SUMMARY
Hepatocellular carcinoma (HCC) is a leading cause of cancer mortality worldwide. HCC a heterogeneous disease occurring on the background of cirrhosis. The presence of cirrhosis limits the sensitivity of conventional imaging modalities in differentiating HCC from surrounding cirrhotic parenchyma. Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) is widely used for assessing a variety of malignancies, however, has poor sensitivity in the evaluation of HCC. This has led to the investigation of other radiotracers such as 11C-acetate and 11C-choline, with improved sensitivity in terms of detection and therapeutic response. In this review, we discuss the emerging field of PET imaging for the detection, staging and assessment of treatment response in HCC. In particular we discuss the role of 18F-FDG-PET in imaging hepatocellular cancer, the limitations of this PET tracer and emerging novel PET tracers being investigated that exploit key metabolic processes including fatty acid and lipid synthesis, choline kinase activity and gene expression.
KEYWORDS: : choline kinase activity, fatty acid and lipid synthesis, hepatocellular carcinoma, positron emission tomography
Practice points.
There is a clinical need for better imaging in hepatocellular carcinoma (HCC) as conventional imaging has low sensitivity in differentiating regenerative nodules from HCC.
The utility of 18F-FDG in detecting HCC is limited because of high background uptake of 18F-FDG by normal hepatocytes.
Clinically, 18F-FDG is limited to detecting extra-hepatic disease and assessing response to poorly differentiated tumors.
The addition of complementary radiotracers to 18F-FDG can improve the overall diagnostic sensitivity of HCC and also predict survival and recurrence following liver resection or transplantation.
Several novel PET tracers are currently under investigation and larger studies are needed to establish their role in HCC.
Hepatocellular carcinoma (HCC) is the most common primary liver tumor worldwide and the third most common cause of cancer-related death [1,2]. It is the fifth most common cancer in men and seventh in women [3], with an increasing incidence rate of 3 per 100,000 in the western world, with up to 15 per 100,000 in areas with prevalent hepatitis B and C infections [4]. More than 80% of newly diagnosed HCCs arise in the context of liver cirrhosis, secondary to alcoholic liver disease, chronic infection by hepatotropic viruses or metabolic derangements like α-1 antitrypsin deficit and haemochromatosis. Cirrhosis is a progressive process that involves diffuse fibrosis of the liver characterized by the development of nodules that range from benign regenerative nodules to dysplastic nodules to HCC. Early diagnosis and staging of HCC is critical in determining long-term outcome in patients. In those patients where HCC is detected at an early stage, 5-year survival rates of at least 70% can be achieved through surgical interventions and transplantation. However, in patients with late-stage disease, 5-year survival rates are less then 10% despite advances in targeted therapies [5]. Therefore, accurate staging of HCC is critical in determining not only therapeutic options but for prognostication.
A wide range of imaging modalities such as ultrasound (USS), multiphase computed tomography (CT), dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) are used in diagnosis, staging and monitoring of treatment response, and although each imaging modality has its own individual merits, universal difficulties arise when characterizing small and hypovascular lesions due to their atypical enhancing patterns. The cirrhotic liver is not homogenous but contains regenerative or dysplastic nodules as well as HCC, which presents a challenge to conventional imaging techniques that have limited sensitivity in differentiating the varying pathological processes. Furthermore, there lie challenges in biopsy of lesions in the cirrhotic liver, with risk of needle track seeding, intraperitoneal bleeding and tumor heterogeneity [6]. Therefore, there is a real need for accurate imaging modalities to better image these tumors [7,8].
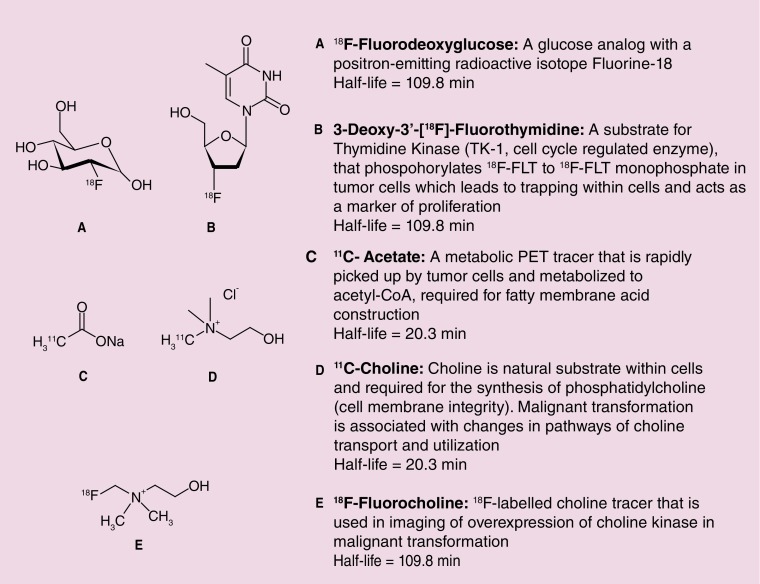
PET imaging has emerged as an important decision-making tool in oncology particular with the advent of targeted therapies, which tend to produce cytostatic responses on standard imaging, with changes in tumor size taking months to develop, such that patients can be exposed to potential adverse side effects of drugs without any therapeutic benefit [9]. PET allows imaging of molecular pathways and biological processes, and several radiotracers have been investigated within oncology exploiting the key hallmarks of tumorigenesis (e.g., cell proliferation, angiogenesis, apoptosis) [10]. PET imaging involves the intravenous administration of trace amounts of radiolabeled isotopes, which are either substrates of normal physiological processes or specifically bind to biological targets, allowing the evaluation of these processes or substrate–target interaction [11]. It is a noninvasive functional imaging technique that can be combined with CT or MRI to improve spatial resolution. In this way, molecular biological processes such as glucose metabolism, choline kinase activity, gene expression as well as lipid synthesis can be imaged. Figure 1 highlights different radiotracers that have shown potential use in HCC.
Figure 1. . Common tracers involved in imaging of hepatocellular carcinoma.
18F-fluorodeoxyglucose
18F-fluorodeoxyglucose (18F-FDG) has emerged as an important noninvasive diagnostic and prognostic tool in several malignancies (e.g., lung, breast and lymphoma) [12]. 18F-FDG is taken up by the cell and phosphorylated by the enzyme hexokinase, it then becomes trapped within the cell. In hepatocytes, however, 18F-FDG is released from the cell due to the high rate of glucose-6-phosphatase, thereby, resulting in reduced accumulation of 18F-FDG in low and intermediate grade HCC. In the primary diagnostic setting, several studies have shown 18F-FDG to have poor sensitivity rates (50–55%) compared with contrast-enhanced CT, USS and MRI[13–16]. It is worth noting, however, that these earlier studies only utilized PET while later scans employ the use of PET/CT that improve resolution. These earlier studies were also limited in the small numbers of patients enrolled. In the largest series of patients with HCC (n = 91), Wudel and colleagues, reported sensitivity rates of 64% with 18F-FDG [17]. Routine use of 18F-FDG, therefore is limited in the primary setting as the rate of gluconeogenesis in well-differentiated HCC and the surrounding liver is similar resulting in almost equivalent uptake of 18F-FDG and therefore poor differentiation of tumors.
18F-FDG has, however, shown promise in the detection of extrahepatic disease, where it can be used in complement with CT. Extrahepatic disease is not uncommon in patients with HCC, reported in up to 37% of patients with the main sites of disease being lung, lymph nodes and bone [18]. Additionally, poorly differentiated HCCs tend to metastasize, and are more likely to be 18F-FDG avid [19]. Sugiyama et al., reported on the use of 18F-FDG in detection of extrahepatic disease in a prospective study involving 19 patients. 18F-FDG had a sensitivity rate of 83% for detecting extrahepatic disease measuring greater than 1 cm, and 13% for lesions less than or equal to 1 cm [20]. On the basis of their results, resection of isolated extrahepatic metastases was carried out in five patients, with two out of five patients being alive and disease free for greater than 12 months at latest followup.
Yoon et al., compared the sensitivity of 18F-FDG PET with MRI and CT prior to treatment for the detection of extrahepatic disease [21]. Out of 87 patients, 24 were found to have extrahepatic disease, all of which were detected with 18F-FDG. In addition, MRI and CT performed poorly, unable to detect lymph node metastases in four patients and bone metastases in six patients. As a result, a change in TNM staging was seen in 5% of patients which led to a change in clinical management. Furthermore, the authors note that those with primary tumors ≥5 cm were more likely to develop extrahepatic metastases. A recent systematic review and meta-analysis by Chun Yi Lin et al. [22], summarized the findings of eight studies (seven retrospective, one prospective), using 18F-FDG in the detection of extrahepatic metastases or recurrent HCC. Pooled estimates of sensitivity and specificity for the detection of extrahepatic disease was reported as 77 and 98%, respectively. The detection of recurrent intrahepatic HCC with 18F-FDG was reported as having a sensitivity of 82% and specificity of 89%. This meta-analysis is important in that it confirms the poor sensitivity of 18F-FDG in the assessment of the primary disease, but suggests a role in the early detection of extrahepatic metastases, which may potentially offer the chance for surgical resection and long-term survival [23,24].
18F-FDG has also shown a promising role in the setting of detecting HCC recurrence. Early detection of recurrent disease is critical, with early surgical resection correlating with better post-recurrent survival rates [7]. Due to high recurrence rates post liver resection (50–60% at 5 years) and liver transplantation (LT; 15–20%) [25,26], 18F-FDG has been investigated as a potential marker of disease recurrence. Yang et al. [27] investigated the role of 18F-FDG in determining tumor recurrence following LT in a retrospective study of 38 patients with HCC meeting Milan criteria [5,25]. They reported a significant association between tumor recurrence and PET positivity (p = 0.016) with patients with PET-positive tumors having an overall higher risk of recurrence than PET-negative patients (OR = 7.6; 95% CI: 1.9–28.9). In addition, the study reported a statistically significant association between PET-positive scans and pre-operative serum alpha feto-protein (AFP), whereby positivity of PET imaging correlated with serum AFP greater than 200 ng/ml and vascular invasion (p < 0.05). The authors concluded that PET positivity may reflect the aggressiveness of HCC supported by several studies that have reported pre-operative AFP levels as well as vascular invasion to be key factors for tumor recurrence post LT [26,28–31].
The utility of 18F-FDG in the assessment of residual, viable tumor following transarterial chemoembolization (TACE) therapy and radiofrequency ablation (RFA) has also been reported [32,33]. TACE is an effective palliative treatment option for patients with intermediate stage disease but follow up with imaging has proven difficult, as CT is unable to distinguish viable tumor, due to the hyperattenuating lipoidal deposition, which is seen post TACE [32]. The predictive value of 18F-FDG post TACE was reported in a recent study by Song et al. [34]. Based on a retrospective review of 83 patients treated with TACE, they evaluated the utility of 18F-FDG in predicting treatment response. All patients underwent 18F-FDG PET within 3 days prior to TACE. They reported that the standardized uptake value ratio (TSUV max, SUV max of tumor/LSUV max, Liver mean SUV; cut-off value 1.90) was significantly associated with overall survival. Those patients whose tumors had a high SUV ratio (≥1.90) had an overall survival of 38 months compared with 10 months in those with a low SUV ratio (<1.90). The results from Song et al., are provocative in suggesting that FDG uptake is a measure of the biological behavior of HCC, and in turn, a marker of treatment response, results that need further exploration in large, prospective, multicenter studies with biological correlates from tumor biopsies.
The use of 18F-FDG has also been reported as a potential tool in detecting earlier recurrence post RFA in HCC [33]. This small retrospective study (n = 24) showed that disease recurrence was detected in more patients using 18F-FDG post RFA in the first 4–9 months compared with CT (8 vs 4 months), and an overall detection rate of 92 versus 75% was observed. Although this was a small study, a significant correlation was noted between SUV values pre-RFA to the time to recurrence detected by 18F-FDG, with patients with high SUV tending to recur earlier than those with lower SUV values. While, several studies have investigated the role of 18F-FDG and its predictive value, it is clear that larger scale studies are needed for further validation of the utility of this tracer, in particular given its limitations in imaging intrahepatic lesions [35].
11C-acetate
The advent of new radiopharmaceuticals has sparked interest in the imaging of HCC, especially given the limitations of 18F-FDG in the primary diagnostic setting. 11C-acetate is used to evaluate fatty acid synthesis which is associated with tumor cell growth and invasiveness [36]. Through an anabolic pathway, acetate is converted into fatty acids by acetyl coenzyme A of the Krebs cycle and then incorporated into the intracellular phosphatidylcholine membrane microdomains. The uptake of acetate is thought to be related to the expression of fatty acid synthetase – a multienzyme that catalyzes the formation of palmitate from acetyl coA and is elevated in well-differentiated HCC [37]. Hence, there is a rationale for using 11C-acetate to better differentiate between neoplastic lesions and inflammation within the liver compared with 18F-FDG. The use of 11C–acetate in the primary diagnosis of HCC, has been reported in a study by Ho et al. where differentiation between liver masses was evaluated [38]. The sensitivity rate 11C-acetate in detecting HCC was 87% with no uptake observed in other liver masses (i.e., metastatic lesions secondary to colon, breast and lung, cholangiocarcinoma and carcinoid tumors).
The differential uptake pattern seen with different tracers due to heterogeneity within tumors can be exploited, and in a further study, Ho et al. reported the use of 11C-acetate in conjunction with 18F-FDG in patients with HCC [19]. They reported that while sensitivity using 11C-acetate alone was good in well-differentiated tumors, 18F-FDG was better in the detection of poorly differentiated tumors. By using both tracers, there was a 100% sensitivity rate in the detection of HCC. Park et al. also evaluated the use of dual tracers (11C-acetate and 18F-FDG) in a prospective study involving 90 patients diagnosed with HCC [39]. They reported a sensitivity rate of 83% using the combination of tracers compared with 60% with 18F-FDG alone, and 75% with 11C-acetate. The study also highlighted that higher sensitivity rates were related to larger tumor size (≥5 cm), and again confirming the results from previously discussed studies that 18F-FDG had higher detection rate for extrahepatic metastases.
In a similar study, Cheung et al., utilized a dual tracer approach with 11C-acetate and 18F-FDG to predict microvascular invasion before LT or surgical resection in 58 patients with HCC [40]. The sensitivity in detecting HCC using a dual tracer approach was 93% compared with 43% using 18F-FDG alone. The addition of 11C-acetate improved the overall sensitivity of 18F-FDG, providing more information on the number of lesions, histological grade of the tumor as well as the probability of microvascular invasion in patients being considered for LT [40]. Overall, 11C-acetate may be of use in primary setting but used in combination with 18F-FDG seems to be of benefit in metastatic HCC. However, routine clinical use of a dual tracer approach is limited given the duration of time to perform, the high overall radiation dose delivered to the patient as well as the short half-life of 11C-acetate (20.4 min) necessitating an onsite cyclotron.
11C-choline
Choline is a substrate for the synthesis of phosphatidylcholine, a major phospholipid in the cell membrane [41]. During malignant transformation, overexpression of key enzymes involved in choline metabolism are seen (e.g., choline kinase-α [CHKα]), leading to increased phosphocholine and total choline containing compounds [41]. Deranged choline metabolism, and in particular, overexpression of CHK-α, has been reported in several cancers such as prostate, colon, ovarian and breast [42,43]. As such PET imaging with choline tracers is used clinically for staging prostate cancer, and is being investigated in several other tumor types. Despite its promising role in imaging malignancy, 11C-choline has not been established in HCC. A recent retrospective study by Yamamoto et al., failed to show statistical significance in evaluation of 11C-choline, with sensitivity rate of 63 vs 50% compared with 18F-FDG in the detection of HCC [44]. A further, small prospective study (n = 12) by Talbot et al. using 18F-fluorocholine (18F-FCH) reported a 100% detection rate on a per-patient analysis in newly diagnosed and recurrent HCC [45]. The authors observed a trend between high SUV and well-differentiated HCC. 18F-FCH may therefore be potentially useful in visualizing HCC, however, this was a small study and patients selected had large lesions, mean size (8.15 ± 3.9 cm).
The complementary role of radiolabeled choline analogs has also been investigated in HCC, as it can preferentially detect well-differentiated lesions that are not 18F-FDG-avid [46]. A prospective study by Wu et al., evaluated 76 patients with HCC who underwent 18F-FDG PET/CT. They reported that 48 out of 76 (61%) patients had positive 18F-FDG PET/CT scans. Those with no uptake seen on 18F-FDG scans (n = 28) were subsequently scanned with 11C-choline PET/CT. The study showed that imaging with 11C-choline increased the sensitivity of 18F-FDG alone from 63 to 90% (p < 0.001), and that 18F-FDG showed a lower sensitivity for well-differentiated HCC (36 vs 67%) compared with 11C-choline [46]. Larger studies are needed to confirm use of these tracers and evaluate their specificity.
Other potential PET tracers in imaging HCC
• 18F-fluorothymidine
Thymidine is a nucleoside utilized in DNA replication by proliferating cells, and both thymidine and its analogs have been extensively studied as markers of cellular proliferation. After injection, 18F-fluorothymidine (18F-FLT) enters the cell by both active transport, via sodium-dependent nucleoside transporters, and by passive diffusion. 18F-FLT follows the salvage pathway of DNA synthesis and like thymidine undergoes phosphorylation by thymidine kinase–1 (TK1) to 18F-FLT-monophosphate [47]. 18F-FLT is a selective substrate for TK1 whereas thymidine is also phosphorylated by TK2. TK1 is virtually absent in quiescent cells but is increased in proliferating cells [48,49]. Phosphorylated 18F-FLT is not incorporated into DNA and is trapped within the cytosol. The rate-limiting step for 18F-FLT accumulation is the initial phosphorylation by TK1; it is also the rate-limiting step in the salvage pathway of DNA synthesis, therefore the handling of 18F-FLT reflects cellular proliferation [50].
The ‘accuracy’ of 18FFLT PET in demonstrating proliferation has been illustrated in a number of studies where 18F-FLT PET parameters have been shown to correlate with the histological marker of proliferation, Ki67 labeling index, in colorectal, breast and lung cancer [51–53]. In a small pilot study, Eckel et al. utilized 18F-FLT PET to visualize HCC [54]. Eighteen untreated patients with clinical suspicion of HCC underwent USS, MRI or CT followed by 18F-FLT PET. The results showed a mixed pattern of uptake on PET and poor sensitivity rates: (69%) in the detection of HCC. However, the sensitivity of 18F-FLT PET in detecting HCC was hampered by high background activity within the normal liver, the result of rapid delivery and metabolism of 18F-FLT to 18F-FLT-glucuronide. This limits the utility of 18F-FLT in assessing proliferation in liver tumors and further studies in HCC have not been pursued.
• 9–(4–18F-Fluoro-3-hydroxymethylbutyl)guanine (18F-FHBG)
18F-FHBG is used to image gene expression of herpes simplex virus type-1 thymidine kinase (HSV1-tk) and is a safe and stable radiotracer with a rapid blood clearance and acceptable radiation doses [55]. Gene therapy offers a new area of treatment for patients with HCC, whereby the introduction of genetic material into tumor tissue produces therapeutic benefit either through the restoration of tumor suppressor genes, the activation of a prodrug, the stimulation of antitumor immune activity or via oncolytic virotherapy [56]. PET offers a safe, sensitive and reproducible imaging modality of monitoring of transgene expression with the aid of a reporter gene and probe that accumulates only in the organ of interest. In a Phase I study performed by Penuelas et al. [57], 18F-penciclovir analog (18F-FHBG) was used to analyze transgene expression of herpes simplex virus thymidine kinase (HSV1-tk) in seven patients with HCC after intratumoral introduction of a recombinant adenoviral vector encoding thymidine kinase (AdCMVtk). This study identified that all patients who displayed accumulation of 18F-FHBG in tumor lesions within the first hours of injecting the viral vector containing HSV1-tk showed stable disease a month later compared with patients without detectable 18F-FHBG tumor lesions who progressed at 1 month. This study suggests that PET imaging can be used to assess transduction efficiency of a viral vector as well as predict the efficacy of gene-therapy strategies, making it a potential tool in early phase clinical trials.
There is still no ideal PET tracer for the assessment of HCC, and the search for potential tracers continues to be in development (Table 1). Recent attempts, with 11C-metomidate (methyl derivative of etomidate), previously used as an imaging tool in detection of adrenocortical tumors, have been disappointing with low sensitivity for detection of HCC compared with 11C-acetate [58]. The tracer binds to GABA (gamma-aminobutyric acid) receptors, which are upregulated in HCC [59]. Further preliminary work has also been carried out investigating the use of (4S)-4-(3–18F-fluoropropyl)-l-glutamate (18F-FSPG), for imaging of xC - transporter activity in HCC [60]. xC -, is a sodium independent transporter system that is responsible for the defense machinery in cells against oxidative stress and mediates uptake of cysteine in exchange for intracellular glutamate [61,62]. Thiol containing molecules such as glutathione are essential for the deactivation of reactive oxygen species, and this defense mechanism, offers a particular advantage for tumor cell growth [63].
Table 1. . Summary of translational studies that have investigated use of positron emission tomography in hepatocellular carcinoma.
| Radiotracer | Study | Number of patients | Sensitivity (%) | Specificity (%) | Accuracy (%) | Ref. |
|---|---|---|---|---|---|---|
| 18F-FDG | Cheung | 58 | 43% detection of primary HCC55% predicting MI | 69% predicting MI | [67] | |
| Wu | 76 | 63% detection of primary HCC83% detection of EM | 95% in EM | 91% in EM | [68] | |
| Ho | 121 | 79% detection of EM | 91% | [19] | ||
| Chen | 31 | 73% detecting HCC recurrence | 100% | [7] | ||
| Wang | 11 | 100% detecting HCC recurrence | 67% | [69] | ||
| Ho | 257 | 62% detection of EM | [70] | |||
| Yamamoto | 12 | 50% detection of primary HCC | [44] | |||
| Khan | 20 | 55% detection of primary HCC | [13] | |||
| Park | 99 | 64% detection of primary HCC; 86% detection of EM | 100% | [39] | ||
| Paudyal | 24 | 92% detection of recurrence | 100% | [33] | ||
| 18F-FLT | Eckel | 16 | 69% detection of primary HCC | [54] | ||
| 11C- Acetate(in combination with 18F-FDG) | Ho | 39 | 87% primary HCC | [38] | ||
| Ho | 257 | 93% alone97% combined with 18F-FDG detection of EM | [70] | |||
| Park | 99 | 84% alone; detection of primary HCC 77% alone; detection of EM86% combined with 18F-FDG detection of EM | [39] | |||
| Cheung | 58 | 93% predicting MI | 0% | [67] | ||
| 11C- Choline | Wu | 76 | 86% detection of primary HCC 90% (dual tracer with 18F-FDG) | [68] | ||
| Yamamoto | 12 | 63% detection of primary HCC | [44] | |||
18F-FDG: 18F-Fluorodeoxyglucose; 18F-FLT: 18F-Fluorothymidine; EM: Extrahepatic metastases; HCC: Hepatocellular carcinoma; MI: Microvascular invasion.
Conclusion & future perspective
The low sensitivity of 18F-FDG in the assessment of intrahepatic HCC patient, limits routine clinical use, and is limited to the evaluation of extrahepatic disease in some centers. The advent of new radiotracers enables the visualization of other metabolic processes apart from glucose metabolism and have improved diagnostic sensitivity both in conjunction with 18F-FDG PET or as when used as single agent for imaging of HCC. While the role of dual tracers in imaging will continue to evolve this strategy is limited because of need for on-site cyclotron facilities, cost and inconvenience for patients. It is also important to consider other limitations of PET, in particular the inability to detect small lesions (<2 cm) due to poor spatial resolution, and partial volume effects as well as additional radiation exposure. PET imaging also involves quite complex scanning protocols and set up and analysis is resource intensive. These limitations mean that we will continue to combine imaging procedures using CT, MRI and PET. This multiparametric imaging approach uses both morphological and molecular information, enabling us to understand the biologic processes and guides us in management decisions for patients [64].
The advent of PET/MRI hybrid imaging systems has the potential for improved accuracy of staging, with MRI providing information on tumor extent and identification of small lesions that are pivotal for treatment decisions and in assessing whether patients are suitable for radical or palliative approach. Treatment response is often difficult to assess using RECIST criteria, especially with the advent of targeted drugs, which often produce cytostatic changes or necrosis. The role of PET, therefore, allowing detection of changes in metabolic processes may lie primarily in response assessment and aid in identifying tumor recurrence. PET imaging has shown to predict response to therapy in other tumor types as well as guide targeted therapy with better understanding of the metabolic processes of liver tumor cells.
Current search for biomarkers in HCC are in development with cross collaboration in imaging and drug development. Galectin-4, a multifunctional lectin present intra- and extra-cellularly, has recently been identified as a potential prognostic marker in HCC [65]. Another avenue of potential research is the application of mathematical modeling techniques using 18F-FLT-PET to enhance the visualization of liver lesions [66].
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 1999;340(10):745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Surveillance for hepatocellular carcinoma: long way to achieve effectiveness. Dig. Dis. Sci. 2012;57(12):3050–3051. doi: 10.1007/s10620-012-2413-z. [DOI] [PubMed] [Google Scholar]
- 4.Verslype C, Rosmorduc O, Rougier P, Group EGW. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl.7):41–48. doi: 10.1093/annonc/mds225. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):s44–s57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 6.Wee A. Fine-needle aspiration biopsy of hepatocellular carcinoma and related hepatocellular nodular lesions in cirrhosis: controversies, challenges, and expectations. Patholog. Res. Int. 2011 doi: 10.4061/2011/587936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen WT, Chau GY, Lui WY, et al. Recurrent hepatocellular carcinoma after hepatic resection: prognostic factors and long-term outcome. Eur. J. Surg. Oncol. 2004;30(4):414–420. doi: 10.1016/j.ejso.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105(2):488–494. doi: 10.1016/0016-5085(93)90724-q. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur. J. Cancer. 2003;39(14):2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Aboagye E. Development of radiotracers for oncology‐‐the interface with pharmacology. Br. J. Pharmacol. 2011;163(8):1565–1585. doi: 10.1111/j.1476-5381.2010.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review of regulations and development of positron emission tomography (PET) radiopharmaceuticals with assessment of pharmacokinetics and pharmacodynamics.
- 12.Rigo P, Paulus P, Kaschten BJ, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur. J. Nucl. Med. 1996;23(12):1641–1674. doi: 10.1007/BF01249629. [DOI] [PubMed] [Google Scholar]
- 13.Khan MA, Combs CS, Brunt EM, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J. Hepatol. 2000;32(5):792–797. doi: 10.1016/s0168-8278(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 14.Teefey SA, Hildeboldt CC, Dehdashti F, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226(2):533–542. doi: 10.1148/radiol.2262011980. [DOI] [PubMed] [Google Scholar]
- 15.Trojan J, Schroeder O, Raedle J, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am. J. Gastroenterol. 1999;94(11):3314–3319. doi: 10.1111/j.1572-0241.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- 16.Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK, Jr, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch. Surg. 1998;133(5):510–515. doi: 10.1001/archsurg.133.5.510. [DOI] [PubMed] [Google Scholar]
- 17.Wudel LJ, Jr., Delbeke D, Morris D, et al. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am. Surg. 2003;69(2):117–124. [PubMed] [Google Scholar]
- 18.Katyal S, Oliver JH, 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 19.Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J. Nucl. Med. 2007;48(6):902–909. doi: 10.2967/jnumed.106.036673. [DOI] [PubMed] [Google Scholar]; • Small study of 56 patients with hepatocellular carcinoma (HCC). Highlights potential use and benefit of dual tracers.
- 20.Sugiyama M, Sakahara H, Torizuka T, et al. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J. Gastroenterol. 2004;39(10):961–968. doi: 10.1007/s00535-004-1427-5. [DOI] [PubMed] [Google Scholar]
- 21.Yoon KT, Kim JK, Kim Do Y, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in detecting extrahepatic metastasis in pretreatment staging of hepatocellular carcinoma. Oncology. 2007;72(Suppl.1):104–110. doi: 10.1159/000111715. [DOI] [PubMed] [Google Scholar]
- 22.Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Eur. J. Radiol. 2012;81(9):2417–2422. doi: 10.1016/j.ejrad.2011.08.004. [DOI] [PubMed] [Google Scholar]; •• Systematic review and meta-analysis of seven retrospective and one prospective study which highlights potential use of 18F-FDG PET/CT in early detection of extrahepatic metastases.
- 23.Lo CM, Lai EC, Fan ST, Choi TK, Wong J. Resection for extrahepatic recurrence of hepatocellular carcinoma. Br. J. Surg. 1994;81(7):1019–1021. doi: 10.1002/bjs.1800810730. [DOI] [PubMed] [Google Scholar]
- 24.Aramaki M, Kawano K, Sasaki A, et al. Prolonged survival after repeat resection of pulmonary metastasis from hepatocellular carcinoma. J. Hepatobiliary Pancreat. Surg. 2002;9(3):386–388. doi: 10.1007/s005340200046. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 26.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann. Surg. 1993;218(2):145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang SH, Suh KS, Lee HW, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006;12(11):1655–1660. doi: 10.1002/lt.20861. [DOI] [PubMed] [Google Scholar]; • Retrospective study of 38 patients diagnosed with HCC, undergoing liver transplant, showing significant association between hepatic tumor recurrence and 18F-FDG positivity.
- 28.Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann. Surg. 1991;214(3):221–228. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 30.Roayaie S, Haim MB, Emre S, et al. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C. A western experience. Ann. Surg. Oncol. 2000;7(10):764–770. doi: 10.1007/s10434-000-0764-8. [DOI] [PubMed] [Google Scholar]
- 31.Shetty K, Timmins K, Brensinger C, et al. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10(7):911–918. doi: 10.1002/lt.20140. [DOI] [PubMed] [Google Scholar]
- 32.Jinpeng Li M, Shao1md W, Jinlong Song M, Shi C, Hua Chen M, Cong N. The therapeutic effect of transcatheter arterial thromboembolization of hepatocellular carcinoma as for residual viable tumors related to lipiodol density areas and detected by 18F-FDG PET/CT and CT. Hell. J. Nucl. Med. 2013;16(1):64–65. [PubMed] [Google Scholar]
- 33.Paudyal B, Oriuchi N, Paudyal P, et al. Early diagnosis of recurrent hepatocellular carcinoma with 18F-FDG PET after radiofrequency ablation therapy. Oncol. Rep. 2007;18(6):1469–1473. [PubMed] [Google Scholar]
- 34.Song MJ, Bae SH, Yoo Ie R, et al. Predictive value of (1)(8)F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J. Gastroenterol. 2012;18(25):3215–3222. doi: 10.3748/wjg.v18.i25.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JD, Yun M, Lee JM, et al. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2004;31(12):1621–1630. doi: 10.1007/s00259-004-1602-1. [DOI] [PubMed] [Google Scholar]
- 36.Grassi I, Nanni C, Allegri V, et al. The clinical use of PET with (11)C-acetate. Am. J. Nucl. Med. Mol. Imaging. 2012;2(1):33–47. [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 38.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J. Nucl. Med. 2003;44(2):213–221. [PubMed] [Google Scholar]
- 39.Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 2008;49(12):1912–1921. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 40.Cheung TT, Chan SC, Ho CL, et al. Can positron emission tomography with the dual tracers [11C] acetate and [18F] fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl. 2011;17(10):1218–1225. doi: 10.1002/lt.22362. [DOI] [PubMed] [Google Scholar]; • Fifty eight patients examined with dual tracer PET during pre-operative assessment prior to liver resection or transplant. 11C-acetate increased sensitivity of 18F-FDG PET/CT, but did not predict for microvascular invasion.
- 41.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat. Rev. Cancer. 2011;11(12):835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez De Molina A, Rodriguez-Gonzalez A, Gutierrez R, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem. Biophys. Res. Commun. 2002;296(3):580–583. doi: 10.1016/s0006-291x(02)00920-8. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez De Molina A, Sarmentero-Estrada J, Belda-Iniesta C, et al. Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: a retrospective study. Lancet Oncol. 2007;8(10):889–897. doi: 10.1016/S1470-2045(07)70279-6. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto Y, Nishiyama Y, Kameyama R, et al. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J. Nucl. Med. 2008;49(8):1245–1248. doi: 10.2967/jnumed.108.052639. [DOI] [PubMed] [Google Scholar]
- 45.Talbot JN, Gutman F, Fartoux L, et al. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2006;33(11):1285–1289. doi: 10.1007/s00259-006-0164-9. [DOI] [PubMed] [Google Scholar]
- 46.Wu Hb WQ, Li by, Li Hs, Zhou Wl, Wang QY. F-18 FDG in conjunction with 11C-choline PET/CT in the diagnosis of hepatocellular carcinoma. Clin. Nucl. Med. 2011 Dec;36(12):1092–1097. doi: 10.1097/RLU.0b013e3182335df4. [DOI] [PubMed] [Google Scholar]
- 47.Seitz U, Wagner M, Neumaier B, et al. Evaluation of pyrimidine metabolising enzymes and in vitro uptake of 3‘- [(18)F]fluoro-3‘-deoxythymidine ([(18)F]FLT) in pancreatic cancer cell lines. Eur. J. Nucl. Med. Mol. Imaging. 2002;29(9):1174–1181. doi: 10.1007/s00259-002-0851-0. [DOI] [PubMed] [Google Scholar]
- 48.Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J. Biol. Chem. 1988;263(17):8350–8358. [PubMed] [Google Scholar]
- 49.Munch-Petersen B, Cloos L, Jensen HK, Tyrsted G. Human thymidine kinase 1. Regulation in normal and malignant cells. Adv. Enzyme Regul. 1995;35:69–89. doi: 10.1016/0065-2571(94)00014-t. [DOI] [PubMed] [Google Scholar]
- 50.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998;4(11):1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 51.Francis DL, Visvikis D, Costa DC, et al. Potential impact of [18F]3‘-deoxy-3‘-fluorothymidine versus [18F]fluoro-2-deoxy-D-glucose in positron emission tomography for colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging. 2003;30(7):988–994. doi: 10.1007/s00259-003-1187-0. [DOI] [PubMed] [Google Scholar]
- 52.Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3‘-deoxy-3‘- [18F]fluorothymidine positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2007;34(9):1339–1347. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 53.Buck AK, Halter G, Schirrmeister H, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J. Nucl. Med. 2003;44(9):1426–1431. [PubMed] [Google Scholar]
- 54.Eckel F, Herrmann K, Schmidt S, et al. Imaging of proliferation in hepatocellular carcinoma with the in vivo marker 18F-fluorothymidine. J. Nucl. Med. 2009;50(9):1441–1447. doi: 10.2967/jnumed.109.065896. [DOI] [PubMed] [Google Scholar]
- 55.Yaghoubi S, Barrio JR, Dahlbom M, et al. Human pharmacokinetic and dosimetry studies of [18F] FHBG: a reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J. Nucl. Med. 2001;42(8):1225–1234. [PubMed] [Google Scholar]
- 56.Sangro B, Prieto J. Gene therapy for liver cancer: clinical experience and future prospects. Curr. Opin. Mol. Ther. 2010;12(5):561. [PubMed] [Google Scholar]
- 57.Peñuelas I, Mazzolini G, Boán JF, et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology. 2005;128(7):1787–1795. doi: 10.1053/j.gastro.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Roivainen A, Naum A, Nuutinen H, et al. Characterization of hepatic tumors using [11C]metomidate through positron emission tomography: comparison with [11C]acetate. EJNMMI Res. 2013;3(1):13. doi: 10.1186/2191-219X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Li YH, Guo FJ, et al. Gamma-aminobutyric acid promotes human hepatocellular carcinoma growth through overexpressed gamma-aminobutyric acid A receptor alpha 3 subunit. World J. Gastroenterol. 2008;14(47):7175–7182. doi: 10.3748/wjg.14.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baek S, Mueller A, Lim YS, et al. (4S)-4-(3–18F-fluoropropyl)-l-glutamate for imaging of xC transporter activity in hepatocellular carcinoma using PET: preclinical and exploratory clinical studies. J. Nucl. Med. 2013;54(1):117–123. doi: 10.2967/jnumed.112.108704. [DOI] [PubMed] [Google Scholar]
- 61.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J. Biol. Chem. 1986;261(5):2256–2263. [PubMed] [Google Scholar]
- 62.Koglin N, Mueller A, Berndt M, et al. Specific PET imaging of xC- transporter activity using a (1)(8)F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin. Cancer Res. 2011;17(18):6000–6011. doi: 10.1158/1078-0432.CCR-11-0687. [DOI] [PubMed] [Google Scholar]
- 63.Ogunrinu TA, Sontheimer H. Hypoxia increases the dependence of glioma cells on glutathione. J. Biol. Chem. 2010;285(48):37716–37724. doi: 10.1074/jbc.M110.161190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256(2):348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 65.Cai Z, Zeng Y, Xu B, et al. Galectin-4 serves as a prognostic biomarker for the early recurrence/metastasis of hepatocellular carcinoma. Cancer Sci. 2014;105(11):1510–1517. doi: 10.1111/cas.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Contractor KB, Kenny LM, Stebbing J, et al. [18F]-3‘Deoxy-3‘-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin. Cancer Res. 2011;17(24):7664–7672. doi: 10.1158/1078-0432.CCR-11-0783. [DOI] [PubMed] [Google Scholar]
- 67.Cheung TT, Chan SC, Ho CL, et al. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl. 2011;17(10):1218–1225. doi: 10.1002/lt.22362. [DOI] [PubMed] [Google Scholar]
- 68.Wu HB, Wang QS, Li BY, Li HS, Zhou WL, Wang QY. F-18 FDG in conjunction with 11C-choline PET/CT in the diagnosis of hepatocellular carcinoma. Clin. Nucl. Med. 2011;36(12):1092–1097. doi: 10.1097/RLU.0b013e3182335df4. [DOI] [PubMed] [Google Scholar]
- 69.Wang XL, Li H, Wang QS, Zhang XL. Clinical value of pre-and postoperative 18F-FDG PET/CT in patients undergoing liver transplantation for hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26(8):1087–1091. [PubMed] [Google Scholar]
- 70.Ho CL, Chen S, Cheng TK, Leung YL. PET/CT characteristics of isolated bone metastases in hepatocellular carcinoma. Radiology. 2011;258(2):515–523. doi: 10.1148/radiol.10100672. [DOI] [PubMed] [Google Scholar]