Abstract
Cholangiocarcinoma (CCA) is the second most common primary hepatic neoplasm and accounts for 10–20% of hepatobiliary cancer-related deaths. The prognosis of patients with CCA is poor with 5-year survival rates of 10%, partially due to the limited effective treatment options that exist for this disease. In this review, we discuss the evolving role of liver transplantation in the management of patients with perihilar CCA (pCCA). We specifically discuss the Mayo Clinic protocol of neoadjuvant chemoradiation followed by liver transplantation in selected patients with pCCA and describe pretransplant, peritransplant, and post-transplant considerations and challenges with this approach. Finally, we review local, national and international outcomes and discuss future directions in optimizing this treatment strategy for patients who otherwise have few therapeutic options and limited survival.
KEYWORDS : cholangiocarcinoma, intrahepatic cholangiocarcinoma, liver transplantation, neoadjuvant therapy, perihilar cholangiocarcinoma, primary sclerosing cholangitis
Practice points.
Mayo Clinic protocol
Structured protocol consisting of neoadjuvant external beam radiation, chemotherapy and brachytherapy followed by liver transplantation.
Pretransplant considerations
Eligible patients have unresectable perihilar cholangiocarcinoma (pCCA) or pCCA associated with PSC.
Diagnosis made with pathological confirmation from biopsy or cytology odds ratio (OR) malignant appearing stricture with suspicious cytology and FISH polysomy OR a mass lesion on cross-sectional imaging OR a malignant appearing stricture with CA-19-9 >100 U/ml in the absence of bacterial cholangitis.
Peritransplant challenges
Wait list drop out is commonly due to progressive disease and occurs in about 30%.
Predictors of drop out include CA-19-9 level ≥ 500 U/ml, mass ≥ 3 cm, malignant brushing or biopsy and MELD ≥ 20.
Surgical considerations & postoperative complications
Post-transplantation vascular complications can occur in up to 40% of patients and are likely a result of neoadjuvant chemoradiotherapy.
Outcomes
Local, national and international experience suggests that 2-year overall survival ranges from 65 to 70% and 5-year recurrence-free survival ranges from 47 to 68%.
Patients with PSC-associated CCA have improved post-transplant survival compared with patients with de novo CCA.
Predictors of post-transplant recurrence include elevated CA-19-9, portal vein encasement, and residual tumor on explant pathology.
Conclusion
Neoadjuvant chemoradiotherapy followed by liver transplantation affords prolonged survival in a highly selected group of patients with pCCA managed under a structured protocol.
Liver transplantation in the management of iCCA is under study, and for the time being remains controversial.
Cholangiocarcinoma (CCA) is the second most common primary hepatic neoplasm after hepatocellular carcinoma (HCC) and is reported to be rising in incidence worldwide [1]. It is estimated that there are approximately 3000–4000 new cases of CCA per year in the USA [2], with the incidence being particularly high in patients with primary sclerosing cholangitis (PSC) at up to 10% within 10 years of diagnosis [3]. The mean age of diagnosis is at 50 years and is uncommon before age 40 – except in the presence of PSC where CCA can occur earlier [4]. Unfortunately, the prognosis of patients with CCA remains dismal with only approximately 10% of patients surviving 5 years after diagnosis [5].
CCA is a highly aggressive malignant neoplasm with features of biliary epithelial differentiation [4]. It is classified by anatomic location with different sites corresponding to different tumor biology and mandating specific treatment approaches. Intrahepatic CCAs (iCCAs) are located within the hepatic parenchyma and are anatomically separated from extrahepatic CCA by the second order bile ducts [6]. Extrahepatic CCA consists of perihilar CCA (pCCA) and distal CCA (dCCA) with the anatomic distinction between these subtypes occurring at the level of the cystic duct [6]. pCCA is the most common subtype accounting for about 50% of cases, followed by dCCA in about 40%, and iCCA in approximately 10% [7]. As will be discussed, most of the literature has focused on liver transplantation in the management of perihilar CCA and as such for the purposes of this review we will focus on this indication. Currently, the role of transplantation in the management of iCCA remains investigational and not widely accepted.
Liver transplantation in patients with pCCA
The rationale for considering liver transplantation as a management option for patients with pCCA arises from features characteristic of this tumor. pCCA occurs in the perihilar bile ducts and typically presents with signs of biliary obstruction such as jaundice, and less commonly, cholangitis [8] allowing it to clinically manifest early in its course. In addition, distant metastases occur uncommonly and thus, disease often remains confined to the liver. Furthermore, surgical resection for localized disease is often limited by bilateral liver involvement, vascular encasement, perineural and lymphatic invasion, and functional hepatic reserve [9]. Resectability rates vary widely in the literature from 28 to 80% leaving a significant proportion of patients with little hope for cure [10–13]. Survival in patients with unresectable CCA is estimated at just 12–16 months after the onset of symptoms [14]; yet, even among patients who do undergo curative R0 resection, 5-year survival rates range between 20 and 40% [10,15–26]. Patients with PSC who have a lifetime risk of CCA on the order of 10–15% are particularly challenging to manage with resection given their underlying parenchymal liver disease, the presence of skip lesions, and the diffuse oncogenic field defect that exists in the biliary tree [4]. Clearly, alternative and effective treatment options for these patients are desperately needed.
Liver transplantation was considered to be the ideal operation for CCA given its ability to provide radical resection, achieve wide surgical margins, circumvent limitations imposed by residual liver volume and function and treat underlying disease in the case of PSC. Unfortunately, the early experience with liver transplantation alone failed to confirm these desired expectations. The Cincinnati Transplant Tumor Registry reported 5-year survival estimates of just 23% with 51% of patients developing tumor recurrence, usually within 2 years of transplantation. Half of the recurrences occurred in the allograft and survival after recurrence was rarely longer than 1 year [27]. Other series from Scandinavia [28], Spain [29] and Canada [30] reported similar results with 3 and 5-year survival rates of about 30%. Even more aggressive approaches with cluster abdominal transplantation failed to improve outcomes – a series from the University of Pittsburgh reported a 20% 3-year survival with this approach [31]. This knowledge combined with scarcity of the donor pool caused most transplant programs to abandon liver transplantation for CCA (Figure 1).
Figure 1. . Number of liver transplants done between 1988 and 2014 with an indication of cholangiocarcinoma as per the United Network for Organ Sharing database.
Data taken with permission from [32].
Though overall outcomes were suboptimal, selected patients with negative surgical margins and absence of regional lymph node metastasis did benefit from transplantation and achieved prolonged survival [33]. Furthermore, adjuvant approaches using radiation with and without chemotherapy also resulted in improved outcomes in a proportion of patients. In a small cohort of patients treated at Mayo Clinic, 22% 5-year survival was achieved in patients with unresectable extrahepatic CCA treated with external beam radiation therapy (EBRT) plus concomitant chemotherapy with infusional 5-FU followed by intraluminal brachytherapy [34]. A similar trimodal approach at the Thomas Jefferson University Hospital demonstrated 2-year survival rates of 30% [35]. Armed with knowledge of potential benefit of chemoradiotherapy and the high rate of locoregional recurrence after surgery, the University of Nebraska [36] and then Mayo Clinic established structured protocols using neoadjuvant chemoradiation therapy followed by liver transplantation in the management of selected patients with pCCA.
Mayo Clinic protocol
In 1993, Mayo Clinic developed a protocol using EBRT plus concomitant infusional 5-FU followed by brachytherapy, prolonged chemotherapy and subsequent liver transplantation for selected patients with early stage pCCA. As of 18 March 2015, 168 patients have been transplanted using this protocol at our center. Eligible patients undergo EBRT at a dose of 1.5 Gy twice daily over 3 weeks for a total of 45 Gy in 30 fractions. 5-FU is administered by continuous venous infusion during radiation therapy at a dose of 225 mg/m2/day [37]. Two weeks after the completion of EBRT intraluminal brachytherapy is administered by transcatheter irradiation with iridium-192 beads delivered endoluminally through an endoscopically (or rarely, percutaneously) placed biliary tube at a target dose of 16 Gy in four fractions at a 1-cm radius over 2 days [37]. If brachytherapy is not possible, an extra boost of EBRT dosed at 1000–1500 cGy is administered [38]. Since 2001, prolonged chemotherapy with oral capecitabine 2000 mg/m2/day in two divided doses 2 out of every 3 weeks is then given until the time of transplantation whereas prior to this infusional 5-FU was used and delivered through an ambulatory infusion pump [39]. After listing and prior to transplantation a staging abdominal exploration is performed to ensure disease remains localized. In the case of deceased donor transplant recipients this is performed as patients approach the top of the waiting list, and for living donor transplant recipients exploration is performed the day before transplantation. During the staging procedure (now done using a hand assisted laparoscopic approach in patients with no prior abdominal operations) a thorough abdominal exploration is performed [39]. At least one lymph node along the hepatic artery proper and common bile duct is excised and examined for metastasis even if it appears grossly normal. Suspicious lymph nodes or nodules are removed and examined pathologically for tumor. The liver is carefully palpated for evidence of intrahepatic metastasis that has gone undetected by imaging. Identification of regional lymph node metastasis, peritoneal metastasis, or locally extensive disease precludes transplantation. During transplantation, a caval sparing approach is used unless there is suspected caudate involvement or atrophy that would threaten the resection margin. A frozen section of the bile duct margin is also obtained and pancreaticoduodenectomy performed if there is microscopic involvement [40]. Standard immunosuppressive regimens are used post transplantation and currently include short-term mycophenolate mofetil and prednisone taper with long-term tacrolimus monotherapy.
Pretransplant considerations: diagnosis & eligibility
• CCA diagnosis
In its early stages, pCCA can be difficult to diagnose, partly owing to its desmoplastic nature [41] and its initial growth pattern along bile ducts rather than away from them, making both histologic confirmation and imaging detection difficult [42]. This tropism for bile often leads to stricturing and biliary obstruction, which most commonly manifests as painless jaundice [8]. Approximately half of patients have nonspecific or systemic symptoms including abdominal discomfort, generalized malaise, nausea and weight loss [43]. On physical examination, occasionally a palpable liver lobe is detected which usually reflects compensatory hypertrophy of the unaffected lobe due to atrophy of the contralateral affected lobe as a result of vascular encasement by tumor – the so called ‘atrophy-hypertrophy complex’ (Figure 2). Laboratory studies often include nonspecific elevations in serum alkaline phosphatase, bilirubin and CA19–9. While helpful when used in combination with other diagnostic tests, isolated elevations of CA19-9 can be found in benign hepatobiliary disease such as cholangitis or PSC. Using a cutoff of 129 U/ml in patients with PSC, the sensitivity and specificity of an elevated CA19–9 in CCA is 79 and 98%, respectively [44]. That said, the positive predictive value is about 56% [45] and two-thirds of PSC patients with elevations above this cutoff do not seem to develop CCA [46]. In patients with de novo CCA, a CA19–9 level >100 U/ml has a sensitivity of 75% and a specificity of 92% when compared with patients with benign biliary strictures [6,47]. Furthermore, CA19-9 levels >1000 U/ml in the absence of cholangitis are associated with unresectable disease and poorer prognosis compared with lower serum levels [48] and should raise suspicion for metastatic disease.
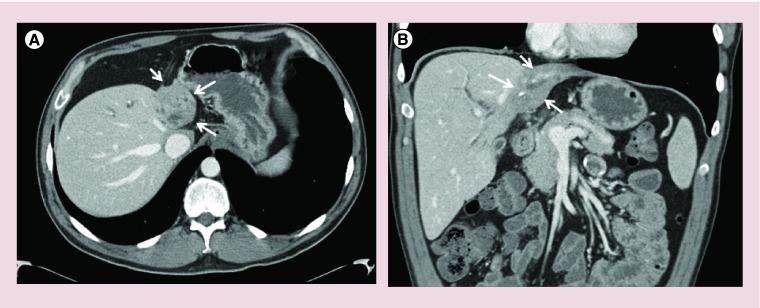
Figure 2. . Axial and coronal CT images demonstrating the ‘atrophy-hypertrophy complex’ in a patient with perihilar cholangiocarcinoma.
(A) Axial section demonstrating marked left lobe atrophy as highlighted by the arrows. (B) Coronal section demonstrating atrophy of the left lobe due to vascular involvement by tumor and compensatory hypertrophy of the unaffected right lobe.
Imaging modalities including CT, MRI, endoscopic retrograde cholangiography (ERC) and endoscopic ultrasound (EUS) are important in the diagnosis and staging of pCCA. CT has a sensitivity of approximately 85% in identifying vascular involvement by pCCA and an overall accuracy of 86% in determining ductal extent of tumor [49]; however, its ability to reliably detect regional lymphadenopathy is limited. Magnetic resonance cholangiography (MRC) is the modality of choice for pCCA as it has an accuracy of up to 95% in assessing locoregional disease extent and resectability, though its assessment of vascular involvement is suboptimal [6,50–53]. MRI and MRC also perform well in patients with PSC with a sensitivity of 89% and accuracy of 76% in detecting CCA [44]; and in fact, its diagnostic accuracy is comparable to that of ERC.
ERC is critical in the diagnosis and management of pCCA as it allows for direct visualization of dominant strictures and ductal abnormalities, sampling of biliary epithelial cells for cytological analysis, and can provide therapeutic relief of biliary obstruction with dilation and/or stenting [8]. Though conventional cytology can confirm the diagnosis, obtaining adequate tissue endoscopically (or percutaneously) is challenging, and often limited by acellular specimens, sampling errors and difficulty with interpretation, particularly in the setting of PSC or cholangitis where inflammation can mimic malignant cytologic changes [54]. Specimens analyzed by conventional cytology can be classified as negative, atypical, suspicious, or diagnostic of malignancy; when only diagnostic specimens are considered, the sensitivity of this technique is just 15% with only slight improvement to 48% when diagnostic or suspicious interpretations are considered for cancer diagnosis [54]. Because of the low sensitivity, many centers now use fluorescence in situ hybridization (FISH) to aid in the diagnosis of CCA. This technique employs fluorescently labeled DNA probes to identify structural chromosomal changes in chromosomes 3, 7, 17, and the 9p21 locus within cells. Compared with conventional cytology, FISH polysomy increases sensitivity for diagnosis of pCCA to 38–58% [55,56] while maintaining specificity. Polysomy is observed in up to 77% of CCA cases [6,57], and at our center, polysomy in the presence of a malignant appearing stricture is sufficient for the diagnosis of pCCA, particularly if this is confirmed over time [58]. Polysomy as detected by FISH can also predict pCCA development in a subset of patients with PSC. A recent study demonstrated that among patients with PSC with no detectable mass by imaging, equivocal cytology (atypical or suspicious), a CA19–9 >129 U/ml and polysomy all went on to develop CCA, frequently within 2 years [59].
EUS is a particularly valuable imaging modality in the evaluation of unresectable pCCA prior to transplantation [60]. It is able to detect tumors at a rate of 94% in patients with CCA, with distal tumors more readily identified than proximal tumors (100 vs 83%) [61]. While EUS-guided fine needle aspiration of the primary tumor does carry a diagnostic sensitivity 73% [61], this practice is strongly discouraged due to a high risk of tumor seeding. In a study of 191 patients evaluated for liver transplantation for CCA at our center, 16 patients underwent EUS-guided fine needle aspiration (FNA) of their primary tumor and malignancy was detected in 6 patients [62]. Peritoneal metastasis was detected at operative staging in 83% of patients in whom malignancy was detected on EUS-FNA compared with 0% of patients without malignancy. Moreover, only 8% (14/175) of patients who did not undergo transperitoneal FNA had peritoneal metastasis detected emphasizing the high risk of seeding associated with this procedure. Due to this risk, at our center transperitoneal FNA of the primary tumor is an absolute contraindication for proceeding with transplantation. EUS is especially valuable in staging of locoregional lymph node involvement prior to transplantation [60]. In a study of 47 patients with unresectable pCCA, EUS identified lymph nodes in all patients and FNA of identified nodes detected metastasis in 17% of patients, thus precluding transplantation and avoiding operative staging. In addition, 91% of lymph nodes identified as histologically benign by EUS-FNA were confirmed as such during operative staging [63].
Given its associated challenges, at our center eligibility for liver transplantation in the setting of pCCA is not limited by a requirement for histologic confirmation. CCA is diagnosed if any of the criteria in Box 1 are met. In a study of 215 patients who received neoadjuvant therapy as part of the liver transplant protocol for pCCA, 98 patients did not have pretreatment pathological confirmation, yet 53% of these patients subsequently had pathologically proven disease either at staging, in the explant, or developed disease recurrence [64]. Thus, though pathological confirmation is ideal, its absence should not be considered an exclusion for transplantation if other criteria are met. As will be discussed later, in this cohort, the survival benefit demonstrated with liver transplantation both in patients with PSC and de novo pCCA remained similar even after excluding all patients without pretreatment pathological confirmation [64].
Box 1. . Diagnostic criteria for eligibility in the liver transplantation for perihilar cholangiocarcinoma protocol at the Mayo Clinic.
Any of
Biopsy or conventional cytology positive for adenocarcinoma
Malignant appearing stricture with suspicious cytology for adenocarcinoma plus FISH polysomy
Mass lesion on cross-sectional imaging
Malignant appearing stricture with CA-19-9 >100 U/ml in absence of bacterial cholangitis
• Eligibility
Eligible patients include those with unresectable pCCA as determined by an experienced hepatobiliary surgeon or pCCA in the setting of PSC [40]. Diagnosis is established as described above. Tumors must have a radial diameter of ≤3 cm though there are no longitudinal limits for bile duct involvement given the difficulty in assessment of this parameter. Clearly, eligible candidates must also otherwise be suitable for neoadjuvant chemoradiotherapy and liver transplantation. All patients undergo staging to assess for intra- and/or extra-hepatic disease using chest CT, abdominal MRI with MRC and bone scan. Whereas detection of distant or metastatic intrahepatic disease precludes transplantation, vascular encasement is not a contraindication. All patients subsequently undergo EUS-FNA of celiac and perihilar nodes and those with negative staging proceed with neoadjuvant therapy, operative staging and, subsequently, transplantation. Exclusion criteria are listed in Box 2 [40].
Box 2. Exclusion criteria for liver transplantation for perihilar cholangiocarcinoma protocol at the Mayo Clinic.
Tumor characteristics
Intrahepatic cholangiocarcinoma
Intrahepatic metastasis
Extrahepatic disease
Gall bladder involvement
Prior therapies or interventions
Prior radiation or chemotherapy
Prior biliary resection or attempted resection
Transperitoneal biopsy including percutaneous and endoscopic ultrasound guided fine needle aspiration of primary tumor
Patient characteristics
Comorbid medical condition that renders patient unsuitable for transplantation
Peritransplant challenges
Once eligibility is established, a unique set of challenges ensue related to tolerability of neoadjuvant treatment, risk of progressive disease and wait list drop out, and recipient prioritization. Complications associated with preoperative chemoradiation therapy are variable and range from mild nausea or vomiting to radiation-associated GI ulceration with hemorrhage, venothromboembolic disease and dose limiting bone marrow suppression. Infectious complications are also of concern and in one series, problems related to cholangitis, hepatic abscess or sepsis related to indwelling biliary stents occurred in 20% of patients [65]. Such infections are often treatable with prompt antibiotic therapy; however, in the aforementioned series 1 of 19 included patients died of biliary sepsis [65]. Furthermore, repeated use of broad-spectrum antibiotics may put these patients at increased risk of infection with antibiotic resistant isolates. Patients with PSC have been shown to have altered biliary bacteriology, with a significantly higher rate of infection with gram positive bacteria compared with those without PSC, potentially increasing the risk of complicated infections in this subgroup of patients [66].
The most important factor leading to wait list drop out in patients with pCCA initially eligible for transplantation is progressive disease. In a recent study of 199 patients at Mayo Clinic, 62 (31%) dropped out of the protocol after a median of 4.7 months with 89% of these patients becoming ineligible due to progressive disease [38]. On multivariable analysis, independent predictors of protocol dropout included a CA19–9 level ≥ 500 U/ml (HR = 2.3; 95% CI: 1.2–4.3), tumor mass ≥ 3 cm (HR = 2.2; 95% CI: 1.2–4.0), malignant brushing or biopsy (HR = 3.6; 95% CI: 1.7–7.9) and MELD score ≥ 20 (HR = 3.5; 95% CI: 1.5–8.3) [38]. The outcome of patients who become ineligible for transplantation is poor and comparable with those with advanced and metastatic CCA [37]. In another study using the same cohort, the median overall survival of patients who fell out of the protocol was 12.3 months (95% CI: 10.7–13.5) with median survival after fallout being just 6.8 months (95% CI: 5.1–8.5) [37].
In 2002 the MELD system was implemented, which allowed prioritization for liver transplant candidates based on their risk of dying on the waiting list within 3 months. Selected patients are granted MELD exception points based on regional agreements and since January 2010, all patients with pCCA that meet highly specified criteria are granted standardized MELD exception scores starting at 22 with a 10% increase every 3 months, corresponding to a 10% waitlist mortality at 3-month intervals [67]. This approach was supported by a recent multicenter study in the USA where 71 of 287 eligible patients (25%) dropped out of the protocol after a median of 4.6 months, corresponding to a dropout rate that increased by 11.5% every 3 months [68].
Surgical considerations & postoperative complications
Liver transplantation for pCCA at our center is most commonly performed with caval sparing hepatectomy. Hilar dissection is avoided to prevent disease spread [69]. The distal bile duct margin is assessed histologically by frozen section and pancreaticoduodenectomy performed if microscopic involvement is detected. After neoadjuvant radiation therapy, the native hepatic artery is damaged and thus an arterial interposition graft using a segment of donor iliac artery is used during deceased donor liver transplant (DDLT) [39]. During live donor liver transplant (LDLT), the native hepatic artery is used for reconstruction. A portal vein interposition graft between the donor right or left portal vein and recipient portal vein is also used in living donor transplantation. Biliary anastomosis is done using a Roux-en-Y choledochojejunostomy [64].
As a result of neoadjuvant therapy, vascular complications are encountered more frequently. In a study of 68 patients who underwent neoadjuvant therapy and subsequent liver transplantation, 40% of patients overall developed vascular problems, with arterial complications occurring in 21% and portal venous complications in 22% [70]. Of the patients who developed arterial complications, 50% occurred within 1 month of transplantation. There was no difference in early or late arterial complications between patients who underwent DDLT for CCA compared with DDLT for other indications; however, there was an increased rate of late hepatic artery complications in patients undergoing LDLT for CCA (18%, 2/11 patients) compared with patients with LDLT for other indications (0%, 0/38 patients). Late portal vein complications including stenosis with or without thrombosis occurred more commonly in in patients with CCA who had either DDLT (18%) or LDLT (27%) compared with patients transplanted for other indications (1% for DDLT and 0% for LDLT) [70]. As a result of these findings, at our center we use low dose aspirin indefinitely after both LDLT and DDLT in patients with pCCA. When they occur, hepatic artery and portal venous stenosis can be effectively managed with transluminal angioplasty and stenting. In the Mayo Clinic experience, no grafts have been lost due to late vascular stenoses [40].
Outcomes
Experience accumulated thus far at the local, national and international level with protocols utilizing neoadjuvant therapy in combination with liver transplantation have demonstrated favorable results as summarized in Table 1. The largest experience comes from Mayo Clinic in Rochester, which enrolled 215 patients into their protocol between 1993 and 2011. Intent to treat survival after the initiation of therapy at 1-, 3- and 5-years was 81% (95% CI: 78–84), 62% (95% CI: 58–66), and 56% (95% CI: 52–60), respectively. In 136 patients who proceeded to transplantation enrolled over the same time period, 1-, 3- and 5-year survival after transplantation was 92% (95% CI: 90–94), 81% (95% CI: 77–85), and 74% (95% CI: 70–78), respectively. Five-year survival post transplantation was better in patients with PSC associated pCCA at 81% (95% CI: 77–85) compared with those with de novo pCCA at 58% (95% CI: 51–65), which may speak to earlier detection in these patients who may be closely clinically monitored. Comparable outcomes have been seen elsewhere. In a large retrospective multicenter review of 287 patients from 12 centers across the USA, overall survival from time of presentation at 2 years was 68% (95% CI: 62–74), at 5 years was 53% (95% CI: 46–60), and at 10 years was 42% (95% CI: 33–51). Recurrence-free survival post transplantation at 2, 5 and 10 years was 78% (95% CI: 72–84), 65% (95% CI: 57–73) and 59% (95% CI: 49–69), respectively. Though 193 patients in this study came from one center, 94 patients were from other transplant programs across the country and no significant differences were identified in intent-to-treat or recurrence free survival between the center that contributed the majority of patients and all other centers combined [68]. Recently, a small series from Ireland that reviewed their experience with neoadjuvant chemoradiotherapy followed by liver transplantation in patients with pCCA reported 1-, 2-, 3- and 4-year post-transplant survival of 94, 73, 73 and 61%, respectively, after excluding operative mortality [71]. Another Scanadanvian series reported a 5-year post-transplant survival of 58% among selected patients transplanted after 1995, with a TNM stage ≤2 and a CA 19–9 <100, though importantly patients did not receive neoadjuvant therapy [72].
Table 1. . Outcomes of patients with perihilar cholangiocarcinoma undergoing neoadjuvant chemoradiation followed by liver transplantation by location.
| n | 1 YS (%) | 2 YS (%) | 3 YS (%) | 4 YS (%) | 5 YS (%) | 10 YS (%) | Notes | |
|---|---|---|---|---|---|---|---|---|
| Mayo Clinic, Rochester, MN, USA | ||||||||
| Survival from presentation (95% CI) | 193 | − | 65% (58–72) | − | − | 53% (45–61) | 42% (32–52) | |
| Recurrence-free survival (95% CI) | 193 | − | 80% (73–87) | − | − | 68% (59–77) | 66% (56–78) | |
| Survival post-transplant (95% CI) | 136 | 92% (90–94) | 81% (77–85) | 74% (70–78) | ||||
| USA – 12 centers | ||||||||
| Survival from presentation (95% CI) | 287 | − | 68% (62–74) | − | − | 53% (46–60) | 42% (33–51) | |
| Recurrence-free survival (95% CI) | 287 | − | 78% (72–84) | − | − | 65% (57–73) | 59% (49–69) | |
| Ireland | ||||||||
| Post LT survival | 20 | 94% | 73% | 73% | 61% | − | − | Operative mortality excluded |
| Mayo Clinic, FL, USA | ||||||||
| Overall survival (95% CI) | 22 | 90% (69–98) | 70% (47–87) | 63% (40–81) | − | − | − | |
| University of California, Los Angeles, CA, USA | ||||||||
| Recurrence-free survival | 11 | − | 88% | 75% | − | 47% | − | iCCA and pCCA |
iCCA: Intrahepatic cholangiocarcinoma; LT: Liver transplant; pCCA: Perihilar cholangiocarcinoma; YS: Year survival.
Absence of histological confirmation does not significantly affect outcomes. In the multicenter US study described above [68], 30% (n = 87) of patients did not have pretransplant pathological confirmation, though 83% (n = 72) of these patients did have either pathological confirmation on explant or a visible tumor mass on imaging and/or CA-19-9 > 100 U/ml in the absence of biliary obstruction. After exclusion of the 15 patients (5% of the entire cohort) without objective evidence of malignancy despite extensive investigations, 5-year intent to treat survival and recurrence free survival was similar to that of the entire cohort at 50 and 62%, respectively. Furthermore, a study from Mayo Clinic, Rochester compared outcomes of patients with and without pretreatment pathological confirmation [64]. In patients with de novo CCA, there was no significant difference in 5-year survival after start of therapy (39% in those with confirmed pathology vs 48% in those without, p = 0.37), survival after transplantation (63 vs 65%, p = 0.77), or recurrence after transplantation (46 vs 34%, p = 0.27). In contrast to those with de novo cancer, outcomes were worse in patients with PSC who had confirmed pathology prior to treatment. Five-year intent to treat survival in PSC patients with pathologic confirmation was 50% compared with 80% in those without confirmed histology (p < 0.01) and 5-year post-transplantation survival was also worse in those with confirmed histology (66 vs 92%, p = 0.01). Interestingly, there was no difference in CCA recurrence in PSC patients with and without pretreatment pathological confirmation (15 vs 14%, p = 0.53). These data suggest that though there is improved survival after liver transplantation in PSC-associated and de novo CCA with and without pathologic confirmation, one should be cautious of the diagnosis of CCA in patients with PSC in the absence of pathologic confirmation.
Recurrent CCA post transplantation is clearly a concern and rigorous selection of patients is essential to avoid this dreaded outcome. In the multicenter US study, 20% of patients developed recurrence and those transplanted outside current UNOS criteria for MELD exception points had a threefold worse recurrence-free survival compared with those transplanted within criteria [68]. An analysis of the Mayo Clinic cohort demonstrated that independent predictors of post-transplant recurrence included an elevated CA19–9 (HR 1.8; 95% CI: 1.1–2.8), portal vein encasement (HR 3.3; 95% CI: 1.4–7.9), and most importantly residual tumor on explant pathology (HR 9.8; 95% CI: 2.9–32.8) [38]. In a time of scarcity of organs, careful selection of patients and compliance with a structured protocol is of utmost importance. That said, for the optimally selected patient, such a regimen offers an option for prolonged survival far superior to the alternative.
Future perspective
The data presented suggest that neoadjuvant therapy followed by liver transplantation affords prolonged survival in a highly selected group of patients with pCCA. However, those eligible make up only a small proportion of patients with CCA leaving a significant proportion of patients with few options. Currently, the role of liver transplantation in patients with iCCA remains controversial. Similar to the initial experience with pCCA early reports of liver transplant for iCCA were disappointing with 5-year patient survival rates of 0–42% [73]. Such results caused many centers to abandon this treatment approach and to date, most transplant centers still consider iCCA a contraindication to OLT.
Recently, however, there has been renewed interest in identifying select patients with iCCA that may benefit from liver transplantation. A Spanish multicenter retrospective study assessed outcomes in 42 cirrhotic patients who underwent liver transplant for presumed HCC within Milan criteria which was subsequently pathologically confirmed to be either mixed HCC-CCA or iCCA on explant compared with 84 matched patients with histologically confirmed HCC. The 1-, 3- and 5-year cumulative risk of recurrence was higher in the subset of patients with iCCA compared with their HCC matched controls (12, 25 and 36% compared with 0, 2 and 2%) though no significant survival difference was seen between the HCC-CC subgroup compared with their HCC matched controls, suggesting a potential benefit with transplantation in patients with HCC-CCA for whom transplantation has historically been contraindicated [74]. Similarly, 1-, 3- and 5-year survival was also lower in the iCCA subgroup but no difference was identified in patients with HCC-CCA. Interestingly, when the analysis was limited to patients with uninodular tumors ≤2 cm no significant difference in tumor recurrence or survival was seen among patients with HCC-CCA or iCCA compared with their HCC matched controls [74], suggesting a potential benefit to transplantation in these ‘very early’ iCCA cases. Another study using the same multicenter Spanish cohort identified risk factors associated with tumor recurrence in patients with iCCA and found that in the small subset of patients with iCCA ≤2 cm, none of the patients presented with disease recurrence after a median follow up of 36.4 months and the 1-, 3- and 5-year actuarial survival of this subset was 100, 73 and 73%, respectively [75]. That said, it is important to note that compared with pCCA which can present early with biliary obstruction, patients with iCCA are much less likely to be symptomatic; thus, unless engaged in a screening program such as in the setting of cirrhosis, few patients with iCCA are likely to be diagnosed with disease at such an early stage.
With the added benefit of neoadjuvant therapy prior to liver transplantation seen in selected patients with pCCA, investigators at the UCLA reported outcomes in 25 patients who had liver transplantation for locally advanced iCCA, 9 of whom received neoadjuvant therapy. Though survival rate for these patients was not reported, disease-free survival was significantly higher in patients who received neoadjuvant therapy compared with those who did not and independent predictors of decreased survival included perineural invasion and multifocal tumors. Interestingly, tumor size (≥5 cm iCCA) was not a predictor of patient survival [76]. This group has since proposed a protocol for liver transplantation in patients with iCCA after neoadjuvant therapy [77]. Inclusion criteria include unresectable iCCA ≤ 8 cm, disease confined to the operative field for total hepatectomy and regional lymphadenectomy for liver transplantation, and absence of distant metastasis. Patients undergo preoperative risk stratification with biopsy of the mass to classify tumors into low, intermediate and high risk of poor postoperative outcomes based on tumor characteristics. Neoadjuvant treatment consists of stereotactic body radiation therapy (SBRT) for tumors <6 cm and transarterial chemoembolization (TACE) for tumors between 6 and 8 cm followed by 5-FU or capecitabine-based chemotherapy until the time of transplantation. Patients in the low and intermediate risk groups subsequently undergo liver transplantation after staging laparotomy and those initially at high risk are rebiopsied to assess for response. In an analysis of 40 patients by risk score (65% of whom had iCCA), 5-year tumor recurrence free survival was 78% in the low-risk group compared with 19% in the intermediate risk and 0% in the high-risk group [78]. Thus, though evidence from small series are emerging that have demonstrated potential benefit of liver transplantation for iCCA in selected patients, further validation and large-scale studies are needed to confirm these findings. Though this holds promise for the future in terms of expanding indications for liver transplantation for hepatobiliary malignancy and improving patient outcomes, for the time being, this approach remains controversial.
Finally, locoregional therapy in the form of EBRT is an important part of the neoadjuvant protocol for pCCA but interest in alternative forms of locoregional control including TACE [79] for iCCA, and SBRT and photodynamic therapy (PDT) for pCCA has also emerged. Recently, Cosgrove et al. reported that ERC directed PDT was reasonably well tolerated and safe in maintaining locoregional control in patients with pCCA awaiting liver transplantation [32]. Another pilot study demonstrated acceptable tolerability with neoadjuvant SBRT followed by chemotherapy in patients with pCCA prior to liver transplantation. As data accumulate, the role that these alternative locoregional options will play in the perioperative management of patients with pCCA will be more clearly defined [80].
In summary, when combined with preoperative neoadjuvant therapy and staging, liver transplantation is an important treatment option for selected patients with pCCA and offers improved survival far beyond what would be expected with nonsurgical therapeutic approaches. However, adherence to strict selection criteria is essential for optimal outcomes. While patients with de novo CCA should be treated with surgical resection when possible, those with unresectable pCCA or pCCA with associated PSC should be considered for liver transplantation.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 2.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66(3):167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 3.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 2004;99(3):523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8(9):512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deoliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013;11(1):13–21. doi: 10.1016/j.cgh.2012.09.009. e11, quiz e13–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin. Surg. Oncol. 2000;19(2):156–176. doi: 10.1002/1098-2388(200009)19:2<156::aid-ssu8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch. Surg. 2004;139(5):514–523. doi: 10.1001/archsurg.139.5.514. discussion 523–515. [DOI] [PubMed] [Google Scholar]
- 11.Petrowsky H, Hong JC. Current surgical management of hilar and intrahepatic cholangiocarcinoma: the role of resection and orthotopic liver transplantation. Transplant. Proc. 2009;41(10):4023–4035. doi: 10.1016/j.transproceed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann. Surg. 2009;250(2):210–218. doi: 10.1097/SLA.0b013e3181afe0ab. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Cherqui D. Operative management of cholangiocarcinoma. Semin. Liver Dis. 2013;33(3):248–261. doi: 10.1055/s-0033-1351784. [DOI] [PubMed] [Google Scholar]
- 14.Farley DR, Weaver AL, Nagorney DM. ‘Natural history’ of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin. Proc. 1995;70(5):425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 15.Kondo S, Hirano S, Ambo Y, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann. Surg. 2004;240(1):95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann. Surg. 1996;224(5):628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann. Surg. 1999;230(5):663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwatsuki S, Todo S, Marsh JW, et al. Treatment of hilar cholangiocarcinoma (klatskin tumors) with hepatic resection or transplantation. J. Am. Coll. Surg. 1998;187(4):358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang JY, Kim SW, Park DJ, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann. Surg. 2005;241(1):77–84. doi: 10.1097/01.sla.0000150166.94732.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todoroki T, Kawamoto T, Koike N, et al. Radical resection of hilar bile duct carcinoma and predictors of survival. Br. J. Surg. 2000;87(3):306–313. doi: 10.1046/j.1365-2168.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- 21.Jarnagin WR, Fong Y, Dematteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001;234(4):507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch. Surg. 1995;130(3):270–276. doi: 10.1001/archsurg.1995.01430030040006. [DOI] [PubMed] [Google Scholar]
- 23.Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma: a 10 year experience of a tertiary referral centre in the UK. Eur. J. Surg. Oncol. 2005;31(5):533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Qin Y, Cui Y, Chen H, Hao X, Li Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig. Surg. 2011;28(3):226–231. doi: 10.1159/000327361. [DOI] [PubMed] [Google Scholar]
- 25.Saxena A, Chua TC, Chu FC, Morris DL. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am. J. Surg. 2011;202(3):310–320. doi: 10.1016/j.amjsurg.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 26.Regimbeau JM, Fuks D, Le Treut YP, et al. Surgery for hilar cholangiocarcinoma: a multi-institutional update on practice and outcome by the AFC-HC Study Group. J. Gastrointest. Surg. 2011;15(3):480–488. doi: 10.1007/s11605-011-1414-0. [DOI] [PubMed] [Google Scholar]
- 27.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69(8):1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 28.Brandsaeter B, Isoniemi H, Broome U, et al. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J. Hepatol. 2004;40(5):815–822. doi: 10.1016/j.jhep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann. Surg. 2004;239(2):265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghali P, Marotta PJ, Yoshida EM, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the canadian experience. Liver Transpl. 2005;11(11):1412–1416. doi: 10.1002/lt.20512. [DOI] [PubMed] [Google Scholar]
- 31.Alessiani M, Tzakis A, Todo S, Demetris AJ, Fung JJ, Starzl TE. Assessment of five-year experience with abdominal organ cluster transplantation. J. Am. Coll. Surg. 1995;180(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Cosgrove ND, Al-Osaimi AM, Sanoff HK, et al. Photodynamic therapy provides local control of cholangiocarcinoma in patients awaiting liver transplantation. Am. J. Transplant. 2014;14(2):466–471. doi: 10.1111/ajt.12597. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda M, Farmer DG, Colquhoun SD, et al. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7(12):1023–1033. doi: 10.1053/jlts.2001.29419. [DOI] [PubMed] [Google Scholar]
- 34.Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997;39(4):929–935. doi: 10.1016/s0360-3016(97)00299-x. [DOI] [PubMed] [Google Scholar]
- 35.Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal ir-192 brachytherapy for bile duct cancer. Int. J. Radiat. Oncol. Biol. Phys. 1994;28(4):945–951. doi: 10.1016/0360-3016(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 36.Sudan D, Deroover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am. J. Transplant. 2002;2(8):774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 37.Sio TT, Martenson JA, Jr., Haddock MG, et al. Outcome of transplant-fallout patients with unresectable cholangiocarcinoma. Am. J. Clin. Oncol. 2014 doi: 10.1097/COC.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In patients who fall out of a transplant protocol, survival is similar to those with locally advanced or metatstatic disease.
- 38.Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56(3):972–981. doi: 10.1002/hep.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Predictors of protocol dropout include elevated CA-19-9, large mass, malignant histology and high MELD and predictors of recurrence post-transplant include elevated CA-19-9, portal vein encasement and residual tumor on explant.
- 39.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann. Surg. 2005;242(3):451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Among candidates for surgical therapy, recurrence-free survival is significantly higher in patients with perihilar cholangiocarcinoma (pCCA) having neoadjuvant chemoradiation and OLT compared with those with surgical resection.
- 40.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl. Int. 2010;23(7):692–697. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 41.Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59(6):2397–2402. doi: 10.1002/hep.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blechacz BR, Sanchez W, Gores GJ. A conceptual proposal for staging ductal cholangiocarcinoma. Curr. Opin. Gastroenterol. 2009;25(3):238–239. doi: 10.1097/MOG.0b013e3283292383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizvi S, Gores GJ. Current diagnostic and management options in perihilar cholangiocarcinoma. Digestion. 2014;89(3):216–224. doi: 10.1159/000360791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19–9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2005;50(9):1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 46.Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19–9 do not have cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2011;9(5):434–439. doi: 10.1016/j.cgh.2011.02.007. e431. [DOI] [PubMed] [Google Scholar]
- 47.Patel AH, Harnois DM, Klee GG, Larusso NF, Gores GJ. The utility of ca 19–9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am. J. Gastroenterol. 2000;95(1):204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 48.Juntermanns B, Radunz S, Heuer M, et al. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur. J. Med. Res. 2010;15(8):357–361. doi: 10.1186/2047-783X-15-8-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruys AT, Van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br. J. Radiol. 2012;85(1017):1255–1262. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopera JE, Soto JA, Munera F. Malignant hilar and perihilar biliary obstruction: use of MR cholangiography to define the extent of biliary ductal involvement and plan percutaneous interventions. Radiology. 2001;220(1):90–96. doi: 10.1148/radiology.220.1.r01jl3990. [DOI] [PubMed] [Google Scholar]
- 51.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin. Liver Dis. 2004;24(2):155–164. doi: 10.1055/s-2004-828892. [DOI] [PubMed] [Google Scholar]
- 52.Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur. Radiol. 2008;18(10):2213–2221. doi: 10.1007/s00330-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 53.Vogl TJ, Schwarz WO, Heller M, et al. Staging of klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur. Radiol. 2006;16(10):2317–2325. doi: 10.1007/s00330-005-0139-4. [DOI] [PubMed] [Google Scholar]
- 54.Barr Fritcher EG, Kipp BR, Slezak JM, et al. Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pancreatobiliary tract malignancy. Am. J. Clin. Pathol. 2007;128(2):272–279. doi: 10.1309/BC6DY755Q3T5W9EE. [DOI] [PubMed] [Google Scholar]
- 55.Gonda TA, Glick MP, Sethi A, et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest. Endosc. 2012;75(1):74–79. doi: 10.1016/j.gie.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am. J. Gastroenterol. 2004;99(9):1675–1681. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 57.Dehaan RD, Kipp BR, Smyrk TC, Abraham SC, Roberts LR, Halling KC. An assessment of chromosomal alterations detected by fluorescence in situ hybridization and p16 expression in sporadic and primary sclerosing cholangitis-associated cholangiocarcinomas. Hum. Pathol. 2007;38(3):491–499. doi: 10.1016/j.humpath.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am. J. Gastroenterol. 2011;106(11):2023–2028. doi: 10.1038/ajg.2011.272. [DOI] [PubMed] [Google Scholar]
- 59.Barr Fritcher EG, Voss JS, Jenkins SM, et al. Primary sclerosing cholangitis with equivocal cytology: fluorescence in situ hybridization and serum CA 19–9 predict risk of malignancy. Cancer Cytopathol. 2013;121(12):708–717. doi: 10.1002/cncy.21331. [DOI] [PubMed] [Google Scholar]
- 60.Levy MJ, Heimbach JK, Gores GJ. Endoscopic ultrasound staging of cholangiocarcinoma. Curr. Opin. Gastroenterol. 2012;28(3):244–252. doi: 10.1097/MOG.0b013e32835005bc. [DOI] [PubMed] [Google Scholar]
- 61.Mohamadnejad M, Dewitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest. Endosc. 2011;73(1):71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 62.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13(5):356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gleeson FC, Rajan E, Levy MJ, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest. Endosc. 2008;67(3):438–443. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Rosen CB, Darwish Murad S, Heimbach JK, Nyberg SL, Nagorney DM, Gores GJ. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J. Am. Coll. Surg. 2012;215(1):31–38. doi: 10.1016/j.jamcollsurg.2012.03.014. discussion 38–40. [DOI] [PubMed] [Google Scholar]; • Similar rates of residual CCA and post-transplant recurrence in patients with and without pretreatment pathologic confirmation.
- 65.De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6(3):309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 66.Negm AA, Schott A, Vonberg RP, et al. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest. Endosc. 2010;72(2):284–291. doi: 10.1016/j.gie.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 67.Gores GJ, Gish RG, Sudan D, Rosen CB. Model for end-stage liver disease (meld) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 2006;12(12 Suppl. 3):S95–97. doi: 10.1002/lt.20965. [DOI] [PubMed] [Google Scholar]
- 68.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98. doi: 10.1053/j.gastro.2012.04.008. e83; quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Large multicenter experience demonstrating 65% 5-year recurrence-free survival post liver transplant in patients with pCCA from 12 US centers.
- 69.Gores GJ, Darwish Murad S, Heimbach JK, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma. Dig. Dis. 2013;31(1):126–129. doi: 10.1159/000347207. [DOI] [PubMed] [Google Scholar]
- 70.Mantel HT, Rosen CB, Heimbach JK, et al. Vascular complications after orthotopic liver transplantation after neoadjuvant therapy for hilar cholangiocarcinoma. Liver Transpl. 2007;13(10):1372–1381. doi: 10.1002/lt.21107. [DOI] [PubMed] [Google Scholar]
- 71.Duignan S, Maguire D, Ravichand CS, et al. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB (Oxford) 2014;16(1):91–98. doi: 10.1111/hpb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friman S, Foss A, Isoniemi H, et al. Liver transplantation for cholangiocarcinoma: selection is essential for acceptable results. Scand. J. Gastroenterol. 2011;46(3):370–375. doi: 10.3109/00365521.2010.533384. [DOI] [PubMed] [Google Scholar]
- 73.Hashimoto K, Miller CM. Liver transplantation for intrahepatic cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2015;22(2):138–143. doi: 10.1002/jhbp.159. [DOI] [PubMed] [Google Scholar]
- 74.Sapisochin G, De Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann. Surg. 2014;259(5):944–952. doi: 10.1097/SLA.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 75.Sapisochin G, Rodriguez De Lope C, Gastaca M, et al. Very early’ intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am. J. Transplant. 2014;14(3):660–667. doi: 10.1111/ajt.12591. [DOI] [PubMed] [Google Scholar]
- 76.Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch. Surg. 2011;146(6):683–689. doi: 10.1001/archsurg.2011.116. [DOI] [PubMed] [Google Scholar]; •• Study demonstrating benefit of neoadjuvant therapy plus liver transplantation in patients with pCCA and iCCA.
- 77.Rana A, Hong JC. Orthotopic liver transplantation in combination with neoadjuvant therapy: a new paradigm in the treatment of unresectable intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol. 2012;28(3):258–265. doi: 10.1097/MOG.0b013e32835168db. [DOI] [PubMed] [Google Scholar]
- 78.Hong JC, Petrowsky H, Kaldas FM, et al. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J. Am. Coll. Surg. 2011;212(4):514–520. doi: 10.1016/j.jamcollsurg.2010.12.005. discussion 520–511. [DOI] [PubMed] [Google Scholar]
- 79.Kuhlmann JB, Blum HE. Locoregional therapy for cholangiocarcinoma. Curr. Opin. Gastroenterol. 2013;29(3):324–328. doi: 10.1097/MOG.0b013e32835d9dea. [DOI] [PubMed] [Google Scholar]
- 80.Welling TH, Feng M, Wan S, et al. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl. 2014;20(1):81–88. doi: 10.1002/lt.23757. [DOI] [PMC free article] [PubMed] [Google Scholar]