-checked against The National Death Index at 2- to 3-year intervals.
Statistical Analysis
The analysis was based on the intent-to-treat approach. The number of person-years (PY) at risk was calculated from the date of randomization to the date of colorectal cancer diagnosis, death or last follow-up (8/31/2014), whichever occurred first. Kaplan-Meier plots were used to illustrate colorectal cancer events over time by randomization status for NSAID users and for non-users. Cox proportional hazard model was employed to assess the effects of DM, medication use and their interaction terms on colorectal cancer events. Medication use was modeled in two ways. The first was based on use at baseline only and the second based on time-dependent covariates using updated information on medication use available at the year 1, 3, 6 and 9 visits. In the latter, the start time of medications that newly appeared after the baseline assessment was retrospectively reconstructed from the duration of use at the survey and the exposure status was carried over to a new start date of the subsequent inventory, the date of the subsequent inventory, or up to 3.5 years, whichever occurred first [20]. The follow up was censored 3.5 years after the last medication data collection and for the any interval periods which were not covered by subsequent inventories until the next inventory data became available as well as any interval periods with indeterminate exposure due to inconsistent information from multiple inventory records [20]. Hazard ratios (HRs) and 95% confidence intervals (CI) were first estimated using Cox proportional hazards models that included a minimal set of basic covariates as described in the original analysis[1], i.e., baseline age groups, prior colorectal cancer, randomization status in other WHI clinical trials, and participation status in the post-intervention (extension) phase. Although DM and comparison groups were well balanced in terms of other risk factors for colorectal cancer[1], we assessed the effects of additional covariates, including use of estrogen plus progesterone at baseline or allocation to active hormonal therapy (HT) and/or calcium-vitamin D (CaD) clinical trial arm, baseline energy intake, alcohol use, cigarette smoking, physical activity and colonoscopy, colorectal polyp histories, family history (1st degree) of colorectal cancer and waist circumference, on the regression coefficient for the interaction term. Since none of the listed variables altered the regression coefficient for the interaction term by at least 10%, the final statistical model remained the same as described above. Overall adherence to DM at year 1 was assessed by proportions of the participants who met individual dietary goals and their interactions with intervention status, as described by Belin et al [21]. P-values were 2-sided and 0.05 was used for the cutoff point for statistical significance.
Results
Frequencies of users of NSAIDs, other anti-inflammatory or analgesic drugs and other common medications at baseline as well as years of 1, 3, 6 and 9 in the WHI DM and comparison groups are presented in Table 1. These medication inventories show that approximately one third to one half of the study population was NSAIDs users of any type. Although some of the differences between the 2 groups were statistically significant, the actual differences were very small (2% or lower). Aspirin was the most common NSAID used, representing 20%−39% of the study population with an increasing trend of use over time. At baseline, other common types of analgesics, Ibuprofen and Acetaminophen, were each used by 11% of the study population, and Ibuprofen users declined over time (reduced by 4% from year 1 to year 9) while Acetaminophen users remained stable. Other NSAIDs, alone or in combination with any other NSAIDS, were also used by about 10% of the study participants, which increased over time partly due to availability of new classes of drugs, such as selective COX-2 inhibitors. There were little differences in baseline dietary patterns (percent total dietary fat, number of servings of fruits/vegetable and grains) between NSAID users and non-users (data not shown). When overall adherence to the DM at year 1 was assessed, there were no differences between NSAID users and non-users in adherence to major dietary goals (total fat, fruits/vegetables and grains), but there was a modest but statistically significant interactions between dietary intervention and NSAID use in dietary goals reducing saturated fat and trans fat, indicating stronger intervention effect among NSAID users (Table 2).
Table 1.
Frequencies (%) of regular use* of non-steroidal anti-inflammatory drugs (NSAID), other related and common medications in Women’s Health Initiative (WHI) dietary modification cohort (DM) by time
| Baseline (N=48834) | Year 1 (N=45036) | Year 3 (N=43323) | Year 6 (N=41610) | Year 9 (N=9865) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | Comparison | DM | Comparison | DM | Comparison | DM | Comparison | DM | Comparison | ||||||||||||||||
| Drug classes/types | N | % | N | % | p-value | N | % | N | % | p-value | N | % | N | % | p-value | N | % | N | % | p-value | N | % | N | % | p-value |
| Any NSAID | 7024 | 35.95 | 10816 | 36.92 | 0.03 | 7024 | 35.95 | 10816 | 36.92 | 0.03 | 6227 | 36.20 | 9804 | 37.53 | 0.005 | 7566 | 46.01 | 11858 | 47.12 | 0.03 | 1999 | 51.34 | 3204 | 53.66 | 0.02 |
| Aspirin | 3922 | 20.07 | 6128 | 20.92 | 0.02 | 3922 | 20.07 | 6128 | 20.92 | 0.02 | 3923 | 22.80 | 6196 | 23.72 | 0.03 | 5239 | 31.86 | 8237 | 32.73 | 0.06 | 1469 | 37.72 | 2390 | 40.03 | 0.02 |
| <325 mg | 967 | 4.95 | 1532 | 5.23 | 0.07 | 967 | 4.95 | 1532 | 5.23 | 0.07 | 1587 | 9.23 | 2471 | 9.46 | 0.07 | 3144 | 19.12 | 4879 | 19.39 | 0.12 | 999 | 25.65 | 1650 | 27.63 | 0.06 |
| ⩾325 mg | 2955 | 15.12 | 4596 | 15.69 | 0.07 | 2955 | 15.12 | 4596 | 15.69 | 0.07 | 2336 | 13.58 | 3725 | 14.26 | 0.07 | 2095 | 12.74 | 3358 | 13.34 | 0.12 | 470 | 12.07 | 740 | 12.39 | 0.06 |
| Ibuprofen | 2117 | 10.83 | 3264 | 11.14 | 0.29 | 2117 | 10.83 | 3264 | 11.14 | 0.29 | 1293 | 7.52 | 1995 | 7.64 | 0.64 | 1143 | 6.95 | 1733 | 6.89 | 0.80 | 267 | 6.86 | 402 | 6.73 | 0.81 |
| Other NSAIDs | 1920 | 9.83 | 2927 | 9.99 | 0.55 | 1920 | 9.83 | 2927 | 9.99 | 0.55 | 1771 | 10.29 | 2872 | 11.00 | 0.02 | 2431 | 14.78 | 3822 | 15.19 | 0.26 | 607 | 15.59 | 975 | 16.33 | 0.33 |
| Other AID and analgesics | |||||||||||||||||||||||||
| Corticosteroids | 44 | 0.23 | 44 | 0.15 | 0.06 | 44 | 0.23 | 44 | 0.15 | 0.06 | 130 | 0.76 | 173 | 0.66 | 0.25 | 175 | 1.06 | 235 | 0.93 | 0.19 | 56 | 1.44 | 66 | 1.11 | 0.14 |
| Acetaminophen | 2168 | 11.10 | 3231 | 11.03 | 0.82 | 2168 | 11.10 | 3231 | 11.03 | 0.82 | 1518 | 8.82 | 2313 | 8.86 | 0.91 | 1685 | 10.25 | 2571 | 10.22 | 0.92 | 454 | 11.66 | 730 | 12.23 | 0.40 |
| Other common medications | |||||||||||||||||||||||||
| Antihyperlipidemics | 1405 | 7.19 | 2170 | 7.41 | 0.37 | 1405 | 7.19 | 2170 | 7.41 | 0.37 | 2330 | 13.54 | 3732 | 14.29 | 0.03 | 3657 | 22.24 | 5836 | 23.19 | 0.02 | 1073 | 27.56 | 1674 | 28.04 | 0.60 |
| Statins | 1207 | 6.18 | 1836 | 6.27 | 0.69 | 1207 | 6.18 | 1836 | 6.27 | 0.69 | 2178 | 12.66 | 3507 | 13.43 | 0.02 | 3387 | 20.60 | 5460 | 21.70 | 0.007 | 984 | 25.27 | 1531 | 25.64 | 0.68 |
| Bile sequestrants | 81 | 0.41 | 123 | 0.42 | 0.93 | 81 | 0.41 | 123 | 0.42 | 0.93 | 46 | 0.27 | 55 | 0.21 | 0.23 | 59 | 0.36 | 100 | 0.40 | 0.53 | 13 | 0.33 | 31 | 0.52 | 0.18 |
| Fibrate | 148 | 0.76 | 247 | 0.84 | 0.30 | 148 | 0.76 | 247 | 0.84 | 0.30 | 122 | 0.71 | 190 | 0.73 | 0.83 | 231 | 1.40 | 298 | 1.18 | 0.05 | 80 | 2.05 | 106 | 1.78 | 0.32 |
| Non-statins | 230 | 1.18 | 368 | 1.26 | 0.44 | 230 | 1.18 | 368 | 1.26 | 0.44 | 185 | 1.08 | 269 | 1.03 | 0.65 | 369 | 2.24 | 527 | 2.09 | 0.30 | 145 | 3.72 | 219 | 3.67 | 0.89 |
| Antidiabetics | 725 | 3.71 | 1139 | 3.89 | 0.31 | 725 | 3.71 | 1139 | 3.89 | 0.31 | 869 | 5.05 | 1463 | 5.60 | 0.01 | 1190 | 7.24 | 1857 | 7.38 | 0.58 | 294 | 7.55 | 461 | 7.72 | 0.76 |
| Antihypertensives | 2136 | 10.93 | 3417 | 11.66 | 0.01 | 2136 | 10.93 | 3417 | 11.66 | 0.01 | 2659 | 15.46 | 4385 | 16.79 | <0.001 | 3917 | 23.82 | 6316 | 25.10 | 0.003 | 1061 | 27.25 | 1707 | 28.59 | 0.15 |
Table 2:
Dietary goal achievement (%) at year 1 by NSAID use status at baseline in the WHI Dietary Modification (DM) trial
| NSAID use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary goals | No | Yes | P* | |||||||
| DM | Comparison | DM | Comparison | |||||||
| N | % | N | % | N | % | N | % | |||
| Percent calories from total fat < 20%, year one | No | 7936 | 68.70 | 16501 | 98.23 | 4460 | 68.55 | 9790 | 98.44 | 0.20 |
| Yes | 3616 | 31.30 | 297 | 1.77 | 2046 | 31.45 | 155 | 1.56 | . | |
| Percent calories from saturated fat < 7 %, year one | No | 6904 | 59.76 | 16087 | 95.77 | 3868 | 59.45 | 9595 | 96.48 | 0.005 |
| Yes | 4648 | 40.24 | 711 | 4.23 | 2638 | 40.55 | 350 | 3.52 | . | |
| Percent calories from Trans fat < 1 %, year one | No | 9488 | 82.13 | 16230 | 96.62 | 5454 | 83.83 | 9702 | 97.56 | 0.02 |
| Yes | 2064 | 17.87 | 568 | 3.38 | 1052 | 16.17 | 243 | 2.44 | . | |
| dietary cholestrol < 200mg, year one | No | 3302 | 28.58 | 8597 | 51.18 | 1957 | 30.08 | 5279 | 53.08 | 0.92 |
| Yes | 8250 | 71.42 | 8201 | 48.82 | 4549 | 69.92 | 4666 | 46.92 | . | |
| Grains (>= 6 servings) | No | 8151 | 70.56 | 13863 | 82.53 | 4518 | 69.44 | 8139 | 81.84 | 0.90 |
| Yes | 3401 | 29.44 | 2935 | 17.47 | 1988 | 30.56 | 1806 | 18.16 | . | |
| combined Fruits / veggies (>= 5 servings) | No | 6053 | 52.40 | 12604 | 75.03 | 3376 | 51.89 | 7366 | 74.07 | 0.47 |
| Yes | 5498 | 47.60 | 4194 | 24.97 | 3130 | 48.11 | 2579 | 25.93 | . | |
P-values for the interaction between dietary intervention and NSAID use.
As shown in Table 3, the incidence rates of colorectal cancer over an average of 14 years of follow-up were little affected by use of most NSAIDs at baseline. The hazard ratio (HR) for the DM among aspirin users was 0.81 (95% confidence interval (CI) 0.60–1.11), whereas the corresponding HR in aspirin non-users was 1.12 (0.97–1.30). The p-value for this interaction was 0.07. In Figure 1, we illustrated Kaplan−Meier curves for colorectal cancer free survival by intervention status for aspirin users (top) and for non-users (bottom). We observed a slight non-significant separation of the two curves among aspirin users after 5 years, but these results were not statistically significant. The overall association was not changed by aspirin strength (HR=0.80, 95%, CI 0.56–1.13 among those with>=325 mg). Additional analysis by duration of aspirin use showed that short term (<5 years) users had the HR of 0.70 (95% CI 0.50–1.11), compared with 0.94 (95% CI 0.57–1.55) in long term users (data not shown). None of these differences was statistically significant.
Table 3.
Annualized incidence (%), and hazard rations and 95% confidence interval (CL) of colorectal cancer for dietary modification (DM) according to baseline NSAID use
| NSAIDs use | DM (14.2 ± 4.8 yrs*) | Comparison (14.4s ± 4.7yrs*) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | case |
Annualized % |
N | case |
Annualized % |
Hazard Ratio |
lower CL | upper CL |
P-value
for interaction |
||
| Any type | No | 12516 | 264 | 0.15% | 18478 | 337 | 0.13% | 1.14 | (0.96, | 1.34) | 0.14 |
| Yes | 7024 | 114 | 0.12% | 10816 | 191 | 0.12% | 0.92 | (0.73, | 1.16) | . | |
| Aspirin | No | 15618 | 318 | 0.14% | 23166 | 412 | 0.12% | 1.12 | (0.97, | 1.30) | 0.07 |
| Yes | 3922 | 60 | 0.11% | 6128 | 116 | 0.13% | 0.81 | (0.60, | 1.11) | . | |
| <325 mg | 967 | 13 | 0.10% | 1532 | 23 | 0.11% | 0.89 | (0.45, | 1.76) | 0.18 | |
| >= 325 mg | 2955 | 47 | 0.11% | 4596 | 93 | 0.14% | 0.80 | (0.56, | 1.13) | . | |
| Ibuprofen | No | 17423 | 336 | 0.14% | 26030 | 471 | 0.13% | 1.05 | (0.91, | 1.21) | 0.72 |
| Yes | 2117 | 42 | 0.14% | 3264 | 57 | 0.12% | 1.14 | (0.76, | 1.69) | . | |
| Other Nsaids | No | 17620 | 354 | 0.14% | 26367 | 490 | 0.13% | 1.07 | (0.93, | 1.23) | 0.66 |
| Yes | 1920 | 24 | 0.09% | 2927 | 38 | 0.09% | 0.95 | (0.57, | 1.58) | . | |
Stratified by age group, HT arm, baseline colorectal cancer, adjusted for time dependent CAD participation
Mean follow up time and its standard deviation
Fig 1.
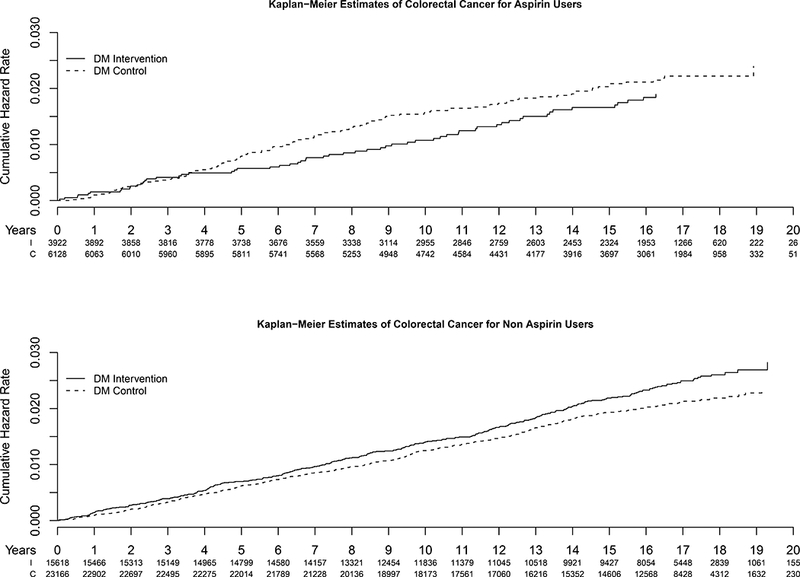
Kaplan-Meier curves for cumulative incidence of colorectal cancer by DM treatment assignment in baseline aspirin users (top) and non-users (bottom) (solid line: active treatment, broken line: comparison).
Next we tested a time-dependent exposure model (Table 4) in which we censored approximately 45% of the events primarily due to missing medication information at 9 year as a result of trial closure and discontinuation of the follow-up medication inventory after the closure, representing average 8–8.5 years of follow-up also due to censored interval periods described in details in the Methods. The HR for the DM among the overall NSAIDs users remained at an equivalent level to that from the baseline analyses. Whereas the HR for the DM increased in aspirin users (HR=1.34, 95% CI 0.63–2.87), it decreased in Ibuprofen (HR= 0.87, 95% CI: 0.47–1.64) and other NSAIDs (HR=0.33, 95% CI 0.07–1.52) users. We observed a somewhat decreased risk for colorectal cancer among high-dose aspirin users (325 mg or greater) (0.68, 95% CI 0.21–2.15). None of these interactions were statistically significant.
Table 4.
Annualized incidence (%), and hazard rations and 95% confidence interval (CL) of colorectal cancer for dietary modification (DM) according to time-dependent NSAID use up to 9 year visit
| DM (Mean 7.9–8.6 yrs*) | Comparison (Mean 8.0–8.7 yrs*) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NSAID use | Cumulative person-years |
Number of Events |
Annualized rate (%) |
Cumulative person-years |
Number of Events |
Annualized rate (%) |
Hazard Ratio |
lower CL |
upper CL |
P-value for interaction |
|
| NSAID | No | 76358.71 | 108 | 0.14 | 112884.4 | 135 | 0.12 | 1.02 | (0.58, | 1.78) | 0.71 |
| Yes | 78221.45 | 87 | 0.11 | 122786.8 | 147 | 0.12 | 0.87 | (0.46, | 1.63) | ||
| Aspirin | No | 110687.12 | 151 | 0.14 | 164969.1 | 192 | 0.12 | 0.90 | (0.56, | 1.42) | 0.37 |
| Yes | 51697.57 | 63 | 0.12 | 81427.34 | 107 | 0.13 | 1.34 | (0.63, | 2.87) | ||
| <325 mg | 25960.94 | 32 | 0.12 | 40860.40 | 43 | 0.11 | 2.66 | (0.87, | 8.16) | 0.16 | |
| >= 325 mg | 25736.63 | 31 | 0.12 | 40566.94 | 64 | 0.16 | 0.68 | (0.21, | 2.15) | ||
| Ibuprofen | No | 146609.39 | 187 | 0.13 | 221176.3 | 278 | 0.13 | 1.15 | (0.76, | 1.75) | 0.30 |
| Yes | 16176.05 | 18 | 0.11 | 24986.4 | 22 | 0.09 | 0.55 | (0.15, | 2.05) | ||
| Other NSAID | No | 144267.63 | 201 | 0.14 | 216217.2 | 290 | 0.13 | 1.04 | (0.70, | 1.55) | 0.15 |
| Yes | 23404.21 | 17 | 0.07 | 37350.48 | 36 | 0.10 | 0.33 | (0.07, | 1.52) | ||
Stratified by age group, HT arm, baseline colorectal cancer, adjusted for time dependent CAD participation
Mean follow up time slightly varies with types of medication because of reconstructed time from duration and censored periods due to irreconcilable exposure information.
Discussion
The present study was designed as an extension of the initial analysis of the randomized WHI DM trial to evaluate the impact of NSAIDS on colorectal cancer incidence. We hypothesized that longer follow-up, which allows a larger number of incident cases and a sufficient incubation time for the intervention effects on cancer development, would strengthen the significant trend for an aspirin-DM interaction observed in the initial analysis. However, current results marginally suggest a possibility of an effect modification by aspirin use, implying that changes in both diet and medication over time may have compromised the effects of the extended follow-up. Alternatively, the original observation may have been a chance finding due to multiple variables tested for the interaction. Other types of NSAIDs did not yield a significant interaction with the low-fat dietary intervention. Furthermore, none of 95% CI of the HRs for the DM excluded unity, although a 20% reduction seen in high dose aspirin users was exactly the target of the intervention effect which was used to determine the sample size of the DM trial [1]. Corroboratively, the most recent analyses of the DM trial including the post-intervention period confirmed that the dietary intervention did not produce any measureable effect on all-cause deaths, cancer deaths or colorectal cancer incidence[2]. However, use of any type of NSAID and aspirin have been associated with a 10–20% reduction in colorectal cancer incidence in WHI observational study and clinical trials combined [22]. The lack of an overall effect of the DM on total mortality [2] also indicates that deaths from other causes were not confounded with the effect of the DM on colorectal cancer incidence, as such comorbidities are differentially distributed between NSAID users and non-users. For example, cardiovascular diseases were more common in NSAID users than non-users in this cohort, and reducing deaths from these conditions by the DM would have led to an increase in incidence of colorectal cancer due to shared risk factors between two diseases.
One of the strengths in this paper includes the exposure assessment of regular medication use, which was based on direct examination of prescription and non-prescription pill bottle/packages that participants brought at each clinic visit [23], rather than relying on participants’ memory and self-report. All listed active ingredients were then coded by research personnel by matching a national pharmacy database. Thus, identification and classification of medications were less prone to error. Yet, it is still possible that some participants did not bring all of their medication bottles/packages that were taken in the past two weeks to clinic visits, as we could not confirm the prescriptions and use by linking them to medical and pharmacy records. However, common medication use identified in this cohort is equivalent to the observed frequencies in a population-based national survey [24] and aspirin use at year 9 (38% in the DM and 40% in the comparison group) was close to the estimated prevalence by a 2005–2011 national survey data in older adults [25],supporting the validity of medication data in this study. Also, although NSAID was not an RCT component and therefore users and non-users were not necessarily balanced in risk factors for colorectal cancers, we confirmed that none of such risk factors listed in the Materials and Methods section were confounded with the interaction between DM and NSAIDs, and that users and non-users had very similar dietary patterns at the baseline and a similar level of adherence to DM treatment at the end of year 1.
Several limitations are also present in our study. The time dependent exposure analysis was affected by a substantial attrition in the number of participants in year 9 clinic visit due to the trial closure, limiting the number of events available for use in the analysis. However, the number of incident cases included in the time-dependent analysis was almost equivalent to the number of cases included in the initial analysis reported by Beresford [1] and this analysis in fact reflected the effects of active DM, minimizing its carry-over effects. In addition, we found that the relative proportion of regular or high dose aspirin users among all aspirin users declined over time, which further complicated the interpretation of results. Regular strength or high dose aspirin is generally used to control specific symptoms such as aches and fever, thus its use is more often episodic, whereas use of low dose aspirin for prophylactic purposes is usually continuous. In fact, a recent report from the WHI cohorts by Brasky [22] revealed that inconsistent use was very common among users of aspirin. Furthermore, in this DM trial the participants were not masked for their treatment assignment, and thus participants who were randomized to the comparison arm could change their dietary behaviors[1], thereby weakening the effect of the DM on the primary outcomes in the study.
Another limitation of this study is lack of information on the number of pills taken and frequency of use, which limited our ability to perform more detailed analyses beyond duration and strength. Also, adherence to the prescribed diet declined over time from years 1 through year 9, narrowing the difference in diet between the active treatment and comparison groups [1], and this trend may have continued after trial closure. Thus, this study was not able to evaluate the full effect of DM when it is implemented and adhered to throughout the intended intervention period.
The present study focused the potential synergistic effect of NSAID with a low fat dietary intervention on the risk of developing colorectal cancer. It is noteworthy that an increasing number of recent studies have pointed to a potential protective effect of post-diagnosis/treatment aspirin use on the outcomes of the disease, i.e., recurrence-free survival, cause-specific mortality or overall survival. The most recent meta-analysis by Elwood et al [26] found a 20–30% reduction in the hazard ratio of cause-specific and all causes mortality, although this meta-analyses contained overlapped and mis-identified studies. A more pronounced reduction was observed when recurrence-free survival was the endpoint [27, 28]. Some studies reported reduced mortality limited to post-diagnosis use only (not including pre-diagnostic users) [13, 29]. These protective effects were primarily seen for aspirin, but a similar level of protection was noted for COX-2 inhibitor [27]. Furthermore, specific molecular markers in cancer tissue, e.g., HLA Class 1 antigen and COX-2 over-expression and PIK3CA mutation that predict response to aspirin therapy have been reported by others [29–31]. Although this study included a small number of participants who were found with an exclusion criterion of a history of colorectal cancer after randomization, as per intent-to-treat principal, the number of these women is too few to perform any survival analysis. Alternatively, WHI data should be further explored for survival of colorectal cancer patients who were diagnosed after cohort enrollment, if post-diagnostic use of NSAIDs can be reliably identified from data derived from repeated surveys and study visits.
In conclusion, although this study represents one of the largest low-fat dietary intervention with a geographically and ethnically diverse study population, extended follow-up beyond the end of the intervention did not confirm the initial findings suggesting a combined protective effect of aspirin and low fat diet on colorectal cancer. We cannot rule out the possible effect of dietary modification in high risk individuals who are treated with high dose aspirin or selective COX-2 inhibitors for a protracted period of time. Further investigation for potential use of dietary modification as an additional treatment to chemoprevention with NSAIDs for individuals who are genetically susceptible to colorectal cancer [32, 33] may be warranted.
Acknowledgements
Authors thank the following:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221, and by the National Cancer Institute, NIH through the Cancer Center Support Grant: P30CA022453.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Beresford SA, Johnson KC, Ritenbaugh C, et al. : Low-fat dietary pattern and risk of colorectal cancer: The women's health initiative randomized controlled dietary modification trial. JAMA 2006, 295(6):643–654. [DOI] [PubMed] [Google Scholar]
- 2.Thomson CA, Van Horn L, Caan BJ, Aragaki AK, Chlebowski RT, Manson JE, Rohan TE, Tinker LF, Kuller LH, Hou L, Lane DS, Johnson KC, Vitolins MZ, Prentice R: Cancer Incidence and Mortality during the intervention and post intervention periods of the Women’s Health Initiative Dietary Modification Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2014, 23(12): 2924–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flossmann E, Rothwell PM: Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. The Lancet 2007, 369(9573): 1603–1613. [DOI] [PubMed] [Google Scholar]
- 4.Algra AM, Rothwell PM: Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The Lancet Oncology 2012, 13(5):518–527. [DOI] [PubMed] [Google Scholar]
- 5.García Rodríguez LA, Huerta-Alvarez C: Reduced Risk of Colorectal Cancer among Long-Term Users of Aspirin and Nonaspirin Nonsteroidal Antiinflammatory Drugs. Epidemiology 2001, 12(1):88–93. [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Beebe-Donk J, Alshafie GA: Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer 2008, 8:237–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasky TM, Potter JD, Kristal AR, Patterson RE, Peters U, Asgari MM, Thornquist MD, White E: Non-steroidal anti-inflammatory drugs and cancer incidence by sex in the VITamins And Lifestyle (VITAL) cohort. Cancer Causes & Control 2012, 23(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z: Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012, 379(9826):1591–1601. [DOI] [PubMed] [Google Scholar]
- 9.IARC: IARC Handbooks of Cancer Prevention, vol. 1 Lyon, France: IARC; 1997. [Google Scholar]
- 10.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B: Celecoxib for the Prevention of Colorectal Adenomatous Polyps. New England Journal of Medicine 2006, 355(9): 885–895. [DOI] [PubMed] [Google Scholar]
- 11.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, Bolognese JA, Oxenius B, Horgan K, Loftus S, Morton DG: A Randomized Trial of Rofecoxib for the Chemoprevention of Colorectal Adenomas. Gastroenterology 2006, 131(6): 1674–1682. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW: Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012, 379(9826):1602–1612. [DOI] [PubMed] [Google Scholar]
- 13.Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJM, van Herk-Sukel MPP, Lemmens V, van den Broek CBM, Coebergh JW, Herings RMC, van de Velde CJH, Fodde R, Liefers GJ: Use of Aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer 2012, 106(9):1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparini G, Longo R, Sarmiento R, Morabito A: Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? The Lancet Oncology 2003, 4(10):605–615. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB: Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004, 80(4):1029–1035. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB: Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. The Journal of nutrition 2005, 135(3):562–566. [DOI] [PubMed] [Google Scholar]
- 17.Bavry AA, Thomas F, Allison M, Johnson KC, Howard BV, Hlatky M, Manson JE, Limacher MC: Non-Steroidal Anti-Inflammatory Drugs and Cardiovascular Outcomes in Women: Results from the Women’s Health Initiative. Circulation Cardiovascular quality and outcomes 2014, 7(4):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wysong A, Ally MS, Gamba CS, Desai M, Swetter SM, Seiffert-Sinha K, Sinha AA, Stefanick ML, Tang JY: Non-melanoma skin cancer and NSAID use in women with a history of skin cancer in the Women’s Health Initiative. Preventive Medicine 2014, 69:8–12. [DOI] [PubMed] [Google Scholar]
- 19.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S: Outcomes ascertainment and adjudication methods in the women’s health initiative. Annals of Epidemiology 2003, 13(9, Supplement):S122–S128. [DOI] [PubMed] [Google Scholar]
- 20.Approximation of medication use history in WHI cohorts [https://www.whi.org/researchers/sigs/diabesity/Supporting_Docs/0929211_WHI_Medication_analysis_group_final.doc]
- 21.Belin RJ, Greenland P, Allison M, Martin L, Shikany JM, Larson J, Tinker L, Howard BV, Lloyd-Jones D, Van Horn L: Diet quality and the risk of cardiovascular disease: the Women’s Health Initiative (WHI). The American Journal of Clinical Nutrition 2011, 94(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brasky TM, Liu J, White E, Peters U, Potter JD, Walter RB, Baik CS, Lane DS, Manson JE, Vitolins MZ, Allison MA, Tang JY, Wactawski-Wende J: Non-steroidal anti-inflammatory drugs and cancer risk in women: Results from the Women’s Health Initiative. International Journal of Cancer 2014, 135(8):1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang C-Y, Stein E, Prentice RL: Implementation of the women’s health initiative study design. Annals of Epidemiology 2003, 13(9, Supplement):S5–S17. [DOI] [PubMed] [Google Scholar]
- 24.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL: TRends in prescription drug use among adults in the united states from 1999–2012. JAMA 2015, 314(17):1818–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qato DM, Wilder J, Schumm L, Gillet V, Alexander G: CHanges in prescription and over-the-counter medication and dietary supplement use among older adults in the united states, 2005 vs 2011. JAMA Internal Medicine 2016, 176(4):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, Kelson M, Dolwani S: Aspirin in the Treatment of Cancer: Reductions in Metastatic Spread and in Mortality: A Systematic Review and Meta-Analyses of Published Studies. PLoS ONE 2016, 11(4):e0152402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng K, Meyerhardt JA, Chan AT, Sato K, Chan JA, Niedzwiecki D, Saltz LB, Mayer RJ, Benson AB, Schaefer PL, Whittom R, Hantel A, Goldberg RM, Venook AP, Ogino S, Giovannucci EL, Fuchs CS: Aspirin and COX-2 Inhibitor Use in Patients With Stage III Colon Cancer. Journal of the National Cancer Institute 2015, 107(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GOH HH, LEONG WQ, CHEW MH, PAN YS, TONY LKH, CHEW L, TAN IBH, TOH HC, TANG CL, FU WPC, CHIA WK: Post-operative Aspirin Use and Colorectal Cancer-specific Survival in Patients with Stage I-III Colorectal Cancer. Anticancer Research 2014, 34(12):7407–7414. [PubMed] [Google Scholar]
- 29.Chan AT, Ogino S, Fuchs CS: Aspirin Use and Survival After Diagnosis of Colorectal Cancer. JAMA : the journal of the American Medical Association 2009, 302(6):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, Davidson B, Kerr DJ, Tomlinson IPM, Midgley R: Evaluation of PIK3CA Mutation As a Predictor of Benefit From Nonsteroidal Anti-Inflammatory Drug Therapy in Colorectal Cancer. Journal of Clinical Oncology 2013, 31(34):4297–4305. [DOI] [PubMed] [Google Scholar]
- 31.Reimers MS, Bastiaannet E, Langley RE, et al. : EXpression of hla class i antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Internal Medicine 2014, 174(5):732–739. [DOI] [PubMed] [Google Scholar]
- 32.Steinbach G, Lynch PM, Phillips RKS, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su L-K, Levin B, Godio L, Patterson S, Rodriguez-Bigas MA, Jester SL, King KL, Schumacher M, Abbruzzese J, DuBois RN, Hittelman WN, Zimmerman S, Sherman JW, Kelloff G: The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. New England Journal of Medicine 2000, 342(26):1946–1952. [DOI] [PubMed] [Google Scholar]
- 33.Burn J, Gerdes A-M, Macrae F, Mecklin J- P, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard M-L, Dunlop MG, Ho JWC, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJW, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT, on behalf of the CI: Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378(9809):2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]


