Abstract
Background and Objectives:
A non-surgical method is being developed for treating female stress urinary incontinence by laser thermal remodeling of subsurface tissues with applied surface tissue cooling. Computer simulations of light transport, heat transfer, and thermal damage in tissue were performed, comparing transvaginal and transurethral approaches.
Study Design/Materials and Methods:
Monte Carlo (MC) simulations provided spatial distributions of absorbed photons in the tissue layers (vaginal wall, endopelvic fascia, and urethral wall). Optical properties (n,μa,μs,g) were assigned to each tissue at λ=1064 nm. A 5-mm-diameter laser beam and incident power of 5 W for 15 seconds was used, based on previous experiments. MC output was converted into absorbed energy, serving as input for finite element heat transfer simulations of tissue temperatures over time. Convective heat transfer was simulated with contact probe cooling temperature set at 0°C. Variables used for thermal simulations (κ,c,ρ) were assigned to each tissue layer. MATLAB code was used for Arrhenius integral thermal damage calculations. A temperature matrix was constructed from ANSYS output, and finite sum was incorporated to approximate Arrhenius integral calculations. Tissue damage properties (Ea,A) were used to compute Arrhenius sums.
Results:
For the transvaginal approach, 37% of energy was absorbed in the endopelvic fascia target layer with 0.8% deposited beyond it. Peak temperature was 71°C, the treatment zone was 0.8-mm-diameter, and 2.4mm of the 2.7-mm-thick vaginal wall was preserved. For transurethral approach, 18% energy was absorbed in endopelvic fascia with 0.3% deposited beyond the layer. Peak temperature was 80°C, treatment zone was 2.0-mm-diameter, and 0.6 mm of 2.4-mm-thick urethral wall was preserved.
Conclusions:
Computer simulations suggest that transvaginal approach is more feasible than transurethral approach.
Keywords: coagulation, incontinence, laser, Monte Carlo, simulations, thermal remodeling
INTRODUCTION
Female Stress Urinary Incontinence and Conventional Therapies
Over 6.5 million women in the U.S. and 10 million women worldwide suffer from Stress Urinary Incontinence (SUI) [1]. Only about 200,000 U.S. women (3%) seek surgical therapy, including Burch (open) colposuspension, insertion of sub-urethral sling, or injection of urethral bulking agents [2]. The need for general anesthesia, prolonged recovery time, incisions, concern about treatment failures with future pregnancies, and procedural morbidity are reasons for patient hesitation to seek SUI therapy. As a result, the remaining 97% of women with SUI use disposable absorbable products, at total cost of billions of dollars, to cope with, but not cure, the symptoms [3]. Given numerous patient concerns about current treatment options for SUI, there is a role for a non-surgical method which can improve patient quality of life, especially for a treatment that is rapidly performed, carries minimal morbidity, and provides brief recovery time.
Non-surgical treatments such as Kegel exercises, biofeedback, and pelvic floor stimulation are plagued by burdensome compliance and treatment requirements as well as by issues of efficacy and durability. Thus, most SUI patients do not select any definitive nonpalliative treatment. Patient surveys demonstrate that for the majority of incontinent women, treatment selection is driven by the desire for a minimally invasive therapy, and expectation of treatment is an improvement in quality of life, with only a minority of patients expecting a cure. Thus, many SUI patients would prefer a minimally invasive therapy, which safely improves their quality of life rather than a more invasive treatment even if associated with a high likelihood of a cure.
Radiofrequency Thermal Treatment of Female Stress Urinary Incontinence
Radiofrequency (RF) energy has been used for transurethral thermal shrinkage and micro-remodeling of submucosal collagen without tissue necrosis, in the bladder neck and proximal urethra as a nonsurgical treatment for SUI, with success rates of up to 80% [4,5]. This therapy produces a functional change in regional tissue compliance, without a gross change in lumenal caliber (thus avoiding strictures). The ability of RF therapy to safely and effectively alter tissue compliance, increase barrier function, and improve patient quality of life has been demonstrated.
However, RF heating decays rapidly with depth as 1/r4 (1/r2 term for resistive heating and 1/r2 term for electric field strength), resulting in a penetration depth due to direct heating of only 1–2 mm [6,7]. Increasing RF power only accelerates thermal coagulation at tissue surface due to a steep temperature gradient, increasing tissue impedance, and further limiting RF penetration. Therefore, RF probes for SUI therapy require needles inserted through the urethral wall and into submucosal tissue for localized heating, collagen denaturation, and shrinkage, with saline irrigation of the mucosa to prevent overheating. Thus, due to limited RF energy penetration depth, RF therapy is more invasive than desired by SUI patients seeking nonsurgical treatment.
Laser Treatment of Female Stress Urinary Incontinence
Noninvasive laser applications utilizing applied cooling have been successfully exploited for skin resurfacing (thermal denaturation and shrinkage of collagen for tissue remodeling), hair removal, tattoo removal, and treatment of vascular birthmarks [8,9]. In dermatology, the targeted tissues are superficial, requiring preservation of only a thin layer of surface tissue (e.g., epidermis and papillary dermis). These laser-based techniques have not yet been extended to other applications where noninvasive laser targeting of deeper tissue structures may be required.
Erbium:YAG (λ=2.94 μm) and CO2 (λ=10.6 μm) skin resurfacing lasers have recently been applied in gynecology for sub-ablative resurfacing of atrophic vaginal tissue in post menopausal women, and for treatment of female SUI [10–14]. Short-term studies appear more promising for vaginal rejuvenation, but also show modest improvements for SUI. However, these wavelengths, directly adopted from the laser skin resurfacing field, are limited to an optical penetration depth of only tens of micrometers and a thermal treatment zone of less than 0.5 mm.
It should also be noted that, unlike the endopelvic fascia, the vaginal wall is not primarily collagen, but instead composed of several layers. The first layer is the stratified squamous non-keratinized epithelium of the mucosa. (The epithelium has folds called transverse epithelial ridges that can becompressed and changes thickness with estrogen content, thinner for pre-pubescent, and post menopause). The second layer is lamina propria of connective tissue, also part of the mucosa. The lamina propria is rich in blood vessels and lymphatic channels. Third is the muscular layer made of smooth muscle fibers, with outer layer of longitudinal muscle, and inner layer of circular muscle. Fourth is adventitial layer, which is connective tissue that blends with endopelvic fascia. This layer contains blood vessels, lymphatic vessels, and nerve fibers [15].
Preliminary studies in our laboratory have demonstrated that subsurface thermal denaturation of tissues can be achieved using a deeply penetrating laser wavelength (λ=1,064 or 1,075 nm) in conjunction with surface cooling, preserving 1–2 mm of tissue surface (in porcine liver and skin both ex vivo and in vivo) from thermal necrosis [16–20]. Thus, laser denaturation and shrinkage of the endopelvic fascia may potentially produce improved tissue remodeling results similar to the RF approach, but in a less invasive manner with preservation of the vaginal mucosa.
The overall goal is to thermally remodel endopelvic fascia without damaging adjacent tissue (e.g., urethra or vaginal wall). The thickness of vaginal wall, endopelvic fascia, and urethral wall measure about 2.7, 4.3, and 2.4 mm [21,22]. Therefore, computer simulations will determine whether a “transvaginal” approach can preserve vaginal wall while treating endopelvic fascia, or whether a “transurethral” approach can preserve urethra while treating endopelvic fascia.
METHODS
Monte Carlo Simulations of Light Transport in Tissue
Monte Carlo (MC) simulations use a statistical “random walk” approach to model light propagation in tissue layers, in which photons experience scattering and absorption events. Each tissue layer is assigned a scattering and absorption coefficient that determines when an event occurs, by expressing the coefficients as probability distributions. During a scattering event, the computer program determines the scattering angle and then carries out multiple events until the photon is extinguished. The number of photons needed to produce an accurate distribution of light absorbed in the tissue layers is dependent on the spatial resolution desired. The MC output provides the radiated energy density distribution as a function of space through the tissue [23].
To simulate the distribution of photons absorbed and deposited in the tissue layers, a standard, and widely used MC program was adapted for these studies [24]. This program modeled photon transport through tissue layers having plane parallel geometry. This assumption was valid in our application because although the tissue structures are cylindrical in their normal state, in practice the contact laser probe would gently compress and hence flatten the tissue layers. One million photons were used in this MC simulation to achieve a sufficient distribution of light absorbed in the tissue. A previously reported convolution program was used to fit the results to an actual laser beam of known power, profile, and beam size [25]. A complete set of values for the optical properties of each tissue layer for an Nd:YAG wavelength of 1064 nm, namely, index of refraction (n), absorption coefficient (μa), scattering coefficient (μs), and anisotropy factor (g), were compiled from the literature (Table 1). An incident laser power of 5 W, laser irradiation time of 15 seconds, and laser spot diameter of 5 mm was used, based on our previous experimental results. During preliminary studies, a wider span of laser parameters was also studied, ranging from 4.2 to 7.2 W and from 15 to 60 seconds. The laser parameters examined in detail in this study (5 W, 15 seconds) were based on the most promising results from these preliminary studies.
TABLE 1.
Optical, Thermal, and Damage Parameters for Computer Simulations of Transvaginal and Transurethral Laser Therapy
| Vaginal wall | Endopelvic fascia | Urethral wall | |
|---|---|---|---|
| Optical properties (Monte Carlo) | |||
| Absorption coefficient (μa) cm−1 | 0.43 | 0.35 | 0.5 |
| Scattering coefficient (μs) cm−1 | 21.6 | 484 | 239 |
| Anisotropy factor (g) | 0.9 | 0.9 | 0.9 |
| Index of refraction (n) | 1.38 | 1.39 | 1.39 |
| Tissue thickness (mm) | 2.7 | 4.3 | 2.4 |
| References | [21,22,26] | [21,22,27,28] | [21,22,29] |
| Thermal properties (heat transfer) | |||
| Thermal conductivity (κ) W/m-k | 0.54 | 0.47 | 0.46 |
| Specific heat (c) J/kg-k | 3,655 | 3,200 | 3,306 |
| Density (ρ) kg/m3 | 1,088 | 1,085 | 1,102 |
| References | [30] | [30] | [31] |
| Damage parameters (damage integral) | |||
| Frequency factor (A) s−1 | 5.73 × 1034 | 1.61 × 1045 | 5.60 × 1063 |
| Activation energy (Ea) J/mol | 2.40 × 105 | 3.06 × 105 | 4.30 × 105 |
| Tcrit (°C) | 88.0 | 80.4 | 78.9 |
| References | [32] | [32] | [32] |
A laser wavelength of 1,064 nm, incident power of 5.0 W, spot diameter of 5.0 mm, and irradiation time of 15 seconds was used.
Heat Transfer Simulations
Tissue temperature simulations were conducted using a finite element software package (Version 14.5, ANSYS, Canonsburg, PA). The heat transfer model was imported from the MC simulation model dimensions with a square mesh of 350☓350 elements equally spaced to be 28.5☓28.5 μm. The tissue layers were represented in the mesh using thermal properties from Table 1. Absorption data from MC simulations was convolved and converted into the input of the heat transfer model to simulate heat created by laser irradiation. Since both MC simulations and heat transfer programs utilize energy density absorbed by tissue (J/cm3) as units, no further manipulation was needed to import data across the two programs. The initial tissue temperature was set at 35°C (308 K) with contact probe cooling temperature set at 0°C (273 K). Pre-cooling and post-cooling times of 15 seconds each were applied to the tissue surface to provide optimal preservation of the tissue surface from excessive temperatures during the procedure.
Arrhenius Integral Thermal Damage Simulations
The Arrhenius integral model, based on the field of chemical reaction kinetics, assumes that damage processes can be modeled as first order rate processes using two experimentally derived coefficients (frequency factor, A, and activation energy barrier, E). Thermal damage is found to be linearly dependent on time and exponentially dependent on temperature. The Arrhenius integral formulation can also be written as a volume fraction model, or the ratio of the fraction of the logarithm of original concentration of native tissue to remaining concentration of native tissue. The damage integral can be related to pathologic endpoints, including birefringence loss in tissue, collagen damage, or cell survival in a cell culture [32].
A standard Arrhenius integral model [33–36] was employed to predict and characterize thermal injury using known values from the literature. A single damage parameter, Ω(t), quantified thermal damage to the tissue:
| (1) |
In equation 1, A (s−1) is a frequency factor determined by an experimentally derived constant; ז(s) is the total heating time; Ea (J/mol) is an activation energy of the transformation, which is also an experimentally derived constant (Table 1), R (8.32 J/K mol) is the universal gas constant, and T(t) is the absolute temperature of the tissue in Kelvin. Using Eq. 1, damage can also be expressed in the form of percent damage to tissue, given by:
| (2) |
Damage is 0% when Ω(t)=0, and at 63.2%, Ω(t)=1. From Eq. 1, Eq. 3 can be derived, which shows the thermal damage rate as well. Damage rate increases exponentially when temperature exceeds Tcrit, defined as the temperature at which the damage accumulation rate is 1. Both damage parameter and percent damage were computed for the tissues, and percent damage was displayed for simplified interpretation of the results.
| (3) |
Monte Carlo, heat transfer, and Arrhenius integral simulations were conducted to compute thermal damage, based on optical, thermal, and damage parameters for urinary tissues (Table 1). Due to limited availability of damage parameter data, vaginal wall was modeled as smooth muscle, endopelvic fascia as collagen, and urethral wall modeled as aortic wall tissue.
RESULTS
Monte Carlo Simulations
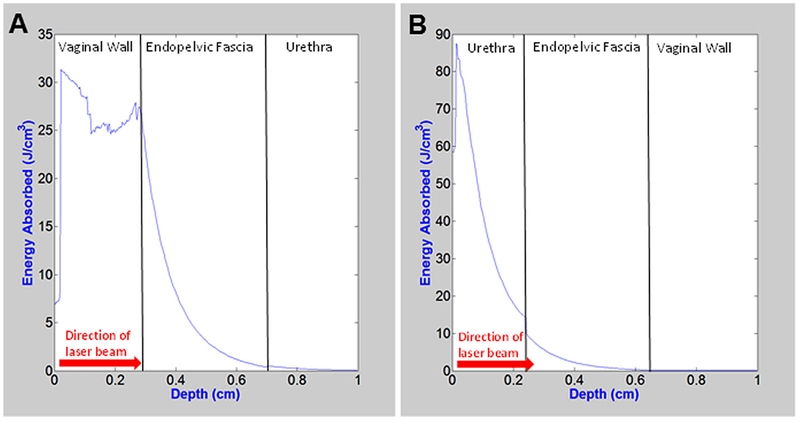
Transvaginal MC simulations predicted that 62% of the energy is absorbed by the vaginal wall, 37% of energy is absorbed in the endopelvic fascia target layer, and only 0.8% of energy is deposited in the urethral wall beyond it (Fig. 1A). These results demonstrate the need for cooling of the tissue surface, both before, during, and after laser irradiation of the tissue. The transurethral approach was also simulated, where laser irradiation propagated from urethra to endopelvic fascia and then to the vaginal wall in reverse order from the transvaginal approach. For the transurethral MC simulations, 82% of energy is absorbed in urethral wall, only 18% energy is absorbed in endopelvic fascia, and 0.3% deposited in vaginal wall beyond (Fig. 1B). These undesirable results for the transurethral approach show an even higher percentage of light absorbed in the surface tissue than for the transvaginal approach.
Fig. 1.
Monte Carlo simulations showing one dimensional spatial absorbed energy distribution along the central axis of laser irradiation for (A) transvaginal and (B) transurethral approaches.
Heat Transfer Simulations
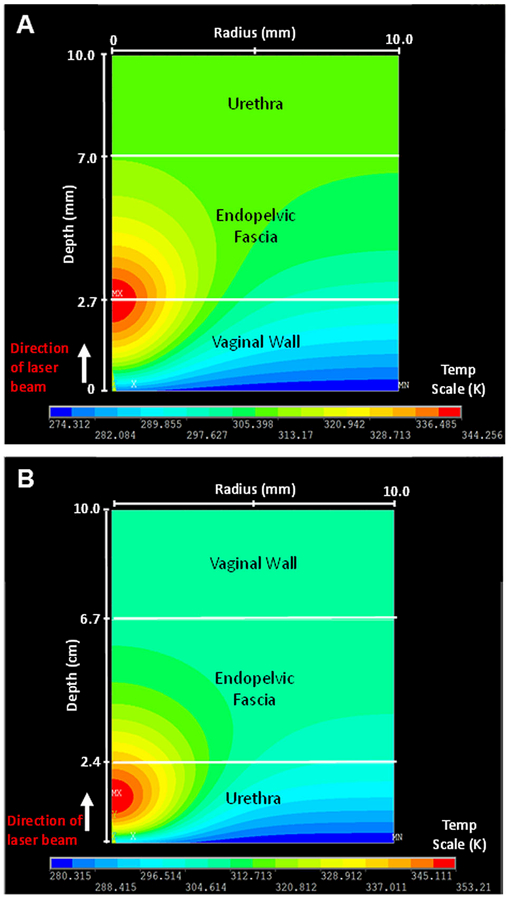
During transvaginal heat transfer simulations, 15 seconds of pre-cooling at a temperature of 0°C was applied to the tissue model prior to laser irradiation at 5.0 W for 15 seconds. After 15 seconds of simultaneous laser irradiation and surface cooling, the internal tissue temperature in the simulation reached 71°C (344°K) at the boundary between the vaginal wall, and endopelvic fascia (Fig. 2A). After laser irradiation, the laser probe was then removed and 15 seconds of post-operative tissue cooling was performed. The maximum temperature decreased to 42.8°C (316°K) at the end of this time period, close to normal body temperature of 37°C. In a similar manner, transurethral heat transfer simulations showed that the maximum temperature reaches 80°C in the urethra (Fig. 2B). Not only are transurethral temperatures much higher, but the peak temperature also occurs closer to the tissue surface, than for the transvaginal approach.
Fig. 2.
(A) Two dimensional temperature distribution (depth☓width), showing maximum temperatures immediately after laser irradiation at 5 W for 15 seconds, for (A) transvaginal, and (B) transurethral approaches.
The maximum temperatures obtained in these simulations (71°C for transvaginal approach versus 80°C for transurethral approach) differ primarily due to differences in the optical properties of tissue layers. The laser energy is incident on different surface tissues for each approach: vaginal wall in transvaginal approach versus urethra in transurethral approach. The scattering coefficient is higher for urethra than vaginal wall, which results in more attenuation, lower penetration depth, and hence higher absorption and temperature increase in this layer.
Arrhenius Integral Tissue Damage Simulations
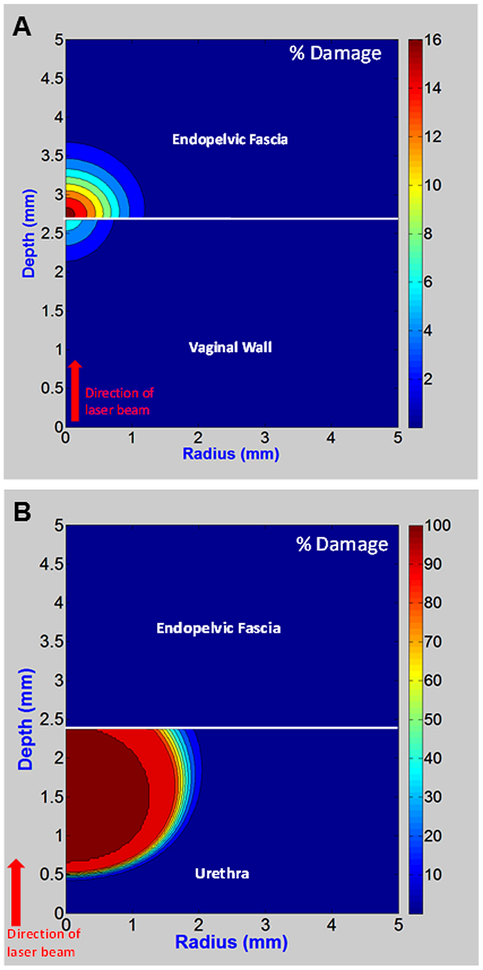
In transvaginal tissue damage simulations, heat distribution data was converted into percent tissue damage using the Arrhenius integral. The treatment zone is 0.8-mm-diameter, and a 2.4-mm-thick surface tissue layer, almost the entire (2.7-mm-thick) vaginal wall, is preserved (Fig. 3A). By contrast, for the transurethral thermal damage simulations, only 0.5 mm of surface tissue out of the entire (2.4-mm-thick) urethral wall is preserved (Fig. 3B).
Fig. 3.
Arrhenius integral output showing thermal damage plotted as percent damage for (A) transvaginal and (B) transurethral approaches.
The disparity in these tissue damage results is because the maximum simulated temperature for the transvaginal approach (71°C) is well below the critical temperature used for irreversible thermal damage of the vaginal wall(88.0°C), endopelvic fascia (80.4°C), and even urethra (78.9°C), listed in Table 1. The maximum temperature achieved during the transvaginal simulations is closer to the current parameters for thermal denaturation and shrinkage of collagenous tissues in general (65°C for 30 seconds), and adopted for successful RF treatment of SUI [37]. While our temperature is slightly higher, treatment time is shorter which compensates in part for this difference. By contrast, the peak temperature simulated during the transurethral approach (80°C), is above the critical temperature used for irreversible thermal damage to the urethra (78.9°C), so that undesirable thermal coagulation and necrosis of the urethra is expected.
DISCUSSION
Computer simulations utilizing optical, thermal, and damage parameters for the vaginal wall, endopelvic fascia, and urethral wall, were conducted for two minimally invasive laser approaches to treatment of female stress urinary incontinence: transvaginal and transurethral. The transurethral approach, based on these simulations did not achieve a desirable outcome. Even when using idealized parameters (for direct comparison with the transvaginal approach), where the laser probe was cooled to 0°C and a 5 mm diameter laser spot size, irreversible thermal damage, and tissue necrosis in the urethral wall was still predicted.
However, it should be noted that use of such parameters in practice may not be possible due to limitations in the physical dimensions of the probe (<6-mm-OD) during a transurethral approach, which would translate into a smaller laser spot diameter and lower coolant flow. A smaller laser spot diameter would in turn limit optical penetration depth, and a low coolant flow rate would hinder efficient cooling of the tissue surface. Therefore, the experimental results with a transurethral probe may be even worse than our simulations currently predict, and with an even greater percentage of the urethral wall exposed to excessive temperatures producing irreversible thermal damage and tissue necrosis. Furthermore, the coolant temperature setting of 0°C used in the simulations may also be unrealistic compared to 7°C actually achieved during our previous preliminary experiments with similar transurethral probe designs in other tissues [38].
On the contrary, the computer simulations utilizing a transvaginal approach predict that the majority of the vaginal wall can be preserved from thermal damage, due to the combination of deeper light penetration (a greater percentage of energy deposited in targeted endopelvic fascia layer), and applied cooling of vaginal wall. Furthermore, a transvaginal approach allows use of a larger diameter probe capable of achieving the laser spot diameter (5 mm) and coolant temperatures (0°C) simulated in these models [20].
From an anatomic approach, the transurethral method is more desirable because it does not require rotational alignment of the probe, since the endopelvic fascia surrounds the urethra on all sides. The transvaginal approach is more challenging, since it requires either image guidance, or precision alignment of the probe with the proper rotational orientation and alignment, so that the endopelvic fascia is accurately targeted. However, a transvaginal approach with ruler markings for precise insertion and rotation of the laser probe has recently been successfully utilized for both laser vaginal resurfacing and SUI applications in the clinic, so the feasibility of this approach has been demonstrated [14].
There were several assumptions and limitations to our computer simulations that need to be briefly discussed. First, as previously mentioned, a rectangular geometry was used for the tissue layers, instead of a cylindrical geometry. This is a reasonable approximation since in practice, the laser probe would be applied in contact mode, slightly compressing the tissue, and hence providing a flattened surface. For convenience, simulations were also performed in two dimensions, with the assumption that results may be extrapolated to a third dimension based on symmetry in the anatomy.
Second, the dynamic optical properties of tissue layers due to temperature changes were not included in the MC simulations. Instead, the values for absorption and scattering coefficients were assumed to be constant. While significant changes in absorption and scattering coefficients are well known to occur at high temperatures (e.g., during tissue coagulation and ablation) [39], the moderate temperatures computed in these studies, especially for the transvaginal approach, may not produce significant changes. While tissue optical properties are also dependent on pressure [40], in practice, the contact probe would only lightly compress the tissue surface to provide adequate and efficient contact cooling, so such effects may be minimal as well. Nevertheless, further improvements in the MC model may be warranted in the future to study the potential effects of dynamic tissue optical parameters.
Third, the effect of blood perfusion on tissue temperatures was not included in the heat transfer simulations. For relatively short times scales considered in this study (seconds), perfusion is not a major factor. Convective heat transfer in tissue due to blood flow only becomes significant on the time scale of minutes, for example, during longer duration thermal therapies such as laser interstitial thermal therapy and hyperthermia applications [41].
Finally, in practice, it may also be possible to preserve an even thicker surface tissue layer (e.g., entire vaginal wall) than demonstrated in these simulations. Optical clearing agents (OCA), most commonly in the form of alcohol and sugar compounds, have been shown to improve optical transmission through a variety of soft tissues [42]. The OCA mechanism is still under active investigation, but it is believed that optical transmission is improved by two different means: temporary dehydration of tissue which lowers water absorption, and refractive index matching through close packing of collagen fibrils which reduces light scattering. Although the vaginal wall is not a highly collagenous tissue, OCA’s may potentially provide a moderate improvement in optical penetration through this surface tissue layer, and thus more efficient deposition of light into the targeted endopelvic fascia layer as well.
CONCLUSIONS
Computer simulations (including optical, thermal, and damage models) suggest that a transvaginal laser based approach to thermal remodeling of the endopelvic fascia and minimally invasive treatment of female stress urinary incontinence is more feasible than a transurethral approach.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health under Award #1R15DK099774. The authors also thank Gino Schweinsberger for his assistance with the simulations.
Contract grant sponsor: National Institutes of Health; Contract grant number: 1R15DK099774.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: L.A. Hardy, C.C. Chang, and E.M. Myers do not have any disclosures. N.M. Fried has received funding from National Institutes of Health and Medtronic Covidien Group. He has consulted for Boston Scientific. M.J. Kennelly has received funding from National Institutes of Health. He has served as a consultant, advisor, or lecturer for Allergan, Amphora Medical, American Medical Systems, Astellas, Boston Scientific, Coloplast, Cook Myosite, Dignify Therapeutics, Novabay, and Hollister.
REFERENCES
- 1.Lightner DJ, Itano NM. Treatment options for women with stress urinary incontinence. Mayo Clin Proc 1999;74: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 2.Pesce F Current management of stress urinary incontinence. BJU Int 2004;94:8–13. [DOI] [PubMed] [Google Scholar]
- 3.Bent AE, McLennan MT. Surgical management of urinary incontinence. Obstet Gynecol Clin North Am 1998;25:883. [DOI] [PubMed] [Google Scholar]
- 4.Davila GW. Nonsurgical outpatient therapies for the management of female stress urinary incontinence: long-term effectiveness and durability. Adv Urol 2011;176498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukban JC. Transurethral radiofrequency collagen denaturation for treatment of female stress urinary incontinence: A review of the literature and clinical recommendations. Obstet Gynecol Int 2012;384234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lustgarten DL, Spector PS. Ablation using irrigated radio-frequency: A hands-on guide. Heart Rhythm 2008;5:899–902. [DOI] [PubMed] [Google Scholar]
- 7.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr Probl Diagn Radiol 2009;38:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson JS, Majaron B, Kelly KM. Active skin cooling in conjunction with laser dermatologic surgery. Semin Cutan Med Surg 2000;19:253–266. [DOI] [PubMed] [Google Scholar]
- 9.Zenzie HH, Altschuler GB, Smirnov MZ, Anderson RR. Evaluation of cooling methods for laser dermatology. Lasers Surg Med 2000;26:130–144. [DOI] [PubMed] [Google Scholar]
- 10.Salvatori S, Nappi RE, Zerbinati N, Calligaro A, Ferrero S, Origoni M, Candiani M, Leone Roberti Maggiori U. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: A pilot study. Climacteric 2014;17:363–369. [DOI] [PubMed] [Google Scholar]
- 11.Salvatore S, Leone Roberti Maggiore U, Athanasiou S, Origoni M, Candiani M, Calligaro A, Zerbinati N. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: An ex vivo study. Menopause 2015;22:845–849. [DOI] [PubMed] [Google Scholar]
- 12.Zerbinati N, Serati M, Origoni M, Candiani M, Iannitti T, Salvatore S, Marotta F, Calligaro A. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci 2015;30:429–436. [DOI] [PubMed] [Google Scholar]
- 13.Vizintin Z, Lukac M, Kazic M, Tettamanti M. Erbium laser in gynecology. Climacteric 2015;18(suppl 1):4–8. [DOI] [PubMed] [Google Scholar]
- 14.Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med 2015;47:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N Development and anatomy: Disorders and development In: Brown L, editor. Pathology of the vulva and vagina. London: Springer-Verlag; 2013. pp 1–11. [Google Scholar]
- 16.Ramli R, Durand D, Fried NM. Subsurface tissue lesions using an Nd:YAG laser and cryogen cooling. J Endourol 2003;17:923–926. [DOI] [PubMed] [Google Scholar]
- 17.Ramli R, Chung CC, Fried NM, Franco N, Hayman MH. Subsurface tissue lesions created using an Nd:YAG laser with a sapphire contact cooling probe. Lasers Surg Med 2004; 35:392–396. [DOI] [PubMed] [Google Scholar]
- 18.Chung CC, Varkarakis IM, Permpongkosol S, Lima G, Franco N, Hayman MH, Nicol TL, Fried NM. Laser probes for noninvasive coagulation of subsurface tissues. Proc SPIE 2006;607822:1–5. [Google Scholar]
- 19.Cilip CM, Scott NJ, Trammell S, Fried NM. Noninvasive thermal coagulation of deep subsurface tissue structures using a laser probe with integrated contact cooling. Proc IEEE Eng Med Biol Soc 2008;1:3657–3660. [DOI] [PubMed] [Google Scholar]
- 20.Chang CH, Wilson CR, Fried NM. Comparison of four lasers (l ¼ 650, 808, 980, and 1075 nm) for noninvasive creation of deep subsurface lesions in tissue. Proc SPIE 2015;95420G: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panayi DC, Digesu GA, Tekkis P, Fernando R, Khullar V. Ultrasound measurement of vaginal wall thickness: A novel and reliable technique. Int Urogynecol J 2010;21:1265–1270. [DOI] [PubMed] [Google Scholar]
- 22.Wieczorek AP, Wozniak MM, Stankiewicz A, Santoro GA, Bogusiewicz M, Rechberger T. 3-D high-frequency endovaginal ultrasound of female urethral complex and assessment of inter-observer reliability. Eur J Radiol 2012;81:e7–e12. [DOI] [PubMed] [Google Scholar]
- 23.Jacques SL. Monte Carlo modeling of light transport in tissues (steady state and time of flight) In: Welch AJ, van Gemert MJC, editors. Optical-Thermal Response of Laser-Irradiated Tissue. 2nd edition Heidelberg: Springer; 2011. pp 109–144. [Google Scholar]
- 24.Wang LH, Jacques SL, Zheng LQ. MCML—Monte Carlo modeling of photon transport in multi-layered tissues. Comput Methods Programs Biomed 1995;47:131–146. [DOI] [PubMed] [Google Scholar]
- 25.Wang LH, Jacques SL, Zheng LQ. CONV—Convolution for responses to a finite diameter photon beam incident on multilayered tissues. Comput Methods Programs Biomed 1997;54:141–150. [DOI] [PubMed] [Google Scholar]
- 26.Huilan A, Xing D, Wei H, Gu H, Lu J. Thermal coagulation-induced changes of the optical properties of normal and adenomatous human colon tissue in vitro in the spectral range 400–1100 nm. Phys Med Biol 2008;53:1. [DOI] [PubMed] [Google Scholar]
- 27.Roggan A, Dorschel K, Minet D, Wolff D, Muller G. The optical properties of biological tissue in the near infrared wavelength range—Review and measurements In: Muller G, Roggan A, editors. Laser-induced interstitial thermo-therapy. Bellingham, WA: SPIE Optical Engineering Press; 1995. pp 10–44. [Google Scholar]
- 28.Tsai CL, Chen JC, Wang WJ. Near-infrared absorption property of biological soft tissue constituents. J Med Biol Eng 2001;21:7–14. [Google Scholar]
- 29.Cheong WF. Summary of optical properties In: Welch AJ, van Gemert MJC, editors. Optical-thermal response of laser-irradiated tissue. 1st edition New York: Plenum; 1995. pp 275–303. [Google Scholar]
- 30.Giering K, Minet O, Lamprecht I, Muller G. Review of thermal properties of biological tissues In: Muller G, Roggan A, editors. Laser-induced interstitial thermotherapy. Bellingham, WA: SPIE Optical Engineering Press; 1995. pp 45–65. [Google Scholar]
- 31.McIntosh RL, Anderson V. Comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys Rev Lett 2010;5:129–151. [Google Scholar]
- 32.Thomsen S, Pearce JA. Thermal damage and rate processes in biological tissues In: Welch AJ, van Gemert MJC, editors. Optical-thermal response of laser-irradiated tissue. 2nd edition Heidelberg: Springer; 2011. pp 487–549. [Google Scholar]
- 33.Henriques FC, Moritz AR. Studies of thermal injury in the conduction of heat to and through skin and the temperatures attained therein: A theoretical and experimental investigation. Am J Pathol 1947;23:531–549. [PMC free article] [PubMed] [Google Scholar]
- 34.Moritz AR, Henriques FC. Studies of thermal injury II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol 1947;23: 695–720. [PMC free article] [PubMed] [Google Scholar]
- 35.Moritz AR. Studies of thermal injury III. The pathology and pathogenesis of cutaneous burns: An experimental study. Am J Pathol 1947;23:915–934. [PMC free article] [PubMed] [Google Scholar]
- 36.Henriques FC. Studies of thermal injury V. The predictability and significance of thermally induced rate processes leading to irreversible epidermal injury. Arch Pathol 1947;43: 489–502. [PubMed] [Google Scholar]
- 37.Wall MS, Deng SH, Torzilli PA, Doty SB, O’Brien SJ, Warren R. Thermal modification of collagen. J Shoulder Elbow Surg 1999;4:339–244. [DOI] [PubMed] [Google Scholar]
- 38.Chang CH, Fried NM. Diffusing, side-firing, and radial delivery laser balloon catheters for creating subsurface thermal lesions in tissue. Proc SPIE (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barton JK. Dynamic changes in optical properties In: Welch AJ, van Gemert MJC, editors. Optical-thermal response of laser-irradiated tissue. 2nd edition Heidelberg: Springer; 2011. pp 321–349. [Google Scholar]
- 40.Chan EK, Sorg B, Protsenko D, O’Neil M, Motamedi M, Welch AJ. Effects of compression on soft tissue optical properties. IEEE J Sel Top Quantum Electron 1996;2: 943–950. [Google Scholar]
- 41.Niemz MH. Interaction mechanisms In: Niemz MH, editor. Laser-tissue interactions: Fundamentals and applications. 3rd edition Heidelberg: Springer; 2004. pp 45–150. [Google Scholar]
- 42.Zhu D, Larin KV, Luo Q, Tuchin VV. Recent progress in tissue optical clearing. Laser Photon Rev 2013;7:732–757. [DOI] [PMC free article] [PubMed] [Google Scholar]