Abstract
Retrosplenial cortex is a region within the posterior neocortical system, heavily interconnected with an array of brain networks, both cortical and subcortical, that is, engaged by a myriad of cognitive tasks. Although there is no consensus as to its precise function, evidence from both human and animal studies clearly points to a role in spatial cognition. However, the spatial processing impairments that follow retrosplenial cortex damage are not straightforward to characterise, leading to difficulties in defining the exact nature of its role. In this article, we review this literature and classify the types of ideas that have been put forward into three broad, somewhat overlapping classes: (1) learning of landmark location, stability and permanence; (2) integration between spatial reference frames; and (3) consolidation and retrieval of spatial knowledge (schemas). We evaluate these models and suggest ways to test them, before briefly discussing whether the spatial function may be a subset of a more general function in episodic memory.
Keywords: Learning, memory, cingulate cortex, primate, hippocampal formation, thalamus, neuroimaging, default mode network, immediate-early genes, electrophysiology
Introduction
Retrosplenial cortex (RSC) has fallen within the scope of memory research for at least 40 years (Vogt, 1976) and yet as Vann et al. (2009) pointed out in their recent comprehensive review, little was discovered about the structure for the first 90 years after Brodmann first identified it. Since the early 1990s, a growing body of evidence has implicated the RSC variously in spatial memory, navigation, landmark processing and the sense of direction, visuospatial imagery and past/future thinking, and episodic memory. Early results were difficult to interpret in the absence of precise neuroanatomical, behavioural, electrophysiological and functional data. However, as a consequence of intense research on the RSC, both across animal models using a variety of methods and also in human neuropsychological and imaging studies, a group of theories is now emerging that highlight the involvement of the RSC in aspects of cognition that go beyond, yet at the same time still underlie, our abilities to process spatial information and retrieve memories. This review will examine the experimental data in light of its contribution to spatial cognition, beginning with a review of the anatomy and connectivity, followed by functional investigations based on lesion studies, imaging and electrophysiology, and concluding with evaluation and classification of the main ideas that have emerged. We suggest that the proposals about RSC function fall into at least three classes: first, it is involved in the setting of perceived landmarks into a spatial reference frame for use in orientation (spatial and directional) as well as evaluation of landmark stability; second, it stores and reactivates associations between different processing modes or reference frames for spatial navigation; and third, it has a time-limited role in the storage and possibly retrieval of hippocampal-dependent spatial/episodic memories. We conclude with some suggestions about how to further refine, and perhaps ultimately synthesise, these models.
Anatomy and connectivity of RSC
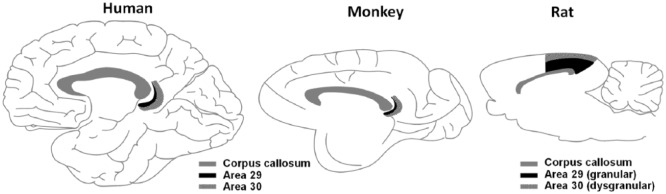
In human and non-human primates, RSC conforms to the cortical regions that Brodmann identified as areas 29 and 30, which – along with areas 23 and 31 – form part of the posterior cingulate cortex, lying immediately posterior to the corpus callosum (Figure 1 – left and middle). Rodents lack areas 23 and 31, and RSC itself is located more dorsally and reaches the brain surface (Figure 1 – right). Its central location makes it pivotally positioned to receive information from, and readily influence, many key brain regions responsible for the processing of spatial information.
Figure 1.
Schematic of the RSC as seen in midsagittal section and located just posterior to the corpus callosum, in humans, rhesus monkeys and rats.
Source: Figure by Jeffery (2017); available at: https://doi.org/10.6084/m9.figshare.5414179.v1 under a CC-BY 4.0 licence.
Typically, structural neural connections have been mainly derived from studies in animal models (rodents and non-human primates), while the majority of neural connections studied in humans have been derived functionally. It is known that in both rats and primates, the majority of RSC (RSC granular A and granular B, and RSC dysgranular) connections (up to 78%) originate in or are received from other parts of RSC and from the posterior cingulate cortex in primates (Kobayashi and Amaral, 2003).
Cortical connections
As shown in Figure 2, neural connections of the RSC from the cortex include the parahippocampal region (postrhinal cortex in rodents) (Suzuki and Amaral, 1994), medial entorhinal cortex (Czajkowski et al., 2013; Insausti and Amaral, 2008; Insausti et al., 1987; Jones and Witter, 2007; Van Hoesen and Pandya, 1975) and cingulate cortex (Jones et al., 2005). RSC receives unidirectional inputs from the CA1 field of the hippocampus (Cenquizca and Swanson, 2007; Miyashita and Rockland, 2007) and from the subiculum (Honda and Ishizuka, 2015; Wyss and Van Groen, 1992). It is also interconnected with the extended hippocampal complex, including the presubiculum, postsubiculum and parasubiculum (Kononenko and Witter, 2012; Wyss and Van Groen, 1992), visuospatial cortical association areas (mainly medial precuneate gyrus, V4 of the occipital lobes and the dorsal bank of the superior temporal sulcus) (Passarelli et al., 2017) and prefrontal cortex (with the heaviest terminations in the dorsolateral prefrontal cortex, frontopolar area 10 and area 11 of the orbitofrontal cortex); these frontal connections are all reciprocal. RSC also receives inputs directly from V2 of the occipital lobes. There are also prominent excitatory reciprocal connections between RSC and posterior secondary motor cortex – namely M2, that have been recently identified in mice (Yamawaki et al., 2016).
Figure 2.
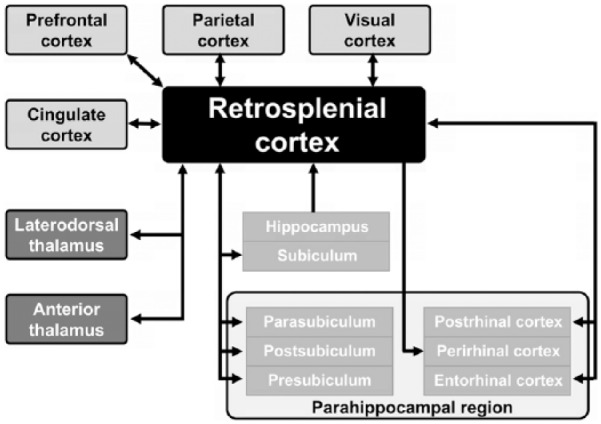
A schematic diagram detailing the gross connectivity of retrosplenial cortex. As depicted in the figure, RSC serves as an interconnected hub for neocortical, hippocampal, parahippocampal and thalamic regions that are functionally involved in the processing of mammalian perceptions important for direction, location, landmarks and navigation. Different shading is used for effect only.
Subcortical connections
In addition, as shown in Figure 2, RSC has major reciprocal subcortical interactions with the anterior (ATN) and laterodorsal thalamic nuclei (Aggleton et al., 2014; Kobayashi and Amaral, 2003, 2007; Van Groen and Wyss, 1990, 1992a and 1992b, 2003; Vogt et al., 1987). While the RSC projections to thalamus mainly arise from layer 6, projections from areas 29 and 30 provide different densities of terminal fields in the three subdivisions – anteroventral, anteromedial and anterodorsal – of the ATN (Aggleton et al., 2014). Given that the ATN and laterodorsal thalamic nuclei provide major RSC inputs, it is of interest to establish where these two thalamic structures receive their inputs. Briefly, the laterodorsal thalamus receives inputs from the postsubiculum, visual association cortex and the lateral mamillary bodies (Shinkai et al., 2005; Sripanidkulchai and Wyss, 1986; Taube, 2007; Vogt and Miller, 1983), while the ATN receives inputs directly from the lateral and medial mamillary bodies and from the hippocampal formation/ subicular complex. Possibly, the key message transmitted from the lateral mamillary bodies to the anterodorsal subdivision of the ATN and the laterodorsal thalamus is information about the position of the head received from the dorsal tegmental nucleus of Gudden located in the midbrain (Cajal, 1955; Guillery, 1956, 1957; Powell et al., 1957; Taube, 1995, 2007). In contrast, we do not yet fully know what information is transmitted to the RSC and cingulate cortex via the anteromedial and anteroventral subdivisions of the ATN, although theta-modulation (Vertes et al., 2001) and theta-modulated head direction (HD)-signalling neurons have been identified in the anteroventral subdivision in rats (Tsanov et al., 2011), and gravity-tuned neurons have been identified in primate ATN (Laurens et al., 2016). In addition to the above major connections, there are also lesser connections with the mediodorsal thalamus and rodent lateral posterior thalamic nucleus (Aggleton et al., 2014; Powell, 1978). RSC also receives inputs from the intralaminar thalamic nuclei (important for arousal) and primate medial pulvinar (supporting visual attention) (Baleydier and Mauguiere, 1985; Buckwalter et al., 2008; Vogt et al., 2006).
In general, the anatomy shows that RSC interacts reciprocally with many brain regions, consistent with its role, described below, in a number of core cognitive competences. In particular, it is clear that the RSC interacts with many visual areas of the brain across mammalian species. Of interest is the more unidirectional relationship with hippocampus and with perirhinal cortex.
Lesion studies
The literature on pure RSC lesions in humans is sparse and mostly from unilateral pathology due to the rarity with which localised infarcts or injury occur to this region, and so most of our knowledge of human RSC comes from neuroimaging, which we discuss later. Most of the identified lesion-induced deficits appear to involve memory and spatial processing. Maguire (2001) conducted a comprehensive review of the literature on RSC extant at the time and concluded that case studies of RSC lesions reveal deficits in episodic memory (memory for life events), occurring particularly following left-sided lesions, but also consistent reports of topographical disorientation (getting lost), with or without concomitant memory deficits, most of which followed right-sided lesions. The area that was most consistently involved in the pure disorientation cases was Brodmann area BA30. Maguire (2001) noted: ‘In every case, the patient was able to recognise the landmarks in their neighbourhoods and retained a sense of familiarity …’. Despite this, none of the patients were able to find their way in familiar environments, and all but one were unable to learn new routes. Studies since then have confirmed the link between RSC lesions and topographic disorientation, with association of left-sided infarct with memory deficits (Kim et al., 2007) and of right-sided lesions with spatial impairment (Hashimoto et al., 2010, 2016), although spatial impairment has also been reported in patients with left-sided lesions (Ino et al., 2007; Ruggiero et al., 2014). Claessen and van der Ham (2017) conducted a review of lesion-related navigation deficits and found that involvement of the RSC was prominent in impairments of landmark processing, particularly when it came to reporting distances and directions between known landmarks or describing the positions of known landmarks or buildings on a map.
Experimental lesion studies in non-human primates can be much more precise, and also bilateral, which has provided new insights into RSC function. In rhesus macaque monkeys, damage to the RSC, which included the most caudal part of the posterior cingulate cortex, selectively impaired the ability to retrieve object-in-place scene discriminations that the monkeys had previously learnt (retrograde memory) (Buckley ad Mitchell, 2016). In these tasks, animals have to learn and remember the location of a discrete object in a spatial scene. In contrast, these same animals were able to learn new object-in-place scene discriminations postoperatively (anterograde memory), so their ability to organise spatial information appeared to remain intact. However, during new learning that involved a 24-h delay period between successive sessions of learning the new set of object-in-place discriminations (i.e. from session 1 to session 2), monkeys with RSC damage made more errors than controls during postoperative session 2 of new learning only. This selective deficit, which was present in all monkeys with RSC damage, comprised a specific impairment in their ability to retrieve these new discriminations which they had seen only 24 h beforehand. The task, object-in-place scene discriminations, incorporates elements of both spatial (e.g. landmark information) and episodic-like memory (unique object-in-place scene discriminations, with one of the objects in each discrimination paired with a reward if it is selected) without being explicitly autobiographical in nature (Gaffan, 1994; Mitchell et al., 2008; Mitchell and Gaffan, 2008; Murray and Wise, 2010). The novel findings observed in the monkeys’ performance led the authors to conclude that an intact RSC is particularly important for the ability to retrieve information that has been previously acquired, regardless of whether these memories are autobiographical, or episodic (in the pure sense of what/ where and when), or actively spatial in nature (Buckley and Mitchell, 2016). Finally, the ability to retrieve this information did not require the monkeys to move around in their environment, although the successful executions of self-generated hand-eye coordinated movements (in order to select the correct object within the scene on the touchscreen) were necessary.
Studies involving smaller mammals have proved vital in furthering our understanding of the contribution of the RSC to cognition, as they afford far greater neuroanatomical precision than is currently possible in primate studies. An early study by Berger et al. (1986) found that rabbits with RSC lesions could acquire a tone-light discrimination, but were profoundly impaired in reversing it, suggesting a failure to modify a recently established memory. Given the dense interconnections between the RSC and the hippocampal spatial system, the majority of subsequent lesion studies have focused on spatial learning.
Some of the early studies into the effects of RSC lesions on spatial tasks produced mixed results. This divergence in findings may be attributable to methodological considerations such as the use of electrolytic or ablation lesions, which destroy fibres of passage and consequently may exaggerate the impact of the RSC damage, while other studies spared the more caudal aspect of the RSC, which is now known to be critically involved in spatial memory (Vann and Aggleton, 2002, 2004). Despite these earlier controversies, there is now very good evidence that RSC lesions in rodents disrupt spatial memory. Deficits are consistently reported on tasks that involve allocentric spatial processing, particularly when – as with the imaging studies – visual cues are needed for orientation (Hindley et al., 2014). Such tasks include learning the fixed or alternating location of a platform in the Morris watermaze (Sutherland et al., 1988; Vann and Aggleton, 2002, 2004; Whishaw et al., 2001), the radial arm maze (Keene and Bucci, 2009; Pothuizen et al., 2008; Vann and Aggleton, 2004) and object-in-place discriminations (Parron and Save, 2004). There is some evidence that the RSC dysgranular region (area 30; see Figure 1 – left) may be particularly important for processing allocentric space, as rats with selective RSC dysgranular lesions were unable to use distal visual cues to guide spatial working memory and relied instead on motor sequence information (Vann and Aggleton, 2005). Furthermore, deficits have also been found on tasks that require the use of directional information (Keene and Bucci, 2009; Pothuizen et al., 2008; Vann and Aggleton, 2004) as well as self-motion cues (Elduayen and Save, 2014; Whishaw et al., 2001). In some instances, the involvement of RSC has been found to be time-limited: for example, Maviel et al. (2004) found that RSC inactivation in mice disrupted the retrieval of a recent 1-day-old spatial memory but not a remote 30-day-old one, while Keene and Bucci (2009) found large impairments on radial maze performance for a 30-s delay relative to a 5-s delay. Findings such as these, combined with the immediate-early gene study findings described later, and the primate studies mentioned above, suggest a particular role for RSC when spatial information needs to be retrieved from memory.
In general, the magnitude of spatial deficits after RSC lesions tends to be smaller and less striking than the spatial impairments associated with either hippocampal or ATN damage. The most striking demonstration of this difference is T-maze alternation performance, which is acutely sensitive to both hippocampal and ATN damage (Aggleton et al., 1986, 1996), but is often spared after RSC lesions (Meunier and Destrade, 1988; Neave et al., 1994; Nelson et al., 2015b; Pothuizen et al., 2008). Indeed, the full impact of RSC lesions often only emerges under specific conditions or when animals are required to shift between different spatial metrics. For example, temporary inactivation of the RSC selectively impairs navigation in the dark, but not the light (Cooper et al., 2001). However, Wesierska et al. (2009) found that rats with RSC dysgranular lesions could learn to avoid the shock zone of a rotating platform if the rotation occurred in the dark, so darkness per se does not seem to be the problem. The rats could also learn to avoid the shock zone if this was defined by allocentric room cues provided there were no conflicting local cues; thus, there was not a straightforward impairment of allocentric cue use either. There was a notable impairment when the animals had to disregard the local cues and focus on the room cues. Thus, as the authors noted, impairments arose when relevant and irrelevant cues needed to be segregated. Similarly, impairments on both the radial arm maze and T-maze often only emerge when intra-maze cues are placed in conflict with extra-maze cues (Nelson et al., 2015b; Pothuizen et al., 2008; Vann and Aggleton, 2004).
A further illustration of the selective nature of RSC lesion-induced spatial deficits comes from an experiment by Nelson et al., 2015a in which the location of a submerged platform in a Morris watermaze was determined by either the geometric properties of the test environment or the juxtaposition of highly salient visual cues. Rats learnt the location of the platform either by actively swimming to the platform or passively, by being repeatedly placed on the platform location. They were then given a test in which they had to swim to the correct location for the first time. RSC-lesioned rats were selectively impaired in the passive condition, indicating that RSC damage did not disrupt navigation per se, but selectively impaired the ability to switch spatial frames of reference and different spatial viewpoints when navigating to the platform from a novel position in the environment (Nelson et al., 2015a). Similarly, complete RSC or selective RSC dysgranular lesions disrupted the ability to recognise the layout of a room from different viewpoints (Hindley et al., 2014).
Taken together, RSC effects appear to depend on the extent to which task performance relies on the retrieval of spatial landmarks for orientation, or the need to switch between different spatial strategies or viewpoints. This is in line with the proposal that key aspects of RSC functioning include integration of the context in which an event occurs, learning about the significance of such stimuli or updating representations as new information comes on-line.
Brain imaging (positron emission tomography, functional magnetic resonance imaging and immediate-early gene activation)
As outlined above, human, primate and rodent RSC lesion studies have pointed to a role in spatial processing: complementary evidence comes from research using metabolic brain imaging, particularly positron emission tomography (PET), functional magnetic resonance imaging (fMRI) and immediate-early gene activation (IEG) studies.
Human neuroimaging studies have been complicated by the lack of agreement about exactly which regions belong to RSC proper. While the scene-selective posterior and ventral bank of the parieto-occipital sulcus is often referred to as RSC, Silson et al. (2016) have suggested that the term be reserved for the region within the callosal sulcus extending onto the isthmus of the cingulate gyrus. Such distinctions are relevant for the issue of the specificity of RSC processing, as well as its cross-species homology, which is still not fully established.
In an early PET study of cerebral glucose metabolism, Minoshima et al. (1997) found reduced activation in the posterior cingulate in patients with mild cognitive impairment and early Alzheimer’s disease, while Nestor et al. (2003) found that the RSC part of the posterior cingulate, was the most consistently hypometabolic region. More recent imaging studies have continued to confirm that changes in glucose metabolism in the posterior cingulate cortex, as well as hippocampal atrophy, are early biomarkers for Alzheimer’s disease and are likely present many years before the clinical symptoms appear (e.g. An et al., 2017; Teipel et al., 2016).
Since the advent of fMRI in cognitive neuroscience, many studies have investigated RSC activation as subjects perform tasks in the scanner. Indeed, RSC is now considered to be part of the so-called default mode network, which consists of a set of brain structures including medial frontal and medial temporal lobe regions, lateral and medial parietal areas and the RSC (Vann et al., 2009), which are active when subjects are not performing a task in the scanner but rather are lying in the scanner at ‘rest’, or actively simulating a situation (particularly one close in time and space to the present (Tamir and Mitchell, 2011), or when they are retrieving a memory (Sestieri et al., 2011)).
Cognitive tasks that reliably activate RSC in fMRI studies include most that have a spatial component, especially when this requires use of the visual environment to retrieve previously learned information in order to orient. These typically involve virtual reality simulations in which subjects navigate, by joystick or sometimes just by imagination, around a virtual environment, such as a town. In one of the earliest studies, Wolbers and Büchel (2005) scanned subjects as they learned a virtual maze-like town and found that RSC activation increased steadily with learning and paralleled increasing map performance. Similarly, in a study of London taxi drivers in a virtual environment based on real maps of London (Spiers and Maguire, 2006), RSC activation occurred during route planning, spontaneous trajectory changes and confirmation of expectations about the upcoming features of the outside environment - but not, interestingly, expectation violations. Another fMRI study confirmed that RSC activity was specifically associated with thoughts of location and orientation, as opposed to context familiarity or simple object recognition (Epstein et al., 2007). In both studies, the overall pattern of RSC activation differed from the one observed for hippocampus (Iaria et al., 2007), with the entire RSC active during both encoding and retrieval of spatial information.
A related line of work has investigated the encoding of location and/or direction by RSC. Marchette et al. (2014) performed multi-voxel pattern analysis (MVPA) of fMRI brain activation patterns on subjects recalling spatial views from a recently learned virtual environment. Because MVPA compares fine-grained patterns of activation, it allows inferences to be made about whether a subject is discriminating stimuli. The virtual environment comprised a set of four museums located near each other in a virtual park. RSC activity patterns were similar when subjects faced in similar directions and/or occupied similar locations within each museum, suggesting that RSC was activating the same representations of local place and local direction, even though the environments were separated and oriented differently in global space. Similarity judgement reaction times were faster for homologous directions or locations, suggesting encoding by local features independent of global relationships. However, it was not demonstrated that subjects had been able to form global maps of the virtual space (i.e. the reference frame in which the local spaces were set), so the question remains unanswered about whether RSC is also involved in relating directions within a global space.
Robertson et al. (2016) also found encoding of local landmarks in a setting in which subjects viewed segments of a 360° panorama that either did or did not overlap. RSC activation was higher when subjects subsequently viewed isolated scenes from the overlap condition and judged whether it came from the left or the right side of the panorama. A study by Shine et al. (2016) did, however, find evidence for global heading representation in RSC. They investigated RSC and thalamus activation in subjects who had learned a virtual environment by walking around with a head-mounted display, which provides vestibular and motor cues to orientation. They found activation of both structures, which both contain directionally sensitive HD cells (discussed below), when subjects were shown stationary views of the environment and had to make orientation judgements (Shine et al., 2016). Furthermore, recent work has also examined RSC activation when participants navigate in a virtual 3D environment (Kim et al., 2017). Interestingly, in this study, the RSC activation was particularly sensitive to the vertical axis of space, which the authors suggest may be supporting processing of gravity, which is a directional cue in the vertical plane and may be useful for 3D navigation. Given that there is evidence for both local and global encoding of direction in RSC, the question arises as to how these might both be accommodated within the one structure; we return to this question later.
While the foregoing studies looked at global spatial environments, work from the Maguire lab has suggested a role for RSC in the processing of individual landmarks. Auger et al. (2012) scanned subjects as they viewed a variety of images with a mixture of large and smaller objects and found that RSC was activated only by the spatially fixed, landmark-like objects, and furthermore that the extent of activation correlated with navigation ability. In a follow-up study using MVPA, Auger and Maguire (2013) showed that decoding of the number of permanent landmarks in view was possible, and more so in better navigators, concluding that RSC, in particular, is concerned with encoding every permanent landmark that is in view. They then showed that this RSC permanence encoding also occurred when subjects learned about artificial, abstract landmarks in a featureless Fog World (Auger et al., 2015), demonstrating that the RSC is involved in new learning of landmarks and their spatial stability and also that such learning correlates with navigation ability (Auger et al., 2017). Puzzlingly, however, the involvement of RSC seems better correlated with the stability per se than with the orientational relevance of the landmarks (Auger and Maguire, 2018a).
Some meta-analyses of human imaging studies have indicated that higher RSC activation occurs when subjects process landmark information (Auger et al., 2012; Auger and Maguire, 2013; Maguire, 2001; Mullally et al., 2012; Spiers and Maguire, 2006) and associate the current panoramic visual scene with memory (Robertson et al., 2016). Further evidence has revealed that RSC is activated when subjects retrieve autobiographical memories (Maddock, 1999; Spreng et al., 2009) or engage in future thinking or imagining (Tamir and Mitchell, 2011), although RSC appears more engaged with past than future spatial/contextual thinking (Gilmore et al., 2017). While the retrieval of autobiographical memories, imagining and future thinking may not explicitly engage spatial processes, they are nonetheless closely allied to the spatial functions of RSC and its identified role in retrieval, as they require self-referencing to spatial contexts and the updating of spatial representations as events are recalled or imagined based on subjective memories.
Animal models, in particular rodent experiments that engage their ability to readily explore their spatial environment, have provided imaging evidence across mammalian species that highlights the importance of the RSC for spatial functioning. One particular experimental approach is to study RSC functioning in the intact rodent brain by investigating the extent, and location, of the activation of learning-induced immediate-early genes (IEGs; e.g. Arc, Fos or Zif268) after animals have performed a behavioural task. Most of these studies have shown increased expression of IEGs in the RSC as a consequence of spatial learning (Maviel et al., 2004; Vann et al., 2000). One of the distinct advantages of this approach is that it allows for far greater anatomical precision, for example, revealing subregional or layer-specific differences in RSC activity after animals have performed a spatial task (Pothuizen et al., 2009).
IEG studies have also revealed the involvement of RSC in spatial memory formation. Tse et al. (2011) investigated the two IEGs, zif268 and Arc, as rats learned flavour-place pairs; they found up-regulation of these genes in RSC when animals added two new pairs to the set. A more recent approach has been to combine IEG mapping with chronic in vivo two-photon imaging to study the dynamics of Fos fluorescent reporter (FosGFP) in RSC dysgranular cortex during acquisition of the watermaze task (Czajkowski et al., 2014). Higher reporter activity was observed when animals relied on a set of distal visual cues (allocentric strategy), as compared to a simple swimming task with one local landmark. Moreover, these observations also revealed a small population of neurons that were persistently reactivated during subsequent sessions of the allocentric task. This study showed that plasticity occurs within RSC during spatial learning and also suggested that this structure is critical for formation of the global representation. Indeed, in another set of experiments, optogenetic reactivation of Fos-expressing neuronal ensembles in mouse RSC led to the replication of context-specific behaviours when the animal was in a safe context, devoid of any features of the original training context (Cowansage et al., 2014).
Taken together, these complementary human and animal studies highlight that RSC functioning is involved in spatial learning and memory, particularly when environmental cues (landmarks) are to be used for re-orientation and perhaps navigation. Studies of the time course of RSC involvement suggest a dissociation between new learning and memory retrieval/updating. The implication is that perhaps RSC is less involved in spatial perception per se, and more involved with visual memory retrieval and editing.
Single neuron studies
Researchers typically turn to rodent single-neuron studies to address fine-grained questions about encoding. Chen et al. (1994a, 1994b) after conducting the first electrophysiological studies of spatial correlates of rodent RSC reported that around 10% of RSC cells in the rat have the properties of HD cells. These are cells that fire preferentially when the animal faces in a particular global direction; cells with these properties are found in a variety of brain regions, and are thought to subserve the sense of direction (Taube, 2007). RSC head direction cells have very similar properties to those in other regions, although interestingly they fire slightly in advance of the actual head direction (Cho and Sharp, 2001; Lozano et al., 2017). However, 90% of the RSC neurons had more complex firing correlates, and no clear hypothesis about overall RSC function emerged.
A later study by Jacob et al. (2017) similarly found a sub-population of HD cells, in the RSC dysgranular cortex only, the firing of which was controlled by the local environmental cues independently of the global HD signal. They also – like Chen et al. – found a further sub-population of directionally tuned cells that showed mixed effects, being influenced both by landmarks and by the global head direction signal. This experiment took place in an environment composed of two local sub-compartments (two connected rectangles) that had opposite arrangements of landmarks within the room as a whole. Some cells behaved like typical HD cells and fired whenever the animal faced in a particular direction in the global space, while others fired in one direction in one compartment and the opposite direction in the other compartment, as if these cells were more interested in local direction than global direction. This observation is thus reminiscent of the fMRI experiment by Marchette et al. (2014) discussed earlier, in which human subjects showed similar RSC activation patterns in local subspaces independent of their global orientation. Together, these results support the idea that RSC might be involved in relating spatial reference frames, with some cells responsible for local orientation and others responsible for the bigger picture.
More broadly, the findings concerning HD cells suggest that RSC neurons may be integrating landmark information coming from the visual cortex, together with the ongoing HD signal being assembled and maintained by more central in the HD network. Such interaction might depend on the strength and/or reliability of the sensory input (i.e. landmarks) to RSC and/or the HD system (Knight et al., 2014), raising the possibility that RSC directional neurons have the task of evaluating landmarks and deciding whether they are stable and/or reliable enough to help anchor the sense of direction (Jeffery et al., 2016).
The above notwithstanding, only around 10% of RSC neurons seem to be HD neurons, the remainder having more complex firing correlates. Many of these seem related to the actions the animal is performing. The first systematic analysis by Chen et al. (1994a, 1994b) reported RSC cells related to body turns in addition to those with spatial firing characteristics. A subsequent study found RSC cells with firing significantly correlated with running speed, location and angular head velocity (Cho and Sharp, 2001). Similarly, cells that respond to specific combinations of location, direction and movement were reported by Alexander and Nitz (2015), who recorded RSC neurons as rats ran on two identical ‘W’-shaped tracks located at different places in a room. As well as ordinary HD cells, they found cells encoding conjunctions of local position, global position and left/right turning behaviour. In a later study (Alexander and Nitz, 2017), some RSC neurons were found to show firing rate peaks that recurred periodically as animals ran around the edge of a plus maze – some cells activated once per circumnavigation, some twice, some four times and so on. Since the environment had fourfold symmetry, this observation again suggests a possible role in relating local and global spatial reference frames. However, recurring activation patterns having fourfold symmetry were also seen when the animal ran on a ring track, with no local substructure, so it is possible that the cells were responding to some type of symmetric feature, such as the corners of the room, that was present in the distal room cues.
In contrast to encoding of route, within which every location that the animal visits along the full trajectory is represented, others have reported encoding of navigational or behaviourally significant cues (e.g. goal-location coding) by RSC in simpler linear environments. In a study by Smith et al. (2012), animals on a plus maze learned to approach the east arm for reward for half of each session and then switched to the west. RSC neurons developed spatially localised activity patches (‘place fields’) that were sensitive to reward-associated locations, and the number of place fields substantially increased with experience. However, unlike co-recorded hippocampal place cells, which produce very focal place fields, RSC place fields were dispersed and sometimes covered the entire arms. One function of RSC place fields could be enabling the rats to discriminate two behavioural contexts.
A recent study by Mao et al. (2017) reported more hippocampal-like activity in RSC cells, finding spatially localised activity (i.e. place fields) on a treadmill during movements in head-fixed mice. Locations on the track were marked by tactile cues on the travelling belt. As with hippocampal place cells, changes in light and reward location cause the cells to alter their firing locations (remap). These observations support the notion that RSC is sensitive to spatially informative cues and contextual changes.
In addition to place-, cue- and reward-location, Vedder et al. (2017) reported conjunctive coding. In a light-cued T-maze task, RSC neurons increased responsiveness to the light cue, mostly irrespective of left–right position, but they also frequently responded to location or to reward. Responses involved both increased firing (on responding) and decreased firing (off responding). Interestingly, responding to the light often slightly preceded the onset of the light cue, an anticipatory response also reported earlier in RSC in rabbits in an associative conditioning task (Smith et al., 2002). The location-sensitive firing on the stem of the T often distinguished forthcoming left and right turns, so-called splitter behaviour also seen in hippocampal place cells (Wood et al., 2000). Thus, although responding is associated with a cue, there seems sometimes to be a supra-sensory component related to a learned expectation.
To summarise, then, the results from electrophysiology studies of RSC neurons provide a mixed picture in which spatial processing dominates, but the nature of the processing is hard to pin down exactly. It is clear that place and heading are represented, but so are other variables, and the nature and function of the conjunctive encoding remains to be elucidated.
The RSC contribution to spatial cognition – consensus and controversies
The experimental literature reviewed above has revealed areas where investigators are in general agreement, and other areas where there is debate or uncertainty. In this section we review these areas and outline some ways forward to resolve these.
There seems to be general agreement that RSC has a role in allocentric spatial processing, as highlighted in the review of Vann et al. (2009), but there are differences in opinion as to the exact contribution it makes, and also in whether it has a broader role in memory, of which space is just a subcomponent. In this regard, the status of RSC research is a little reminiscent of hippocampal research 30 years ago. Our conclusion is that the literature has yielded three broad, somewhat related views concerning RSC’s spatial function, which we explore further below:
It processes landmarks and landmark stability/permanence, possibly in service of spatial/directional orientation or perhaps more broadly.
It mediates between spatial representations, processing modes or reference frames.
It is involved in consolidation and retrieval of spatial schemas, for example to support episodic memory.
Landmark processing
The first set of views is that RSC has a specific function in the encoding of the spatial and directional characteristics, as well as stability, of landmarks, independent of their identity. This view emerges from such findings as that HD-cell sensitivity to landmarks is reduced following RSC lesions (Clark et al., 2010), that some RSC directionally tuned cells respond to environmental landmarks in preference to the main HD network signal (Jacob et al., 2017), that RSC is active when humans process landmark permanence (Auger et al., 2012, 2017; Auger and Maguire, 2013) and that lesions to RSC in human subjects cause them to lose the ability to use landmarks to orient (Iaria et al., 2007). It is also supported by findings that rats with RSC lesions are poor at using allocentric spatial cues to navigate (Vann and Aggleton, 2005). By this view, the function of RSC is to process landmarks as currently perceived and use them to update an already established spatial framework so that in future they can be used for better self-localisation and re-orientation. This viewpoint supposes a particular role for landmarks in the ongoing formation and updating of spatial representations and is consistent with the close relationship of RSC to visual areas as well as to the hippocampal spatial system. Recently, Auger and Maguire suggested that RSC processing of landmarks may be more to do with permanence and stability than orientation relevance, and indeed that RSC’s role in permanence may extend beyond landmarks to other domains (Auger and Maguire 2018a, 2018b).
Spatial representations
The second view, which could be regarded as an extension of the first to information beyond landmarks, is that this area serves to mediate between spatial representations, as detailed in the review by Vann et al. (2009). For some investigators, this has meant between egocentric and allocentric processing, although egocentric suggests different things to different researchers, meaning self-motion-updated to some and viewpoint-dependent to others. Chen et al. (1994a) made the specific proposal that the egocentric information processed by RSC concerns self-motion, a position also taken by Alexander and Nitz (2015); this is supported by their and others’ observations that directionally tuned neurons in RSC are updated by self-motion (sometimes called idiothetic) cues and that navigation in RSC-lesioned animals is affected by darkness (Cooper and Mizumori, 1999). Other authors have taken “egocentric” more broadly to mean spatial items encoded with respect to the body versus with respect to the world. For example, Burgess and colleagues have suggested that RSC is part of the progressive cortical transform of parietal egocentrically to hippocampal allocentrically encoded information (Byrne et al., 2007): the hypothesis of egocentric-allocentric transformation by RSC recurs repeatedly in the literature (see Vann et al. (2009) for discussion).
Another, not dissimilar view is that RSC is involved in constructing and relating allocentric spatial reference frames more generally, not necessarily egocentric/allocentric ones. This view is supported by studies such as the museums study of Marchette et al. (2014), which found similarities in the encoding of local spaces even though these were separated in global space, and the similar findings of Jacob et al. (2017) that some RSC neurons constructed a directional signal based on local cues while others used the global space. A related notion is that RSC is involved in switching between different modes (as opposed to frames) of spatial processing, such as from light to dark (Cooper et al., 2001; Cooper and Mizumori, 1999), or distal to proximal cues and so on.
These models all share the underlying feature that RSC has access to the same spatial information represented in different ways, and is needed in order to switch between these.
Spatial schema consolidation/retrieval
The final view, which is broadest of all, is that RSC is involved in formation and consolidation of hippocampus-dependent spatial/episodic memories. What differentiates these models from the foregoing, and also from standard theories of hippocampal function, is the incorporation of a temporal dimension to the encoding. By this is meant that RSC is not needed for de novo spatial learning, but is required when the animal needs to draw on a previously learned set of spatial relationships, in order to execute a task or acquire new information to add to its stored representation.
These ideas draw on two sets of theoretical work already extant in the literature: the idea proposed by Marr (1971) that rapidly formed hippocampal memories are slowly consolidated in neocortex, and the idea that spatial learning entails the formation not just of task- or item-specific memories, but also of a more general framework within which the memories are situated, which has sometimes been called a schema (Morris, 2006; Ghosh and Gilboa, 2014). An example of a schema might be the watermaze task, in which a rat is faced with needing to learn a new platform location: learning of the new location is faster than the original because the rat already knows the layout of the room and the watermaze, and the procedures required to learn the platform location – learning the location for today requires just a small updating.
Support for this consolidation/updating idea comes from multiple observations in the literature that the role of RSC in behavioural tasks is frequently time-limited in that its effects occur later in training rather than immediately. In particular, there seems to be a 24-h time window after training, below which RSC is engaged less, but after which it becomes involved: see the experiment by Buckley and Mitchell (2016), and the observation by Bontempi and colleagues that IEGs are up-regulated when mice retrieve a 30-day-old memory, but not a 1-day-old one (Maviel et al., 2004). IEG studies have also revealed greater engagement for RSC in spaced versus massed training (Nonaka et al., 2017), consistent with the need, in spaced training, for reactivation of a partly consolidated (10-min-old) memory rather than a completely newly formed (30-s-old) one.
This time-dependency has led several investigators to propose that RSC is part of a primacy system (Bussey et al., 1996; Gabriel, 1990), the function of which is to retrieve and process information learned earlier. Ranganath and Ritchey (2012) put forward one of the most detailed of such models that proposed that RSC, together with parahippocampal cortex, form part of a posterior cortical network that functions to support episodic memory. They suggest that this network matches incoming cues about the current behavioural context to what they call situation models, which are internally stored representations of the relationships among the entities and the environment. According to their view, the parahippocampal cortex identifies contexts and the RSC compares these external cues with internal models of the situation, including input regarding self-motion.
Open questions
Resolving the above ideas into a single, inclusive model of RSC function (if this is possible) will require the answering of some of the outstanding questions raised by the studies to date. Below, we outline some of these questions.
Does RSC have a specific interest in landmarks, as a subclass of spatial cue?
A finding that has emerged from multiple studies of spatial processing is that RSC is particularly involved in the processing of landmarks, which is to say discrete objects or visual discontinuities in the panorama that serve, by virtue of their distant location and spatial stability, to orient the sense of direction. However, the specific hypothesis that it is interested in landmarks as discrete objects as opposed to, say, visual panoramas, has not been fully tested. An unanswered question then is whether RSC is engaged during spatial processing in the absence of landmarks; for example, in an environment devoid of discrete spatial cues in which geometry or smooth visual shading provides the only cues to direction. It should be noted that most types of geometric environment (squares, rectangles, teardrops, etc) have corners, which could in principle act as discrete landmarks, so care would have to be taken with the environmental design to ensure the absence of all such discrete visual stimuli. The general question to be answered here is whether the brain, via the RSC, treats landmarks as a special category of object or whether the interest of RSC in landmarks stems solely from their spatial utility, derived from the constant spatial relations between them, or from their permanent nature, irrespective of their status as landmarks (Auger and Maguire, 2018 a and b).
Does RSC mediate between spatial representations?
The core idea here is that RSC may not be needed for spatial learning per se, but is needed when the subject moves between representational modes. This may entail switching from egocentric to allocentric encoding of cues, or relating an interior space to an exterior one (e.g. deciding which door one needs to exit through to reach the carpark). This view is an extension of the local idea discussed above, that RSC is needed to be able to use spatial landmarks to retrieve current location and heading. The important new ingredient supplied by the reference frame framework, as it were, is that at least two representations have had to be activated: for example, being in one place and thinking about another, or navigating in the dark and remembering where things are based on experience in the light. The question to be answered, therefore, is whether RSC is indeed needed for a subject to activate two representations simultaneously.
Testing this idea is complicated by the demonstration discussed earlier that RSC is also needed to use local spatial cues to retrieve a previously learned spatial layout. Since it is required for current self-localisation, the idea that it is also needed for spatial imagination, or route planning, or future thinking, or other similar imagination-based functions, is hard to test directly because a lesioned subject can’t even get past the initial orientation problem. However, more temporally focused interventions such as optogenetic (in)activation may be of help here. For example, once intact animals have learned a radial maze task, it may be found that RSC is needed if the lights are turned off halfway through a trial, forcing a switch from one processing mode to another. Similarly, rats familiarised with a small space inside a larger one may be able to navigate between the two when RSC is operating, but not when it is inactivated. These types of task probe retrieval and manipulation of already-stored spatial representations.
What is the time course of RSC involvement in spatial learning?
It remains an open question exactly when RSC comes into play during formation and use of spatial memories. IEG studies have found that activation occurs rapidly (within minutes) of modifying a spatial schema (Tse et al., 2011), and the massed/spaced learning experiment from the same group suggests a role for RSC during at least the first few minutes (Nonaka et al., 2017). However other animal studies have found that RSC dependence of a task does not appear until memories are reactivated again after a delay of up to 24 h (e.g. Buckley and Mitchell, 2016), presumably to allow an epoch of time to pass before re-engagement of the RSC memory can occur. Consequently, as our current understanding lies it is difficult to distinguish if there is a critical time window in which RSC is engaged by spatial tasks or whether methodological issues, such as divergences in experimental design or even species specific differences, can explain the discrepancies in the literature. Nevertheless, this issue can readily be addressed empirically. One approach would be to take a task that is known to induce the expression of IEG within RSC and compare the effects of blocking IEG expression with antisense oligonucleotides at different stages of task acquisition (early versus late stage). Pharmacological or chemogenetic silencing of RSC neuronal activity could similarly be used to assess whether the RSC is differentially involved in remote or recent spatial memory (Corcoran et al., 2011). Studies in rodents can be complemented by imaging studies in humans that compare RSC activity in participants navigating in new or previously learnt virtual or real environments (Patai et al., 2017).
What is the relationship between RSC and hippocampus?
RSC first attracted attention because of its links with the hippocampal memory system, and as discussed here, many of the deficits arising from RSC damage resemble those of hippocampal lesions, with some notable differences. Of interest is the asymmetric relationship with hippocampus, in that RSC receives more direct connections (from CA1 and subiculum) than it sends, although it does project indirectly to hippocampus via entorhinal cortex and the subicular complex. It will thus be important to determine the interaction between these structures, during memory formation, retrieval and updating.
Targeted combinations of anterograde and retrograde transported opsins and optogenetic and chemogenetic interventions will be useful in these studies, as they allow more precise interruption of selective neurons. Given the well-known connectivity of these structures, several experimental schemes can be proposed. For example, the general population of hippocampal neurons sending projections to the RSC could be targeted by both chemogenetic and optogenetic approaches with a retrogradely transported vector. Since the effective illumination of the entire hippocampus would be technically challenging given its subcortical location, the chemogenetic approach would be currently preferred in this case. The presynaptic terminals of the CA1 projection neurons could be optically activated within RSC after hippocampal vector injections. In this case, an implantable, light-emitting diode (LED)-based module could be positioned on top of dysgranular RSC to stimulate areas 30 and 29c. With the development of efficient orange LEDs, a similar approach could be used for inhibition. Red-shifted opsins could further increase the range, potentially allowing the illumination of the entire RSC and enabling optogenetic intervention in the hippocampus. Finally, transsynaptic circuit labelling with rabies virus could single-out even more specific sub-populations of projection neurons (Callaway and Luo, 2015). In all cases, temporally precise interruption of processing epochs could be achieved.
The extent to which RSC and hippocampus are functionally coupled could also be examined by combining temporary modulation techniques with electrophysiology: for example, assessing the effect of hippocampal inactivation on neuronal firing within RSC or vice versa. Extant data suggests that RSC inactivation causes changes in hippocampal place fields (Cooper and Mizumori, 2001) and the hippocampal inactivation alters experience-dependent plasticity in RSC (Kubik et al., 2012), but many questions remain open. By combining the latest optogenetic and chemogenetic techniques with electrophysiological recordings and behavioural assays, researchers will be able to address questions about the nature of functional interactions between RSC and hippocampus with far greater anatomical and temporal precision.
Future studies could also explore whether RSC and hippocampus are engaged by different navigational strategies, such as map-based, route planning, and scene construction. Furthermore, RSC may work separately from the hippocampus in processing previously consolidated spatial information (see above), as evident in a recent work by Patai et al. (2017). This study reported higher RSC activity during distance coding in familiar environments, in contrast to higher hippocampal activity seen in newly learned environments, where more route planning might have occurred.
What is the role of RSC in episodic memory more broadly?
A review of the human literature reveals a difference between left and right RSC in both lesion findings and imaging; in particular, the left seems to be more implicated in general episodic memory, while the right is more implicated in spatial processing. Is RSC also involved in episodic memory in animals? We still lack a good animal model of this form of memory, because most animal tasks require training whereas episodes are, by their nature, transient. Nevertheless it will be important to determine, in future, the extent to which RSC has a role in memory that extends beyond space. Indeed, evidence is now emerging implicating RSC in mnemonic processes that do not contain any obvious spatial component including the processing or retrieval of temporal information (Powell et al., 2017; Todd et al., 2015) as well as learning the inter-relationship between sensory stimuli in the environment (Robinson et al., 2014), processes that are likely to be central to our ability to remember an event. Reconciling these seemingly disparate spatial and non-spatial roles, therefore, represents a key challenge for understanding RSC function.
Summary and conclusion
In conclusion, we have reviewed the literature on the RSC contribution to spatial memory and have found that there are three broad classes of models which differ in their focus but have significant overlaps. It remains unclear whether RSC has more than one function, or whether some overarching model that can explain the current findings better describes these three classes of function. We have outlined some open questions, the answers to which will require an interaction between multiple different approaches, in a variety of species.
Over 100 years have passed since Brodmann first identified the RSC, and, while in the intervening years significant advances have been made in elucidating the role RSC plays in cognition, the precise functions of the RSC still remain somewhat of an enigma. It is hoped that the framework set out in this review will provide a basis for subsequent endeavours to probe the underlying function(s) of this most fascinating of brain structures.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: This work was supported by a Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences to A.S.M. (110157/Z/15/Z), a grant from National Science Centre (Poland) Sonata Bis 2014//14/E/NZ4/00172 to R.C., a scholarship from Chinese Scholarship Council to N.Z. (201608000007), a Wellcome Trust Investigator Award to K.J. (WT103896AIA) and A.J.D.N by grants from the BBSRC (BB/H020187/1 and BB/L021005/1).
ORCID iDs: Anna S Mitchell  https://orcid.org/0000-0001-8996-1067
https://orcid.org/0000-0001-8996-1067
Kate Jeffery  https://orcid.org/0000-0002-9495-0378
https://orcid.org/0000-0002-9495-0378
References
- Aggleton JP, Hunt PR, Rawlins JN. (1986) The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioural Brain Research 19(2): 133–146. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, et al. (1996) The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioural Brain Research 81(1–2): 189–198. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Saunders RC, Wright NF, et al. (2014) The origin of projections from the posterior cingulate and retrosplenial cortices to the anterior, medial dorsal and laterodorsal thalamic nuclei of macaque monkeys. The European Journal of Neuroscience 39(1): 107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA. (2015) Retrosplenial cortex maps the conjunction of internal and external spaces. Nature Neuroscience 18(8): 1143–1151. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA. (2017) Spatially periodic activation patterns of retrosplenial cortex encode route sub-spaces and distance travelled. Current Biology 27(11): 1551–1560. [DOI] [PubMed] [Google Scholar]
- An Y, Varma VR, Varma S, et al. (2017) Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s & Dementia. Epub ahead of print 19 October DOI: 10.1016/j.jalz.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Maguire EA. (2013) Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex 49(10): 2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, Maguire EA. (2012) Retrosplenial cortex codes for permanent landmarks. PLoS ONE 7(8): e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Zeidman P, Maguire EA. (2015) A central role for the retrosplenial cortex in de novo environmental learning. Elife 18(4). DOI: 10.7554/eLife.09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Zeidman P, Maguire EA. (2017) Efficacy of navigation may be influenced by retrosplenial cortex-mediated learning of landmark stability. Neuropsychologia 104: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Maguire EA. (2018. a) Dissociating landmark stability from orienting Value Using functional magnetic resonance imaging. Journal of Cognitive Neuroscience (in press), doi: 10.1162/jocn_a_01231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Maguire EA. (2018. b) Retrosplenial cortex indexes stability beyond the spatial domain. Journal of Neuroscience, 38(6):1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. (1985) Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. The Journal of Comparative Neurology 232(2): 219–228. [DOI] [PubMed] [Google Scholar]
- Berger TW, Weikart CL, Bassett JL, et al. (1986) Lesions of the retrosplenial cortex produce deficits in reversal learning of the rabbit nictitating membrane response: Implications for potential interactions between hippocampal and cerebellar brain systems. Behavioral Neuroscience 100(6): 802–809. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Mitchell AS. (2016) Retrosplenial cortical contributions to anterograde and retrograde memory in the monkey. Cerebral Cortex 26(6): 2905–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA, Parvizi J, Morecraft RJ, et al. (2008) Thalamic projections to the posteromedial cortex in the macaque. The Journal of Comparative Neurology 507(5): 1709–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, et al. (1996) Dissociable effects of anterior and posterior cingulate cortex lesions on the acquisition of a conditional visual discrimination: Facilitation of early learning vs. impairment of late learning. Behavioural Brain Research 82(1): 45–56. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. (2007) Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychological Review 114(2): 340–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. (1955) Studies on the Cerebral Cortex: Limbic Structures. Chicago, IL: Year Book Publishers. [Google Scholar]
- Callaway EM, Luo L. (2015) Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. The Journal of Neuroscience 35(24): 8979–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. (2007) Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Research Reviews 56(1): 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Barnes CA, et al. (1994. a) Head-direction cells in the rat posterior cortex, II, contributions of visual and ideothetic information to the directional firing. Experimental Brain Research 101(1): 24–34. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, et al. (1994. b) Head-direction cells in the rat posterior cortex, I, anatomical distribution and behavioral modulation. Experimental Brain Research 101(1): 8–23. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. (2001) Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behavioral Neuroscience 115(1): 3–25. [DOI] [PubMed] [Google Scholar]
- Claessen MH, Van der Ham IJ. (2017) Classification of navigation impairment: A systematic review of neuropsychological case studies. Neuroscience and Biobehavioral Reviews 73: 81–97. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, et al. (2010) Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. The Journal of Neuroscience 30(15): 5289–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. (1999) Retrosplenial cortex inactivation selectively impairs navigation in darkness. NeuroReport 10(3): 625–630. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. (2001) Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. The Journal of Neuroscience 21(11): 3986–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Manka TF, Mizumori SJ. (2001) Finding your way in the dark: The retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behavioral Neuroscience 115(5): 1012–1028. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, et al. (2011) NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. The Journal of Neuroscience 31(32): 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BCC, et al. (2014) Direct reactivation of a coherent neocortical memory of context. Neuron 84(2): 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski R, Jayaprakash B, Wiltgen B, et al. (2014) Encoding and storage of spatial information in the retrosplenial cortex. Proceedings of the National Academy of Sciences of the United States of America 111(23): 8661–8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski R, Sugar J, Zhang SJ, et al. (2013) Superficially projecting principal neurons in layer v of medial entorhinal cortex in the rat receive excitatory retrosplenial input. The Journal of Neuroscience 33(40): 15779–15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elduayen C, Save E. (2014) The retrosplenial cortex is necessary for path integration in the dark. Behavioural Brain Research 272: 303–307. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM. (2007) Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. The Journal of Neuroscience 27(23): 6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M. (1990) Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. Progress in Brain Research 85: 467–483. [PubMed] [Google Scholar]
- Gaffan D. (1994) Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transection. Journal of Cognitive Neuroscience 6(4): 305–320. [DOI] [PubMed] [Google Scholar]
- Ghosh VE, Gilboa A. (2014) What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia. Epub ahead of print 23 November 2013. DOI: 10.1016/j.neuropsychologia.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, Chen HY, et al. (2017) Task-related and resting-state fMRI identify distinct networks that preferentially support remembering the past and imagining the future. Neuropsychologia. Epub ahead of print 15 June DOI: 10.1016/j.neuropsychologia.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Guillery RW. (1956) Degeneration in the post-commissural fornix and the mamillary peduncle of the rat. Journal of Anatomy 90(3): 350–370. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. (1957) Degeneration in the hypothalamic connexions of the albino rat. Journal of Anatomy 91(1): 91–115. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Tanaka Y, Nakano I. (2010) Heading disorientation: A new test and a possible underlying mechanism. European Neurology 63(2): 87–93. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Uechi M, Yumura W, et al. (2016) Egocentric disorientation and heading disorientation: Evaluation by a new test named card placing test. Rinsho Shinkeigaku = Clinical Neurology 56(12): 837–845. [DOI] [PubMed] [Google Scholar]
- Hindley EL, Nelson AJD, Aggleton JP, et al. (2014) The rat retrosplenial cortex is required when visual cues are used flexibly to determine location. Behavioural Brain Research 263: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Ishizuka N. (2015) Topographic distribution of cortical projection cells in the rat subiculum. Neuroscience Research 92: 1–20. [DOI] [PubMed] [Google Scholar]
- Iaria G, Chen JK, Guariglia C, et al. (2007) Retrosplenial and hippocampal brain regions in human navigation: Complementary functional contributions to the formation and use of cognitive maps. The European Journal of Neuroscience 25(3): 890–899. [DOI] [PubMed] [Google Scholar]
- Ino T, Doi T, Hirose S, et al. (2007) Directional disorientation following left retrosplenial hemorrhage: A case report with fMRI studies. Cortex 43(2): 248–254. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. (1987) The entorhinal cortex of the monkey: II. Cortical afferents. The Journal of Comparative Neurology 264(3): 356–395. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. (2008) Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. The Journal of Comparative Neurology 509(6): 608–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob PY, Casali G, Spieser L, et al. (2017) An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nature Neuroscience 20(2): 173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Page HJI, Stringer SM. (2016) Optimal cue combination and landmark-stability learning in the head direction system. The Journal of Physiology 594(22): 6527–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Witter MP. (2007) Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus 17(10): 957–976. [DOI] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. (2005) Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience 133(1): 193–207. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. (2009) Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiology of Learning and Memory 91(4): 408–414. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park KY, Seo SW, et al. (2007) Reversible verbal and visual memory deficits after left retrosplenial infarction. Journal of Clinical Neurology 3(1): 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Jeffery KJ, Maguire EA. (2017) Multivoxel pattern analysis reveals 3D place information in the human hippocampus. The Journal of Neuroscience 37(16): 4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Piette CE, Page H, et al. (2014) Weighted cue integration in the rodent head direction system. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 369(1635): 20120512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. (2003) Macaque monkey retrosplenial cortex: II, cortical afferents. The Journal of Comparative Neurology 466(1): 48–79. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. (2007) Macaque monkey retrosplenial cortex: III, cortical efferents. The Journal of Comparative Neurology 502(5): 810–833. [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Witter MP. (2012) Presubiculum layer III conveys retrosplenial input to the medial entorhinal cortex. Hippocampus 22(4): 881–895. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Kubik-Zahorodna A, et al. (2012) Loss of activity-dependent arc gene expression in the retrosplenial cortex after hippocampal inactivation: Interaction in a higher-order memory circuit. Neurobiology of Learning and Memory 97(1): 124–131. [DOI] [PubMed] [Google Scholar]
- Laurens J, Kim B, Dickman JD, et al. (2016) Gravity orientation tuning in macaque anterior thalamus. Nature Neuroscience 19(12): 1566–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano YR, Page HI, Jacob PY, Lomi E, Street J, Jeffery KJ. (2017) Retrosplenial and postsubicular head direction cells compared during visual landmark discrimination. Brain and Neuroscience Advances, 10.1177/2398212817721859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. (1999) The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences 22(7): 310–316. [DOI] [PubMed] [Google Scholar]
- Maguire EA. (2001) The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scandinavian Journal of Psychology 42(3): 225–238. [DOI] [PubMed] [Google Scholar]
- Mao D, Kandler S, McNaughton BL, et al. (2017) Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nature Communications 8(1): 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette SA, Vass LK, Ryan J, et al. (2014) Anchoring the neural compass: Coding of local spatial reference frames in human medial parietal lobe. Nature Neuroscience 17(11): 1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. (1971) Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 262(841): 23–81. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, et al. (2004) Sites of neocortical reorganization critical for remote spatial memory. Science 305(5680): 96–99. [DOI] [PubMed] [Google Scholar]
- Meunier M, Destrade C. (1988) Electrolytic but not ibotenic acid lesions of the posterior cingulate cortex produce transitory facilitation of learning in mice. Behavioural Brain Research 27(2):161–172. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, et al. (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals of Neurology 42(1): 85–94. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Gaffan D. (2008) The magnocellular mediodorsal thalamus is necessary for memory acquisition, but not retrieval. The Journal of Neuroscience 28(1): 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Browning PGF, Wilson CRE, et al. (2008) Dissociable roles for cortical and subcortical structures in memory retrieval and acquisition. The Journal of Neuroscience 28(34): 8387–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Rockland KS. (2007) GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. The European Journal of Neuroscience 26(5): 1193–1204. [DOI] [PubMed] [Google Scholar]
- Morris RG. (2006) Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 23(11):2829-46. [DOI] [PubMed] [Google Scholar]
- Mullally SL, Hassabis D, Maguire EA. (2012) Scene construction in amnesia: An fMRI study. The Journal of Neuroscience 32(16): 5646–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP. (2010) What, if anything, can monkeys tell us about human amnesia when they can’t say anything at all? Neuropsychologia 48(8): 2385–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave N, Lloyd S, Sahgal A, et al. (1994) Lack of effect of lesions in the anterior cingulate cortex and retrosplenial cortex on certain tests of spatial memory in the rat. Behavioural Brain Research 65(1): 89–101. [DOI] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Pearce JM, et al. (2015. a) The effect of retrosplenial cortex lesions in rats on incidental and active spatial learning. Frontiers in Behavioral Neuroscience 9: 11 DOI: 10.3389/fnbeh.2015.00011. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Powell AL, Holmes JD, et al. (2015. b) What does spatial alternation tell us about retrosplenial cortex function? Frontiers in Behavioral Neuroscience 9: 126 DOI: 10.3389/fnbeh.2015.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, et al. (2003) Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease). The European Journal of Neuroscience 18(9): 2663–2667. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Fitzpatrick R, Lapira J, et al. (2017) Everyday memory: Towards a translationally effective method of modelling the encoding, forgetting and enhancement of memory. The European Journal of Neuroscience 46(4): 1937–1953. [DOI] [PubMed] [Google Scholar]
- Parron C, Save E. (2004) Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiology of Learning and Memory 82(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Passarelli L, Rosa MGP, Bakola S, et al. (2017) Uniformity and diversity of cortical projections to precuneate areas in the macaque monkey: What defines area PGm? Cerebral Cortex. Epub ahead of print 25 March DOI: 10.1093/cercor/bhx067. [DOI] [PubMed] [Google Scholar]
- Patai EZ, Javadi AH, Ozubko JD, et al. (2017) Long-term consolidation switches goal proximity coding from hippocampus to retrosplenial cortex. Available at: http://www.biorxiv.org/content/early/2017/07/25/167882.1.abstract
- Pothuizen HHJ, Aggleton JP, Vann SD. (2008) Do rats with retrosplenial cortex lesions lack direction? The European Journal of Neuroscience 28(12): 2486–2498. [DOI] [PubMed] [Google Scholar]
- Pothuizen HHJ, Davies M, Albasser MM, et al. (2009) Granular and dysgranular retrosplenial cortices provide qualitatively different contributions to spatial working memory: Evidence from immediate-early gene imaging in rats. The European Journal of Neuroscience 30(5): 877–888. [DOI] [PubMed] [Google Scholar]
- Powell AL, Vann SD, Olarte-Sánchez CM, et al. (2017) The retrosplenial cortex and object recency memory in the rat. The European Journal of Neuroscience 45(11): 1451–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EW. (1978) The cingulate bridge between allocortex, isocortex and thalamus. The Anatomical Record 190(4): 783–7–93. [DOI] [PubMed] [Google Scholar]
- Powell TP, Guillery RW, Cowan WM. (1957) A quantitative study of the fornixmamillo-thalamic system. Journal of Anatomy 91(4): 419–437. [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. (2012) Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience 13(10): 713–726. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Hermann KL, Mynick A, et al. (2016) Neural representations integrate the current field of view with the remembered 360° panorama in scene-selective cortex. Current Biology 26(18): 2463–2468. [DOI] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, et al. (2014) Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. The Journal of Neuroscience 34(33): 10982–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero G, Frassinetti F, Iavarone A, et al. (2014) The lost ability to find the way: Topographical disorientation after a left brain lesion. Neuropsychology 28(1): 147–160. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, et al. (2011) Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. The Journal of Neuroscience 31(12): 4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JP, Valdés-Herrera JP, Hegarty M, et al. (2016) The human retrosplenial cortex and thalamus code head direction in a global reference frame. The Journal of Neuroscience 36(24): 6371–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai M, Yokofujita J, Oda S, et al. (2005) Dual axonal terminations from the retrosplenial and visual association cortices in the laterodorsal thalamic nucleus of the rat. Anatomy and Embryology 210(4): 317–326. [DOI] [PubMed] [Google Scholar]
- Silson EH, Steel AD, Baker CI. (2016) Scene-selectivity and retinotopy in medial parietal cortex. Frontiers in Human Neuroscience 10: 412 DOI: 10.3389/fnhum.2016.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Barredo J, Mizumori SJY. (2012) Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus 22(5): 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Freeman JH, Jr, Nicholson D, et al. (2002) Limbic thalamic lesions, appetitively motivated discrimination learning, and training-induced neuronal activity in rabbits. The Journal of Neuroscience 22(18): 8212–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. (2006) Thoughts, behaviour, and brain dynamics during navigation in the real world. NeuroImage 31(4): 1826–1840. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. (2009) The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience 21(3): 489–510. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. (1986) Thalamic projections to retrosplenial cortex in the rat. The Journal of Comparative Neurology 254(2): 143–165. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. (1988) Contributions of cingulate cortex to two forms of spatial learning and memory. The Journal of Neuroscience 8(6): 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. (1994) Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. The Journal of Comparative Neurology 350(4): 497–533. [DOI] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. (2011) The default network distinguishes construals of proximal versus distal events. Journal of Cognitive Neuroscience 23(10): 2945–2955. [DOI] [PubMed] [Google Scholar]
- Taube JS. (1995) Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. The Journal of Neuroscience 15(1 Pt 1): 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. (2007) The head direction signal: Origins and sensory-motor integration. Annual Review of Neuroscience 30: 181–207. [DOI] [PubMed] [Google Scholar]
- Teipel S, Grothe MJ. and Alzheimer’s Disease Neuroimaging Initiative (2016) Does posterior cingulate hypometabolism result from disconnection or local pathology across preclinical and clinical stages of Alzheimer’s disease? European Journal of Nuclear Medicine and Molecular Imaging 43(3): 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Meyer HC, Bucci DJ. (2015) Contribution of the retrosplenial cortex to temporal discrimination learning. Hippocampus 25(2): 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Vann SD, et al. (2011) Theta-modulated head direction cells in the rat anterior thalamus. The Journal of Neuroscience 31(26): 9489–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, et al. (2011) Schema-dependent gene activation and memory encoding in neocortex. Science 333(6044): 891–895. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss MJ. (1990) Connections of the retrosplenial granular a cortex in the rat. The Journal of Comparative Neurology 300(4): 593–606. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss MJ. (1992. a) Connections of the retrosplenial dysgranular cortex in the rat. The Journal of Comparative Neurology 315(2): 200–216. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss MJ. (1992. b) Projections from the laterodorsal nucleus of the thalamus to the limbic and visual cortices in the rat. The Journal of Comparative Neurology 324(3): 427–448. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss MJ. (2003) Connections of the retrosplenial granular B cortex in the rat. The Journal of Comparative Neurology 463(3): 249–263. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey, I, temporal lobe afferents. Brain Research 95(1): 1–24. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. (2009) What does the retrosplenial cortex do? Nature Reviews Neuroscience 10(11): 792–802. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. (2002) Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behavioral Neuroscience 116(1): 85–94. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. (2004) Testing the importance of the retrosplenial guidance system: Effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behavioural Brain Research 155(1): 97–108. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. (2005) Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behavioral Neuroscience 119(6): 1682–1686. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, et al. (2000) Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. The Journal of Neuroscience 20(7): 2711–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder LC, Miller AMP, Harrison MB, et al. (2017) Retrosplenial cortical neurons encode navigational cues, trajectories and reward locations during goal directed navigation. Cerebral Cortex 27(7): 3713–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Albo Z, Viana Di, Prisco G. (2001) Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez’s circuit. Neuroscience 104(3): 619–625. [DOI] [PubMed] [Google Scholar]
- Vogt BA. (1976) Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. The Journal of Comparative Neurology 169(1): 63–97. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. (1983) Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. The Journal of Comparative Neurology 216(2): 192–210. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. (1987) Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. The Journal of Comparative Neurology 262(2): 256–270. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. (2006) Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage 29(2): 452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesierska M, Adamska I, Malinowska M. (2009) Retrosplenial cortex lesion affected segregation of spatial information in place avoidance task in the rat. Neurobiology of Learning and Memory 91(1): 41–49. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Maaswinkel H, Gonzalez CL, et al. (2001) Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behavioural Brain Research 118(1): 67–76. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Büchel C. (2005) Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. The Journal of Neuroscience 25(13): 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, et al. (2000) Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27(3): 623–633. [DOI] [PubMed] [Google Scholar]
- Wyss J, Van Groen T. (1992) Connections between the retrosplenial cortex and the hippocampal formation in the rat: A review. Hippocampus 2(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Radulovic J, Shepherd GMG. (2016) A corticocortical circuit directly links retrosplenial cortex to M2 in the mouse. The Journal of Neuroscience 36(36): 9365–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]