SUMMARY
Cholangiocarcinomas are often locally advanced or have metastasized, and at the time of diagnosis individuals often have a poor prognosis. Endoscopic treatment options traditionally include biliary decompression via stenting to allow for systemic chemotherapy and radiotherapy, with self-expanding metal biliary stents being preferred. Recent developments in locoregional therapy delivered endoscopically, such as photodynamic therapy and radiofrequency abalation, have shown promising results in improving patient survival.
Practice Points.
Most cholangiocarcinomas are locally advanced or have metastasized at the time of diagnosis.
Endoscopic treatment options have previously been limited to biliary decompression via stenting. Self-expanding metal stents are preferred for distal malignant biliary obstruction.
Recent developments in locoregional therapy, such as photodynamic therapy and, more recently, radiofrequency ablation, have shown promising results, particularly in improving patient outcomes for those with locally advanced or unresectable disease.
Further studies are needed to confirm the benefits of photodynamic therapy and radiofrequency ablation on long-term biliary stent patency and survival rates.
Cholangiocarcinoma is an epithelial carcinoma arising from the intra- and extra-hepatic biliary tree. Since the 1970s, the incidence of cholangiocarcinoma has increased steadily in western societies and now accounts for 3% of all gastrointestinal malignancies [1,2]. In the USA, the incidence rate has been reported as one to two cases per 100,000 patients, with most patients being older than 65 years of age [3]. Cholangiocarcinomas are classified into three different subgroups based on their anatomic location: intrahepatic, perihilar and distal cholangiocarcinoma [4].
The only curative treatment for cholangiocarcinoma is surgical resection with negative margins. Unfortunately, more than 80% of patients present with unresectable disease at the time of diagnosis [5]. The prognosis at this stage is poor, as the median survival for nonsurgical candidates is 4–7 months [6,7]. Successful palliation of biliary obstruction is the main goal, but alleviating jaundice through biliary stenting alone has not been proven to prolong survival. Systemic chemotherapy and radiotherapy have also been relatively ineffective for prolonging survival or relieving jaundice for patients with inoperable tumors. This has led to a push for novel locoregional therapies directed at improving patient survival and palliation of symptoms.
Biliary stenting
Biliary obstruction develops in 90% of patients with unresectable disease, ultimately leading to jaundice, pruritus, abdominal pain and cholangitis [8]. Biliary decompression palliates these symptoms but has not been shown to affect survival [9]. Surgical bypass used to be the primary method of palliation of jaundice in patients with unresectable cholangiocarcinoma but in the last 20 years has been largely replaced by less invasive nonsurgical procedures such as percutaneous or endoscopic biliary stent placement. Endoscopic stenting costs significantly less and was associated with longer survival than surgical treatment (19 vs 16.5 months), suggesting that endoscopic stenting is the procedure of choice for palliative biliary drainage [10]. In patients who are operative candidates, however, preoperative biliary drainage is controversial. A meta-analysis did not show any significant differences in mortality, morbidity or complications in patients who received preoperative biliary drainage versus the patients who received surgery directly [11], and currently, the majority of data on preoperative drainage pertain to obstructive jaundice in malignancies other than cholangiocarcinoma. Currently, preoperative drainage is not recommended on a routine basis except in cases of acute suppurative cholangitis [12].
▪ Plastic versus metal stents
Both plastic and metal stents can be used for the palliation of malignant bile duct obstruction. Plastic stents have been used since the 1980s and are made from materials including Teflon®, polyurethane and polyethylene, ranging in multiple diameters and sizes. They are effective and inexpensive compared with their metallic counterparts. However, plastic stents have a shorter duration of stent patency (about 3 months), often requiring repeat endoscopic retrograde cholangiopancreatogarphies (ERCP) for stent occlusion that can develop in the setting of sludge, tumor overgrowth or biofilm formation [13,14].
Self-expanding metal stents (SEMS) have been made from three materials: stainless steel, nitinol (a nickel and titanium alloy) and platinol (platinum and nitinol alloy). SEMS are more expensive than plastic stents and may be difficult to remove because of tumor and tissue ingrowth. SEMS, however, are larger in diameter, thus leading to an extended duration of stent patency (approximately 6–12 months) [10]. Metal stents are also associated with fewer ERCPs, a shorter hospital stay and fewer complications than plastic stents in patients who survive more than 6 months [10–15]. Moss et al. evaluated the outcomes of surgical bypass, endoscopically placed plastic stents and endoscopically placed metal stents in 24 studies including almost 2500 patients in a large meta-analysis that showed similar rates of biliary decompression but improved patency rates for SEMS [13]. Numerous studies have compared plastic stents with SEMS for distal malignant biliary strictures and confirm longer patency, decreased hospitalizations, decreased number of endoscopic procedures and reduced overall cost in the SEMS group (Table 1). Thus, SEMS are thought to be the treatment of choice in patients with unresectable distal malignant biliary obstruction.
Table 1. Plastic versus metal stents (self-expanding metal stents) for distal malignant stricture.
| Study (year) | Plastic stents | SEMS | p-value | Ref. |
|---|---|---|---|---|
| Choi et al. (2012) | Stent occlusion: median 94.6 days | Stent occlusion: median 233.6 days | 0.006 | [16] |
| Yoon et al. (2009) | Stent occlusion: mean 133 days | Stent occlusion: mean 278 days | 0.0004 | [14] |
| Soderlund et al. (2006) | Stent occlusion: median 1.1 month | Stent occlusion: median 3.5 months | 0.007 | [17] |
| Kaassis et al. (2003) | Stent occlusion: median 5 months | Median not reached | 0.007 | [18] |
| Prat et al. (1998) | Stent occlusion: median 3 months | Stent occlusion: median 4.8 months | NS | [19] |
| Knyrim et al. (1993) | 43% stent failure rate | 22% stent failure rate | 0.0035 | [20] |
NS: Not significant; SEMS: Self-expanding metal stents.
▪ Uncovered SEMS versus covered SEMS
SEMS can be uncovered, partially covered, or fully covered. The covering is a silicone, polycaprolactone, polyether polyurethane, polyurethane or expanded polytetrafluoroethylene fluorinated ethylene propylene lining. Disadvantages of uncovered metal stents include being difficult to remove endoscopically and tumor ingrowth through the stent mesh, thus causing further problems with biliary obstruction and sepsis (Figure 1). The benefits of covered SEMS are removability and theoretically longer patency rate due to reduced tumor ingrowth. Covered SEMS also have also been reported to have a higher migration rate [21]. Multiple studies have compared the patency of covered versus uncovered SEMS in distal pancreaticobiliary malignancy (Table 2). In a meta-analysis including five trials, Saleem et al. showed that covered SEMS have prolonged stent patency (weighted difference plus 60.56 days) and prolonged survival (weighted mean difference plus 68.76 days) compared with uncovered SEMS [27]; however, in a more recent meta-analysis, Almadi et al. calculated the mean difference in the stent patency duration on two studies to be 67.9 days longer for covered SEMS than for uncovered SEMS (95% CI: 60.3–75.5) with a summary analysis showing no difference in patency of covered versus uncovered SEMS after 6 and 12 months [28]. The choice of covered versus uncovered SEMS in patients with distal malignant biliary obstruction should be made on an individual basis.
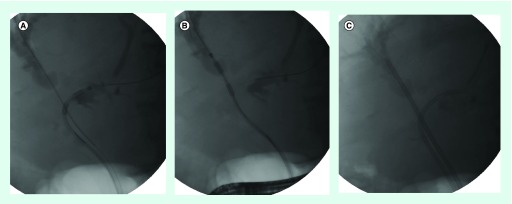
Figure 1. Metal biliary stenting in cholangiocarcinoma.
(A) Tumor ingrowth in a previously placed uncovered self-expandable metallic stent in a 67-year-old man with advanced cholangiocarcinoma. (B & C) Insertion of a partially covered self-expandable metallic stent inside the previously placed stent to relieve the obstruction.
Table 2. Covered versus uncovered self-expanding metal stents for distal malignant stricture.
| Study (year) | Covered SEMS patency | Uncovered SEMS patency | p-value | Ref. |
|---|---|---|---|---|
| Isayama et al. (2004) | Mean 304 days | Mean 106 days | 0.0032 | [22] |
| Yoon et al. (2006) | Mean 398 days | Mean 319 days | 0.578 | [23] |
| Park et al. (2006) | Mean 148 days | Mean 143 days | 0.531 | [24] |
| Telford et al. (2010) | Median 357 days | Median 711 days | 0.530 | [21] |
| Kullman et al. (2010) | 154 days (when a quarter of stents failed) | 199 days (when a quarter of stents failed) | 0.348 | [25] |
| Lee et al. (2013) | Median 15.4 months | Median 26.3 months | 0.61 | [26] |
SEMS: Self-expanding metal stents.
Similarly, for nonresectable hilar tumors, SEMS are preferred over plastic stents, due to increased patency rates and a decrease in the number of endoscopic sessions (Table 3). Uncovered SEMS are recommended for hilar and intrahepatic regions as to avoid blocking the drainage of other intrahepatic segments with covered SEMS, as it would lead to segmental cholangitis [33].
Table 3. Plastic versus self-expanding metal stents for hilar lesions.
| Study (year) | Plastic | SEMS | p-value | Ref. |
|---|---|---|---|---|
| Knyrim et al. (1993) | Median patency: 5 months Cholangitis incidence 36% |
Median patency: 5 months Cholangitis incidence 15% |
NS <0.05 |
[20] |
| Perdue et al. (2008) | Complication rate at day 30: 32.1% Adverse outcome: 39% |
Complication rate at day 30: 8.8% Adverse outcome: 12% |
0.027 0.017 |
[29] |
| Raju et al. (2011) | Median patency: 1.86 months Complication rate: 7.7% |
Median patency: 5.56 months Complication rate: 8.3% |
<0.0001 0.79 |
[30] |
| Sangchan et al. (2012) | Successful drainage: 46.3% Complication rate: 33.3% |
Successful drainage: 70.4% Complication rate: 46.3% |
0.011 0.246 |
[31] |
| Liberato et al. (2012) | Median patency: 20 weeks Complication rate: 56.4% |
Median patency: 27 weeks Complication rate: 24.4% |
<0.0001 <0.001 |
[32] |
NS: Not significant; SEMS: Self-expanding metal stents.
Unilateral versus bilateral drainage: hilar cholangiocarcinoma
There is currently no consensus on whether bilateral or unilateral (single vs multiple) drainage is more effective in the setting of hilar lesions, as various studies have demonstrated conflicting data. Approximately 55–60% of the liver volume is drained via the right hepatic duct, 30–35% by the left hepatic duct, and 10% from the caudate lobe. Only 25% of the liver volume requires drainage to see improvement in jaundice [34]. Earlier studies found that bilateral stent placement was associated with improved outcomes compared with unilateral stenting, but contrast material was injected into both lobes, leading to bacterial contamination of undrained areas and an increased risk of cholangitis [35]. In the only randomized trial to address this question, De Palma et al. reported that unilateral SEMS insertion was effective in biliary palliation in unresectable hilar cholangiocarcinoma, with higher rates of successful endoscopic stent insertion than bilateral stents (88.6 vs 76.9%; p = 0.041) [36] and lower rates of complication (18.9 vs 26.9%; p = 0.026) [37]. Unilateral drainage has also been associated with a lower incidence of liver abscess as well as a comparable outcome with regard to stent patency and complication-free survival compared with bilateral drainage [38]; however, more recent studies have found bilateral stenting may still be needed to allow for adequate drainage of liver volume (Figure 2). Chang et al. demonstrated that the survival rate was highest in patients who underwent bilateral drainage, with the lowest survival rate in patients who had cholangiographic filing of both lobes but drainage of only one [35]. Cumulative stent patency was also higher in patients who had bilateral stents placed [39]. A recent retrospective study of 107 patients with hilar tumors found that the most important predictor of effective drainage (defined as a decrease in serum bilirubin by 50% after 1 month) was stents draining a volume greater than 50%, which often did require bilateral stents. A drainage of >50% was also associated with a longer median survival (119 vs 59 days; p = 0.005), whereas intubating an atropic sector (<30%) was not helpful and increased the risk of cholangitis (odds ratio: 3.04; p = 0.01) [40]. Therefore, in malignant hilar obstruction, cross-sectional imaging is important to identify the dominant biliary system before endoscopic decompression of malignant hilar obstruction, and biliary drainage should be maximized, either through unilateral or bilateral stenting.
Figure 2. Cholangiogram demonstrating placement of bilateral self-expanding metal stents allowing for maximum drainage in a patient with advanced hilar cholangiocarcinoma.
Reproduced with permission from BSCI, Inc. (MA, USA).
▪ Complications
Complications are inherent in the performance of endoscopy, arising from the procedure itself (e.g., bleeding, pancreatitis, perforation or cardiopulmonary events) or are stent related (e.g., occlusion, migration, infection, cholangitis or cholecystitis). Selective endoscopic stenting into the appropriate bile duct is still a challenge, and is one of the technical limitations to performing effective biliary drainage in hilar cholangiocarcinoma.
Stent migration is usually seen in covered metal stents, with reported rates ranging from 6 to 8% [14,41,42]. Uncovered metal stents have a much lower migration rate, approximately 1% [27], likely secondary of tumor and tissue growth within the stent interstices. Stents can become occluded from debris, tumor ingrowth or overgrowth, and plastic stents are particularly vulnerable. As mentioned earlier, patency of plastic stents is reported to be around 3 months, and they require frequent replacement, whereas SEMS have patency rates of nearer to 9 months. Uncovered SEMS are prone to tissue or tumor ingrowth (Figure 1), and may be difficult to remove, thus necessitating placement of a second stent within the lumen of the occluded stent [43]. Covered SEMS have also been associated with cholecystitis by causing cystic duct occlusion, although two retrospective studies have shown no significant differences in the rate of cholecystitis between uncovered SEMS and covered SEMS [44,45]. However, tumor involvement at the orifice of the cystic duct is a significant risk factor for cholecystitis [46,47]. Rates of pancreatitis with SEMS have been reported to be approximately 6% [48], and cholangiocarcinoma, contrast injection into the pancreatic duct [44] and hilar cholangiocarcinoma [49] have been independent predictive factors for pancreatitis after SEMS placement.
Locoregional therapies
▪ Photodynamic therapy
Photodynamic therapy (PDT) is an investigational procedure using a photosensitizing agent (such as Photosan-3® and Photofrin®) activated by a laser (at a specific wavelength) introduced via ERCP (Figure 3). The activation leads to creation of reactive oxygen species that subsequently causes necrosis of tumor cells to a depth of 4–6 mm and an antiangiogenic effect [50]. PDT has been shown to increase survival in patients with unresectable cholangiocarcinoma compared with historic controls in multiple studies (Table 4).
Figure 3. Photodynamic therapy light probe.
(A) A photodynamic therapy light diffuser probe for use in the bile duct. (B) Uniform illumination can be achieved despite curvature of the probe.
Reproduced with permission from Medlight, Switzerland.
Table 4. Results of photodynamic therapy in cholangiocarcinoma.
| Study (year) | Patients (n) | Study type | Serum bilirubin before and after PDT | Median survival (months) | Adverse events; n (%)† | Ref. |
|---|---|---|---|---|---|---|
| Ortner et al. (1998) | 9 | Single arm | 18.6, 6 | 14.6 | 1(11), 0(0) | [51] |
| Berr et al. (2000) | 23 | Single arm | 11.2, 1 | 11.1 | 3(13), 8(35) | [52] |
| Rumalla et al. (2001) | 6 | Single arm | 2.7, 1.3 | 6 | 2(33), 2(33) | [53] |
| Dumoulin et al. (2003) | 24 | Single arm | 13.3, 2.6 | 9.9 | 2(8), 5(21) | [54] |
| Ortner et al. (2003) | 20 | Randomized | Decreased | 16.4 | 2(10), 5(25) | [50] |
| Harewood et al. (2005) | 8 | Single arm | 7.7, 1.1 | 9.2 | 2(25), 2(25) | [55] |
| Zoepf et al. (2006) | 32 | Randomized | Decreased | 21 | NR | [56] |
| Witzigmann et al. (2006) | 68 | Single arm | Decreased | 12.0 | 8(12), 38(56) | [57] |
| Prasad et al. (2007) | 25 | Single arm | 6.1, 3.5 | 13.4 | 1(4), 2(8) | [58] |
| Kahaleh et al. (2008) | 19 | Comparative | 6.3, 3.5 | 16.2 | 3(16), 7(37) | [59] |
| Quyn et al. (2009) | 23 | Comparative | Decreased | 14.2 | 4(17), 5(21) | [60] |
| Cheon et al. (2012) | 72 | Comparative | 9.9, 1.8 | 9.8 | 10(14), NR | [61] |
| Lee et al. (2012) | 33 | Comparative | 8.5, 1.3 | 11.7 | 1(6), 1(6) | [62] |
†Photoxicity, cholangitis.
NR: Not reported; PDT: Photodynamic therapy.
The procedure technique has become standardized, as PDT is typically offered to patients as part of a neoadjuvant regimen or those with unresectable cholangiocarcinoma. Two to four days after administration of the photosensitizing agent, the patient undergoes ERCP. A cholangiogram is performed to delineate the distribution of malignant disease, and stricture dilation is performed to facilitate the subsequent placement of the cylindrical diffuser (with a laser) within the stricture. Photoactivation is performed at 630 nm with a light dose of 10 J/cm2, fluence of 0.250 W/cm2 and irradiation time of 750 s [63]. Multiple segments can be treated at once, and placement of an endoprosthesis is performed after PDT to prevent cholangitis. Photosensitivity effects of the drug last for 4–6 weeks [58], and as these agents can also deposit in the skin, phototoxicity is a side effect and all patients must be educated regarding sun exposure. PDT can be repeated at 3-month intervals, at which time all stents should be replaced [48].
The first successful report of PDT in unresectable cholangiocarcinioma was by McCaughan in 1991, where a patient was treated over the course of 4 years with seven sessions of PDT [64]. Many subsequent studies (Table 4) have had promising results, particular when used in conjunction with metal stenting. At this time, there have been two prospective randomized controlled trials comparing PDT after stent insertion compared with stents alone. Ortner et al. demonstrated that median survival was 493 days in the PDT group versus 98 days in the non-PDT group (p < 0.0001), and was thus terminated early (although this study was limited to enrollment in patients in whom biliary decompression was not successful via stents alone) [50]. Zoepf‘s study of 32 patients also demonstrated higher survival in patients who underwent PDT compared with stents alone (median survival 630 vs 210 days; p < 0.01) [56]. The presence of a visible mass on imaging, and increased time between diagnosis and PDT treatment session was associated with a lower survival rate after PDT [65], suggesting that early treatment with PDT after diagnosis may be preferred. Two small cases series (totaling 17 patients) have also demonstrated some promising results for adjuvant and neoadjuvant use of PDT [66,67]. In a systemic review of 20 studies published in 2010, Gao et al. concluded that PDT offered benefit on survival and quality of life with few adverse effects and should be offered to patients with unresectable cholangiocarcinoma [68].
More recently, PDT has been performed under direct visualization with single-operator cholangioscopy and peroral cholangioscopy. Cholangioscopy-guided PDT allows for the identification of tumor margins and determination of the appropriate location for placement of the diffuser (Figure 4); it also allows for evaluation of the response to therapy (Figure 5). Talreja et al. compared patients undergoing PDT with those undergoing cholangioscopy with direct PDT [69]. While the median survival time between the groups was not different, there was a statistically significant difference between the groups in terms of fluoroscopy time, as median time was 21.1 min with PDT and 11.1 min with cholangioscopy with direct PDT, thus allowing for decreased radiation exposure for both patients and healthcare professionals. Choi et al. also reported on their preliminary experience with peroral cholangioscopy with an ultraslim upper endoscope for patients with inoperable cholangiocarcinoma and an 88% success rate in performing PDT (Figure 6) [70]. In addition, PDT has also been found in a retrospective study to prolong metal stent patency (median 244 ± 66 days for PDT and metal stent vs 177 ± 45 days for metal stent alone; p = 0.002) [59].
Figure 4. Photodynamic therapy with choldeochoscopy.
(A) Radiograph of SpyGlass™ (BSCI, Inc., MA, USA) choledochoscope insertion. (B) Choledochoscopic-guided insertion of photodynamic therapy probe.
Reproduced with permission from Michel Kahaleh (Weill Cornell Medical Center, NY, USA).
Figure 5. Effects of photodynamic therapy as seen with choledochoscopy.
Endoscopic (SpyGlass™ choledochoscope; BSCI, Inc., MA, USA) evaluation of the photodynamic therapy effect 3 months after treatment.
Reproduced with permission from Michel Kahaleh (Weill Cornell Medical Center, NY, USA).
Figure 6. Endoscopic evaluation of photodynamic therapy with a patient with hilar cholangiocarcinoma.
(A) Before and (B) after 3 months of photodynamic therapy.
Reproduced with permission from Young Koon Cheon (Digestive Disease Center, Konkuk University School of Medicine, Seoul, Korea).
Although many studies have been published about PDT use in cholangiocarcinoma, questions remain whether PDT in combination with chemoradiation therapy is beneficial with regard to long-term survival and whether this modality can be used as a bridge to definitive therapy, such as surgery or transplantation.
▪ Radiofrequency ablation
Radiofrequency ablation (RFA) has been used effectively for years in the management of primary and secondary hepatic malignancies by causing tissue necrosis. RFA involves the delivery of alternating currents that leads to thermal injury to abnormal cells, thus causing necrosis, and an endoscopic bipolar RFA catheter has been developed for the palliation of human malignant biliary obstruction. The Habib Endo HPB (EMcision UK, London, UK) catheter is a bipolar 8F catheter with a 180 cm working length, with two electrodes 8 mm in length and 8 mm apart, and has a 5-mm leading tip (Figure 7). The catheter can be introduced over a 0.035-in. guidewire into the biliary system. The catheter is activated at 10 W of energy for 90 s at a time, causing coagulation necrosis over a 2.5-cm length, and then the device can be removed and subsequently, stents can be inserted. There have been animal studies to assess the power and duration of treatment [71].
Figure 7. The Habib EndoHPB biopolar radiofrequency ablation catheter, designed for endoscopic treatment of biliary tumors.
Reproduced with permission from EMcision, Ltd (Imperial College London, London, UK).
There have been two published clinical studies on the efficacy of RFA in cholangiocarcinoma published in full. Steel et al., in an open-label pilot cohort study, treated 22 patients with malignant biliary obstruction (16 with pancreatic cancer and six with cholangiocarcinoma) with RFA [72]. Patients were treated with RFA and had stents placed. Initial successful decompression was achieved in 21 out of 22 patients. Thirty-day stent patency was preserved in 20 patients, and 90-day stent patency was seen in 16 out of 19 living patients. Complications included biochemical but asymptomatic pancreatitis (amylase 1450 IU/l), and two patients developed cholecystitis that required percutaneous drainage. Both patients were septic originally and had evidence of cystic duct involvement of the tumor. Figueroa-Barojas et al. treated 20 patients with biliary stents and RFA, 11 of whom had unresectable cholangiocarcinoma (total of 25 strictures) [73]. Mean stricture diameter before RFA was 1.7 mm while the mean diameter after RFA was 5.2 mm, with a significant increase of 3.5 mm (p = <0.0001). Five patients presented with pain after the procedure, but only one developed mild post-ERCP pancreatitis and cholecystitis.
Most recently, Sharaiha et al. have also reported, in abstract form, their experience on endobiliary RFA versus stenting alone [74]. A total of 15 patients underwent RFA with metal stenting, while ten patients received stents alone. Overall, 16 of the patients had cholangiocarcinoma, and technical success was reported at 100% for both groups; survival was significantly improved in patients undergoing RFA therapy. One patient in the RFA group had an adverse event (cholecystitis). Strand et al. also recently reported their experience with RFA in cholangiocarcinoma and compared it to patients who received PDT [75]. In total, 16 patients who received RFA and 32 patients who received PDT were included, with similar baseline characteristics. Median survival time was 7.5 months (95% CI: 4.3–16.0) for the PDT cohort and 9.6 months (95% CI:5.1–11.7) for the RFA cohort, and survival was not statistically different in patients who received endobiliary RFA and those who received PDT. Kahaleh et al. have also recently reported their interim results from using RFA in a multicenter registry (Figure 8); the 25 patients with unresectable cholangiocarcinoma had an increase in stricture diameter after RFA treatment (mean diameter before treatment: 2.21 mm, mean diameter after RFA: 5.26 mm; p < 0.0001) [76]. Cholangioscopy-directed RFA has also been used to confirm tissue necrosis and abalation [73,77].
Figure 8. Radiographic images of radiofrquency ablation in the treatment of cholangiocarcinoma.
(A) Left and (B) right intrahepatic systems, (C) followed by bilateral stent placement.
Reproduced with permission from Michel Kahaleh (Weill Cornell Medical Center, NY, USA).
Until recently, PDT was the only evidence-based endoscopic treatment other than stenting that improved the quality of life for patients with cholangiocarcinoma. Endobiliary RFA has demonstrated some promising results, and further controlled trials are needed to establish survival advantages, improved stent patency and cost–effectiveness.
Conclusion
Most cholangiocarcinomas are locally advanced or have metastasized at the time of diagnosis. Endoscopic treatment options have previously been limited to biliary decompression via stenting, but recent developments in locoregional therapy, such as PDT and, more recently, radiofrequency ablation, have shown promising results, particularly in improving patient outcomes for those with locally advanced or unresectable disease. Further studies are needed to confirm the benefits of PDT and RFA on long-term biliary stent patency and survival rates.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest
- 1.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Tyson GL, El-Serag HB. Risk factors of cholangiocarcinoma. Hepatology. 2011;54(1):173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9:43–57. doi: 10.1634/theoncologist.9-1-43. [DOI] [PubMed] [Google Scholar]
- 4.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroentrol. Hepatol. 2013;11(1):13–21. doi: 10.1016/j.cgh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Highlights the latest advances in the diagnosis and management of cholangiocarcinoma.
- 5.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001;234(4):507–519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaib YH, Davila JA, Henderson L, et al. Endoscopic and surgical therapy for intrahepatic cholangiocarcinoma in the United States: a population-based study. J. Clin. Gastroenterol. 2007;41(10):911–917. doi: 10.1097/MCG.0b013e31802f3132. [DOI] [PubMed] [Google Scholar]
- 7.Farley DR, Weaver AL, Nagorney DM. ‘Natural history’of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin. Proc. 1995;70(5):425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Moss AC, Morris E, Leyden J, et al. Malignant distal biliary obstruction: a systemic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat. Rev. 2007;33:213–221. doi: 10.1016/j.ctrv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Levy MJ, Baron TH, Gostout CJ, et al. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: an evidence based approach. Clin. Gastroenterol. Hepatol. 2004;2:273–285. doi: 10.1016/s1542-3565(04)00055-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin RC, 2nd, Vitale GC, Reed DN, et al. Cost comparison of endoscopic stenting vs surgical treatment for unresectable cholangiocarcinoma. Surg. Endosc. 2002;16:667–670. doi: 10.1007/s004640080006. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Gurusamy KS, Lin H, et al. Preoperative drainage for obstructive jaundice. Cochrane Database Syst. Rev. 2008;3:CD005444. doi: 10.1002/14651858.CD005444.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment for cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 13.Moss AC, Morris E, Leyden J, et al. Do benefits of metal stents justify the cost? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur. J. Gastroenerol. Hepatol . 2007;19(12):1119–1124. doi: 10.1097/MEG.0b013e3282f16206. [DOI] [PubMed] [Google Scholar]
- 14.Yoon WJ, Ryu JK, Yang KY. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost–effectiveness in a country with a lower ERCP cost. Gastrointest. Endosc. 2009;70:284–289. doi: 10.1016/j.gie.2008.12.241. [DOI] [PubMed] [Google Scholar]
- 15.Yeoh KG, Zimmerman MJ, Cunningham JT. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest. Endosc. 1999;49:466–471. doi: 10.1016/s0016-5107(99)70044-1. [DOI] [PubMed] [Google Scholar]
- 16.Choi JM, Kim JH, Kim SS, et al. A comparative study on the efficacy of covered metal stent and plastic stent in unresectable malignant biliary obstruction. Clin. Endosc. 2012;45:78–83. doi: 10.5946/ce.2012.45.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest. Endosc. 2006;63(7):986–995. doi: 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Kaassis M, Boyer J, Dumar R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest. Endosc. 2003;57(2):178–182. doi: 10.1067/mge.2003.66. [DOI] [PubMed] [Google Scholar]
- 19.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest. Endosc. 1998;47(1):1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 20.Knyrim K, Wagner HJ, Pausch J, Vakil N, et al. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25(3):207–212. doi: 10.1055/s-2007-1010294. [DOI] [PubMed] [Google Scholar]
- 21.Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized trial comparing uncovered and partially covered self-expandeableexpandable metal stents in palliation of distal malignant biliary obstruction. Gastrointest. Endosc. 2010;72:907–914. doi: 10.1016/j.gie.2010.08.021. [DOI] [PubMed] [Google Scholar]; ▪ One of the most pivotal trials in defining the use of stents in malignant biliary obstruction.
- 22.Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of ‘covered’versus ‘uncovered’diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–734. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal biliary obstruction. Gastrointest. Endosc. 2006;63(7):996–1000. doi: 10.1016/j.gie.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 24.Park DH, Kim MH, Choi JS, et al. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: a cohort comparative analysis. Clin. Gastroenterol. Hepatol. 2006;4(6):790–796. doi: 10.1016/j.cgh.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Kullman E, Frozanpor F, Soderlund C, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized multicenter study. Gastrointest. Endosc. 2010;72:915–923. doi: 10.1016/j.gie.2010.07.036. [DOI] [PubMed] [Google Scholar]; ▪ One of the most pivotal trials in defining the use of stents in malignant biliary obstruction.
- 26.Lee JH, Krishna SG, Signh A, et al. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest. Endosc. 2013;78(2):312–324. doi: 10.1016/j.gie.2013.02.032. [DOI] [PubMed] [Google Scholar]; ▪ Compares stent choice in cholangiocarcinoma.
- 27.Saleem A, Leggett CL, Murad MH, et al. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest. Endosc. 2011;74(2):321–327. doi: 10.1016/j.gie.2011.03.1249. [DOI] [PubMed] [Google Scholar]
- 28.Alamdi MA, Barkun AN, Martel M. No benefit of covered vs. uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin. Gastroenterol. Hepatol. 2013;11(1):27–37. doi: 10.1016/j.cgh.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Perdue DG, Freeman ML, DiSario JA, et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. J. Clin. Gastroenterol. 2008;42(9):1040–1046. doi: 10.1097/MCG.0b013e31815853e0. [DOI] [PubMed] [Google Scholar]
- 30.Raju RP, Jaganmohan SR, Ross WA, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig. Dis. Sci. 2011;56:1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 31.Sangchan A, Kongkasame W, Pugkhem A, et al. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest. Endosc. 2012;76(1):93–99. doi: 10.1016/j.gie.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 32.Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012;12:103. doi: 10.1186/1471-230X-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng JL, Bruno MJ, Bergman JJ, et al. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic wallstents. Gastrointest. Endosc. 2002;56(1):33–39. doi: 10.1067/mge.2002.125364. [DOI] [PubMed] [Google Scholar]
- 34.Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96(4):1180–1186. doi: 10.1016/0016-5085(89)91639-9. [DOI] [PubMed] [Google Scholar]
- 35.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest. Endosc. 1998;47(5):354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]; ▪ Compares drainage in hilar cholangiocarcinoma.
- 36.De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest. Endosc. 2001;53(6):547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 37.De Palma GD, Pezzullo A, Rega M, et al. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest. Endosc. 2003;58(1):50–53. doi: 10.1067/mge.2003.310. [DOI] [PubMed] [Google Scholar]; ▪ Compares drainage in hilar cholangiocarcinoma.
- 38.Iwano H, Ryozawa S, Ishigaki N, et al. Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Dig. Endosc. 2011;23(1):43–48. doi: 10.1111/j.1443-1661.2010.01036.x. [DOI] [PubMed] [Google Scholar]
- 39.Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J. Gastroenterol. Hepatol. 2009;24(4):552–557. doi: 10.1111/j.1440-1746.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- 40.Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest. Endosc. 2010;72(4):728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 41.Ornellas LC, Stefanidis G, Chuttani R, et al. Covered wallstents for palliation of malignant biliary obstruction: primary stent placement versus reintervention. Gastrointest. Endosc. 2009;70(4):676–683. doi: 10.1016/j.gie.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Kahaleh M, Tokar J, Conaway MR, et al. Efficacy and complications of covered wallstents in malignant distal biliary obstruction. Gastrointest. Endosc. 2005;61(4):528. doi: 10.1016/s0016-5107(04)02593-3. [DOI] [PubMed] [Google Scholar]
- 43.Rogart JN, Boghos A, Rossi F, et al. Analysis of endoscopic management of occluded metal biliary stents at a single tertiary care center. Gastrointest. Endosc. 2008;68(4):676. doi: 10.1016/j.gie.2008.03.1064. [DOI] [PubMed] [Google Scholar]
- 44.Gosain S, Bonatti H, Smith L, et al. Gallbladder stent placement for prevention of cholecystitis in patients receiving covered metal stents for malignant obstructive jaundice: a feasibility study. Dig. Dis. Sci. 2010;55(8):2406–2411. doi: 10.1007/s10620-009-1024-9. [DOI] [PubMed] [Google Scholar]
- 45.Kawakubo K, Isayama H, Sasahira N. Endoscopic transpapillary gallbladder drainage with replacement of a covered self-expandable metal stent. World J. Gastrointest. Endosc. 2011;3:46–48. doi: 10.4253/wjge.v3.i2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suk KT, Kim HS, Kim JW, et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastronintest. Endosc. 2006;64(4):522–529. doi: 10.1016/j.gie.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu S, Naitoh I, Nakazawa T, et al. Digestive Diseases Week 2013. Orlando, Florida, USA: 18–21 May 2013. Predictive factors for pancreatitis and cholecysitis in endoscopic covered metal stenting for distal malignant obstruction. Presented at. [DOI] [PubMed] [Google Scholar]
- 48.Kawakubo K, Isayama H, Nakai Y, et al. Risk factors of pancreatitis following transpapillary self-expandable metal stent placement. Surg. Endosc. 2012;26(3):771–776. doi: 10.1007/s00464-011-1950-4. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto S, Fujita N, Ito K, et al. Digestive Diseases Week 2013. Orlando, Florida, USA: 18–21 May 2013. Risk factors for post ERCP pancreatitis and stent dysfunction after preoperative biliary drainage in patients with malignant biliary stricture. Presented at. [DOI] [PubMed] [Google Scholar]
- 50.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]; ▪ Only prospective randomized trial on the benefits of photodynamic therapy.
- 51.Ortner MA, Liebetruth J, Schreiber S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology. 1998;114:536–542. doi: 10.1016/s0016-5085(98)70537-2. [DOI] [PubMed] [Google Scholar]
- 52.Berr F, Wiedmann M, Tannapfel A, et al. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31:291–298. doi: 10.1002/hep.510310205. [DOI] [PubMed] [Google Scholar]
- 53.Rumalla A, Baron TH, Wang KK, et al. Endoscopic application of photodynamic therapy for cholangiocarcinoma. Gastrointest. Endosc. 2001;53:500–504. doi: 10.1067/mge.2001.113386. [DOI] [PubMed] [Google Scholar]
- 54.Dumoulin FL, Gerhardt T, Fuchs S, et al. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest. Endosc. 2003;57:860–867. doi: 10.1016/s0016-5107(03)70021-2. [DOI] [PubMed] [Google Scholar]
- 55.Harewood GC, Baron TH, Rumalla A, et al. Pilot study to assess patient outcomes following endoscopic application of photodynamic therapy for advanced cholangiocarcinoma. J. Gastroentrol. Hepatol. 2005;20:415–420. doi: 10.1111/j.1440-1746.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- 56.Zoepf T, Jakobs R, Arnold JC, et al. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am. J. Gastroenterol. 2005;100(11):2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 57.Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to R1/R2 resection. Ann. Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webb K, Saunders M. Endoscopic management of malignant bile duct strictures. Gastrointest. Endosc. Clin. N. Am. 2013;23(2):313–331. doi: 10.1016/j.giec.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting along versus stenting with photodynamic therapy. Clin. Gastroenterol. Hepatol. 2008;6:290–297. doi: 10.1016/j.cgh.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Quyn AJ, Ziyaie D, Polignano FM, et al. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford) 2009;11(7):570–577. doi: 10.1111/j.1477-2574.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheon YK, Lee TY, Lee SM, et al. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford) 2012;14(3):185–193. doi: 10.1111/j.1477-2574.2011.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee TY, Cheon YK, Shim CS, et al. Photodynamic therapy prolongs metal stent patency in patients with unresectable hilar cholangiocarcinoma. World J. Gastroenterol. 2012;18(39):5589–5594. doi: 10.3748/wjg.v18.i39.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richter JA, Kahaleh M. Photodynamic therapy: palliation and endoscopic technique in cholangiocarcinoma. World J. Gastrointest. Endosc. 2010;2(11):357–361. doi: 10.4253/wjge.v2.i11.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCaughan JS, MErtens BF, Cho C. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch. Surg. 1991;126:111–113. doi: 10.1001/archsurg.1991.01410250119022. [DOI] [PubMed] [Google Scholar]
- 65.Prasad GA, Wang KK, Baron TH, et al. Factors associated with increased survival after photodynamic therapy for cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2007;5:743–748. doi: 10.1016/j.cgh.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Nanashima A, Yamaguchi H, Shibasaki S, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J. Gastroenterol. 2004;39:1095–1101. doi: 10.1007/s00535-004-1449-z. [DOI] [PubMed] [Google Scholar]
- 67.Widemann M, Caca K, Berr F, et al. Neoadjuvant photodyanmic therapy as a new approach to treating hilar cholangiocarcinoma: a Phase II pilot study. Cancer. 2003;97:2783–2790. doi: 10.1002/cncr.11401. [DOI] [PubMed] [Google Scholar]
- 68.Gao F, Bai Y, Ma SR, Liu F, Li ZS. Systematic review: photodynamic therapy for unresectable cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2010;17:125–131. doi: 10.1007/s00534-009-0109-3. [DOI] [PubMed] [Google Scholar]
- 69.Talreja JP, DeGaetani M, Sauer BG, et al. Photodynamic therapy for unresectable cholangiocarcinoma: contribution of single operator cholangioscopy for target treatment. Photochem. Photobiol. Sci. 2011;10:1233–1238. doi: 10.1039/c0pp00259c. [DOI] [PubMed] [Google Scholar]
- 70.Choi HJ, Moon JH, Ko BM, et al. Clinical feasability of direct peroral cholangioscopy-guided photodynamic therapy for inoperable cholangiocarcinoma performed using an ultra-slim upper endoscope (with videos) Gastrointest. Endosc. 2011;73:808–813. doi: 10.1016/j.gie.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 71.Daglilar ES, Yoon WJ, Mino-Kenudson M, et al. Controlled swine bile duct ablation with a bipolar radiofrequency catheter. Gastrointest. Endosc. 2013;77(5):815–819. doi: 10.1016/j.gie.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest. Endosc. 2011;73(1):149–153. doi: 10.1016/j.gie.2010.09.031. [DOI] [PubMed] [Google Scholar]; ▪ Most cited trial to date regarding the use of radiofrequency ablation in malignant biliary strictures.
- 73.Figueroa-Barojas P, Bakhru MR, Habib NA, et al. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J. Oncol. 2013;2013:910897. doi: 10.1155/2013/910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharaiha RZ, Widmer JL, Sarkaria S, et al. Digestive Diseases Week 2013. Orlando, FL, USA: 18–21 May 2013. Comparison of self expanding metal stenting with radiofrequency ablation versus stenting alone in the treatment of malignant biliary strictures: is there an added benefit? Presented at. [Google Scholar]
- 75.Strand DS, Cosgrove N, Patrie JT, et al. Digestive Diseases Week 2013. Orlando, Florida, USA: 18–21 May 2013. ERCP-directed radiofrequency abalation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarinoma. Presented at. [DOI] [PubMed] [Google Scholar]
- 76.Kahaleh M, Sharaiha RZ, Widmer JL, et al. 291 radiofrequency ablation of malignant biliary strictures: results of a collaborative registry. Gastrointest. Endosc. 2013;77(5 Suppl.) Abstract 141. [Google Scholar]
- 77.Monga A, Gupta R, Ramchandani M, et al. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (videos) Gastrointest. Endosc. 2011;74(4):935–937. doi: 10.1016/j.gie.2010.10.018. [DOI] [PubMed] [Google Scholar]