Highlights
-
•
Neonatal handling affects the developmental course of cannabinoid receptors in rat brain.
-
•
In adult handled rats, [3H]CP55,940 binding levels are reduced in CPu, NAc and BLA.
-
•
Dorsal hippocampal [3H]CP55,940 binding levels are reduced only in female handled rats.
-
•
Handling increases adolescent and decreases adult [3H]CP55,940 binding levels in mPFC.
Abbreviations: 2-AG, 2-arachidonoylglycerol; ANOVA, analysis of variance; BLA, basolateral nucleus of amygdala; BSA, bovine serum albumin; CA1, dorsal field 1 of Ammon’s horn; CA3, dorsal field 3 of Ammon’s horn; CB1, cannabinoid receptor 1; CeA, central amygdaloid nucleus; Cg1, anterior cingulate cortex; CPu-DL, dorsolateral striatum; CPu-VM, ventromedial striatum; DG, dentate gyrus; eCB, endocannabinoid; GR, glucocorticoid receptors; GrDG, dentate gyrus granule cell layer; HPA, hypothalamic-pituitary-adrenal; IL, infralimbic cortex; LTD, long-term depression; mPFC, medial prefrontal cortex; MO, medial orbital cortex; MoDG, dentate gyrus molecular layer; NAc, nucleus accumbens; NS, not significant; PFC, prefrontal cortex; PND, postnatal day; PrL, prelimbic cortex; ROD, relative optical density; RT, room temperature
Keywords: Neonatal handling, Maternal separation, CB1 cannabinoid receptors, Adolescence, Male rat brain, Female rat brain
Abstract
Neonatal handling is an experimental model of early life experience associated with resilience in later life challenges, altering the ability of animals to respond to stress. The endocannabinoid system of the brain modulates the neuroendocrine and behavioral effects of stress, while this system is also capable of being modulated by stress exposure itself. The present study has addressed the question of whether neonatal handling in rats could affect cannabinoid receptors, in an age- and sex-dependent manner, using in situ hybridization and receptor binding techniques. Different effects of neonatal handling were observed in adolescent and adult brain on CB1 receptor mRNA and [3H]CP55,940 binding levels, which in some cases were sexually dimorphic. Neonatal handling interfered in the developmental trajectories of CB1 receptor mRNA levels in striatum and amygdaloid nuclei, as well as of [3H]CP55,940 binding levels in almost all regions studied. Adult handled rats showed reduced [3H]CP55,940 binding levels in the prefrontal cortex, striatum, nucleus accumbens and basolateral amygdala, while binding levels in prefrontal cortex of adolescent handled rats were increased. Finally, handling resulted in decreases in female [3H]CP55,940 binding levels in the striatum, nucleus accumbens, CA3 and DG of dorsal hippocampus and basolateral amygdala. Our results suggest that a brief and repeated maternal separation during the neonatal period induces changes on cannabinoid receptors differently manifested between adolescence and adulthood, male and female brain, which could be correlated to their stress response.
1. Introduction
Neonatal handling is an experimental model of early life experience, originally developed by Levine, in which pups are separated from their mother briefly (15 min daily) during the first 21 days of life (Levine, 1957). This brief separation is considered as an early life experience associated with resilience in later life challenges, altering the hypothalamic-pituitary-adrenal axis function and the ability of animals to respond to stress. Specifically, neonatally handled adult animals have increased numbers of glucocorticoid receptors (GR) in the brain (Meaney and Aitken, 1985; Meaney et al., 1985b; Wilber et al., 2008) and consequently they tend to secrete less corticotropin-releasing hormone, adrenocorticotropic hormone and corticosterone following exposure to stressful stimuli, due to enhanced sensitivity of the negative feedback loop (Plotsky and Meaney, 1993; Bhatnagar and Meaney, 1995; Vallée et al., 1996, 1997; Liu et al., 1997). In addition, neonatally handled animals show increased explorative behavior, decreased fear and/or anxiety and an enhanced ability to cope with stressful events (Chapillon et al., 2002; Meaney et al., 1991; Fernandez-Teruel et al., 1997; Vallée et al., 1997; Meerlo et al., 1999). Several studies have shown that neonatal handling enhances spatial learning and memory (Escorihuela et al., 1995; Pryce et al., 2003; Beane et al., 2002; Wong and Jamieson, 1968; Huot et al., 2002; Fenoglio et al., 2005). There is strong evidence that neonatal handling has a number of sexually dimorphic effects, such as on the serotoninergic system (Smythe et al., 1994; Stamatakis et al., 2006), stress reactivity (Papaioannou et al., 2002; Park et al., 2003,) learning and memory (Kosten et al., 2007; Stamatakis et al., 2008) and extinction of fear (Stevenson et al., 2009).
The endocannabinoid system is a neuromodulatory system that consists of cannabinoid receptors [mainly cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2)], endogenous cannabinoid ligands, among which the best characterized are anandamide and 2-arachidonoylglycerol (2-AG), a putative membrane transporter, and enzymes involved in the synthesis and inactivation of the endogenous ligands (Di Marzo, 2006; Hillard, 2015; Piomelli, 2003). The endocannabinoid system of the brain represents an important regulator of the adult hypothalamic-pituitary-adrenal (HPA) axis stress response, modulating the neuroendocrine and behavioral effects of stress, while this system is also capable of being modulated by stress exposure itself (Hill et al., 2010a,b; Hill and Tasker, 2012; Riebe and Wotjak, 2011). Furthermore, it is an important substrate for the control of emotional behavior and mood (Marsicano et al., 2002; Valverde and Torrens, 2012), it is involved in brain reward processes and drug addiction (Solinas et al., 2007, 2008) and it plays a specific role in neural development, guiding the establishment of cortical-subcortical connections (Belue et al., 1995; Mato et al., 2003; Rodríguez de Fonseca et al., 1993). There is evidence that early life experiences of maternal deprivation and social isolation have an effect on several parameters of the endocannabinoid system of the neonatal (Suárez et al., 2009), adolescent (Marco et al., 2014) and adult rat brain (Robinson et al., 2010), while the effect of maternal deprivation is gender dependent.
Given that the endocannabinoid system is important in the regulation of stress and is also being modulated by stress exposure, the present study addressed the question of whether neonatal handling in rats, which is known to confer resilience to stress, could affect in the long-term the endocannabinoid system. In particular, we were interested to investigate whether handling-induced changes in cannabinoid receptors (CB1 mRNA expression and [3H]CP55,940 binding levels) are differently manifested between adolescence and adulthood, as well as between male and female brain. Our findings indicate that the consequences of neonatal handling on rat brain CB1 receptors are age and sex-dependent.
2. Materials and methods
2.1. Animals
Wistar rats of both sexes were reared in the Animal Facility of the Medical School of the University of Patras (Patras, Greece) under standard conditions (21 ± 1 °C; 12 h light/dark cycle, lights on at 08:00 h) and received food and water ad libitum. Two or three virgin females were housed with one stud male rat. Pregnant females were caged separately and litters were randomly distributed to either the handled or non-handled groups. A total of 9 litters were used for the experiment (4–5 litters in each of the two groups: handled, non-handled). The litter size and the sex ratio did not significantly differ between the litters employed in the two groups [average litter size [mean ± SEM (standard error of the mean): non-handled litters 11.75 ± 0.19 (range, 11–12), handled litters 10.6 ± 0.32 (range, 9–12); average sex ratio (males:females, mean ± SEM): non-handled litters 2.15 ± 0.64; handled litters 1.06 ± 0.10]. Culling of litters was not performed since it has been shown that litter size within the range employed does not affect maternal behavior (Champagne et al., 2003; Deviterne et al., 1990). The day of birth was determined as postnatal day 0. Following weaning, three to four animals of the same sex, litter, and group (handled or non-handled) were placed per cage and were kept under standard housing conditions in the same room. A total of 56 animals were used in the present study: 28 mid-adolescent (PND 39–40) and 28 adult animals (PND 89–90). For each age group seven male non-handled, seven male handled, seven female non-handled, and seven female handled rats were used. In each group, animals from all litters were employed. Experiments were carried out in agreement with the ethical recommendation of the European Communities Council Directives of November 24, 1986 (86/609/EEC) and of September 22, 2010 (2010/63/EU). All efforts were made to minimize the number of animals used and their suffering.
2.2. Neonatal handling
We employed a neonatal handling protocol similar to that originally described by Levine (1957) lasting from PND1 until weaning (PND21) and recently described by Katsouli et al. (2014). In particular, every day between 9:00–10:00 a.m. the mothers of the pups were removed from their home cages and placed separately into cages left in the same room (always the same cage for each mother throughout the handling period). All offsprings of a litter were then removed, placed together in a clean plastic container and heated by a lamp so that the temperature close to the pups was 28–29 °C. After 15 min, the pups and then their mothers were returned to their home cages. Non-handled litters were left completely undisturbed until weaning.
2.3. Tissue preparation
On PND39-PND40 or on PND89-PND90 rats were deeply anesthetized with isofluorane, decapitated and the brains were isolated and flash-frozen in −50 °C isopentane. Brain tissue was kept at −80 °C, until use. Brain tissue was cut into coronal 15 μm sections on a cryostat (Leica CM1500, Germany) at −18 °C, thaw mounted onto 0.01% poly-l-lysine-coated slides (for in situ hybridization experiments) or on acid-clean gelatin-coated slides (for in vitro receptor binding experiments). Sections were allowed to air-dry at room temperature and stored at −80 °C until further processing.
Four coronal sections were collected non-consecutively on each slide, so both rostral and caudal parts of each brain region were represented. Sections were collected separately at three different brain levels based on a brain atlas (Paxinos and Watson, 2007) which included the prefrontal cortex (AP 4.68–AP 3), the striatum (AP 2.04 to AP −0.24) and the hippocampus (−2.52 to AP −3.36).
2.4. In vitro receptor binding
In vitro receptor binding for cannabinoid receptors was performed as previously described (Dalton and Zavitsanou, 2010). Cannabinoid receptor binding levels were evaluated using quantitative in vitro receptor autoradiography and the radioligand [3H]CP55,940 (specific activity 141.2 Ci/mg, PerkinElmer, USA), a potent, non-selective agonist, which activates both CB1 and CB2 receptors with equal potency (Howlett et al., 2002).
Sections were preincubated in 50 mM Tris-HCl containing 5% BSA, pH7.4 at RT; air-dried and incubated in the same buffer containing [3H]CP55,940 at final concentration 7.37 nM (prefrontal cortex), 7.24 nM (striatum), 6.75 nM (hippocampus). Non-specific binding was determined by incubating adjacent sections to the buffer containing the tritiated ligand plus 10 μM CP55,940. Three post-incubation washes at 4 °C were performed as follows: 1 h in 50 mM Tris HCl (pH 7.4) plus 1% BSA; 3 h in 50 mM Tris HCl (pH 7.4) plus 1% BSA; 5 min in 50 mM Tris HCl (pH 7.4). In each assay brain sections from all four categories of either male or female animals (handled, non-handled and adult, adolescent) were processed concurrently.
2.5. In situ hybridization
The oligodeoxyribonucleotide probe used in the present study was 48 base-long and was complementary to the mRNA encoding the rat CB1 subtype of cannabinoid receptors. The sequence of the synthetic oligonucleotide was:
5′GGTGATGGTACGGAAGGTGGTGTCTGCAAGGCCATCTAGGATCGACTT-3′ (Microchemistry Laboratory, FORTH, Crete, Greece). Using the National Centre of Biotechnology Information BLAST network service no significant homology of the probe with any other than the target sequence was found. The oligonucleotide probe was diluted to a concentration of 3 pmol/μl and was labeled with 35S-dATP at the 3′ end (Perkin–Elmer, USA, specific activity 1250 Ci/mmol) by terminal transferase (Roche Applied Science, Germany). Unincorporated nucleotides were removed by chromatography with Bio-Spin 6 columns (Bio-Rad). The specific activity of the labeled probes used were 5.2 × 106 cpm/pmol (prefrontal, male), 3.2 × 106 cpm/pmol (striatum, male), 5.7 × 106 cpm/pmol (hippocampus, male), 8.4 × 106 cpm/pmol (prefrontal, female), 3.1 × 106 cpm/pmol (striatum, female), 5.0 × 106 cpm/pmol (hippocampus, female).
In situ hybridization was performed as previously described (Katsouli et al., 2014). Frozen sections were allowed to thaw at room temperature (RT) and were then fixed for 5 min in 4% paraformaldehyde in phosphate-buffered saline (0.1 M PBS; pH 7.4, containing 0.1% diethylpyrocarbonate), washed in PBS and after dehydration through a series of graded alcohols (70% and 95%) the sections were allowed to air-dry at RT. Hybridization was performed in a solution containing 50% formamide (v/v), 4× standard saline citrate (SSC, 1× SSC: 0.15 M sodium chloride, 0.015 sodium citrate), 10 mM dithiothreitol and 10% dextran sulfate (w/v) with 1:100 labeled probe. Tissue sections on each slide were covered with 100–120 μl of hybridization solution and a strip of parafilm and incubated overnight (17–18 h) in humidified chambers at 42 °C. The non-specific signal was determined by the addition of 100-fold excess of unlabeled probe to the hybridization solution on separate slides. Following hybridization, the parafilm was removed and sections were rinsed in 1 × SSC at RT, washed two times (20 min each) in 1 × SSC at 60 °C, dipped in 1 × SSC at RT, washed in 0.1 x SSC for 3 min at RT, dehydrated in 70%, 95% and 100% ethanol and allowed to air-dry at RT. In each assay brain sections from all four categories of either male or female animals (handled, non-handled and adult, adolescent) were processed concurrently.
2.6. Autoradiography
BioMax MR film (Kodak) was exposed to labeled dried sections in x-ray film cassettes. Autoradiographic standards ([3H] microscales from Amersham) were exposed along with sections from the receptor binding experiments. Exposure times were: 29–30 weeks for the receptor binding experiments and 7–12 weeks for the in situ hybridization experiments. After exposure, the films were developed and fixed using Kodak GBX developer and fixer.
2.7. Quantitative analysis of the autoradiographic images
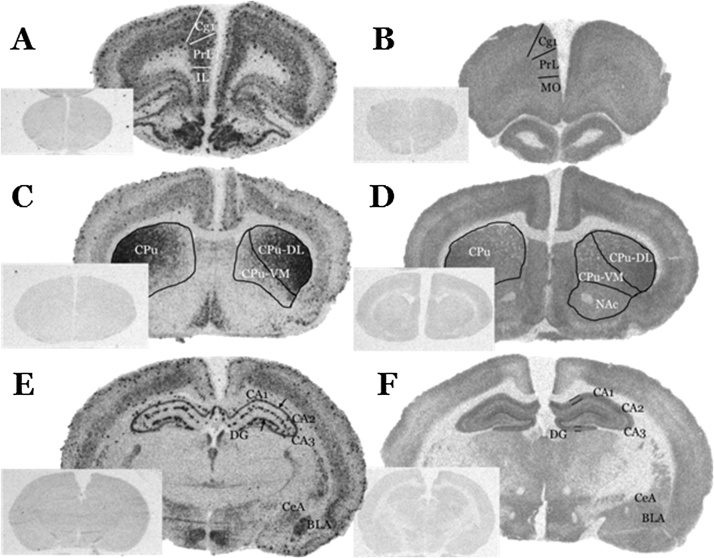
All films were analyzed by using a computer-assisted image analysis system (MCID Core, Interfocus Imaging Ltd, Linton, Cambridge, UK). As shown in Fig. 1, CB1 receptor mRNA levels and [3H]CP55,940 binding levels were quantified in regions of prefrontal cortex: anterior cingulate (Cg1), prelimbic (PrL), infralimbic (IL), medial orbital (MO) prefrontal cortex; in striatum (CPu) and nucleus accumbens (NAc); in dorsal hippocampal regions: CA1, CA3, dentate gyrus (DG); as well as in amygdaloid nuclei: central amygdaloid nucleus (CeA) and basolateral nucleus of amygdala (BLA). In hippocampus, CB1R mRNA levels were quantified from the pyramidal layer of CA1 and CA3 and from the granule cell layer of DG (GrDG) (Fig. 1E), while [3H]CP55,940 binding levels were quantified from the three layers of CA1 and CA3 (oriens, pyramidal, radiatum) and from the molecular layer of DG (Fig. 1F). The brain regions were defined according to a rat brain atlas (Paxinos and Watson, 2007) and cresyl violet stained sections collected along with the sections processed for the assays.
Fig. 1.
Representative autoradiograms of coronal male rat brain sections. Left: distribution of CB1 mRNA levels using in situ hybridization of 35S-labelled oligodeoxynucleotide probe specific for the CB1 mRNA. Right: distribution of [3H]CP55,940 binding levels. The boundaries or the width of quantified brain regions are shown at the level of prefrontal cortex (A, B), at approximate 3.20 mm and 4.20 mm anterior to Bregma, respectively, at the level of striatum (C, D), at approximate 0.96 mm anterior to Bregma, and at the level of dorsal hippocampus (E, F), at approximate -3.00 mm posterior to Bregma according to Paxinos and Watson (2007). Insets indicate the non-specific hybridization signal (left) or non-specific binding (right) in adjacent sections. Cg1: anterior cingulate cortex, PrL: prelimbic cortex; IL: infralimbic cortex; MO: medial orbital cortex; CPu: caudate-putamen, or striatum; CPu-DL: dorsolateral striatum; CPu-VM: ventromedial striatum; NAc: nucleus accumbens; CA1: hippocampal region CA1; CA3: hippocampal region CA3; DG: dentate gyrus; BLA: basolateral amygdala; CeA: central amygdaloid nucleus.
Quantification of [3H]CP55,940 binding levels or CB1 mRNA levels in each brain region was performed by measuring the relative optical density (ROD) in 8–12 brain sections, depending on brain region, from each animal. Non-specific signal (for both receptor binding and in situ hybridization experiments) was quantified from 8 sections (from each experimental group) and was subtracted from the total signal in order to determine the specific signal for each brain region in each animal. Optical density measurements for specific binding for the in vitro receptor binding experiments were converted into fmoles of [3H]CP55,940 per mg tissue equivalent according to the calibration obtained from the tritium standards. For in situ hybridization, mRNA levels were normalized between males and females for all brain areas examined. A correction factor has been calculated based on the specific activity of the probes used (cpm/ml) and the exposure time for the production of the autoradiograms.
2.8. Statistical analysis
Data were analyzed by three-way analysis of variance (ANOVA) with neonatal handling, age, and sex as the independent factors. When interactions between the independent factors were detected, planned pair-wise comparison have been performed in order to identify the specific effect of neonatal handling in each age and sex group. The level of statistical significance was set at 0.002. All tests were performed with the SPSS software.
3. Results
The effects of neonatal handling on cannabinoid receptors were studied in adolescent and adult male and female rat brain. in vitro receptor binding and in situ hybridization were used in order to study cannabinoid receptor binding and CB1 mRNA levels, respectively, in brain regions involved in the regulation of the stress axis, such as the prefrontal cortex, hippocampus and amygdala and in brain regions involved in reward processes, i.e. the striatum and nucleus accumbens.
Figs. 1(A, C, E) and 3 show representative autoradiograms of CB1 mRNA levels at three rostrocaudal levels of rat brain from in situ hybridization experiments. We observed that CB1 mRNA showed high expression signal in dorsolateral CPu, a moderate signal in mPFC, in the pyramidal layer of dorsal hippocampus and in BLA and a low signal in CeA. Furthermore, the distribution of CB1 mRNA was not homogenous in the prefrontal cortex with higher hybridization signal detected in layers I, V and VI (Fig. 1A). We also observed islets of higher intensity scattered in the prefrontal cortex (Fig. 1A), as well as in hippocampal area (Fig. 1E), attributed by other investigators to CB1 expression in GABAergic interneurons (Marsicano and Lutz, 1999; Mailleux and Vanderhaeghen, 1992). In addition, higher hybridization signal was observed in dorsolateral striatum, a lower signal in ventromedial striatum and no detectable signal in nucleus accumbens (Fig. 1C).
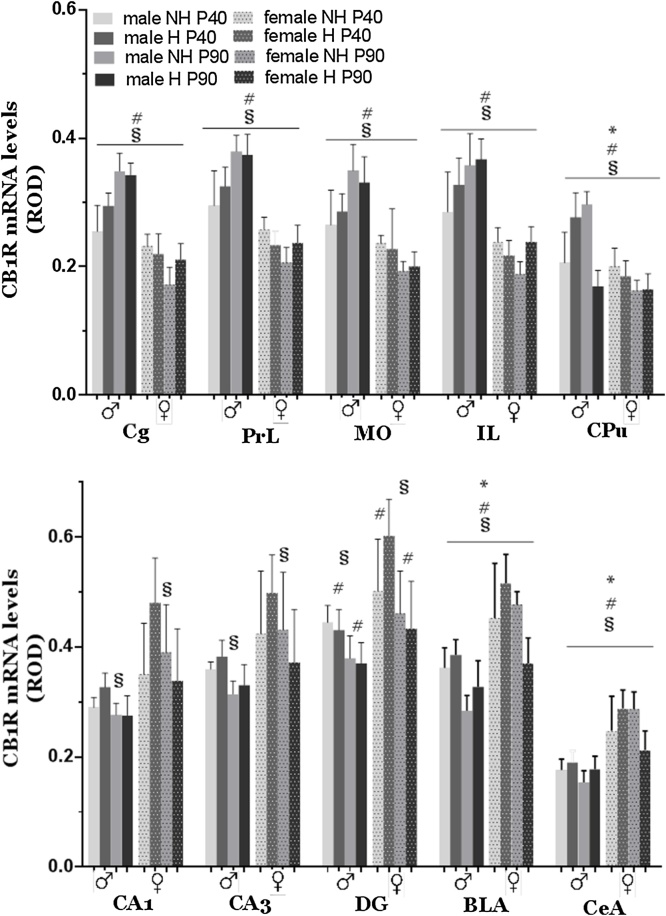
Fig. 3.
Effects of neonatal handling on CB1 receptor mRNA levels of male and female adolescent and adult rat brain. Top: Bar graphs depicting CB1 mRNA levels in male and female rat brain regions of medial prefrontal cortex and caudate-putamen. Bottom: Bar graphs depicting CB1 mRNA levels in male and female rat brain regions of dorsal hippocampus and amygdaloid nuclei. Bars represent mean ± SEM (n = 6–7 animals in each group). Means are expressed in ROB (Relative Optical Density). * statistically significant handling effects; # statistically significant sex effects; § statistically significant age effects (p ≤ 0.002 three-way ANOVA with handling, age and sex as independent factors). Cg: anterior cingulate cortex, PrL: prelimbic cortex; IL: infralimbic cortex; MO: medial orbital cortex; CPu: caudate-putamen, or striatum; NAc: nucleus accumbens; CA1: hippocampal region CA1; CA3: hippocampal region CA3; DG: dentate gyrus; BLA: basolateral amygdala; CeA: central amygdaloid nucleus.
Representative autoradiograms of [3H]CP55,940 binding levels, reflecting levels of functional cannabinoid receptors, from receptor binding autoradiography experiments are shown in Figs. 1 (B, D, F) and 2 . The autoradiographic signal of [3H]CP55,940 binding was homogenously distributed in the layers of prefrontal cortex (Fig. 1B), as well as in dorsal hippocampus (Fig. 1F). In the striatum, we observed a dorsolateral to ventromedial decreasing gradient of [3H]CP55,940 binding levels, similar to the in situ hybridization signal distribution in this region (Herkenham et al., 1990; Jansen et al., 1992). In nucleus accumbens, [3H]CP55,940 receptor binding was also observed, while no CB1 mRNA levels were detected in this region. Finally, regarding the amygdaloid nuclei, we have observed that [3H]CP55,940 binding levels were higher in BLA than in CeA (Fig. 1F).
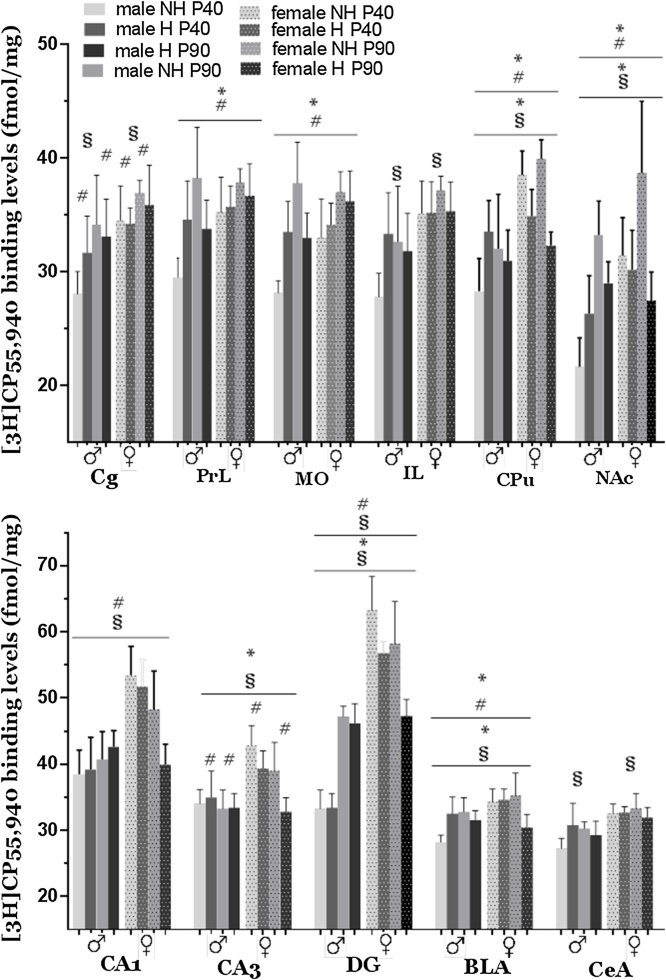
Fig. 2.
Effects of neonatal handling on cannabinoid receptor binding levels, measured with [3H]CP55,940, of male and female adolescent and adult rat brain. Top: Bar graphs depicting specific [3H]CP55,940 binding levels in male and female rat brain regions of medial prefrontal cortex, caudate-putamen and nucleus accumbens. Bottom: Bar graphs depicting specific [3H]CP55,940 binding levels in male and female rat brain regions of dorsal hippocampus and amygdaloid nuclei. Bars represent mean ± SEM (n = 5–7 animals in each group). Mean values are expressed in fmol/mg. * statistically significant handling effects; # statistically significant age effects; § statistically significant sex effects (p ≤ 0.002 three-way ANOVA with handling, age and sex effect as independent factors). Cg: anterior cingulate cortex, PrL: prelimbic cortex; IL: infralimbic cortex; MO: medial orbital cortex; CPu: caudate-putamen, or striatum; NAc: nucleus accumbens; CA1: hippocampal region CA1; CA3: hippocampal region CA3; DG: dentate gyrus; BLA: basolateral amygdala; CeA: central amygdaloid nucleus.
3.1. Effect of neonatal handling on [3H]CP55,940 binding
Analysis of the data for [3H]CP55,940 binding levels did not demonstrate a significant handling × age × sex interaction in any of the regions studied. A significant handling x age interaction was observed in PrL and MO of mPFC, in CPu and in NAc, as well as in BLA (Fig. 2) (three-way ANOVA, F1,53 = 14.165, p < 0.001 for PrL; F1,53 = 19.659, p < 0.001 for MO; F1,52 = 11.740, p = 0.001 for CPu; F1,51 = 24.988, p < 0.001 for NAc; F1,54 = 21.452, p < 0.001 for BLA). As shown in Table 1, in adolescent rats (P40), [3H]CP55,940 binding levels in both PrL and MO of handled rats were higher by approximately 10% compared to non-handled rats, regardless of sex (p = 0.034 for PrL; p = 0.008 for MO). In adult rats, neonatal handling resulted in a significant decrease of [3H]CP55,940 binding levels by 20.2% in NAc (p < 0.001), by 9.2% in BLA (p = 0.003) and by 9% in CPu (p = 0.019), compared to non-handled. Minor decreases were also observed in PrL (p < 0.001) and MO (p < 0.001) of adult rats (Table 1).
Table 1.
The effect of neonatal handling on [3H]CP55,940 binding levels in adolescence and in adulthood, regardless of sex.
| Brain region | Adolescent (P40) | Adult (P90) |
|---|---|---|
| PrL | ↑10% | ↓6.5% |
| MO | ↑10.8% | ↓4.5% |
| CPu | ns | ↓9% |
| NAc | ns | ↓20.2% |
| BLA | ns | ↓9.2% |
Data are expressed as percentage of change of [3H]CP55,940 binding levels of handled compared to non-handled rats. ns: not significant; see abbreviation list for brain regions.
Furthermore, a significant handling x sex interaction was observed in CPu, NAc, CA3, DG as well as in BLA (three way ANOVA, F1,52 = 26.128, p < 0.001 for CPu; F1,51 = 11.692, p = 0.001 for NAc; F1,54 = 11.179, p = 0.002 for CA3; F1,53 = 18.144, p < 0.001 for DG; F1,54 = 10.753, p = 0.002 for BLA) (Fig. 2). Further analysis indicated that handling resulted in significant decreases in [3H]CP55,940 binding levels only in female rats, regardless of age (Table 2). In particular, decreases were detected in CPu (15.1%, p = 0.005), NAc (15.2%, p = 0.005), CA3 (15.2%, p < 0.004), DG (16.6%, p < 0.001) and BLA (8.4%, p = 0.003). Moreover, female non-handled rats demonstrated significantly higher [3H]CP55,940 binding levels compared to male non-handled in all above regions (for CPu: p < 0.001; for NAc: p = 0.005; for CA3: p < 0.001; for DG: p < 0.001; for BLA: p < 0.001), while female handled rats exhibited a decrease towards male levels.
Table 2.
The effect of neonatal handling on [3H]CP55,940 binding levels in female rat brain, regardless of age.
| Brain region | Female |
|---|---|
| CPu | ↓15.1% |
| NAc | ↓15.2% |
| CA3 | ↓15.2% |
| DG | ↓16.6% |
| BLA | ↓8.4% |
Data are expressed as percentage of change of [3H]CP55,940 binding levels of handled compared to non-handled rats; see abbreviation list for brain regions.
It is worth noting that, regardless of sex, binding levels of non-handled rats increased from adolescence to adulthood by 37% (p = 0.001) in NAc and by 18% (p < 0.001) and 19.5% (p < 0.001) in PrL and MO, respectively. However, in handled rats decreases were noticed in BLA (11.1%, p = 0.003) and in CPu (8.6%, p = 0.007) (Table 3).
Table 3.
Developmental changes from adolescence to adulthood of [3H]CP55,940 binding levels in non-handled and handled rats, regardless of sex.
| Brain region | NH | H |
|---|---|---|
| PrL | ↑18% | ns |
| MO | ↑19.5% | ns |
| CPu | ns | ↓8.6% |
| NAc | ↑37% | ns |
| BLA | ↑6.7% | ↓11.1% |
Data are expressed as percentage of change of [3H]CP55,940 binding levels of P90 compared to P40 rats. NH: non-handled rats, H: neonatally handled rats; see abbreviation list for brain regions.
3.2. Effect of neonatal handling on CB1 mRNA
Analysis of the data for CB1 mRNA levels demonstrated a significant handling x age x sex interaction for CPu and for the amygdaloid nuclei BLA and CeA (Fig. 3) (three-way ANOVA, F1,53 = 44.953, p < 0.001 for CPu; F1,52 = 11.447, p = 0.001 for BLA; F1,52 = 11.167, p = 0.002 for CeA). Further analysis showed that CB1 mRNA levels of male handled rats decreased with age in CPu by 36.6% (p < 0.001) and in BLA by 26.6% (p = 0.019). In female handled rats significant decreases with age were observed in both amygdaloid nuclei studied, BLA (29.9%, p < 0.001) and CeA (28.2%, p = 0.002) (Table 4). In non-handled animals, decreases of CB1 mRNA levels with age were also noticed in BLA of male rats (21.2%, p = 0.001) and in CPu of female rats (14.3%, p = 0.015). However, a 49.9% increase (p = 0.001) of CB1 mRNA levels with age was observed in CPu of male non-handled animals (Table 4).
Table 4.
Developmental changes from adolescence to adulthood of CB1 mRNA levels in male and female, non-handled and handled rats.
| Brain region | Male |
Female |
||
|---|---|---|---|---|
| NH | H | NH | H | |
| CPu | ↑49.9% | ↓36.6% | ↓14.3% | ns |
| BLA | ↓21.2% | ↓26.6% | ns | ↓29.9% |
| CeA | ns | ns | ns | ↓28.2% |
Data are expressed as percentage of change of CB1 mRNA levels of P90 compared to P40 rats. ns: not significant; NH: non-handled rats, H: neonatally handled rats; see abbreviation list for brain regions.
In contrast to striatum and amygdala, in mPFC, we did not observe a significant effect of neonatal handling on CB1 mRNA levels. However, in all mPFC areas studied (Cg1, PrL, MO, IL) we observed a significant age x sex interaction (three-way ANOVA, F1,55 = 53.714, p < 0.001 for Cg1; F1,55 = 30.580, p < 0.001 for PrL; F1,55 = 24.071, p < 0.001 for MO; F1,55 = 12.692, p = 0.001 for IL). Male CB1 mRNA levels in mPFC showed an increase from P40 to P90, while female levels showed a decrease with age (Fig. 2).
4. Discussion
In the present study, we investigated the effects of neonatal handling on cannabinoid receptors in adolescent (PND40) and adult (PND90) male and female rat brain. We observed that a brief and repeated maternal separation during the neonatal period leads to alterations in CB1 mRNA receptor expression and [3H]CP55,940 binding levels which are age and sex dependent, in almost all brain regions studied. In particular, we observed that neonatal handling interferes with the developmental changes of cannabinoid receptors from PND40 to PND90 in certain corticolimbic and striatal brain regions. A sex-dependent effect of neonatal handling on the developmental course of CB1 mRNA levels was observed in striatum and amygdala. Moreover, a sex-independent effect of neonatal handling on the PND40-PND90 course of [3H]CP55,940 binding levels was observed in almost all regions studied. We further observed a different effect of neonatal handling on [3H]CP55,940 binding levels between adolescent and adult, regardless of sex, and between male and female rats, regardless of age.
It is well known that endogenous cannabinoids act as retrograde messengers released from depolarized postsynaptic neurons onto CB1 receptors which are localized on presynaptic terminals (Ohno-Shosaku and Kano, 2014). Thus, in projection neurons, CB1 mRNA and presynaptic CB1 receptors are not localized in the same brain nuclei (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). Therefore, this is most likely the reason why the region-specific changes in CB1 mRNA levels induced by neonatal handling that were observed in the present study were not followed by similar changes in cannabinoid receptor binding levels in most regions.
Since CP55,940 activates both CB1 and CB2 receptors with equal potency (Howlett et al., 2002), changes of [3H]CP55,940 binding levels in the present study can reflect changes in both CB1 and CB2 receptors. In rodent brain, CB1 receptors are ubiquitously expressed in high levels in several brain regions (Herkenham et al., 1990), while the expression of CB2 receptors is significantly lower and restricted in microglia, endothelial cells and a sub-population of neurons within the central nervous system (Roche and Finn, 2010). Most findings suggest that CB2 receptors are expressed predominantly in activated microglia during neuroinflammation (Atwood and Mackie, 2010). However, two recent reports refer to the effects of neonatal handling on microglia. In particular, Delpech et al. (2016) have reported that a brief daily maternal separation (similar to neonatal handling) dysregulates microglial function in the developing mouse hippocampus at PND14 and PND28, and Schwarz et al. (2011) have reported that neonatal handling increases selectively the mRNA expression of cytokine IL-10 in nucleus accumbens of Sprague-Dawley rats at PND60, via an epigenetic modification of this gene within microglia. Furthermore, recent reports have shown that CB2 receptors modulate DA neuronal activities and cocaine self-administration behavior in VTA dopamine neurons in both mice and rats (Zhang et al., 2014, 2017). Taking into account all the above, we assume that some of the changes in cannabinoid receptors observed in the present study might be attributed to CB2 receptors in microglia and/or neurons.
Previous studies have shown that CB1 receptor expression can undergo dynamic changes following exposure to a variety of experiences during certain critical developmental periods. For example, CB1 receptor expression changes have been reported following chronic cannabinoid administration (Oviedo et al., 1993), social isolation (Robinson et al., 2010), environmental enrichment (El Rawas et al., 2011), as well as neonatal lipopolysaccharide treatment (Zavitsanou et al., 2013). Furthermore, early maternal deprivation at PND9 induces changes on the expression of hippocampal CB1 as well as CB2 cannabinoid receptors of neonatal rats (Suárez et al., 2009).
There are several reports indicating that the brain endocannabinoid system changes in a temporal-specific and region-dependent manner (Lee and Gorzalka, 2015; Lee et al., 2016 for reviews). In our study, in control (non-handled) rats, we observed alterations in cannabinoid receptors from PND40 to PND90 which were different between brain regions and also between male and female. Most notably, CB1 mRNA levels in CPu increased in male and decreased in the female from adolescence to adulthood, while in BLA CB1 mRNA decreased only in the male. Furthermore, regardless of sex, [3H]CP55,940 binding levels increased from adolescence to adulthood in PrL, MO, NAc, and BLA, while no changes were detected in other regions studied. It is interesting to point out that studies on CB1 receptor mRNA expression and CB1 immunoreactivity in human and monkey dorsolateral prefrontal cortex report decreased or stable levels between adolescence and adulthood (Long et al., 2012; Eggan et al., 2010; Choi et al., 2012). Furthermore, in Sprague-Dawley rats CB1 mRNA expression levels decline from PND40 to PND70 in the striatum and prefrontal cortex (Heng et al., 2011; Van Waes et al., 2012). However, in Wistar rats a comparison of cannabinoid receptor binding in adolescent and adult rats, using PET (with [18F]MK-9470) and in vitro autoradiography (with [3H]CP55,940) showed increased levels in cortex, hippocampus and cerebellum and stable levels in striatum, amygdala, and other regions studied (Verdurand et al., 2011). It appears that the development of cannabinoid receptors follows different trajectories depending on brain region, sex as well as strain and species.
In the present study, rats exposed to neonatal handling exhibited a reduction in [3H]CP55,940 binding levels in NAc in adults, regardless of sex. A similar effect was not seen in NAc of the adolescent rat. It has been reported that within the NAc, the vast majority of CB1 receptors are localized on GABAergic axon terminals (Matyas et al., 2006) and their activation increases dopamine release (Covey et al., 2015). Furthermore, their activation enhances properties of drug-induced (Caillé et al., 2007) and natural rewards, such as social play and feeding behavior (Mahler et al., 2007; Shinohara et al., 2009; Trezza et al., 2012; Wei et al., 2015). We could hypothesize that the downregulation of cannabinoid receptors observed in NAc of adult rats as a result of neonatal handling might contribute to alterations in reward behaviors, through changes in dopamine release. Indeed, previous studies have reported that neonatal handling alters reward behaviors in rats, such as social play behaviors in adolescent rats (Karkow and Lucion, 2013), and feeding behavior of both male and female adult rats by increasing their consumption of palatable food (Silveira et al., 2004).
A major finding in our results is that rats exposed to neonatal handling showed a decrease of both CB1 mRNA and [3H]CP55,940 binding levels in CPu in adults. It is well established that in the striatum the induction of long-term depression (LTD) is dependent on the activation of CB1 cannabinoid receptors both at GABAergic and glutamatergic synapses (Adermark et al., 2009; Gerderman et al., 2002). It is interesting to point out that chronic Δ9-THC administration downregulates CB1 receptors (Breivogel et al., 1999; Sim-Selley and Martin, 2002) and that persistent activation of the endocannabinoid (eCB) pathway impairs eCB-mediated LTD in the dorsolateral striatum (Nazzaro et al., 2012). The downregulation of cannabinoid receptors observed in handled adult rats raises the question of whether neonatal handling might lead to altered eCB-dependent striatal plasticity.
According to our results, in dorsal hippocampus, neonatal handling affected [3H]CP55,940 binding levels of female rats, regardless of age, while this early life experience had no effect on male hippocampal [3H]CP55,940 binding levels. Growing evidence suggests a role of the endocannabinoid system in the regulation of emotional behaviors (Valverde and Torrens, 2012). CB1 receptor-deficient mice have been postulated as a model for depression, given that they display enhanced despair behavior in the tail suspension test, dysregulation of the serotonergic system and impairment of neurotrophic factors, including reduced 5-HT transporter and BDNF in the hippocampus, compared to wild-type (Valverde and Torrens, 2012; Aso et al., 2008, 2009). The effect of neonatal handling on cannabinoid receptor levels in the dorsal hippocampus may correlate to the depressive-like behavior of female handled rats that has been reported. In particular, neonatally handled females, contrary to male handled rats, showed decreased learned helplessness and immobility time in the forced swim test (an index of depressive behavior) and reduced serotonergic activity compared to female non-handled rats (Papaioannou et al., 2002).
In the present study, we observed that in BLA of the amygdala, neonatal handing reduces the PND40-PND90 trajectory of [3H]CP55,940 binding levels and affects adult but not adolescent [3H]CP55,940 binding levels, regardless of sex. The amygdala has been known to play a central role in the acquisition and expression of fear. More recently convergent evidence has implicated the basolateral complex of the amygdala in the extinction of fear, as well (Amano et al., 2010; Barad et al., 2006). Recent evidence indicates that CB1 receptors play an essential role in this process (Fittzerald et al., 2014; Kemorah et al., 2006; Lafenette et al., 2007). CB1 receptors have been localized in lateral and basolateral nuclei of the amygdala in neuronal terminals of a subpopulation of GABAergic interneurons, corresponding to large cholecystokinin-positive cells. Furthermore, CB1 receptor agonists reduced the GABA-A receptor-mediated evoked and spontaneous IPSPs in the region (Katona et al., 2001). Our results showing an effect of neonatal handling on cannabinoid receptors in BLA underscore the possible involvement of CB1 receptors in the fear extinction processes that take place in this brain region. Furthermore, the alterations of cannabinoid receptors in BLA of the adult but not adolescent handled rats may contribute to a different stress response of handled adult rats compared to adolescent. This is based on multiple lines of evidence indicating that endocannabinoid signaling in amygdala (particularly in the BLA) plays a key role in the regulation of both basal- and stress-induced HPA axis activity, functioning as a “gatekeeper” over the HPA axis (Hill and Tasker, 2012) and is a critical regulator of HPA axis stress habituation (Hill et al., 2010a,b). Local administration into the BLA of a CB1 receptor agonist significantly reduced stress-induced corticosterone secretion, whereas administration of a CB1 receptor antagonist increased corticosterone secretion (Hill et al., 2009).
Endocannabinoid signaling in medial prefrontal cortex seems to play a critical role in the termination of the stress response, regulating the negative feedback on stress axis (HPA) through corticosteroids. In particular, Hill et al. (2011) have shown that local administration of the CB1R antagonist AM251 into the medial prefrontal cortex of male rats prolonged corticosterone secretion following exposure to a 30 min restraint stress. A similar prolongation in corticosterone secretion was observed in CB1 knock-out mice compared to controls. Furthermore, Hill et al. (2011) have shown that pre-administration of a glucocorticoid receptor antagonist blocked the elevation of 2-AG which followed the exposure to restrain stress within the medial prefrontal cortex.
In the present study, neonatal handling resulted in age-dependent changes of cannabinoid receptors in mPFC (PrL, MO), unlike to what was seen in other regions; [3H]CP55,940 binding levels were increased in adolescent and slightly but significantly decreased in adult rats and this effect was independent of sex. The downregulation of cannabinoid receptors in adult rats exposed to neonatal handling is contrary to the upregulation of CB1 receptors in the medial prefrontal cortex following chronic stress exposure. In particular, chronic stress exposure, which is strongly associated with depressive-like behavior (Farhan et al., 2014; Logan et al., 2015), leads to decreased glucocorticoid receptor protein levels in PFC (Chiba et al., 2012) and increased [3H]CP55,940 binding sites (Bmax) in ventromedial PFC (McLaughlin et al., 2013, 2014). On the contrary, it is well established that adult neonatally handled rats show increased glucocorticoid receptor density, a shorter duration of stress response (Fenoglio et al., 2004; Meaney et al., 1985a,b) and less vulnerability for depressive behavior in males (Papaioannou et al., 2002). It appears that several parameters of the neonatal handling adult profile are opposite to that of chronic stress, supporting the role of cannabinoid receptors in resilience (Meerlo et al., 1999; Plotsky and Meaney, 1993; Pryce et al., 2003). On the other hand, the upregulation of cannabinoid receptors observed in neonatally handled adolescent rats suggests a different effect of handling on the stress response in adolescence compared to adulthood.
The question raised by our findings is how neonatal handling triggers changes in cannabinoid receptors. The endocannabinoid (ECB) system has recently emerged as a fundamental component of the neuroendocrine response, with a key role in governing glucocorticoid-mediated negative feedback processes in both hypothalamic (Di et al., 2003; Malcher-Lopes et al., 2006) and extrahypothalamic (Hill et al., 2011) brain regions (Hill and Tasker, 2012; Tasker and Herman, 2011 for reviews). The handling procedure has been found to increase pup-directed maternal behavior in rats, particularly licking and grooming (LG) (Liu et al., 1997). Several studies have described the cellular and molecular pathways linking handling and high maternal LG to changes in hippocampal GR and reduced stress reactivity in the offspring (Weaver et al., 2004 for review). In studies of either handling or natural variations in maternal LG, it is apparent that the effects of LG are long-term and persist into adulthood and suggest that GRs are responsible for maintaining the effect of LG on the stress axis response (Champagne, 2013). Taking into account the above findings we propose that changes in GR of the hippocampus and frontal cortex after neonatal handling, that have been reported by Meaney et al. (1985b), may be responsible for some of the observed changes in cannabinoid receptor expression. This hypothesis is based on observations showing that prolonged corticosterone treatment reduces [3H]CP55,940 binding sites in hippocampus and amygdala (Hill et al., 2008; Bowles et al., 2012), as well as CB1 protein levels in hippocampus and primary sensory neurons (Hill et al., 2008; Hong et al., 2011). However, CB1 gene expression changes in response to handling may not be solely the product of corticosterone activation of GR-dependent transcription. Recent research into stress resilience is looking beyond glucocorticoid signaling (McEwen et al., 2015).
In conclusion, the present study demonstrates that a brief and repeated maternal separation during the neonatal period, modeled by the neonatal handling paradigm, is capable of affecting the cannabinoid receptors of the brain endocannabinoid system in a sex- and age-dependent manner. Our findings indicate the importance of studying the long-term consequences of an early life experience on neurobiological parameters taking into consideration age and sex. Future studies are needed to explore the specific role of these alterations in the behavioral changes invoked by neonatal handling.
Conflict of interest statement
The Authors declare no conflict of interests.
Acknowledgement
This work was supported by Polembros Shipping Limited (grant 27720000, Research Committee, University of Patras).
References
- Adermark L., Talani G., Lovinger D.M. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur. J. Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T., Unal C., Pare D. Synaptic correlates of fear extinction in the amygdale. Nat. Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E., Ozaita A., Valdizán E.M., Ledent C., Pazos A., Maldonado R., Valverde O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- Aso E., Renoir T., Mengod G., Ledent C., Hamon M., Maldonado R., Lanfumey L., Valverde O. Lack of CB1 receptor activity impairs serotonergic negative feedback. J. Neurochem. 2009;109:935–944. doi: 10.1111/j.1471-4159.2009.06025.x. [DOI] [PubMed] [Google Scholar]
- Atwood B.K., Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M., Gean P.-W., Lutz B. The role of the amygdale in the extinction of conditioned fear. Biol. Psychiatry. 2006;60:322–328. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Beane M.L., Cole M.A., Spencer R.L., Rudy J.W. Neonatal handling enhances contextual fear conditioning and alters corticosterone stress responses in youngrats. Horm. Behav. 2002;41:33–40. doi: 10.1006/hbeh.2001.1725. [DOI] [PubMed] [Google Scholar]
- Belue R.C., Howlett A.C., Westlake T.M., Hutchings D.E. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol. Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S., Meaney M.J. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non-handled rats. J. Neuroendocrinol. 1995;7:97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Bowles N.P., Hill M.N., Bhagat S.M., Karatsoreos I.N., Hillard C.J., McEwen B.S. Chronic, noninvasive glucocorticoid administration suppresses limbic endocannabinoid signaling in mice. Neuroscience. 2012;204:83–89. doi: 10.1016/j.neuroscience.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel C.S., Childers S.R., Deadwyler S.A., Hampson R.E., Vogt L.J., Sim-Selley L.J. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors andcannabinoid receptor-activated G proteins in rat brain. J. Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Caillé S., Alvarez-Jaimes L., Polis I., Stouffer D.G., Parsons L.H. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J. Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A. Early environments, glucocorticoid receptors, and behavioral epigenetics. Behav. Neurosci. 2013;127:628–636. doi: 10.1037/a0034186. [DOI] [PubMed] [Google Scholar]
- Champagne F.A., Francis D.D., Mar A., Meaney M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Chapillon P., Patin V., Roy V., Vincent A., Caston J. Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev. Psychobiol. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- Chiba S., Numakawa T., Ninomiya M., Richards M.C., Wakabayashi C., Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Choi K., Le T., McGuire J., Xing G., Zhang L., Li H., Parker C.C., Johnson L.R., Ursano R.J. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J. Psychiatr. Res. 2012;46:882–889. doi: 10.1016/j.jpsychires.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Covey D.P., Wenzel J.M., Cheer J.F. Cannabinoid modulation of drug reward and the implications of marijuana legalization. Brain Res. 2015;1628:233–243. doi: 10.1016/j.brainres.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton V.S., Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse. 2010;64:845–854. doi: 10.1002/syn.20801. [DOI] [PubMed] [Google Scholar]
- Delpech J.-C., Wei L., Hao J., Yu X., Madore C., Butovsky O., Kaffman A. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 2016;57:79–93. doi: 10.1016/j.bbi.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deviterne D., Desor D., Krafft B. Maternal behavior variations and adaptations, and pup development within litters of various sizes in Wistar rat. Dev. Psychobiol. 1990;23:349–360. doi: 10.1002/dev.420230406. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol. Sci. 2006;27:134–150. doi: 10.1016/j.tips.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan S.M., Mizoguchi Y., Stoyak S.R., Lewis D.A. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb. Cortex. 2010;20:1164–1174. doi: 10.1093/cercor/bhp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Rawas R., Thiriet N., Nader J., Lardeux V., Jaber M., Solinas M. Early exposure to environmental enrichment alters the expression of genes of the endocannabinoid system. Brain Res. 2011;1390:80–89. doi: 10.1016/j.brainres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Escorihuela R.M., Tobeña A., Fernández-Teruel A. Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman high-avoidance) and hyperemotional (Roman lowavoidance) rats. Learn. Mem. 1995;2:40–48. doi: 10.1101/lm.2.1.40. [DOI] [PubMed] [Google Scholar]
- Farhan M., Ikram H., Kanwal S., Haleem D.J. Unpredictable chronic mild stress induced behavioral deficits: a comparative study in male and female rats. Pak. J. Pharm. Sci. 2014;27:879–884. [PubMed] [Google Scholar]
- Fenoglio K.A., Brunson K.L., Avishai-Eliner S., Chen Y., Baram T.Z. Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology. 2004;145:2702–2706. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio K.A., Brunson K.L., Avishai-Eliner S., Stone B.A., Kapadia B.J., Baram T.Z. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Teruel A., Escorihuela R.M., Castellano B., Gonzalez B., Tobena A. Neonatal handling and environmental enrichment effects on emotionality novelty/reward seeking, and age-related cognitive and hippocampal impairments: focus on the Roman rat lines. Behav. Genet. 1997;27:513–525. doi: 10.1023/a:1021400830503. [DOI] [PubMed] [Google Scholar]
- Fittzerald P.J., Seaman J.R., Maren S. Can fear extinction be enhanced? A review of pharmacological and behavior findings. Brain Res. Bull. 2014;105:45–46. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerderman G.L., Ronesi J., Lovinger D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Heng L., Beverley J.A., Steiner H., Tseng K.Y. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Carrier E.J., Ho W.S., Shi L., Patel S., Gorzalka B.B., Hillard C.J. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Bingham B., Shrestha L., Lee T.T., Gray J.M., Hillard C.J., Gorzalka B.B., Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Morrish A.C., Viau V., Floresco S.B., Hillard C.J., Gorzalka B.B. Suppression of amygdala endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Pan B., Fitzgerald M.L., Roberts C.J., Lee T.T., Karatsoreos I.N., Mackie K., Viau V., Pickel V.M., McEwen B.S., Liu Q.S., Gorzalka B.B., Hillard C.J. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S., Campolongo P., Tasker J.G., Wotjak C.T., Bains J.S. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral outpout. J. Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Tasker J.G. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J. The endocannabinoid signaling system in the CNS: a primer. Int. Rev. Neurobiol. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Zheng G., Wu X., Snider N.T., Owyang C., Wiley J.W. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140:627–637. doi: 10.1053/j.gastro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A.C., Barth F., Bonner T.I., Cabral G., Casellas P., Devane W.A., Felder C.C., Herkenham M., Mackie K., Martin B.R., Mechoulam R., Pertwee R.G. International union of pharmacology. Xxvii. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huot R.L., Plotsky P.M., Lenox R.H., McNamara R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Jansen E.M., Haycock D.A., Ward S.J., Seybold V.S. Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res. 1992;1992(575):93–102. doi: 10.1016/0006-8993(92)90428-c. [DOI] [PubMed] [Google Scholar]
- Karkow A.R.M., Lucion A.B. Mild environmental interventions in mother-infant interactions reduces social play behavior in rats. Psychol. Neurosci. 2013;6:39–44. [Google Scholar]
- Katona I., Ranez E.A., Ascady L., Ledent C., Mackie K., Hajos N., Freund T.F. Cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouli S., Stamatakis A., Giompres P., Kouvelas E.D., Stylianopoulou F., Mitsacos A. Sexually dimorphic long-term effects of an early life experience on AMPA receptor subunit expression in rat brain. Neuroscience. 2014;257:49–64. doi: 10.1016/j.neuroscience.2013.10.073. [DOI] [PubMed] [Google Scholar]
- Kemorah K., Morsicano G., Tany J., Monory K., Bisoano J., Marzo V., Lutz B., Wotlak C.T. CannabinoidCB1 receptor mediates fear extinction via habituation-like processes. J. Neurosci. 2006;26 doi: 10.1523/JNEUROSCI.0153-06.2006. 6977–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.A., Lee H.J., Kim J.J. Neonatal handling alters learning in adult male and female rats in a task-specific manner. Brain Res. 2007;1154:144–153. doi: 10.1016/j.brainres.2007.03.081. [DOI] [PubMed] [Google Scholar]
- Lafenette P., Chaouloff F., Morsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharm. Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lee T.T., Gorzalka B.B. Evidence for a role of adolescent endocannabinoid signaling in regulating HPA axis stress responsivity and emotional behavior development. Int. Rev. Neurobiol. 2015;125:49–84. doi: 10.1016/bs.irn.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Lee T.T., Hill M.N., Lee F.S. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav. 2016;15:108–124. doi: 10.1111/gbb.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Meaney M.J. Maternal care, hippocampal glucocorticoid receptors and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Logan R.W., Edgar N., Gillman A.G., Hoffman D., Zhu X., McClung C.A. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol. Psychiatry. 2015;78:249–258. doi: 10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L.E., Lind J., Webster M., Weickert C.S. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Smith K.S., Berridge K.C. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Mailleux P., Vanderhaeghen J.J. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R., Di S., Marcheselli V.S., Weng F.J., Stuart C.T., Bazan N.G., Tasker J.G. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J. Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E.M., Echeverry-Alzate V., López-Moreno J.A., Giné E., Peñasco S., Viveros M.P. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav. Pharmacol. 2014;25:547–556. doi: 10.1097/FBP.0000000000000068. [DOI] [PubMed] [Google Scholar]
- Marsicano G., Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Hermann H., Tang J., Hofmann C., Zieglgänsberger W., Di Marzo V., Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mato S., Del Olmo E., Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Matyas F., Yanovsky Y., Mackie K., Kelsch W., Misgeld U., Freund T.F. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gray J., Nasca C. Recognizing resilience: learning from the effects of stress on the brain. Neurobiol. Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R.J., Hill M.N., Dang S.S., Wainwright S.R., Galea L.A., Hillard C.J., Gorzalka B.B. Upregulation of CB₁ receptor binding in the ventromedial prefrontal cortex promotes proactivestress-coping strategies following chronic stress exposure. Behav. Brain Res. 2013;237:333–337. doi: 10.1016/j.bbr.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R.J., Hill M.N., Gorzalka B.B. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 2014;42:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Aitken D.H. The effects of early postnatal handing on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Aitken D.H., Bodnoff S.R., Iny L.J., Sapolsky R.M. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1985;7:731–734. doi: 10.1016/0278-5846(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Aitken D.H., Bodnoff S.R., Iny L.J., Tatarewicz J.E., Sapolsky R.M. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav. Neurosci. 1985;99:765–770. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Mitchell J.B., Aitken D.H., Bhatnagar S., Bodnoff S.R., Iny L.J., Sarrieau A. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Horvath K.M., Nagy G.M., Bohus B., Koolhaas J.M. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J. Neuroendocrinol. 1999;12:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Nazzaro C., Greco B., Cerovic M., Baxter P., Rubino T., Trusel M., Parolaro D., Tkatch T., Benfenati F., Pedarzani P., Tonini R. SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat. Neurosci. 2012;15:284–293. doi: 10.1038/nn.3022. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Maejima T., Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Oviedo A., Glowa J., Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- Papaioannou A., Gerozissis K., Prokopiou A., Bolaris S., Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal’s response to stress and the vulnerability for depressive behaviour. Behav. Brain Res. 2002;129:131–139. doi: 10.1016/s0166-4328(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Park M.K., Hoang T.A., Belluzzi J.D., Leslie F.M. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J. Neuroendocrinol. 2003;15:289–295. doi: 10.1046/j.1365-2826.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Plotsky P.M., Meaney M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Bettschen D., Nanz-Bahr N.I., Feldon J. Comparison of the effects of early handling and early deprivation on conditioned stimulus, context, and spatial learning and memory in adult rats. Behav. Neurosci. 2003;117:883–893. doi: 10.1037/0735-7044.117.5.883. [DOI] [PubMed] [Google Scholar]
- Riebe C.J., Wotjak C.T. Endocannabinoids and stress. Stress. 2011;14:384–397. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- Robinson S.A., Loiacono R.E., Christopoulos A., Sexton P.M., Malone D.T. The effect of social isolation on rat brain expression of genes associated with endocannabinoid signaling. Brain Res. 2010;1343:153–167. doi: 10.1016/j.brainres.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Roche M., Finn D.P. Brain CB2 receptors: implications for neuropsychiatric disorders. Pharmaceuticals (Basel) 2010;3:2517–2553. doi: 10.3390/ph3082517. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F., Ramos J.A., Bonnin A., Fernández-Ruiz J.J. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Shinohara Y., Inui T., Yamamoto T., Shimura T. Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. NeuroReport. 2009;20:1382–1385. doi: 10.1097/WNR.0b013e3283318010. [DOI] [PubMed] [Google Scholar]
- Sim-Selley L.J., Martin B.R. Effect of chronic administration of R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl] pyrol [1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J. Pharm. Exp. Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Silveira P.P., Portella A.K., Clementte Z., Bassani E., Tabajar A.S., Gamaro G.D., Dalmaz C. Neonatal handling alters feeding behavior of adult rats. Physiol. Behav. 2004;80:739–745. doi: 10.1016/j.physbeh.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Smythe J.W., Rowe W.B., Meaney M.J. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Brain Res. Dev. Brain Res. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Solinas M., Goldberg S.R., Piomelli D. The endocannabinoid system in brain reward processes. Br. J. Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M., Yasar S., Goldberg S.R. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol. Res. 2007;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., Mantelas A., Papaioannou A., Pondiki S., Fameli M., Stylianopoulou F. Effect of neonatal handling on serotonin 1A sub-type receptors in the rat hippocampus. Neuroscience. 2006;140:1–11. doi: 10.1016/j.neuroscience.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Stamatakis A., Pondiki S., Kitraki E., Diamantopoulou A., Panagiotaropoulos T., Raftogianni A., Stylianopoulou F. Effect of neonatal handling on adult rat spatial learning and memory following acute stress. Stress. 2008;11:148–159. doi: 10.1080/10253890701653039. [DOI] [PubMed] [Google Scholar]
- Stevenson C.W., Meredith J.P., Spicer C.H., Mason R., Marsden C.A. Early life programming of innate fear and fear learning in adult female rats. Behav. Brain Res. 2009;198:51–57. doi: 10.1016/j.bbr.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Suárez J., Llorente R., Romero-Zerbo S.Y., Mateos B., Bermúdez-Silva F.J., de Fonseca F.R., Viveros M.P. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus. 2009;19:623–632. doi: 10.1002/hipo.20537. [DOI] [PubMed] [Google Scholar]
- Schwarz J.M., Hutchinson M.R., Bilbo S.D. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory LI-10 expression. J. Neurosci. 2011;31(49) doi: 10.1523/JNEUROSCI.3297-11.2011. 17847–17835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker J.G., Herman J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Damsteegt R., Manduca A., Petrosino S., Van Kerkhof L.W., Pasterkamp R.J., Zhou Y., Campolongo P., Cuomo V., Di Marzo V., Vanderschuren L.J. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J. Neurosci. 2012;32:14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M., Mayo W., Dellu F., Le Moal M., Simon H., Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J. Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M., Mayo W., Maccari S., Le Moal M., Simon H. Long-term effects of prenatal stress and handling on metabolic parameters: relationship to corticosterone secretion response. Brain Res. 1996;712:287–292. doi: 10.1016/0006-8993(95)01459-4. [DOI] [PubMed] [Google Scholar]
- Valverde O., Torrens M. CB1 receptor-deficient mice as a model for depression. Neuroscience. 2012;204:193–206. doi: 10.1016/j.neuroscience.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Van Waes V., Beverley J.A., Siman H., Tseng K.Y., Steiner H. CB1 cannabinoid receptor expression in the striatum: association with corticostriatal circuits and developmental regulation. Front. Pharmacol. 2012;3:21. doi: 10.3389/fphar.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdurand M., Nguyen V., Stark D., Zahra D., Gregoire M.C., Greguric I., Zavitsanou K. Comparison of cannabinoid CB(1) receptor binding in adolescent and adult rats: a positron emission tomography study using [F]MK-9470. Int. J. Mol. Imaging. 2011;2011:548123. doi: 10.1155/2011/548123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C., Diorio J., Seckl J.R., Szyf M., Meaney M.J. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann. N. Y. Acad. Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- Wei D., Lee D., Cox C.D., Karsten C.A., Peñagarikano O., Geschwind D.H., Gall C.M., Piomelli D. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc. Natl. Acad. Sci. U. S. A. 2015;112:14084–14089. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A.A., Southwood C.J., Wellman C.L. Brief neonatal maternal separation alters extinction of conditioned fear and corticolimbic glucocorticoid and NMDA receptor expression in adult rats. Dev. Neurobiol. 2008;69:73–87. doi: 10.1002/dneu.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.I., Nicoll R.A. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wong R., Jamieson J.L. Infantile handling and the facilitation of discrimination and reversal learning. Q. J. Exp. Psychol. 1968;20:197–199. doi: 10.1080/14640746808400149. [DOI] [PubMed] [Google Scholar]
- Zhang H.-Y., Gao M., Liu Q.-R., Bi G.-H., Li X., Yang H.-J., Gardner E.L., Wu J., Xi Z.-X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. U. S. A. 2014;111(46):E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Gao M., Shen H., Bi G.H., Yang H.J., Liu Q.R., Wu J., Gardner E.L., Bonci A., Xi Z.-X. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict. Biol. 2017;22(3):752–765. doi: 10.1111/adb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K., Dalton V.S., Walker A.K., Weickert C.S., Sominsky L., Hodgson D.M. Neonatal lipopolysaccharide treatment has long-term effects on monoaminergic and cannabinoid receptors in the rat. Synapse. 2013;67:290–299. doi: 10.1002/syn.21640. [DOI] [PubMed] [Google Scholar]