Abstract
BACKGROUND
It is currently unclear if the superior normal organ sparing effect of Intensity Modulated Radiation Therapy (IMRT) as compared to three-dimensional radiation therapy (3D) has clinical impact on survival and cardiopulmonary mortality in esophageal cancer (EC) patients.
METHODS
We identified 2,553 patients older than age 65 years from the SEER/Texas Cancer Registry-Medicare databases who had non-metastatic EC diagnosed between 2002 and 2009 and were treated with either 3D (n=2,240) or IMRT (n=313) within 6 months of diagnosis. The outcomes of the two cohorts were compared using Inverse Probability of Treatment Weighting (IPTW) adjustment.
RESULTS
Except for marital status, year of diagnosis, and SEER region, both radiation cohorts were well balanced for various patient, tumor, and treatment characteristics, including the use of IMRT vs. 3D in urban/metro or rural areas. IMRT use increased from 2.6% in 2002 to 30% in 2009, while 3D use decreased from 97.4% in 2002 to 70% in 2009. On propensity score IPTW-adjusted multivariate analysis, IMRT was not associated with EC-specific mortality (HR 0.93, 95%CI 0.80-1.10) or pulmonary mortality (HR 1.11, 95%CI 0.37-3.36) but was significantly associated with lower all-cause mortality (HR 0.83, 95%CI 0.72-0.95), cardiac mortality (HR 0.18, 95%CI 0.06-0.54) and other cause mortality (HR 0.54, 95%CI 0.35-0.84). Similar associations were seen after adjusting for the type of chemotherapy, physician experience, and sensitivity analysis removing hybrid radiation claims.
CONCLUSIONS
In this population-based analysis, IMRT use was significantly associated with lower all-cause mortality, cardiac mortality, and other-cause mortality in EC patients.
Keywords: Esophageal cancer, IMRT, 3D conformal radiation therapy, SEER, propensity score, cardiopulmonary mortality
CONDENSED ABSTRACT
It is currently unclear if the dosimetric advantages of organ sparing by Intensity Modulated Radiation Therapy (IMRT) compared to three dimensional conformal radiation therapy (3D) can translate to survival and cardiopulmonary mortality benefit in esophageal cancer patients. This SEER/Texas Cancer Registry-Medicare population-based study found that while there were no differences in cancer or pulmonary-specific mortality, all-cause and cardiac-specific mortality were significantly reduced in IMRT-treated patients under a propensity score adjusted multivariate analysis.
INTRODUCTION
Radiation technologies have evolved substantially over time, from 2-dimensional (2D) planning on plain x-ray films to 3-Dimensional (3D) computerized tomography (CT)-based treatment planning. Intensity Modulated Radiation Therapy (IMRT) is the next level of advancement that delivers better prescription dose conformality to the tumor but increases the low dose spread to surrounding tissues. For some sites of disease, IMRT is an accepted standard based on evidence showing toxicity reduction compared to conventional radiotherapy methods, including 3D conformal radiotherapy1–3. However for many sites of disease, including esophageal cancer (EC), 3D remains the standard approach due to the uncertain benefits of the more expensive and technically demanding IMRT.
For newly diagnosed EC, chemoradiation, either preoperative or definitive, is done as a standard of care4. However, given the location of most tumors, the heart dose can be substantial, particularly when standard 3D technique is used. Planning studies have shown that IMRT preferentially spares the heart over the lungs5–7. How this dosimetric advantage translates to clinical benefit for patients is still not convincingly proven, since there are no large randomized trials comparing IMRT to 3D in EC. Previously, a propensity matched analysis of single institution data comparing the long term outcomes of patients treated with either IMRT or 3D radiotherapy from 1998 to 2010 was reported8. The authors found significantly improved overall survival and cardiac-specific mortality for IMRT, but no differences in distant recurrence rate, cancer-specific survival, or pulmonary-related deaths. However, another single institution data found no difference in overall survival but only reduced short term toxicity for patients treated with IMRT9. The benefit of IMRT, particularly in improving long term clinical outcomes, remains unclear.
For this study, we evaluated the Surveillance, Epidemiology, and End Results (SEER)-Medicare and the Texas Cancer Registry-Medicare-linked databases to assess the overall and cause-specific mortality rates of EC patients treated with radiotherapy. On the basis of the dosimetric advantages of IMRT, we hypothesized that IMRT may produce clinical benefit by reducing cardiopulmonary mortality in EC patients treated with radiotherapy.
PATIENTS AND METHODS
Data source
Patients older than age 65 years were identified from the National Cancer Institute (NCI)-supported SEER-Medicare database and the Texas Cancer Registry (TCR), Medicare-linked database. The SEER provided information from 17 geographic locations in the United States, representing approximately 25% of the nation’s incident cancers linked to Medicare claims. The TCR, as a legislative mandate of the Texas Department of State and Health Services in 1979, is the fourth largest state population-based registry. Data on vital statistics and cause-specific deaths are obtained through linkage with the Texas vital statistics and mortality data, the Social Security Death Index, and the National Death Index. Data collection follows standard registry rules, and core data items are similar to that collected on the SEER-Medicare database. The TCR data have been linked to Medicare claims using the same algorithm as the SEER-Medicare linkage. The files from the cancer registries were used to identify patients diagnosed with EC and the vital status of these patients. Subsequent treatment was identified from Medicare claims using billing codes. The relevant codes used are summarized in supplement table 1. This research was reviewed by the Institutional Review Board and granted an exemption.
Cohort selection
A multistep process (supplement table 2) was used to select the patients from the two databases based on their first diagnosis of EC (31,101: SEER 1973-2009, 6,856: TCR 1995-2007), with the histologically and microscopically confirmed diagnosis of squamous or adenocarcinoma but not diagnosed at the time of autopsy. Our patients were aged > 65 with stage I-III EC, had enrolled in Medicare parts A and B for 12 months prior to diagnosis without Health Maintenance Organization insurance, and stayed enrolled until 12 months after diagnosis or death if the patient died within 12 months of diagnosis. Patients must also not have had a second cancer within 1 year of diagnosis.
Radiation use selection
All patients must have started radiotherapy within 6 months after diagnosis based on radiation claims. Patients who had brachytherapy within 12 months of diagnosis were excluded. For IMRT, we used the Healthcare Common Procedure Coding System (HCPCS) codes 77418 and G0174, and for 3D we used codes 77290, 76370, 77014, 77295. We excluded any 2D patients (77280, 77285) and patients who had radiation but were not categorized using these codes. There were 173 patients that were hybrids: having both IMRT and 3D delivery claims in their Medicare records. To categorize these into either IMRT or 3D, we formulated a stepwise approach to segregate these patients using criteria involving radiation course delivery time, the number of fractions between the two types of radiation treatment claims, and the first treatment delivery dates (supplement table 3). Using this stepwise approach, we further were able to define 138 patients into either IMRT or 3D. Sensitivity analysis was performed to evaluate if inclusion of these patients affected the multivariable analysis. The rest of 35 Patients who couldn’t be stratified using this approach were considered inevaluable and excluded from this study.
Baseline patient, tumor, and treatment characteristics
Demographic information included age, gender, race/ethnicity, marital status (not available in TCR), SEER regions, urban/rural setting, educational attainment, and income level. Tumor characteristics included stage (localized vs regional (node positive)), grade, and year of diagnosis. Treatment characteristics included the use of chemotherapy within 6 months of diagnosis and the performance of esophagectomy after radiation treatment. Comorbidities were recorded as either the Klabunde adaptation of the Charlson comorbidity index, or as individual comorbid illnesses existing within 12 months prior to EC diagnosis, such as congestive heart failure, hypertension, other heart diseases (CAD, MI), diabetes, or pulmonary diseases (COPD).
Physician experience
To document physician demographics and experience in the utilization of the radiation technologies, we collected information from physicians who performed the radiation claims using the Unique Physician Identification Number (UPIN) or the National Provider Identifier (NPI) (for claims made after June 2007). For physicians having both UPIN and NPI numbers, redundancy was eliminated by crosslinking the NPI to the UPIN numbers. We collected information regarding the physicians’ age (by 2010), gender, primary and secondary specialties, board certification status, US trained (Yes/No), number of years in practice after training, and EC case load based on the number of yearly claims by the said physician.
Statistical analysis
We used χ2 analysis to compare the proportion of 3D vs IMRT use among the baseline characteristics. Propensity score (PS) was calculated to predict the conditional probability of patients receiving IMRT vs. 3D based on their pre-treatment variables. We calculated the propensity score as a continuous covariate using logistic regression to predict the patients’ possibility of receiving IMRT or 3D. The covariates adjusted in the logistic regression include the patients’ demographics, comorbidities, tumor characteristics, physician characteristics, the type of chemotherapy, and the type of radiation technology used. We also calculated the Inverse Probability of Treatment Weights (IPTW) using the propensity score obtained from the logistic regression. The IPTW-adjusted Kaplan-Meier (KM) survival curves were generated for overall, EC-specific, cardiac, pulmonary, or other non-cancer, non-cardiopulmonary cause deaths (“other deaths”). Statistical analysis was carried out using SAS software program version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patients, treatment, and physician characteristics
We initially identified 3,403 patients aged 66 years or older diagnosed with non-metastatic EC from 1997 to 2009 who met our inclusion criteria. We further confined our analysis to the patients treated between 2002-2009, with 2,240 treated with 3D and 313 with IMRT. IMRT use increased from a rate of 2.6% in 2002 to 31.2% in 2009 (Figure 1). Table 1 summarizes the baseline characteristics of the study cohort. For the most part the two groups were well balanced excepted for the marital status, the SEER region, and the year of diagnosis. The use of IMRT was only slightly higher in metro vs. rural areas, but the difference was not significant. There were no differences in the income or education levels of the two cohorts. The median number of radiation treatment fractions is 26 for 3D and 28 for IMRT, but the difference is not statistically significant.
Figure 1.
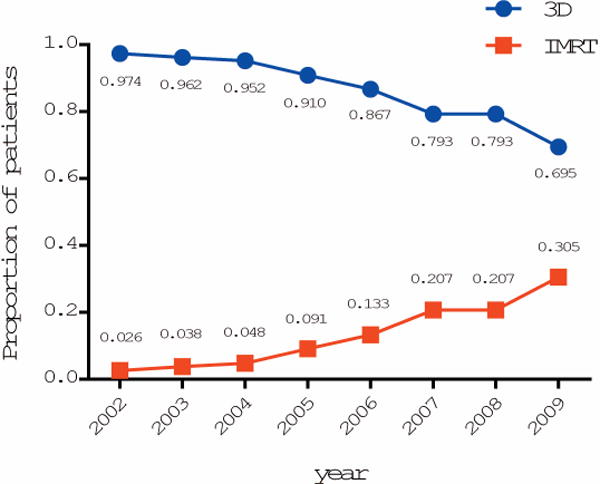
The utilization of 3D and IMRT for the treatment of EC from 2002 to 2009.
Table 1.
Patient demographic, clinical and tumor characteristics
| Overall Cohort | 3D | IMRT | Chi-sq P | |
|---|---|---|---|---|
| N = 2553 (100%) | N=2240(100%) | N=313 (100%) | ||
| Age | ||||
| 66-70 | 729(28.6) | 634(28.3) | 95(30.4) | 0.7698 |
| 71-75 | 669(26.2) | 584(26.1) | 85(27.2) | |
| 76-80 | 560(21.9) | 496(22.1) | 64(20.5) | |
| >80 | 595(23.3) | 526(23.5) | 69(22.0) | |
| Years of Diagnosis | ||||
| 2002-2003 | 695(27.2) | 672(30) | 23(7.4) | <.0001 |
| 2004 | 369(14.5) | 351(15.7) | 18(5.8) | |
| 2005 | 313(12.3) | 284(12.7) | 29(9.3) | |
| 2006 | 349(13.7) | 302(13.5) | 47(15.0) | |
| 2007 | 362(14.2) | 287(12.8) | 75(24.0) | |
| 2008 | 216(8.7) | 171(7.6) | 45(14.4) | |
| 2009 | 249(9.8) | 173(7.7) | 76(24.3) | |
| Marital Status | ||||
| Married | 1230(48.2) | 1097(49.0) | 133(42.5) | 0.0403 |
| Not married | 771(30.2) | 674(30.1) | 97(31.0) | |
| Unknown* | 552(21.6) | 469(20.9) | 83(26.5) | |
| Histology | ||||
| Adeno | 1423(55.7) | 1255(56.0) | 168(53.7) | 0.4325 |
| SCCA | 1130(44.3) | 985(44.0) | 145(46.3) | |
| Race/Ethnicity | ||||
| White | 2095(82.1) | 1834(81.9) | 261(83.4) | 0.0619 |
| Hispanic | 144(5.6) | 120(5.4) | 24(7.7) | |
| Black/Other | 314(12.3) | 286(12.8) | 28(9.0) | |
| Stage | ||||
| Localized | 991(38.8) | 862(38.5) | 129(41.2) | 0.3529 |
| Regional | 1562(61.2) | 1378(61.5) | 184(58.8) | |
| Gender | ||||
| Female | 744(29.1) | 657(29.3) | 87(27.8) | 0.5757 |
| Male | 1809(70.9) | 1583(70.7) | 226(72.2) | |
| Patients receiving Surgery After Radiation Treatment | ||||
| No | 2107(82.5) | 1855(82.81) | 252(80.5) | 0.3152 |
| Yes | 446(17.5) | 385(17.19) | 61(19.5) | |
| Tumor Grade | ||||
| Well differentiated | 123(4.8) | 108(4.8) | 15(4.8) | 0.9662 |
| Moderately differentiated | 957(37.5) | 839(37.5) | 118(37.7) | |
| Poorly differentiated | 1033(40.5) | 910(40.6) | 123(39.3) | |
| Unknown | 440(17.2) | 383(17.1) | 57(18.2) | |
| Charlson Score | ||||
| 0 | 1510(59.2) | 1313(58.6) | 197(62.9) | 0.2957 |
| 1 | 664(26.0) | 593(26.5) | 71(22.7) | |
| 2+ | 379(14.9) | 334(14.9) | 45(14.34) | |
| Regions (SEER + Texas) | ||||
| California + Hawaii | 563(22.1) | 484(21.6) | 79(25.2) | 0.0632 |
| 6 SEER regions combined** | 636(24.9) | 576(25.7) | 60(19.2) | |
| Greater Georgia | 256(10.0) | 232(10.4) | 24(7.7) | |
| Kentucky | 161(6.3) | 141(6.3) | 20(6.4) | |
| Louisiana | 134(5.3) | 116(5.2) | 18(5.8) | |
| New Jersey | 324(12.7) | 284(12.7) | 40(12.8) | |
| Texas | 479(18.8) | 407(18.2) | 72(23) | |
| Use of Chemotherapy | 0.0881 | |||
| No | 411(16.1) | 371(16.6) | 40(12.8) | |
| Yes | 2142(83.9) | 1869(83.4) | 273(87.2) | |
| Urban/Rural | ||||
| Big Metro | 1285(50.3) | 1124(50.2) | 161(51.4) | 0.5954 |
| Less Urban/Rural | 411(16.1) | 369(16.5) | 42(13.4) | |
| Metro | 805(31.5) | 701(31.3) | 104(33.2) | |
| Urban | 168(6.6) | 148(6.6) | 20(6.4) | |
| % of adults with <12 y of Education | ||||
| Lowest Quartile | 625(24.5) | 544(24.3) | 81(25.9) | 0.627 |
| 2nd Quartile | 607(23.8) | 529(23.6) | 78(24.9) | |
| 3rd Quartile | 640(25.1) | 560(25.0) | 80(25.6) | |
| Highest Quartile | 681(26.7) | 607(27.1) | 74(23.6) | |
| % of Family living below poverty line | ||||
| Lowest Quartile | 629(24.6) | 548(24.5) | 81(25.9) | 0.5019 |
| 2nd Quartile | 627(24.6) | 561(25.0) | 66(21.1) | |
| 3rd Quartile | 641(25.1) | 560(25.0) | 81(25.9) | |
| Highest Quartile | 656(25.7) | 571(25.5) | 85(27.2) | |
| Pre-CHF*** | ||||
| No | 2048(80.2) | 1798(80.27) | 250(79.87) | 0.8693 |
| Yes | 505(19.8) | 442(19.73) | 63(20.13) | |
| Pre-Other Heart Disease*** | ||||
| No | 2038(79.8) | 1786(79.73) | 252(80.51) | 0.7477 |
| Yes | 515(20.2) | 454(20.27) | 61(19.49) | |
| Pre-Hypertension*** | ||||
| No | 1313(51.4) | 1161(51.83) | 152(48.56) | 0.2785 |
| Yes | 1240(48.6) | 1079(48.17) | 161(51.44) | |
| Pre-Diabetes*** | ||||
| No | 2100(82.3) | 1845(82.37) | 255(81.47) | 0.6974 |
| Yes | 453(17.7) | 395(17.63) | 58(18.53) | |
| Pre-Respiratory Dz *** | ||||
| No | 2031(79.6) | 1780(79.46) | 251(80.19) | 0.765 |
| Yes | 522(20.5) | 460(20.54) | 62(19.81) | |
| Number of Fractions | ||||
| Mean ± SD | 24±8.5 | 24±8.5 | 25±8.1 | 0.1433 |
| Median | 26 | 26 | 28 |
All TCR Patients Marital Status were Unknown
The six SEER regions include: Conneticut, Detroit, Iowa, New Mexico, Seattle, Utah
Pre-disease within one-year before Esophageal Cancer Diagnosis, parts of Comorbidity disease, therefore not included into Cox Modeling that has adjusted for Charlson Score.
Physician experience or hospital volume have been shown to be influential factors in the clinical outcomes of surgical patients10, 11. We therefore included physician characteristics in the context of the radiation technologies utilized (Table 2). While board certification, gender, and the type of medical degree (MD vs DO) did not differ among physicians using the two radiation modalities, younger physicians (which correlated with being more recent graduates from medical schools, having fewer years in practice and having lower clinical volumes) used IMRT significantly more frequently than older, more seasoned physicians. US trained physicians also used IMRT less often than non-US trained physicians.
Table 2.
The Characteristics of the Physicians Associated with the Treated Patients
| Overall Cohort | 3D | IMRT | Chi-sq P | |
|---|---|---|---|---|
| N = 2553 (100%) | N=2240(100%) | N=313 (100%) | ||
| Board Certified | ||||
| Yes | 2190(85.8) | 1928(86.1) | 262(83.7) | 0.5293 |
| No/Unknown | 363(14.2) | 312(13.9) | 51(16.3) | |
| Graduation Years | ||||
| Prior to 1980 | 657(25.7) | 594(26.5) | 63(20.1) | 0.0421 |
| 1980-1989 | 947(37.1) | 833(37.2) | 114(36.4) | |
| After 1990 | 652(25.5) | 558(24.9) | 94(30.0) | |
| Unknown | 297(11.6) | 255(11.4) | 42(13.4) | |
| Physician Gender | ||||
| F | 393(15.4) | 343(15.3) | 50(16.0) | 0.5145 |
| M | 1863(73.0) | 1642(73.3) | 221(70.6) | |
| Unknown | 297(11.6) | 255(11.4) | 42(13.4) | |
| US Trained | ||||
| No | 376(14.7) | 313(14.0) | 63(20.1) | 0.0111 |
| Yes | 1901(74.5) | 1687(75.3) | 214(68.4) | |
| Unknown | 276(10.8) | 240(10.7) | 36(11.5) | |
| Physician Type | ||||
| MD | 2244(87.9) | 1969(87.9) | 275(87.9) | 0.5126 |
| DO/Unknown | 309(12.1) | 271(12.1) | 38(12.1) | |
| Physician Age | ||||
| 34-46 | 407(15.9) | 341(15.2) | 66(21.1) | 0.0099 |
| 46-52 | 619(24.3) | 537(24.0) | 82(26.2) | |
| 52-60 | 673(26.4) | 606(27.1) | 67(21.4) | |
| 60-85 | 557(21.8) | 501(22.4) | 56(17.9) | |
| Unknown | 297(11.6) | 255(11.4) | 42(13.4) | |
| Physician Training Years | ||||
| 3-13 | 470(18.4) | 388(17.3) | 82(26.2) | 0.0017 |
| 13-19 | 563(22.1) | 503(22.5) | 60(19.2) | |
| 19-28 | 652(25.5) | 579(25.9) | 73(23.3) | |
| 28-61 | 521(20.4) | 470(21.0) | 51(16.3) | |
| Unknown | 347(13.6) | 300(13.4) | 47(15.0) |
A total of 1124 physicians by Upin had seen the cohort of 2553 patients, among them, 136 physicians’ information were missing, resulting up to 297 patients’ physicians’ demographic not identified.
To compare the outcomes of the two groups, we applied the IPTW Cox model analysis, in which each patient is weighted to create a pseudopopulation that mimics what would be attained in a randomized trial. The propensity score, IPTW-adjusted baseline patient, tumor, and physician characteristics are listed in supplemental tables 4 and 5. This was applied to generate the fitted multivariate IPTW-adjusted Cox model for survival analysis comparing 3D and IMRT (Table 3). The IPTW-adjusted KM survival analysis for all-cause, EC-specific, cardiac-specific, pulmonary-specific, or other-cause mortality is shown in Figure 2. IMRT was significantly associated with lower all-cause mortality, cardiac-specific mortality, and other-cause mortality compared to 3D, but not for EC-specific or pulmonary mortality. We found no relationship between the board certification of the physicians, graduation years, physicians’ gender, US vs. non-US training, or physician type, on any of the mortality outcomes of patients.
Table 3.
Fitted Cox Model Using Inverse Probability of Treatment Weights (IPTWs)
| Parameter
|
All cause deaths
|
EC-specific deaths
|
Cardiac-specific deaths
|
Pulmonary-specific deaths
|
Other cause deaths
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | Pr > ChiSq | HR | 95% CI | Pr > ChiSq | HR | 95% CI | Pr > ChiSq | HR | 95% CI | Pr > ChiSq | HR | 95% CI | Pr > ChiSq | ||||||
| Radiation Treatment | ||||||||||||||||||||
| 3D | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| IMRT | 0.831 | 0.725 | 0.952 | 0.0075 | 0.934 | 0.795 | 1.097 | 0.4063 | 0.183 | 0.062 | 0.544 | 0.0022 | 1.111 | 0.367 | 3.363 | 0.8525 | 0.543 | 0.351 | 0.842 | 0.0064 |
| Age | ||||||||||||||||||||
| 66-70 | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 71-75 | 1.026 | 0.907 | 1.16 | 0.6871 | 0.952 | 0.816 | 1.11 | 0.5276 | 0.893 | 0.512 | 1.558 | 0.6893 | 0.674 | 0.245 | 1.853 | 0.4442 | 1.292 | 0.935 | 1.784 | 0.1202 |
| 76-80 | 1.201 | 1.056 | 1.366 | 0.0054 | 1.18 | 1.008 | 1.382 | 0.04 | 0.935 | 0.512 | 1.709 | 0.8279 | 1.043 | 0.393 | 2.768 | 0.9318 | 1.383 | 0.976 | 1.959 | 0.0679 |
| >80 | 1.324 | 1.158 | 1.514 | <.0001 | 1.208 | 1.024 | 1.426 | 0.025 | 1.939 | 1.11 | 3.386 | 0.02 | 0.917 | 0.286 | 2.941 | 0.8835 | 1.285 | 0.877 | 1.881 | 0.1983 |
| Year of Diagnosis | ||||||||||||||||||||
| 2002 | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 2003 | 0.962 | 0.818 | 1.13 | 0.6348 | 0.952 | 0.79 | 1.148 | 0.6053 | 0.761 | 0.412 | 1.405 | 0.382 | 0.163 | 0.029 | 0.909 | 0.0386 | 1.151 | 0.751 | 1.765 | 0.5189 |
| 2004 | 1.033 | 0.876 | 1.219 | 0.6974 | 0.921 | 0.76 | 1.117 | 0.4037 | 1.109 | 0.599 | 2.053 | 0.7416 | 0.863 | 0.279 | 2.668 | 0.7982 | 1.257 | 0.812 | 1.945 | 0.3057 |
| 2005 | 0.981 | 0.826 | 1.166 | 0.8311 | 0.918 | 0.751 | 1.122 | 0.4043 | 0.473 | 0.218 | 1.026 | 0.0582 | 1.276 | 0.436 | 3.735 | 0.6563 | 1.162 | 0.736 | 1.832 | 0.5197 |
| 2006 | 0.982 | 0.826 | 1.167 | 0.8338 | 0.878 | 0.717 | 1.074 | 0.206 | 0.685 | 0.339 | 1.386 | 0.2933 | 0.99 | 0.318 | 3.078 | 0.9856 | 0.869 | 0.536 | 1.408 | 0.568 |
| 2007 | 1.123 | 0.945 | 1.333 | 0.1877 | 0.877 | 0.713 | 1.078 | 0.2113 | 0.827 | 0.42 | 1.628 | 0.583 | 0.437 | 0.1 | 1.904 | 0.2705 | 1.37 | 0.876 | 2.142 | 0.1679 |
| 2008 | 1.165 | 0.955 | 1.421 | 0.1312 | 0.793 | 0.621 | 1.013 | 0.0634 | 0.494 | 0.198 | 1.236 | 0.1317 | 0.787 | 0.157 | 3.931 | 0.77 | 1.4 | 0.801 | 2.448 | 0.2376 |
| 2009 | 1.046 | 0.859 | 1.273 | 0.6545 | 0.255 | 0.181 | 0.359 | <.0001 | 0.085 | 0.013 | 0.536 | 0.0087 | - | - | - | - | 0.575 | 0.28 | 1.179 | 0.1307 |
| Marital Status | ||||||||||||||||||||
| Married | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Not married | 1.155 | 1.037 | 1.287 | 0.0088 | 1.139 | 0.998 | 1.301 | 0.0538 | 1.338 | 0.844 | 2.122 | 0.216 | 1.656 | 0.723 | 3.794 | 0.2333 | 0.904 | 0.653 | 1.251 | 0.5421 |
| Unknown | 1.076 | 0.833 | 1.389 | 0.5747 | 0.925 | 0.653 | 1.311 | 0.6617 | 0.965 | 0.321 | 2.905 | 0.9498 | - | - | - | - | 0.986 | 0.476 | 2.042 | 0.9706 |
| Race/Ethnicity | ||||||||||||||||||||
| White | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Hispanic | 0.766 | 0.622 | 0.944 | 0.0122 | 0.733 | 0.562 | 0.955 | 0.0215 | 0.964 | 0.441 | 2.107 | 0.9269 | 0.634 | 0.123 | 3.256 | 0.5849 | 0.702 | 0.412 | 1.197 | 0.194 |
| Black | 0.999 | 0.848 | 1.178 | 0.9931 | 1.015 | 0.831 | 1.241 | 0.8814 | 1.263 | 0.637 | 2.502 | 0.5038 | 0.659 | 0.158 | 2.757 | 0.5683 | 0.845 | 0.537 | 1.33 | 0.4674 |
| Other | 1.088 | 0.826 | 1.432 | 0.5485 | 1.029 | 0.742 | 1.427 | 0.8641 | 0.775 | 0.176 | 3.425 | 0.7372 | 2.988 | 0.684 | 13.045 | 0.1455 | 1.012 | 0.419 | 2.443 | 0.979 |
| Histology | ||||||||||||||||||||
| Adeno | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| SCCA | 1.064 | 0.963 | 1.176 | 0.2238 | 1.086 | 0.96 | 1.229 | 0.1911 | 0.952 | 0.613 | 1.479 | 0.8262 | 1.48 | 0.668 | 3.275 | 0.3339 | 0.944 | 0.717 | 1.242 | 0.6815 |
| Stage | ||||||||||||||||||||
| Localized | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Regional | 1.261 | 1.149 | 1.383 | <.0001 | 1.258 | 1.122 | 1.411 | <.0001 | 0.855 | 0.581 | 1.257 | 0.4248 | 1.285 | 0.62 | 2.662 | 0.5003 | 1.235 | 0.961 | 1.586 | 0.099 |
| Gender | ||||||||||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Male | 1.094 | 0.984 | 1.216 | 0.0972 | 1.112 | 0.975 | 1.268 | 0.1124 | 0.988 | 0.636 | 1.533 | 0.9558 | 0.956 | 0.417 | 2.193 | 0.9152 | 0.938 | 0.704 | 1.251 | 0.6633 |
| Surgery After Radiation | ||||||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Yes | 0.581 | 0.509 | 0.663 | <.0001 | 0.494 | 0.416 | 0.588 | <.0001 | 0.543 | 0.276 | 1.068 | 0.0769 | 0.507 | 0.167 | 1.54 | 0.2309 | 0.872 | 0.635 | 1.197 | 0.3957 |
| Grade | ||||||||||||||||||||
| Well differentiated | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Moderately differentiated | 0.97 | 0.788 | 1.194 | 0.7716 | 1.051 | 0.811 | 1.363 | 0.7063 | 0.764 | 0.337 | 1.731 | 0.5189 | 0.715 | 0.162 | 3.151 | 0.6581 | 0.844 | 0.508 | 1.401 | 0.5111 |
| Poorly differentiated | 1.114 | 0.841 | 1.476 | 0.4522 | 1.157 | 0.815 | 1.643 | 0.4133 | 0.804 | 0.248 | 2.601 | 0.7154 | 0.716 | 0.092 | 5.555 | 0.7495 | 0.952 | 0.511 | 1.772 | 0.8757 |
| Undifferentiated | 1.146 | 0.927 | 1.416 | 0.2074 | 1.254 | 0.964 | 1.633 | 0.0922 | 0.902 | 0.395 | 2.058 | 0.8056 | 1.434 | 0.331 | 6.209 | 0.6297 | 0.733 | 0.427 | 1.259 | 0.2608 |
| Unknown | 0.999 | 0.799 | 1.249 | 0.9931 | 1.014 | 0.767 | 1.342 | 0.9211 | 1.104 | 0.464 | 2.628 | 0.8223 | 0.25 | 0.034 | 1.845 | 0.1739 | 0.892 | 0.514 | 1.547 | 0.6834 |
| Charlson Score | ||||||||||||||||||||
| 0 | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 1 | 1.136 | 1.024 | 1.259 | 0.0157 | 1.025 | 0.901 | 1.166 | 0.7061 | 1.777 | 1.113 | 2.838 | 0.0161 | 3.887 | 1.732 | 8.723 | 0.001 | 1.333 | 1.021 | 1.739 | 0.0345 |
| 2+ | 1.451 | 1.279 | 1.646 | <.0001 | 1.253 | 1.069 | 1.47 | 0.0055 | 4.288 | 2.716 | 6.769 | <.0001 | 4.534 | 1.748 | 11.76 | 0.0019 | 1.311 | 0.912 | 1.885 | 0.1441 |
| SEER Region | ||||||||||||||||||||
| California + Hawaii | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 6 SEER Regions Combined** | 0.94 | 0.822 | 1.076 | 0.3697 | 0.846 | 0.719 | 0.996 | 0.044 | 1.159 | 0.651 | 2.065 | 0.6152 | 1.075 | 0.344 | 3.363 | 0.9006 | 1.462 | 0.95 | 2.249 | 0.0842 |
| Greater Georgia | 1.269 | 1.069 | 1.506 | 0.0065 | 1.156 | 0.938 | 1.424 | 0.1731 | 1.254 | 0.577 | 2.726 | 0.5672 | 1.17 | 0.247 | 5.548 | 0.843 | 2.175 | 1.312 | 3.607 | 0.0026 |
| Kentucky | 1.273 | 1.04 | 1.558 | 0.0192 | 1.244 | 0.975 | 1.588 | 0.0787 | 1.355 | 0.562 | 3.269 | 0.4988 | 1.744 | 0.366 | 8.302 | 0.4846 | 1.546 | 0.81 | 2.952 | 0.1863 |
| Louisiana | 1.029 | 0.834 | 1.27 | 0.7883 | 0.872 | 0.668 | 1.139 | 0.3144 | 1.042 | 0.407 | 2.665 | 0.9315 | 1.699 | 0.299 | 9.645 | 0.5496 | 1.813 | 0.984 | 3.341 | 0.0563 |
| New Jersey | 0.833 | 0.706 | 0.984 | 0.0312 | 0.709 | 0.576 | 0.874 | 0.0012 | 1.091 | 0.53 | 2.247 | 0.8135 | 0.533 | 0.14 | 2.033 | 0.3569 | 1.449 | 0.881 | 2.383 | 0.1442 |
| Texas | 0.99 | 0.73 | 1.343 | 0.9502 | 0.941 | 0.629 | 1.406 | 0.7649 | 0.968 | 0.257 | 3.65 | 0.9616 | - | - | - | - | 2.657 | 1.147 | 6.155 | 0.0226 |
| Chemotherapy | ||||||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Yes | 0.631 | 0.558 | 0.713 | <.0001 | 0.625 | 0.539 | 0.724 | <.0001 | 0.663 | 0.399 | 1.103 | 0.1138 | 1.113 | 0.322 | 3.852 | 0.8654 | 0.54 | 0.384 | 0.76 | 0.0004 |
| Rural/Urban | ||||||||||||||||||||
| Big metro | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Less Urban | 0.953 | 0.804 | 1.13 | 0.5803 | 0.987 | 0.801 | 1.217 | 0.905 | 0.607 | 0.266 | 1.389 | 0.2374 | 0.499 | 0.107 | 2.334 | 0.3769 | 0.824 | 0.527 | 1.286 | 0.3936 |
| Metro | 1.175 | 1.06 | 1.302 | 0.0021 | 1.174 | 1.035 | 1.331 | 0.0127 | 1.025 | 0.659 | 1.596 | 0.9116 | 0.361 | 0.136 | 0.958 | 0.0408 | 1.116 | 0.84 | 1.483 | 0.4487 |
| Rural | 1.202 | 0.874 | 1.653 | 0.2586 | 1.022 | 0.668 | 1.565 | 0.9197 | 0.343 | 0.042 | 2.774 | 0.3159 | 3.051 | 0.59 | 15.775 | 0.1832 | 1.82 | 0.909 | 3.646 | 0.0909 |
| Urban | 1.066 | 0.883 | 1.287 | 0.508 | 1.066 | 0.845 | 1.346 | 0.5895 | 1.891 | 0.971 | 3.683 | 0.0612 | 0.799 | 0.193 | 3.303 | 0.7565 | 0.828 | 0.485 | 1.415 | 0.49 |
| Education | ||||||||||||||||||||
| Lowest Quartile | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 2nd Quartile | 1.17 | 1.022 | 1.34 | 0.0228 | 1.164 | 0.985 | 1.375 | 0.0754 | 1.749 | 0.921 | 3.32 | 0.0876 | 2.132 | 0.72 | 6.316 | 0.1718 | 1.003 | 0.676 | 1.486 | 0.99 |
| 3rd Quartile | 1.063 | 0.913 | 1.238 | 0.4319 | 1.088 | 0.903 | 1.311 | 0.3777 | 1.384 | 0.68 | 2.82 | 0.3702 | 2.522 | 0.747 | 8.521 | 0.1363 | 1.174 | 0.761 | 1.813 | 0.4679 |
| Highest Quartile | 1.096 | 0.913 | 1.315 | 0.3264 | 0.971 | 0.775 | 1.218 | 0.8004 | 1.124 | 0.495 | 2.551 | 0.7792 | 2.028 | 0.441 | 9.314 | 0.3634 | 1.659 | 1.003 | 2.743 | 0.0486 |
| Poverty | ||||||||||||||||||||
| Lowest Quartile | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 2nd Quartile | 0.9 | 0.788 | 1.027 | 0.1188 | 0.874 | 0.742 | 1.029 | 0.1065 | 0.567 | 0.295 | 1.089 | 0.0882 | 1.059 | 0.396 | 2.829 | 0.9089 | 0.853 | 0.585 | 1.244 | 0.409 |
| 3rd Quartile | 1.048 | 0.897 | 1.223 | 0.5572 | 0.979 | 0.808 | 1.187 | 0.8319 | 1.605 | 0.83 | 3.102 | 0.1595 | 0.322 | 0.079 | 1.309 | 0.1133 | 0.828 | 0.528 | 1.301 | 0.4135 |
| Highest Quartile | 0.998 | 0.824 | 1.208 | 0.9816 | 0.954 | 0.754 | 1.208 | 0.6984 | 1.135 | 0.506 | 2.548 | 0.7589 | 0.728 | 0.168 | 3.158 | 0.672 | 0.927 | 0.546 | 1.575 | 0.7801 |
| Physicians Board Certified | ||||||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Unknown | 1.213 | 0.6 | 2.453 | 0.5914 | 0.858 | 0.356 | 2.068 | 0.7338 | 19.84 | 1.717 | 229.262 | 0.0167 | - | - | - | - | 0.667 | 0.099 | 4.504 | 0.6773 |
| Yes | 1.072 | 0.814 | 1.413 | 0.6209 | 1.003 | 0.722 | 1.392 | 0.986 | 2.788 | 0.641 | 12.122 | 0.1714 | 0.608 | 0.092 | 4.02 | 0.6053 | 0.982 | 0.436 | 2.214 | 0.9655 |
| Graduation Years | ||||||||||||||||||||
| Prior to 1980 | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 1980-1989 | 1.031 | 0.823 | 1.292 | 0.7904 | 1.072 | 0.809 | 1.421 | 0.6299 | 1.011 | 0.424 | 2.409 | 0.9807 | 11.87 | 0.529 | 266.14 | 0.119 | 0.98 | 0.501 | 1.918 | 0.9526 |
| After 1990 | 1.097 | 0.825 | 1.458 | 0.5253 | 1.135 | 0.798 | 1.614 | 0.4825 | 1.5 | 0.45 | 5.002 | 0.5091 | 12.59 | 0.359 | 442.04 | 0.163 | 0.766 | 0.333 | 1.764 | 0.5315 |
| Physician Gender | ||||||||||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Male | 1.007 | 0.886 | 1.145 | 0.9119 | 0.934 | 0.799 | 1.092 | 0.3915 | 1.609 | 0.881 | 2.939 | 0.1219 | 0.987 | 0.346 | 2.815 | 0.9802 | 1.169 | 0.793 | 1.722 | 0.4308 |
| Physician US Trained | ||||||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Unknown | 1.602 | 0.794 | 3.229 | 0.1879 | 1.634 | 0.685 | 3.896 | 0.268 | 0.045 | 0.005 | 0.385 | 0.0047 | - | - | - | - | 11.651 | 0.891 | 152.4 | 0.0612 |
| Yes | 0.981 | 0.854 | 1.127 | 0.7826 | 0.984 | 0.83 | 1.165 | 0.8477 | 0.648 | 0.375 | 1.121 | 0.1209 | 1.383 | 0.372 | 5.149 | 0.6286 | 1.196 | 0.809 | 1.769 | 0.3685 |
| Physician Type | ||||||||||||||||||||
| DO | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| MD | 1.352 | 0.874 | 2.092 | 0.1756 | 1.212 | 0.736 | 1.995 | 0.4505 | 0.402 | 0.088 | 1.831 | 0.2386 | 0.149 | 0.025 | 0.91 | 0.0391 | 5.734 | 0.65 | 50.54 | 0.1158 |
| Physician Training Years | ||||||||||||||||||||
| 3-13 | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| 13-19 | 1.028 | 0.857 | 1.233 | 0.7661 | 0.983 | 0.789 | 1.225 | 0.8788 | 1.293 | 0.529 | 3.163 | 0.5732 | 0.753 | 0.141 | 4.034 | 0.7409 | 1.199 | 0.714 | 2.014 | 0.4918 |
| 19-28 | 1.158 | 0.925 | 1.451 | 0.2001 | 1.185 | 0.902 | 1.556 | 0.2226 | 1.925 | 0.664 | 5.579 | 0.2275 | 0.711 | 0.094 | 5.379 | 0.7416 | 1.091 | 0.586 | 2.03 | 0.7845 |
| 28-61 | 0.959 | 0.699 | 1.315 | 0.7944 | 0.902 | 0.613 | 1.328 | 0.6026 | 1.457 | 0.396 | 5.363 | 0.5716 | 8.283 | 0.183 | 375.59 | 0.2773 | 1.104 | 0.456 | 2.669 | 0.8268 |
| Unknown | 0.915 | 0.627 | 1.336 | 0.6469 | 0.945 | 0.602 | 1.483 | 0.8054 | 1.518 | 0.434 | 5.306 | 0.5132 | - | - | - | - | 1.42 | 0.473 | 4.265 | 0.5318 |
| Number of Fractions | ||||||||||||||||||||
| number of Fractions | 0.971 | 0.965 | 0.976 | <.0001 | 0.964 | 0.958 | 0.97 | <.0001 | 0.983 | 0.96 | 1.006 | 0.1519 | 0.997 | 0.95 | 1.047 | 0.9131 | 0.985 | 0.97 | 1 | 0.0538 |
The 6 SEER Regions are: Connecticut, Detroit, Iowa, New Mexico, Seattle, Utah.
Figure 2.
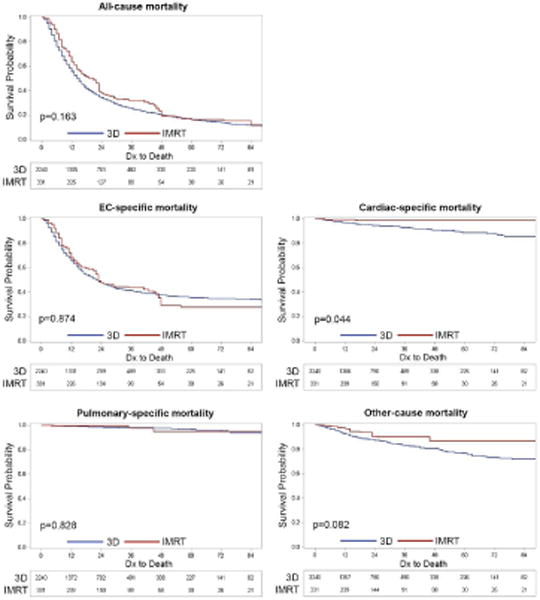
IPTW-adjusted overall survival and cause-specific survival of patients treated with 3D versus IMRT. P value is by log-rank testing.
The most common chemotherapy doublets used were cisplatin/5FU (37.2%), carboplatin/paclitaxel (22.6%), and Docetaxel/5FU (5.9%) (supplemental table 6). For the patients who had chemotherapy, 52% were treated with a 5FU-based regimen. We evaluated if the chemotherapy regimen influenced survival and cause-specific mortality. Under multivariable analysis, the use of any chemotherapy was significantly associated with an improved overall survival and EC-specific and other cause mortality, but not for pulmonary and cardiac mortality (Table 3). We performed a separate multivariable analysis to determine if there was influence of 5FU-based regimen on any of the clinical outcomes. We found 5FU-based regimen to exert similar protective effects on overall and EC-specific survival, including cardiac mortality, compared to either no-chemotherapy use or non-5FU based regimen. Even after adjusting for the type of chemotherapy used, IMRT remained significantly associated with better overall survival, lower cardiac-specific mortality, and other-cause mortality, but was not associated with EC-specific and pulmonary-specific mortality (data not shown).
There is the possibility that with better understanding of radiation planning dose constraints that the cardiac mortality rate may decrease over time for the 3D patients. We evaluated this possibility by examining the cardiac mortality rate in the 3D group between 2002 and 2008, but not in 2009 since the treatment claims data and death record is not mature. The overall cardiac mortality rate for the entire cohort of 3D patients is 5.5%. The average yearly cardiac mortality for the 3D group is 5.7% (± 1.4%), and did not change over the years (Chisq p=0.391). This rate is nearly 5 fold higher in comparison to the rate for IMRT (data not shown).
There were about 5% of patients who had billing claims in which both 3D and IMRT were used. We used a multistep process to segregate these patients into either the 3D or IMRT groups (supplemental table 3). We also performed sensitivity analysis to exclude these patients from the Cox multivariate analysis and found no influence in the multivariate model even after excluding these patients (data not shown). Interestingly, when we evaluated the cardiac mortality risk for 3D, IMRT, and hybrid treatment, the risk for the hybrid treatment was intermediate between 3D and IMRT patients (Fisher’s exact test, p=0.0016).
Discussion
In this population-based analysis of non-metastatic EC patients treated with radiotherapy, we found that the use of IMRT was associated with lower all-cause mortality, cardiac-specific mortality and other-cause mortality, but not cancer-specific and pulmonary mortality. This effect is seen regardless of the experience of the physicians, either based on the number of years in practice or the patient volume, factors known to be critical for surgical outcomes10, 11, or by the type of chemotherapy used.
These results are in line with a previously reported single-institution retrospective analysis of the long-term outcomes of EC patients treated with chemoradiation8. In that report, the authors found overall survival to be significantly better in IMRT-treated patients compared to 3D. However, there was no difference in cancer-specific or pulmonary-related deaths, but only in cardiac-specific deaths and “other deaths”. The “other deaths” in that report were not the same as the “other-cause deaths” in the present study. Previously, the “other deaths” were all unknown deaths due to lost follow up. The “other-cause deaths” for the current study were all other causes reported in the claims data that were not cancer, pulmonary, or cardiac-related. Interestingly, we still saw significant difference in these deaths comparing IMRT and 3D. These studies provide consistent evidence that IMRT may influence the overall health, and importantly, cardiac health of patients who may be cured of EC.
It is widely known that radiation to the thorax can exert long term cardiac morbidities and mortality. Low dose radiation to the chest for the treatment of lymphoma in young people can greatly increase the risk to the development of future myocardial infarction12, 13. In one SEER analysis in 558,871 women treated for breast cancer, left sided breast cancer had a higher cardiac mortality ratio that was evident within 10 years, and the ratio increases over time14. A more detailed population-based case-control study in 2,168 women treated with radiotherapy for breast cancer was conducted in the Netherlands and Sweden15. The study evaluated major coronary events such as myocardial infarction, coronary revascularization, and ischemic heart disease related deaths. The overall average of the mean heart dose was only 4.9 Gray (Gy), yet the probability of developing a major cardiac event increased linearly with the mean heart dose, with an average increase of 7.4% per Gy within the span of 20 years with no threshold. Interestingly, when compared to case-matched controls, the greatest increase in the rate of major coronary events was actually seen in the first 9 years, at 16.3% per Gy from 0 to 4 years and 15.5% per Gy from 5 to 9 years. Despite the low mean heart dose, it’s likely that most of the dose is concentrated at the anterior portion of the heart, origin of many of the coronary vessels. The caveat is that these results are based on outdated, non-image guided treatment approaches. Using modern techniques such as IMRT and breathhold16, it is expected that cardiac morbidity and mortality will be greatly reduced.
Based on some comparative planning studies for EC, IMRT reduces heart dose without a difference in the lung dose compared to 3D, with volumes of heart getting 30 Gy (or V30) to be ~60% for 3D and ~20% for IMRT7, 17 and V45 to be 35% for 3D and 0% for IMRT7. Since V45 significantly predicts for radiation-induced ischemic changes in the heart18, 19, patients treated with 3D likely had heart doses that were substantially above this clinically relevant level compared to IMRT. Tumor location may also be an important factor, since mid to distal esophageal tumors (which accounts for most of the cases in the US) traverse the entire segment of the heart, compared to more proximal tumors. However, the billing coding for tumor location was not precise enough to allow us explore this aspect.
The clinical benefit of IMRT for cancer treatment has been shown for many sites of diseases, such as reducing xerostomia risk for head and neck cancers20, bowel toxicities for cancers within the pelvis such as cervical cancer, prostate cancer, and anal cancer21–23, and esophagitis and pneumonitis risk for lung cancer24. For EC, a previously published single institutional analysis of postoperative morbidity after chemoradiation demonstrated that IMRT significantly improved postoperative pulmonary and GI complications as compared to 3D. The critical factor associated with pulmonary complications is the mean lung dose (MLD), as IMRT was able to significantly reduce the MLD compared to the 3D approach25.
Our study is limited by Medicare claims data, which are largely dependent on the reliability of the billing practices. A number of patients (about 5%) had treatment with both 3D and IMRT within the same time frame and therefore it was difficult to decipher the modality to assign these patients to. We managed to place the majority of these hybrid patients into different treatment bins based on an algorithm we developed. However, using sensitivity analysis, we found that the effect seen for IMRT was the same regardless of these hybrid patients. There is also the limitation of determining precisely if the cause of death was truly cardiac or cancer in origin in a patient with a history of cancer. A patient with treated disease who dies several months later with cardiac arrest could either be scored as being cancer-related or cardiac-related. The definition could be vague and difficult to determine.
In conclusion, our findings from this population-based analysis suggest that the use of IMRT may be associated with reduced all-cause mortality, cardiac-related mortality, and other-cause mortality. Taken together, along with the previously published large single-institutional data, the theoretical dosimetric advantage of IMRT appears to translate to clinically meaningful improvements in the outcomes of patients. In the absence of high quality prospective randomized trial comparing IMRT to 3D, these data provide the evidence that IMRT should be the preferred choice for the treatment of esophageal cancer, and that the current standard-of-care approach using 3D-CRT should be re-evaluated.
Supplementary Material
Acknowledgments
This study used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. The interpretation and reporting of these data are solely the responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services Inc.; and the SEER Program tumor registries in creating the SEER-Medicare linked database. In addition to SEER-Medicare, some of the cancer incidence data used in this study was supported by the Texas Department of State Health Services and CPRIT, as part of the statewide cancer reporting program, and the Centers for Disease Control and Prevention‘s National Program of Cancer Registries Cooperative Agreement #5U58/DP000824-05. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the DSHS, CPRIT, or CDC.
Funding support: This work was supported in part by a grant from the center for Comparative Effectiveness Research on Cancer in Texas, a multi-university consortium funded by the Cancer Prevention and Research Institute of Texas (CPRIT) (RP101207) and the Duncan Family Institute.
S. H. Lin is funded by research contracts with STCube Pharmaceuticals and Roche/Genentech.
Footnotes
Conflict of Interest: No potential conflicts of interest were disclosed by the other authors.
References
- 1.Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008;9(4):367–75. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 2.Lohia S, Rajapurkar M, Nguyen SA, Sharma AK, Gillespie MB, Day TA. A comparison of outcomes using intensity-modulated radiation therapy and 3-dimensional conformal radiation therapy in treatment of oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(4):331–7. doi: 10.1001/jamaoto.2013.6777. [DOI] [PubMed] [Google Scholar]
- 3.Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104(3):343–8. doi: 10.1016/j.radonc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Nutting CM, Bedford JL, Cosgrove VP, Tait DM, Dearnaley DP, Webb S. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiotherapy and Oncology. 2001;61(2):157–63. doi: 10.1016/s0167-8140(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiotherapy and Oncology. 2005;77(3):247–53. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Chen YJ, Liu A, Han C, et al. Helical Tomotherapy for Radiotherapy in Esophageal Cancer: A Preferred Plan With Better Conformal Target Coverage and More Homogeneous Dose Distribution. Medical Dosimetry. 2007;32(3):166–71. doi: 10.1016/j.meddos.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1078–85. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus. 2014 doi: 10.1111/dote.12203. [DOI] [PubMed] [Google Scholar]
- 10.Swisher SG, DeFord L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119(6):1126–34. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 11.Patti MG, Corvera CU, Glasgow RE, Way LW. A Hospital’s Annual Rate of Esophagectomy Influences the Operative Mortality Rate. Journal of Gastrointestinal Surgery. 1998:186–92. doi: 10.1016/s1091-255x(98)80011-5. [DOI] [PubMed] [Google Scholar]
- 12.Boivin JF, Hutchison GB, Lubin JH, Mauch P. Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer. 1992;69(5):1241–47. doi: 10.1002/cncr.2820690528. [DOI] [PubMed] [Google Scholar]
- 13.Cosset JM, Henry-Amar M, Pellae-Cosset B, Carde P, Girinski Tubiana TM, Hayat M. Pericarditis and myocardial infarctions after Hodgkin’s disease therapy. Int J Radiat Oncol Biol Phys. 1991;21(2):447–49. doi: 10.1016/0360-3016(91)90794-5. [DOI] [PubMed] [Google Scholar]
- 14.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108(1):179–82. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 16.Mast ME, van Kempen-Harteveld L, Heijenbrok MW, et al. Left-sided breast cancer radiotherapy with and without breath-hold: does IMRT reduce the cardiac dose even further? Radiother Oncol. 2013;108(2):248–53. doi: 10.1016/j.radonc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83(5):1580–6. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 18.Gayed IW, Liu HH, Yusuf SW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47(11):1756–62. [PubMed] [Google Scholar]
- 19.Gayed I, Gohar S, Liao Z, McAleer M, Bassett R, Yusuf SW. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. International Journal of Cardiovascular Imaging. 2009;25(5):487–95. doi: 10.1007/s10554-009-9440-7. [DOI] [PubMed] [Google Scholar]
- 20.Kohler RE, Sheets NC, Wheeler SB, Nutting C, Hall E, Chera BS. Two-year and lifetime cost-effectiveness of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87(4):683–9. doi: 10.1016/j.ijrobp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Poorvu PD, Sadow CA, Townamchai K, Damato AL, Viswanathan AN. Duodenal and other gastrointestinal toxicity in cervical and endometrial cancer treated with extended-field intensity modulated radiation therapy to paraaortic lymph nodes. Int J Radiat Oncol Biol Phys. 2013;85(5):1262–8. doi: 10.1016/j.ijrobp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Sharma NK, Li T, Chen DY, Pollack A, Horwitz EM, Buyyounouski MK. Intensity-modulated radiotherapy reduces gastrointestinal toxicity in patients treated with androgen deprivation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80(2):437–44. doi: 10.1016/j.ijrobp.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Chuong MD, Freilich JM, Hoffe SE, et al. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Gastrointest Cancer Res. 2013;6(2):39–45. [PMC free article] [PubMed] [Google Scholar]
- 24.Liao ZX, Komaki RR, Thames HD, Jr, et al. Influence of Technologic Advances on Outcomes in Patients With Unresectable, Locally Advanced Non-Small-Cell Lung Cancer Receiving Concomitant Chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775–81. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86(5):885–91. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


