Abstract
In a fusion of two ubiquitous organometallic reagents, triphenylphosphine (PPh3) and tetraphenylborate (BPh4−), the 9-phosphatriptycene-10-phenylborate (PTB) anion has been prepared for the first time. This borato species has been fully characterized by a suite of spectroscopic methods, and initial reactivity studies introduce it as a competent ligand for transition metals, including Co(II) and Fe(II).
Graphical abstract

Owing to low-cost, high availability, and ease of synthesis and handling, triphenylphosphine (PPh3) and tetraphenylborate (BPh4−) have evolved to become prevailing reagents in the synthetic chemist’s toolbox. Congruent with interest in developing zwitterionic organometallic complexes for catalysis,1 including examples from our group featuring P,B- and N,B-containing ligands,2 we envisioned a tethering strategy between these two partners in the form of a triptycene. Some years ago, we explored the use of conceptually related 3- and 4-substituted acyclic diphenylphosphino(tetraphenyl)borates as electron-releasing ligands for coordination to platinum-group metals, along with some preliminary C-C cross coupling investigations (Chart 1A).2a,b It is noteworthy that a host of other phosphine-containing ligands incorporating group 13 elements have also been reported.3
Chart 1.
Representative borate ligands
Since the early report of parent 9-phosphatriptycene in 1974 by Bickelhaupt et al. (Chart 1B),4 several research groups have reported related geometrically constrained compounds containing group 14 or 15 elements, though the synthesis of anionic group 13 analogues has not been achieved.5 In this work, we present the formal joining of PPh3 and BPh4− in the form of the 9-phosphatriptycene-10-phenylborate anion. To fully characterize this phosphino(borate), several derivatives, including cis-dimethylplatinum(II) and pentakis(carbonyl)tungsten(0) PTB complexes, have been prepared. We also show that the PTB anion can be employed for the synthesis of paramagnetic, Co(II) and Fe(II) complexes, serving roles as both σ-donating ligand and halide abstracting equivalent.
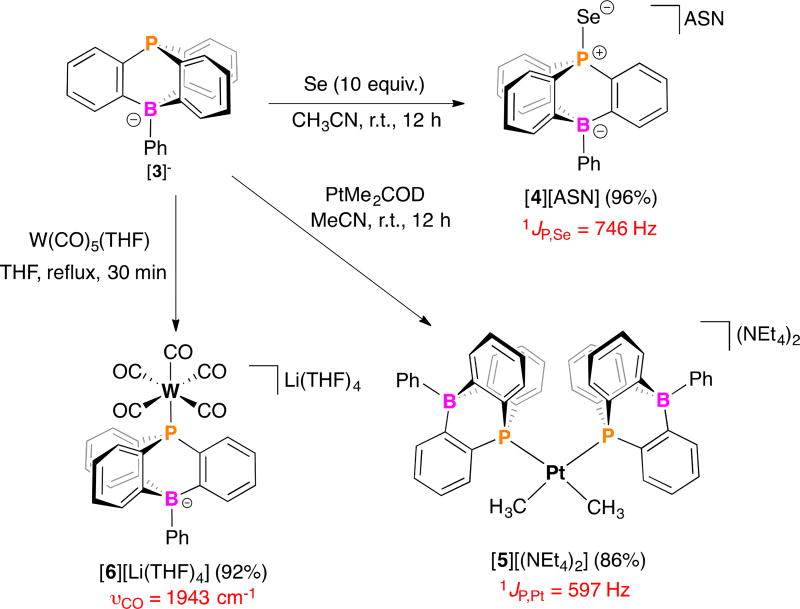
The route used to prepare the 9-phosphatriptycene-10-phenylborate (PTB) anion [3]− is shown in Scheme 1. Treatment of tris(o-bromophenyl)phosphine5a with tBuLi followed by slow addition of PhBCl2 at −78 °C and careful warming to room temperature furnishes tetrakis(tetrahydrofuran) lithium 9-phosphatriptycene-10-phenylborate, [3][Li(THF)4] as a white solid in 64% yield after work-up. The countercation of [3]− is straightforwardly tailored by treatment of a CH3OH solution of [3][Li(THF)4] with [Y]Br (Y = ASN; ASN = 5-azoniaspiro[4.4]nonane or NEt4) giving [3][ASN] or [3][NEt4] as white solids in 97 and 87% yield, respectively.
Scheme 1.
Preparation of 9-phosphatriptycene-10-phenylborate and Mercury depiction of the solid-state molecular structure of [3][ASN] (displacement ellipsoids are shown at the 50% probability, hydrogens and ASN+ counterion omitted for clarity). Selected bond lengths [Å] and angles (°). P(1)-C(1) 1.827(2), P(1)-C(3) 1.839(2), P(1)-C(5) 1.837(2), B(1)-C(2) 1.649(2), B(1)-C(4) 1.655(2), B(1)-C(6) 1.647(2), B(1)-P(1) 3.020(2), <C-P(1)-C 96.5 (avg.), <C-B(1)-C 104.4 (avg.), <(C-P-C) 289.4.
The 31P{1H} NMR spectrum of [3][Li(THF)4] (CD3CN, 298 K) displays a singlet at δ = − 43.7 ppm (d, 3JP-B = 3.8 Hz), while 11B{1H} NMR spectroscopy provides a sharp resonance at δ = − 8.75 ppm (d, 3JP-B = 3.8 Hz); c.f. δB = − 6.7 ppm for Li[BPh4].6 Notably, this is a rare case where three-bond scalar coupling between phosphorus and boron (I = 3/2) is experimentally observed. Analogous NMR spectroscopic parameters are observed for [3][ASN] and [3][NEt4] (see ESI) -all of which are significantly upfield-shifted compared to PPh3 (δP = −3.1 ppm), but in the same range as 1 (δP = −64.8 ppm) and 2 (δP = −44.4 ppm). Additionally, the 7Li{1H} NMR spectrum of [3][Li(THF)4]provides a signal at δLi = − 1.2 ppm.
Crystalline material of [3][Li(THF)4] was obtained from a saturated THF solution at −35 °C, while crystals of [3][ASN] or [3][NEt4] could be grown from a saturated CH3OH solution at −35 °C. The crystal structure of [3][ASN] is shown in Scheme 1 and features a constrained phosphorus atom having an average <C-P-C bond angle of 96.5° c.f. 106.3° for PPh3 (Table 1) and an average <C-B-C bond angle of 104.4°. The <C-P-C bond angle for [3][ASN] is the smallest reported among the known 9-phosphatriptycenes (<C-P-C = 98.0° for 1 and 99.3° for 2; Table 1).
Table 1.
Characterizing the 9-phosphatriptycene-10-phenylborate (PTB) anion.
| Eox/V | Lone pair orbitalc |
1JP,Se (Hz) Ar3P=Se |
1JPt,C (Hz) cis-PtMe2L2 |
ν(CO) (cm−1) W(CO)5L |
<C-P-C (°) by XRDh |
|
|---|---|---|---|---|---|---|
| PPh3 | 1.05a | HOMO | 736d | 616d | 1943f | 106.3 |
| 1 | 1.68a | HMMO-5 | 828d | -- | 1946f | 99.3 |
| 2 | -- | -- | 795d | 607d | -- | 98.0 |
| [3]− | 0.97b | HMMO-4 | 746e | 597e | 1943g | 96.5 |
Irreversible peak potential vs. Ag/Ag+ in CH2Cl2 with 0.1 M [NnBu4][ClO4].
Irreversible peak potential vs. Fc/Fc+ in CH2Cl2 with 0.1 M [NnBu4][ClO4] for NEt4+ counterion.
Single-point calculations and geometry optimization were performed using DFT: B3LYP/6-311G(d) for P, and 6-31G(d) for all other atoms.
in CDCl3.
in MeCN-d3 for ASN+ counterion.
in hexane.
solid-state for Li(THF)4+ counterion.
Average of three bonds.
Having prepared PTBs [3]−, we next assessed phosphine s-character and σ-donating ability (Table 1 & Scheme 2). In agreement with incorporation of an insulated borate, the cyclic voltammogram of [3][NEt4] shows an irreversible oxidative feature at 0.97 V that is cathodically-shifted compared to related phosphatriptycene 1 and PPh3 (Figure 1A). Compound [3]− was further evaluated based on the following characteristics: a) 1JP,Se = 746 Hz for the phosphine selenide, [4][ASN] (X-ray, Figure S30);7 b) 1JPt,C = 597 Hz for the Pt(II) complex, cis-[Pt(CH3)2(PTB)2][NEt4]2 ([5][NEt4]2; Figure 1B), and c) ν(CO) = 1943 cm−1 for the W(0) complex, [W(CO)5(PTB)][Li(THF)4] ([6][Li(THF)4]). Despite having the smallest <C-P-C bond angle of the series, these data point toward [3]− as having lower s-character and similar σ-donating ability when compared with 1 or 2. Of note, oxidation of PTB to the phosphine selenide, [4][ASN], also results in increased pyramidalization at phosphorus (<(C-P-C) = 301.8° (c.f. 289.4°)) and a decreased P(1)-B(1) distance of 2.911(5) Å (c.f. 3.020(2) Å) corresponding to a five-fold increase in 3JP-B to 19.3 Hz.
Scheme 2.
Assessing the s-character and σ-donating properties of [3]−.
Figure 1.
A) The CV for [3]− with the oxidative feature highlighted (using an internal Fc/Fc+ reference). B) Mercury depiction of the solid-state molecular structure for [5][NEt4]2 (50% ellipsoid probability, hydrogens and counterions omitted for clarity).
Protonation of [3][NEt4] was also shown to be facile. Treatment of an MeCN solution of [3][NEt4] with [H-OEt2][BArF4] results in formation of the neutral zwitterion, PTB-H (3-H) along with [NEt4][BArF4]. Protonation is evidenced by 31P NMR spectroscopy that shows a broad doublet at δ = −25.3 ppm (1JP,H = 512 Hz), which collapses to a singlet on 1H decoupling. Notably this value of 1JP,H is close to that observed for [H-PPh3]+ (1JP,H = 510 Hz) and lower than that observed for the [H-PH3]+ cation (1JP,H = 548 Hz), which is near ideally sp3−hybridized.8
Next, we studied the coordination chemistry and spectroscopic features of [3]− with Co and Fe. Combination of two equiv. of [3][NEt4] with CoBr2 in CH3CN at ambient temperature produced a dark turquoise solution (Scheme 3). Metalation of [3][NEt4] was confirmed by 1H NMR spectroscopy, which displayed paramagnetically shifted resonances, while no discernable signal was obtained by 31P NMR spectroscopy (CD3CN, 298 K). In an effort to provide material suitable for single crystal X-ray diffraction, cooling a CH3CN solution of this reaction mixture to −35 °C resulted in a color change from turquoise to forest green, while cooling a saturated THF solution caused no change in color, indicative of a solvent-dependent coordination equilibrium. This process was later studied by variable temperature UV-Visible spectroscopy (vide infra).
Scheme 3.
Generation of Co(II) and Fe(II) PTB complexes
Ultimately, the identity of the constituent Co(II) species was obtained by layering the aforementioned CH3CN solution with THF at −35 °C, which gave three different crystal morphologies (ESI, Figure S26): i) thin blue sheets, ii) turquoise blocks, and iii) orange blocks (Figure 2). Analysis by single crystal X-ray diffraction confirmed the identity of each of these species to be: the mono(PTB) complex [Co(PTB)Br3][NEt4]2 ([7][NEt4]2) and bis(PTB) complexes [Co(PTB)2Br2][NEt4]2 ([8][NEt4]2) and [Co(PTB)2(NCCH3)2Br][NEt4] ([9][NEt4]). The Co(1)-P(1) bond length for [7][NEt4]2 [2.358(4) Å] and [8][NEt4]2 [2.386(4)/2.383(5) Å] agree with those reported for Co(PPh3)2Br2 [2.349(2) Å],9 while for the Co(II) zwitterion, [9][NEt4], a shorter distance of 2.248(4) Å is noted. For the pseudo-tetrahedral complex [8][NEt4], the angles, <Br(1)-Co(1)-Br(2) (117.54(9)°) and <P(1)-Co(1)-P(2) (121.1(2)°) are also in line with those reported for Co(PPh3)2Br2 (117.27(3)° and 115.88(2)°). Finally, for the trigonal bipyramidal complex [9][NEt4], the P(1)-Co(1)-P(2) bond angle (177.58°) is close to 180°, while the sum of angles in the equatorial plane is equal to 360°.
Figure 2.
Mercury depiction of the solid-state molecular structure of the anions: [7]2−, [8]2−, and [9]1− (displacement ellipsoids are shown at the 50% probability, hydrogens and counterions omitted for clarity).
Probing the sample by optical spectroscopy allowed for the study of complex speciation in CH3CN as a function of temperature. At 25 °C, the UV-Vis spectrum showed the presence of several bands between 600 and 700 nm [617, 642, 668, 681, and 696 nm] (Figure 3A). Related absorptions have been documented for the PPh3 analogues, [Co(PPh3)Br3][NEt4] and Co(PPh3)2Br2.10 Cooling the sample to −40 °C, however, gradually revealed the formation of a new species, exhibiting clear isosbestic behavior with growth of bands at 310 and 376 nm. We posit that these data result from a low-temperature metathesis equilibrium of [8][NEt4]2 (turquoise) to give the bis(acetonitrile) adduct [9][NEt4] (orange) and [NEt4]Br; these species account for the forest green color observed at low temperature. This result highlights the ability of [3]− to undergo metathesis with an M-X (X = halide) bond, providing an entryway into coordinatively unsaturated metal complexes that can react with L-type donor ligands (e.g., NCMe).
Figure 3.
A) Variable temperature UV-Visible absorbance data collected on a mixture of [7]2−, [8]2−, and [9]1− in CH3CN. Inset shows expansion of 550 to 750 nm window and the frozen cuvette at −40 °C. Temperature increments are in 5 °C. B) Zero-field 57Fe Mössbauer spectra of a mixture of [10]2− (blue, 43%), [11]2− (green, 47%), and an unknown species (yellow, 10%) as a frozen CH3CN solution collected at 80 K.
A related set of Fe(II) PTB complexes were also obtained with coordination being substantiated by NMR spectroscopy (Scheme 3). Layering of a CH3CN solution with THF and cooling to −35 °C gave colorless blocks – one of which was analyzed by X-ray diffraction as [Fe(PTB)Br3][NEt4]2, [10][NEt4]2 (X-ray, Figure S32). The Fe(1)-P(1) bond length for [10][NEt4]2 [2.4320(6) Å] is similar to that noted for [Fe(PPh3)Br3][HIPr] (HIPr = 1,3-bis(2,6-diisopropylphenyl)imidazolium) [2.463(2) Å] and is otherwise comparable to the Co derivative [7][NEt4].11
The 57Fe Mössbauer spectrum of a frozen CH3CN solution revealed the presence of three Fe-containing species with parameters suggesting the presence of two four- and one higher-coordinate Fe(II) centers (Figure 3B).12 For the two major species, isomer shifts of δ = 0.87 and 0.79 mm/s, with quadrupole splittings of ΔEQ = 2.34 and 2.87 mm/s, respectively were observed. These doublets are assigned to the four-coordinate species [Fe(PTB)Br3][NEt4]2 (43%, [10][NEt4]2) and [Fe(PTB)2Br2][NEt4]2 (47%, [11][NEt4]2). For comparison, values of δ = 0.86 and 0.83 mm/s and ΔEQ = 2.10 and 2.71 mm/s were reported for [Fe(quinoline)Br3][NEt4] and Fe(quinoline)2Br2.13 A third species, accounting for the remaining 10% of the overall fit, displayed an unusually large isomer shift, δ = 1.72 mm/s with ΔEQ = 2.52 mm/s. We presume this species to be a higher-coordinate Fe(PTB)xBry(NCCH3)z complex, such as [Fe(PTB)2(NCCH3)2Br][NEt4], akin to [9][NEt4]. Given the low known isomer shift for [Fe(PEt3)2(NCCH3)4]2+ (δ = 0.37 mm/s), it is unlikely that this doublet results from the neutral zwitterion, Fe(PTB)2(NCCH3)4.14
Inspired by the success of PPh3 and BPh4− as reagents of broad utility, we have provided here the first synthesis of the 9-phosphatriptycene-10-phenylborate anion. The s-character and σ-donating capability of this ligand have been assessed, and preliminary forays into coordination chemistry with Co and Fe demonstrate that this anion can be used to advantage as both a ligand and halide-abstracting agent. Future work will focus on the small-molecule reactivity of these and other PTB-ligated transition metal complexes.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM070757), NSERC (Banting PDF award to MWD), and the Resnick Sustainability Institute at Caltech (Postdoctoral award to MWD). KN thanks the Japan Society for the Promotion of Science (JSPS) for an overseas postdoctoral fellowship. We thank Larry Henling and Dr. Mike Takase for assistance with X-ray crystallography.
Footnotes
Experimental details including 1H, 31P, 11B, and 13C NMR spectra for all complexes as well as crystallographic data for [3][NEt4], [3][ASN], [4][ASN], [5][NEt4]2, [7][NEt4]2, [8][NEt4][Na(THF)4(NCMe)2], [9][NEt4], and [10][NEt4]2. CCDC 1824471–1824478 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
- 1.a) Stradiotto M, Hesp KD, Lundgren RJ. Angew. Chem. Int. Ed. 2010;49:494–512. doi: 10.1002/anie.200904093. [DOI] [PubMed] [Google Scholar]; b) Chauvin R. Eur. J. Inorg. Chem. 2000:577–591. [Google Scholar]; c) Kolychev EL, Kronig S, Brandhorst K, Freytag M, Jones PG, Tamm M. J. Am. Chem. Soc. 2013;135:12448–12459. doi: 10.1021/ja406529c. [DOI] [PubMed] [Google Scholar]; d) Schrock RR, Osborn JA. Inorg. Chem. 1970;9:2339–2343. [Google Scholar]; e) Westcott SA, Blom HP, Marder TB, Baker RT. J. Am. Chem. Soc. 1992;114:8863–8869. [Google Scholar]; f) Mazet C, Köhler V, Pfaltz A. Angew. Chem. 2005;117:4966–4969. doi: 10.1002/anie.200501111. [DOI] [PubMed] [Google Scholar]; g) Komon ZJA, Bu X, Bazan GC. J. Am. Chem. Soc. 2000;122:1830–1831. [Google Scholar]
- 2.a) Thomas CM, Peters JC. Inorg. Chem. 2004;43:8–10. doi: 10.1021/ic0350234. [DOI] [PubMed] [Google Scholar]; b) Thomas CM, Peters JC. Organometallics. 2005;24:5858–5867. [Google Scholar]; c) Luand CC, Peters JC. J. Am. Chem. Soc. 2002;124:5272–5273. doi: 10.1021/ja017011s. [DOI] [PubMed] [Google Scholar]; d) Betley TA, Peters JC. Angew. Chem. Int. Ed. 2003;42:2385–2389. doi: 10.1002/anie.200250378. [DOI] [PubMed] [Google Scholar]; e) Thomas JC, Peters JC. J. Am. Chem. Soc. 2003;125:8870–8888. doi: 10.1021/ja0296071. [DOI] [PubMed] [Google Scholar]; f) Thomas JC, Peters JC. J. Am. Chem. Soc. 2001;123:5100–5101. doi: 10.1021/ja0058987. [DOI] [PubMed] [Google Scholar]
- 3.a) Boone MP, Stephan DW. J. Am. Chem. Soc. 2013;135:8508–8511. doi: 10.1021/ja403912n. [DOI] [PubMed] [Google Scholar]; b) Courtemanche M–A, Légaré M–A, Maron L, Fontaine F-G. J. Am. Chem. Soc. 2013;135:9326–9329. doi: 10.1021/ja404585p. [DOI] [PubMed] [Google Scholar]; c) Fischbach A, Bazinet PR, Waterman R, Tilley TD. Organometallics. 2008;27:1135–1139. [Google Scholar]; d) Bontemps S, Bouhadir G, Miqueu K, Bourissou D. J Am. Chem. Soc. 2006;128:12056–12057. doi: 10.1021/ja0637494. [DOI] [PubMed] [Google Scholar]; e) Staubitz A, Robertson APM, Sloan ME, Manners I. Chem. Rev. 2010;110:4023–4078. doi: 10.1021/cr100105a. [DOI] [PubMed] [Google Scholar]; f) Gutsulyak DV, Gott AL, Piers WE, Parvez M. Organometallics. 2013;32:3363–3370. [Google Scholar]; g) Xu X, Kehr G, Daniliuc CG, Erker G. Organometallics. 2013;32:7306–7311. [Google Scholar]
- 4.Jongsma C, de Kleijn JP, Bickelhaupt F. Tetrahedron. 1974;30:3465–3469. [Google Scholar]
- 5.a) Tsuji H, Inoue T, Kaneta Y, Sase S, Kawachi A, Tamao K. Organometallics. 2006;25:6142–6148. [Google Scholar]; b) Tomaschautzky J, Neumann B, Stammler H-G, Mix A, Mitzel NW. Dalton Trans. 2017;46:1645–1659. doi: 10.1039/c6dt04293g. [DOI] [PubMed] [Google Scholar]; c) Agou T, Kobayashi J, Kawashima T. Chem. Lett. 2004;33:1028–1029. [Google Scholar]; d) Iwai T, Konishi S, Miyazaki T, Kawamorita S, Yokokawa N, Ohmiya H, Sawamura M. ACS Catal. 2015;5:7254–7264. [Google Scholar]
- 6.Hermanek S. Chem. Rev. 1992;92:325–362. [Google Scholar]
- 7.Allen DW, Taylor BF. J. Chem. Soc., Dalton Trans. 1982:51–54. [Google Scholar]
- 8.Olah GA, McFarland CW. J. Org. Chem. 1969;34:1832–1834. [Google Scholar]
- 9.Carlin RL, Chirico RD, Sinn E, Mennenga G, De Jongh LJ. Inorg. Chem. 1982;21:2218–2222. [Google Scholar]
- 10.Sestili L, Furlani C, Festuccia G. Inorg. Chim. Acta. 1970;4:542–548. [Google Scholar]
- 11.Li Z, Liu L, Sun H-M, Shen Q, Zhang Y. Dalton Trans. 2016;45:17739–17747. doi: 10.1039/c6dt02995g. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood NN, Gibb TC. Mössbauer Spectroscopy. Springer Netherlands: Dordrecht; 1971. [Google Scholar]
- 13.Burbridge CD, Goodgame DM. J. Chem. Soc. A. 1968:1074–1079. [Google Scholar]
- 14.Han J, Koutmos M, Al Ahmad S, Coucouvanis D. Inorg. Chem. 2001;40:5985–5999. doi: 10.1021/ic0104914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.