SUMMARY
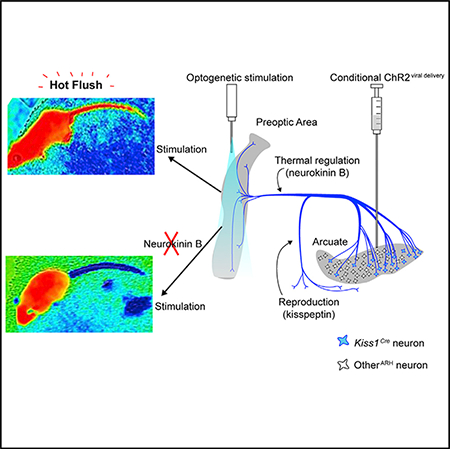
Hot flushes are a sudden feeling of warmth commonly associated with the decline of gonadal hormones at menopause. Neurons in the arcuate nucleus of the hypothalamus that express kisspeptin and neurokinin B (Kiss1ARH neurons) are candidates for mediating hot flushes because they are negatively regulated by sex hormones. We used a combination of genetic and viral technologies in mice to demonstrate that artificial activation of Kiss1ARH neurons evokes a heat-dissipation response resulting in vasodilation (flushing) and a corresponding reduction of core-body temperature in both females and males. This response is sensitized by ovariectomy. Brief activation of Kiss1ARH axon terminals in the preoptic area of the hypothalamus recapitulates this response, while pharmacological blockade of neurokinin B (NkB) receptors in the same brain region abolishes it. We conclude that transient activation of Kiss1ARH neurons following sex-hormone withdrawal contributes to the occurrence of hot flushes via NkB release in the rostral preoptic area.
Graphical Abstract
In Brief
The underlying cause of hot flushes is poorly understood. Padilla et al. provide evidence that a subpopulation of hypothalamic neurons can generate hot flush symptoms in mice. Establishing the mechanism of hot flush generation may allow for the development of therapies.
INTRODUCTION
Hot flushes, also described in the literature as hot flashes or menopausal vasomotor symptoms, are a periodic and often overwhelming sensation of heat, sweating, and flushing that affect millions of individuals (Freeman and Sherif, 2007). Hotflush symptoms negatively impact quality of life and are the predominant reason women seek medical intervention during menopause (Hessetal., 2012). Hot flushes are generally thought to be an inappropriate recruitment of thermoregulatory heat- effector responses to a perceived warmth; namely, an acute episode of vasodilation and a resultant drop in core body temperature as heat is lost to the environment through convection. Decades of research into mammalian thermoregulation has provided a map of brain regions involved in the maintenance of core body temperature, with the preoptic area of the hypothalamus (POA) emerging as the central hub of integration and control (Morrison and Nakamura, 2011). The median POA receives environmental temperature information from peripheral sensory neurons via the parabrachial nucleus, and it is sufficient to modulate vasomotor responses such as those underlying hot flushes (Morrrison and Nakamura, 2011; Nakamura and Morrison, 2010). However, the mechanism by which sex-hormone status is relayed to POA has not been elucidated.
Hot flushes are often coincident with a burst of luteinizing hormone (LH) release, which peaks immediately following an acute rise in skin temperature (Casper et al., 1979). When circulating estrogen is low, the pulsatile activity of GnRH neurons (expressing gonadotrophin regulatory hormone) and corresponding release of LH is thought to be determined by kiss-peptin (Kiss1)-expressing neurons in the arcuate nucleus of the hypothalamus (Kiss1ARH) (Clarkson et al., 2017; Navarro et al., 2009; Qiu et al., 2016). A growing body of evidence from Rance and colleagues (Rance et al., 2013) suggests that Kiss1ARH neurons may be a link between sex hormone fluctuations and hot flushes. Importantly, Kiss1ARH neurons project some of their axons to the median POA, and they co-express neurokinin B (NkB) (Goodman et al., 2007; Krajewski et al., 2010; Navarro et al., 2009; Yeo and Herbison, 2011). Systemic injection of an NkB receptor agonist (senktide), or microinfusion of senktide directly into the POA, is sufficient to evoke hypothermia in rodents (Dacks et al., 2011; Krull et al., 2017), and pharmacological blockade of NkB receptors markedly reduces hot-flush frequency in menopausal women (Prague et al., 2017).
Kiss1ARH neurons are negatively regulated by estrogen as part of a normal feedback mechanism that is necessary to sustain fertility (Goubillon et al., 2000; Han et al., 2015; Mittelman-Smith et al.,2016; Smith et al., 2005). The drop in estrogen levels during menopause leads to the hypertrophy of Kiss1ARH neurons and significant increases in Kiss1 and NkB expression levels in humans (Rance and Young, 1991; Rometo et al., 2007; Sheehan and Kovacs, 1966) or following ovariectomy in mice (Smith et al., 2005), rats (Roa et al., 2006), sheep (Goodman et al., 2007), and primates (Rometo et al., 2007). Altered activity of Kiss1ARH neurons during states of estrogen withdrawal has, therefore, been proposed as a mechanism that predisposes women to hot-flush generation, specifically via disrupted communication to the median POA (Rance et al., 2013). While this is an attractive hypothesis, hot flushes are an episodic phenomenon, and there has been no direct evidence that transient activation of Kiss1 neurons can elicit hot flush-like activity.
RESULTS AND DISCUSSION
Contemporary technologies provide a means to functionally manipulate genetically defined neurons and circuits in mice. These tools allowed us to directly test the impact of Kiss1ARH neuron activity and circuitry on vasomotor thermoregulation. We generated a new mouse line that expresses Cre recombinase from the Kiss1 gene locus (Kiss1Cre mice) to allow for selective manipulation of Kiss1-expressing neurons (Figure S1). We verified that Cre expression in the Kiss1Cre mouse line is consistent with published literature on the central expression of Kiss1, particularly in the arcuate hypothalamus (ARH) and in the rostral periventricular area of the hypothalamus, two of the most studied populations of Kiss1-expressing neurons (Figures S1 and S2). Kiss1Cre homozygous mice are infertile (Figure S2), similar to previous Kiss1 knockout mice (d’Anglemont de Tassigny et al., 2007).
We co-injected adeno-associated virus, serotype 1 (AAV1) viruses containing fluorescently tagged Cre-dependent versions of the excitatory channelrhodopsin (DIO-ChR2:YFP) and the excitatory designer receptor exclusively activated by designer drugs (DREADD), hM3Dq (DIO-hM3Dq:mCherry), into the ARH of female Kiss1Cre mice and implanted a fiber-optic cannula above the ARH to allow artificial activation of KisslARH neurons (Figures 1A and 1B). We hypothesized that activating Kiss1ARH neurons would initiate a hot-flush-like vasodilation, resulting in increased skin temperature and consequent drop in core body temperature, consistent with the clinical presentation of hot flushes (Freedman, 1998). Indeed, we found that chemogenetic activation of Kiss1ARH neurons with clozapine N-oxide (CNO, the ligand for hM3Dq) produced hot-flush-like symptoms in experimental female mice compared to controls. Figure 1C shows the fluctuation in core body temperature of a typical mouse over 5 days, while Figure 1D shows the average of all the mice for 24 hr with CNO injection indicated by the arrow. Figure 1E shows the decrease in core body temperature compared to controls during the first 2 hr after CNO administration, while Figure 1F reveals the rise in tail-skin temperature of the same female mice. Artificial stimulation of Kiss1ARH neurons also suppressed home-cage locomotor activity, a response similar to that observed when activating warm-sensitive POA neurons (Yu et al., 2016) (Figure 1G). Optogenetic activation of channelrhodopsin (ChR2)-expressing Kiss1ARH cell bodies for 1 hr similarly evoked a pronounced increase in tail-skin temperature (Figure 1H).
Figure 1. Artificial Activation of Kiss1ARH Neurons Is Sufficient to Drive Heat Dissipation in Both Female and Male Mice.
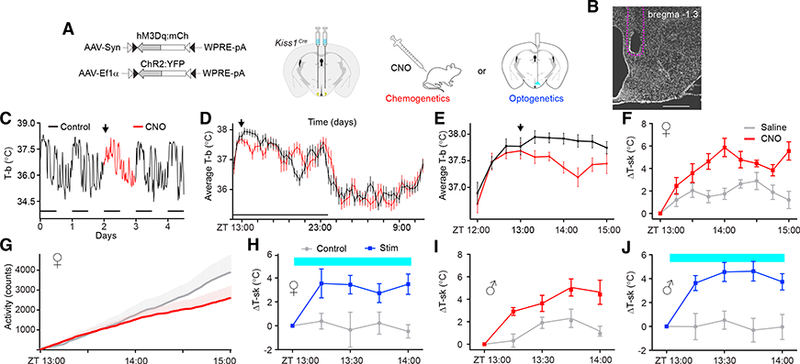
(A) Schematic representation of the viral vectors and injection targets. A unilateral fiber-optic ferrule was implanted above the ARH.
(B) DAPI staining of a coronal brain section indicates the fiber-optic terminal in the ARH. Scale bar, 500 μm.
(C—E) Telemetry recordings of core body temperature (T-b) show that Kiss1ARH neuron stimulation (CNO, 1 mg/kg) was sufficient to transiently decrease T-b.
(C) 5-day T-b recording of a single mouse, with CNO injected on day 2 at ZT= 13:00 (arrow). The animals were housed on a 12-hr:12-hr lightdark cycle (horizontal bars); Zeitgeber time (ZT) = 12:00 refers to the onset of the dark.
(D) Average T-b comparing baseline versus CNO treatment on a scale of 24 hr.
(E) Average T-b comparing baseline vs. CNO-treatment on a scale of 2 hr; n = 9; F(1, 144) = 7.50, p = 0.0146.
(F) Thermal imaging of tail-skin temperature (T-sk), saline versus CNO-treated females; n = 9; F(1, 128) = 17.07, p < 0.00001.
(G) Telemetry recordings of locomotor activity; n = 9; F(1,2178) = 46.48, p < 0.00001.
(H) Optogenetic stimulation (2 Hz) of Kiss1ARH neurons was sufficient to increase T-sk in females; n = 6; F(1,40) = 11.23, p < 0.00001.
(I) T-sk in saline versus CNO-treated males; n = 7; F(1,48) = 26.35, p < 0.00001.
(J) Optogenetic stimulation (2 Hz) of Kiss1ARH neurons was sufficient to increase T-sk in males; n = 9; F(1,64) (male) = 12.97, p = 0.0024.
F statistics represent the main effect of treatment by two-way repeated measures (RMs)-ANOVA. Error bars represent ± SEM. Crossover design with randomized starts.
While the vast majority of patients who suffer from hot flushes are menopausal women, hot flushes can affect both sexes in response to rapid decreases in sex hormone levels, such as that which occurs in men undergoing androgen deprivation therapy for prostate cancer or after castration (Karling et al., 1994; Kumar et al., 2005). To examine whether Kiss1ARH neurons may underlie hot-flush-like symptoms in general, we co-injected AAV1 viruses containing Cre-dependent hM3Dq and ChR2 into the ARH of male Kiss1Cre mice. We found that activation of Kiss1ARH neurons using either CNO administration or light delivery in male mice increased tail-skin temperature to the same extent as that in female mice (Figures 1I and 1J).
To confirm that our viral strategy was efficiently transducing Kiss1ARH neurons, we verified the presence of hM3Dq:mCherry-expressing neurons in the ARH (Figures 2A and 2B). Because Kiss1ARH neuronal signaling can be excitatory via co-release of Kiss1, NkB, and glutamate, we then asked whether artificial activation of Kiss1ARH neurons is sufficient to stimulate downstream neurons in and around the predicted temperature-effector region in the POA. We examined Fos, an immediate-early gene used as a marker of activity, throughout the POA following stimulation of Kiss1ARH neurons compared to controls (Figures 2C–2E). Stimulation of Kiss1ARH neurons significantly increased Fos expression in the POA (Figure 2F).
Figure 2. Activation of Kiss1ARH Neurons Induces Fos Expression in the Rostral POA.
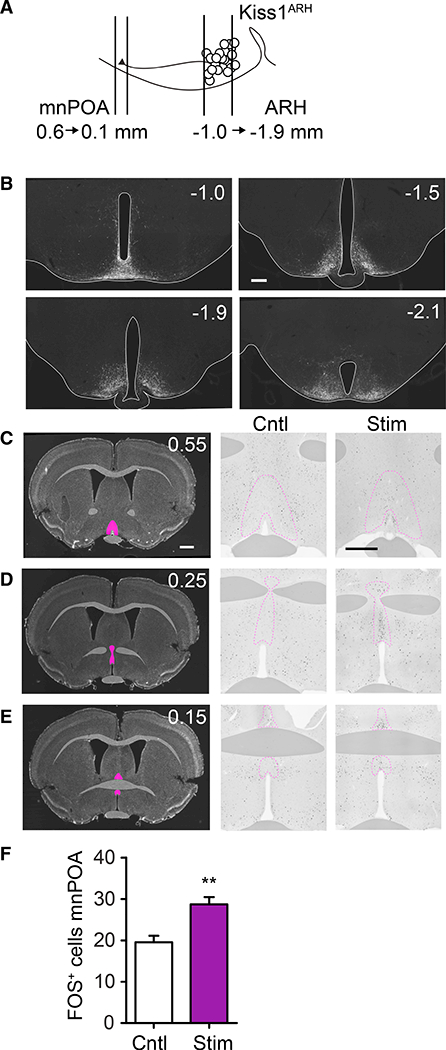
(A) Schematic diagram of Kiss1ARH projections to the POA (numbers indicate distance from Bregma in mm).
(B) Serial, coronal sections of hM3Dq:mCherry-transduced Kiss1Cre neurons with immunohistochemistry for mCherry.
(C-E) Representative Fos staining in POA from rostral to caudal (top to bottom). 0.55 (C), 0.25 (D), and 0.15 (E) from Bregma. Left panels: DAPI-stained coronal sections, with pink highlights indicating the POA. Middle and right panels: Fos immunoreactivity in the POA comparing control versus stimulated mice, respectively.
(F) There were more Fos-positive cells in the rostral POA of stimulated mice; for Cntl, M = 19.57, SD = 7.56; for Stim, M = 28.73, SD = 13.56; t(83) = 3.06; **p = 0.003.
All scale bars, 500 μm.
Based on the hypothesis that Kiss1ARH neurons relay infomation to warm-sensing neurons in the POA (Rance et al., 2013), we predicted that selective activation of Kiss1ARH neuron terminals in the POA would recapitulate the hot- flush-like response seen after cell-body stimulation. To test this idea, we injected an AAV1 virus containing Cre-dependent ChR2 into the ARH of Kiss1Cre mice as before, but we implanted a fiber-optic cannula over the Kiss1ARH fibers in the rostral POA (Figure 3A). Figures 3B and 3C show the typical 6-day and average 24-hr fluctuations of core body temperature, with the photoactivation of Kiss1ARH neurons shown as a vertical blue bar. There was a significant decrease in core body temperature during the 2 hr after photoactivation of the Kiss1 axon terminals in the POA (Figure 3D). Activation of ChR2 in Kiss1ARH-neuron fibers in the POA also increased tail-skin temperature (Figure 3E) and decreased home-cage locomotor activity (Figure 3F), as was observed following cell-body stimulation. The POA contains neurons that are intrinsically temperature sensitive (Nakayama et al., 1961). To test whether our laser stimulation paradigm alone (2 Hz, 10 mW) was sufficient to induce hot-flush-like symptoms, a conditional mCherry virus (AAV1-DIO-mCherry) was injected into the ARH of Kiss1Cre female mice and the fiber-optic cannula implanted above the rostral POA. Laser exposure in the POA of these mice failed to evoke physiological responses, confirming that the responses obtained with ChR2 photoactivation were not induced by heating temperature-sensitive neurons within the POA (Figure S3).
Figure 3. Optogenetic Stimulation of Kiss1ARH Axons in the Rostral POA Promotes a Heat-Dissipation Response in Female Mice.
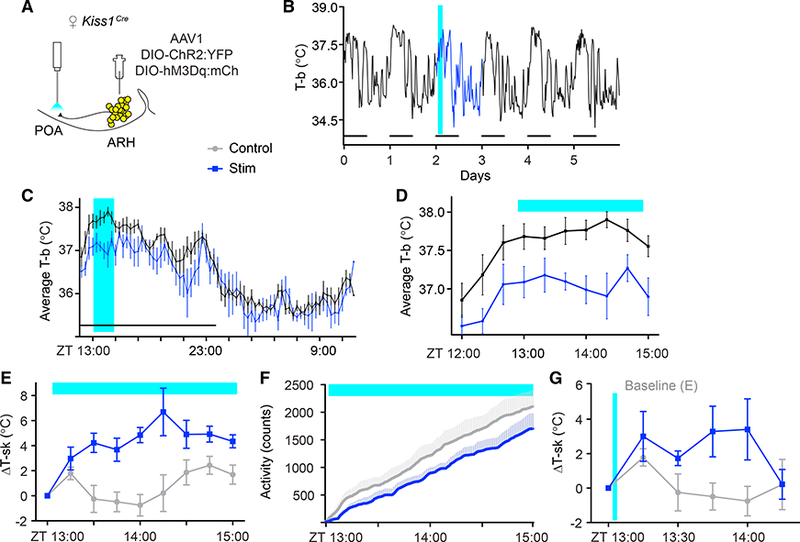
(A) Schematic representation of the targeted viral injection into the ARH and fiber-optic placement 0.5 mm above the POA.
(B-D) Optogenetic stimulation (2 Hz) of Kiss1ARH neuron fibers in the POA was sufficient to transiently decrease T-b. Within-subject comparison of unstimulated baseline (black trace) vs. stimulated (blue trace).
(B) 6-day T-b recording of a single mouse, subjected to blue-light stimulation from ZT = 13:00–15:00 on day 2 (blue).
(C) Average T-b comparing baseline versus stimulation on a scale of 24 hr.
(D) Average T-b comparing baseline vs. stimulation on a scale of 2 hr; n = 5; F(1,72) = 9.589, p = 0.0147.
(E) T-sk in control versus terminal-stimulated females; n = 5; F(1,64) = 17.83, p = 0.0029.
(F) Telemetry recording of locomotor activity, n = 5, F(1,960) (activity) = 185.8, p < 0.00001.
(G) T-sk following a brief (2-min, 2 Hz) stimulation; n = 5; F(1,48) = 9.41, p = 0.0035.
F statistics represent the main effect of treatment by two-way RM-ANOVA. Error bars represent ± SEM. Crossover design with randomized starts.
Hot flushes in humans are typically brief, lasting anywhere from seconds to 10 min (Freedman, 1998). To examine a more physiologically relevant time course, we optogenetically stimulated ChR2 in Kiss1ARH-neuron fibers in the POA for only 2 min. Tail-skin temperature rose significantly above baseline for over 45 min before dropping back down to baseline (Figure 3G). These data confirm that even brief activation of this circuit is sufficient to engage a prolonged, thermoregulatory heat- effector response.
Because clinical hot flushes typically present after acute sex hormone withdrawal, we next examined the influence of sex-hormone status on Kiss1ARH-mediated flushing. It has been shown that Kiss1ARH neurons are more sensitive to artificial activation following gonadectomy (Han et al., 2015), so we predicted that the vasomotor response to Kiss1ARH stimulation would be sensitized. To test this hypothesis, we first established a graded tail-skin temperature response to activation of hM3Dq-expressing Kiss1ARH neurons by administering a range of CNO concentrations from 0.05 to 1 mg/kg. Intact females demonstrated a dose-dependent increase of tail-skin temperature in response to increasing concentrations of CNO, with a partial effect at 0.3 mg/kg and a full effect at 1 mg/kg (Figure 4A). Based on these data, we then ovariectomized the mice and re-tested them with a dose of 0.3 mg/kg. After ovariectomy, this lower dose of CNO was now sufficient to induce a significant increase in tail-skin temperature (Figure 4A), a result consistent with the hypothesis that altered properties of Kiss1ARH neurons after sex-hormone withdrawal could contribute to hot-flush susceptibility.
Figure 4. Evoked Hot Flushes Are Sensitive to Ovarian Estrogens and Are Blocked by Neurokinin Receptor Antagonists Delivered to the POA.
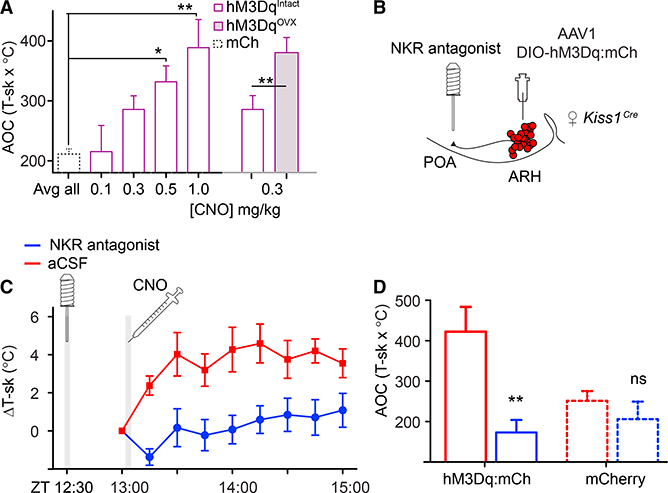
(A) Dose response of T-sk to CNO. CNO was injected at ZT = 13:00, and T-sk was monitored for 2 hr; data are presented as the average area under the curve (AOC; hours x °C). mCherry-expressing controls were treated with CNO (0.05 to 1 mg/kg) and averaged. One-way ANOVA: for mCh, n = 8; for hM3Dq, n = 6; **p = 0.0006, asterisks indicate Bonferroni post-test results. Following ovariectomy, females were significantly more sensitive to 0.3 mg/kg CNO. Paired t test, n = 6. Intact: M = 285.6, SD = 22.74; ovari- ectomized, M = 393.1, SD = 29.31. t(5) = 2.605; *p = 0.0480.
(B) Schematic representation of targeted viral injection into the ARH and placement of infusion cannula in the POA.
(C) Infusion of the cocktail of antagonists was sufficient to block CNO-induced flushing. 30 min prior to recording, either saline or antagonists were infused into the POA of h M3Dq-expressi ng females. T-sk was recorded every 15 min starting immediately prior to CNO delivery at ZT = 13:00. Two-way RM-ANOVA, n = 6; F(1, 45) = 84.05, p < 0.00001.
(D) T-sk from ZT = 13:00–15:00 represented as AOC. hM3Dq data were extracted from (B) and compared to mCherry-expressing controls that were treated in the same manner. Two-way RM-ANOVA main effect of antagonists, n = 6 or 8 per group (hM3Dq or mCh); F(1, 12) = 16.49, **p = 0.0016; Bonferroni post-tests compare artificial cerebral spinal fluid (ACSF) with antagonists for paired animals in each group. ns, not significant.
Experiments were conducted using a crossover design with randomized start times. All error bars represent SEM.
It has been proposed that NkB signaling from Kiss1ARH neurons may mediate hot-flush responses (Rance et al., 2013), because NkB-receptor-expressing neurons are in the median POA (Shughrue et al., 1996), and engaging those receptors through injection of a NkB receptor agonist into the median POA induces hypothermia in rats (Dacks et al., 2011). To test this hypothesis directly, we infused neurokinin receptor antagonists into the POA before chemogenetically activating Kiss1ARH neurons (Figure 4B). Because there are three neurokinin receptors (NK1R, NK2R, and NK3R), and NkB has some affinity for all of them, we infused a cocktail of antagonists for all three (de Croft et al., 2013). Infusion of this antagonist cocktail 30 min prior to chemogenetic activation of Kiss1ARH neurons prevented the increase in tail-skin temperature (Figures 4C and 4D).
These data are consistent with the idea that Kiss1ARH neurons contribute directly to the initiation of hot-flush-like symptoms, specifically via NkB signaling to the POA. Complete abolishment of this effect by infusion of neurokinin receptor antagonists into the POA suggests that NkB release from this projection of Kiss1 neurons is the most relevant for generating hot-flush-like activity (Yeo and Herbison, 2011). The functional relevance of Kiss1ARH innervation of the POA for normal thermoregulation has not been elucidated, although cyclic changes in core body temperature that correlate with fertility and stages of menstrual cycles in women are implicated (Sanchez-Alavez et al., 2011). The process of implantation and carrying offspring to term may require an ability to modulate core body temperature that is related to, but separate from, circadian body temperature fluctuations (Smarr et al., 2016). Whether this circuit represents an independent pathway linking core body temperature to the reproductive axis or is one facet of a larger network of thermoregulatory circuits remains to be established. Alternatively, the POA is involved in a number of homeostatic mechanisms beyond thermoregulation (McKinley et al., 2015), and it is possible that the hypertrophic state of Kiss1ARH neurons when sex-hormone levels fall activates NkB receptors in the POA without having functional significance under normal conditions.
Hot flushes are sporadic with an irregular frequency, and some women do not experience vasomotor symptoms during menopause. Both Kissl expression and NkB expression are elevated in Kiss1ARH neurons after menopause or gonadectomy (Goodman et al., 2007; Rance and Young, 1991; Roa et al., 2006; Rometo et al., 2007; Sheehan and Kovâcs, 1966; Smith et al., 2005), priming these neurons to evoke GnRH-mediated LH release as well as NkB-mediated hot flushes. Kiss1ARH neuron activity is synchronized by local autoregulation mechanisms (Qiu et al., 2016), and following gonadectomy in mice, Kiss1ARH neurons demonstrate a pulsatile activity pattern with a fixed period that is correlated with peaks of LH release (Clarkson et al., 2017). This pulsatile activity pattern, however, is not sufficient to explain the sporadic, episodic nature of hot flushes. Epidemiological data indicate that numerous sensory and interoceptive cues can evoke flushing in menopausal women, including: higher ambient temperature, consumption of spicy foods, anxiety, altitude, and acute physiological stressors (Federici et al., 2016; Hunter et al., 2013; Swartzman et al., 1990). Thus, we hypothesize that flushes occur when excitatory external cues converge on Kiss1ARH neurons during an episode of endogenous activity. Our experiments demonstrate that transient activation of Kiss1ARH neurons by either chemogenetic or optogenetic means is sufficient to elicit hot-flush-like vasomotor responses in mice and that this effect is enhanced by ovariectomy.
The most prevalent current therapy for hot flushes in the United States is estrogen replacement, the long-term safety of which has been questioned (Rossouw et al., 2013). The identity and function of POA neurons innervated by Kiss1ARH neurons, specifically those containing the NkB receptor, may provide additional targets, e.g., receptors, for the development of drugs that can treat hot-flush symptoms. Additionally, the identification of genetically defined neuronal subpopulations in the POA that mediate heat-effector responses is an area of growing interest (Song et al., 2016; Tan etal., 2016; Yu et al., 2016), and how neurons expressing NkB receptors fit into this larger picture remains to be seen.
EXPERIMENTAL PROCEDURES
Additional information and resources are included in the Supplemental Experimental Procedures.
Study Approval
All experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with approval from the University of Washington’s Animal Care and Use Committee. Adult mice (ages 2–8 months) had ad libitum access to standard rodent chow and water and were housed under a 12-hr:12-hr light:dark cycle at 21.51 ± 0.09oC. Zeitgeber time (ZT) of 0:00 indicates the onset of the light cycle each day.
Data Analysis
Statistical comparisons were performed with GraphPad’s Prism software and are reported in detail in the figure legends. A p value of 0.05 was the threshold for significance (*p < 0.05, **p < 0.01, and ***p < 0.001), and all error bars represent SEM. The ns represent either individual mice or histological sections, as indicated in the text.
Supplementary Material
Highlights.
Activation of Kiss1ARH neurons evokes a hot flush-like response in mice
Ovariectomy sensitizes the hot flush response of Kiss1ARH neurons
Kiss1ARH transmission to the preoptic area (POA) is sufficient to evoke a hot flush
Neurokinin B signaling in the POA is required for a hot flush
ACKNOWLEDGMENTS
We thank Dr. Robert Steiner for inspiration and helpful scientific discussions, Ms. Megan Chiang for maintaining our breeding the mice, Mrs. Serina Tsang and Ms. Kathy Kafer for helping to generate the Kiss1Cre mice, and Dr. Diane Durnam for editing. This work was supported by funds from the NIH (Ruth L. Kirschstein National Research Service Award 1F31AG056033 from the National Institute on Aging to C.W.J. and R01DA024908 to R.D.P.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.06.037.
REFERENCES
- Casper RF, Yen SS, and Wilkes MM (1979). Menopausal flushes: a neuroendocrine link with pulsatile luteninizing hormone secreation. Science 205, 823–825. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, and Herbison AE (2017). Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. USA 114, E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny, X., Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, et al. (2007). Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc. Natl. Acad. Sci. USA 104, 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Krajewski SJ, and Rance NE (2011). Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology 152, 4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Croft S, Boehm U, and Herbison AE (2013). Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 154, 2750–2760. [DOI] [PubMed] [Google Scholar]
- Federici LM, Roth SD, Krier C, Fitz SD, Skaar T, Shekhar A, Carpenter JS, and Johnson PL (2016). Anxiogenic CO2 stimulus elicits exacerbated hot flash-like responses in a rat menopause model and hot flashes in postmenopausal women. Menopause 23, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR (1998). Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil. Steril 70, 332–337. [DOI] [PubMed] [Google Scholar]
- Freeman EW, and Sherif K (2007). Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric 10, 197–214. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, and Clarke IJ (2007). Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148, 5752–5760. [DOI] [PubMed] [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, and Herbison AE (2000). Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141, 4218–4225. [DOI] [PubMed] [Google Scholar]
- Han SY, McLennan T, Czieselsky K, and Herbison AE (2015). Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc. Natl. Acad. Sci. USA 112, 13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R, Thurston RC, Hays RD, Chang C-C, Dillon SN, Ness RB, Bryce CL, Kapoor WN, and Matthews KA (2012). The impact of menopause on health-related quality of life: results from the STRIDE longitudinal study. Qual. Life Res 21, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MS, Gupta P, Chedraui P, Blümel JE, Tserotas K, Aguirre W, Palacios S, and Sturdee DW (2013). The international menopause study of climate, altitude, temperature (IMS-CAT) and vasomotor symptoms. Climacteric 16, 8–16. [DOI] [PubMed] [Google Scholar]
- Karling P, Hammar M, and Varenhorst E (1994). Prevalence and duration of hot flushes after surgical or medical castration in men with prostatic carcinoma. J. Urol 152, 1170–1173. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, and Rance NE (2010). Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166, 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull AA, Larsen SA, Clifton DK, Neal-Perry G, and Steiner RA (2017). A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist senktide. Endocrinology 158, 3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RJ, Barqawi A, and Crawford ED (2005). Adverse events associated with hormonal therapy for prostate cancer. Rev. Urol 7 (Suppl 5), S37–S43. [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, and Martelli D (2015). The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol. (Oxf.) 214, 8–32. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, and Rance NE (2016). Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology 157, 2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, and Nakamura K (2011). Central neural pathways for thermoregulation. Front. Biosci 16, 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, and Morrison SF (2010). A thermosensory pathway mediating heat-defense responses. Proc. Natl. Acad. Sci. USA 107, 8848–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, and Hardy JD (1961). Single unit activity of anterior hypothalamus during local heating. Science 134, 560–561. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, and Steiner RA (2009). Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci 29, 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, et al. (2017). Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 389,1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, and Ronnekleiv OK (2016). High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 5, e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, and Young WS 3rd. (1991). Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128, 2239–2247. [DOI] [PubMed] [Google Scholar]
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, and Kra- jewski-Hall SJ (2013). Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front. Neuroendocrinol 34, 211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, and Tena-Sempere M (2006). Hypothalamic expression of KiSS-1 system and gonadotropinreleasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology 147, 2864–2878. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, and Rance NE (2007). Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab 92, 2744–2750. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Manson JE, Kaunitz AM, and Anderson GL (2013). Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet. Gynecol 121, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Alboni S, and Conti B (2011). Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr.) 33, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan HL, and Kovacs K (1966). The subventricular nucleus of the human hypothalamus. Brain 89, 589–614. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, and Merchenthaler I (1996). In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervoussystem. J. Comp. Neurol 372, 395–414. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Zucker I, and Kriegsfeld LJ (2016). Detection of successful and unsuccessful pregnancies in mice within hours of pairing through frequency analysis of high temporal resolution core body temperature data. PLoS ONE 11, e0160127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, and Steiner RA (2005). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692. [DOI] [PubMed] [Google Scholar]
- Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, and Siemens J (2016). The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzman LC, Edelberg R, and Kemmann E (1990). Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychol. 9, 529–545. [DOI] [PubMed] [Google Scholar]
- Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, and Knight ZA (2016). Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SH, and Herbison AE (2011). Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152, 2387–2399. [DOI] [PubMed] [Google Scholar]
- Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud HR, Morrison CD, Derbenev AV, Zsombok A, and Munzberg H (2016). Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J. Neurosci 36, 5034–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


