Abstract
Background
Exposure to ambient air pollution has been associated with lower lung function in adults, but few studies have investigated associations with radiographic lung and airway measures.
Methods
We ascertained lung volume, mass, density, visual emphysema, airway size, and airway wall area by computed tomography (CT) among 2,545 non-smoking Framingham CT sub-study participants. We examined associations of home distance to major road and PM2.5 (2008 average from a spatiotemporal model using satellite data) with these outcomes using linear and logistic regression models adjusted for age, sex, height, weight, census tract median household value and population density, education, packyears of smoking, household tobacco exposure, cohort, and date. We tested for differential susceptibility by sex, smoking status (former vs never), and cohort.
Results
The mean participant age was 60.1 y (standard deviation 11.9). Median PM2.5 level was 9.7 μg/m3 (interquartile range 1.6). Living < 100 m from a major road was associated with a 108 mL (95% CI 8, 207) higher lung volume compared to ≥ 400 m away. There was also a log-linear association between proximity to road and higher lung volume. There were no convincing associations of proximity to major road or PM2.5 with the other pulmonary CT measures. In subgroup analyses, road proximity was associated with lower lung density among men, and higher odds of emphysema among former smokers.
Conclusions
Living near a major road was associated with higher average lung volume, but otherwise we found no association between ambient pollution and radiographic measures of emphysema or airway disease.
Keywords: air pollution, traffic, chronic obstructive pulmonary disease (COPD), chest computed tomography (CT), lung volume
Introduction
A large body of research has demonstrated that short-term exposure to ambient pollution is acutely harmful to adult lung function and respiratory health1–4. A modest number of studies, including our previous work in the Framingham Heart Study (FHS), have found that longer-term yearly exposure to higher levels of pollution is associated with lower lung function and faster lung function decline among adults5–10. It remains unclear if these associations of longer-term pollution with lung function are accompanied by structural differences in the pulmonary parenchyma or airways.
Some recent studies have examined associations between ambient pollution and quantitative computed tomography (CT) measures of emphysema, air trapping and airway thickening. The Multi-Ethnic Study of Atherosclerosis (MESA) examined associations of particulate matter less than 2.5 microns in diameter (PM2.5) and nitrogen oxides (NOx) with percent emphysema-like lung by CT, and found positive associations between annual pollutant exposure and percent emphysema, but these associations became less precise and reversed direction after adjustment for study site11. In COPDGene, a study of current and former smokers, occupational exposures to dust and fumes were associated with higher percent emphysema and gas trapping on chest CT, and greater airway wall area among men12.
In our previous work6, residential proximity to a major road, and recent annual exposure to PM2.5, were associated with lower forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) in a proportional, restrictive pattern in adults. Chronic exposure to traffic and PM2.5 may cause inflammation in the distal airways, resulting in reversible airway closure and a restrictive-pattern lung function decrement13. It has also been proposed that long-term pollution exposure could destroy or damage alveoli, resulting in emphysema11,14, or damage the pulmonary interstitium, resulting in interstitial lung disease15. To further investigate how ambient pollution exposure may affect the lung and airways, we examined associations of proximity to major roadway and annual PM2.5 at home address with previously-determined radiographic measures of inspiratory lung volume, mass, density, emphysema, airway size and wall area, among adult FHS participants.
Methods
Study population
The study population consists of Framingham Offspring and Third Generation Cohort participants, which have been previously described16,17. From 2008-2011, 2,749 of these participants underwent volumetric CT scans of the lung as part of FHS-MDCT2 (Multidetector Computed Tomography 2) sub-study. This sub-study had the following exclusion criteria: weighing ≥350 pounds, age <35 years (men) or <40 years (women), and pregnancy. We included 2,545 sub-study participants who do not currently smoke, had a valid, geocoded home address, and attended the corresponding Framingham Offspring Exam 8 (2005-2008) or Third Generation Exam 2 (2008–2011), during which questionnaire and address information was obtained. For 12 CT sub-study participants who did not attend the corresponding FHS exam, the most recent earlier exam was used for questionnaire and address data. There were also 204 current smokers (7.4% of 2,749) who were excluded from primary analyses, but were included in sensitivity analyses described below. The Institutional Review Boards of the Boston University Medical Center, Massachusetts General Hospital, and Beth Israel Deaconess Medical Center approved this study. All participants provided written consent.
Proximity to the nearest major roadway
We geocoded participants’ home address using ArcGIS 10(ESRI, Redlands, CA) and calculated residential distance to the nearest major (A1, A2 or A3) roadway, defined by the US Census feature classification system as a primary highway with limited-access (A1), a primary road without limited access (A2), or a secondary or connecting road (A3). Based on previous work showing that particles levels diminish to neighborhood background levels 100 to 300 meters from major roads18,19, we examined associations using categories of distance: <100, 100 to <200, 200 to <400, and 400 to <1000 m. These categories of doubling distance were selected to reflect the decay function of traffic pollution as distance to roadway increases. We have also previously observed that the natural log of residential proximity to a major roadway and lung function and mortality are linearly associated6,20. We therefore also tested the natural log of distance to roadway as an exposure. As in previous work, we excluded those living > 1 km from a major road (321 participants, or 11.7% of 2,749) when examining proximity to roadway as the exposure because, beyond 1 km, the distance to nearest major road is more likely to be an indicator of semi-rural or rural exposures rather than traffic6,21.
PM2.5 assessment
We estimated ambient PM2.5 concentrations at each residential address using a spatio-temporal model. The PM2.5 model utilized satellite-based aerosol optical depth data, retrieved using the multi-angle implementation of atmospheric correction (MAIAC) algorithm at 1 × 1 km resolution, and ground daily PM2.5 mass measurements, land use terms, and meteorological covariates to estimate daily PM2.5 at 200 × 200 m resolution22. Predictions from this model had an excellent mean out-of-sample R2 (0.88) and excellent fit of predictions when compared with withheld measurements (slope=0.99)22.
We first fit a model regressing monitor-based PM2.5 concentrations against aerosol optical depth, adjusting for land use terms and meteorological variables. We used inverse probability weighting to deal with non-random missingness of daily aerosol optical depth data. Second, we predicted concentrations for grid cells that only had aerosol optical depth available using the above fitted model. Third, for grid cells/days that had missing aerosol optical depth measurements, we imputed data using a generalized additive model with smoothing and a random intercept for each grid cell. Last, for each ground-level PM2.5 monitoring site, we regressed residuals (differences between actual monitor-based measurement and predicted values for each grid cell) against spatial and temporal variables, as previously described22. We then used this model to estimate a daily localized residual (difference between grid cell and local value) at each residential location at 200 m resolution. The total PM2.5 daily estimates were calculated as the sum of grid and localized residual predictions.
We used the annual PM2.5 concentration of an index year as a measure of recent, longer-term PM2.5 exposure, using the address recorded at the time of their Framingham exam. All Framingham exam visits took place in the same year or in a year preceding the CT scan. We selected 2008 as the index year because it overlapped best with the timing of the chest CT’s (2008-2011) and the study visits when address location was ascertained (2005-2008 for Offspring Exam 8 and 2008-2011 for Third Generation Exam 2). Other exposure averages (2003-2008) were also tested in sensitivity analyses. We did not assign the PM2.5 average for the year of the CT test, because of the downward trend in PM2.5 levels over time. Assigning the same index year to all participants avoids confusing differences in exposure due to home location with difference in exposure due to choice of year. This approach is consistent with our previous work and that of others in the field6,23,24.
Questionnaire and Census Data
At each exam, data were collected on demographics, medication use, smoking history, and respiratory symptoms and diagnoses. Neighborhood-level socioeconomic characteristics were assigned at the census tract level from US Census 2000 data.
Chest CT Acquisition and Analysis
Inspiratory chest CT scans were obtained in the supine position with no administration of contrast using the 64-detector-row CT scanner (Discovery, GE Healthcare, Waukesha, WI) with 120 kV, 300–350 mA (optimized with body weight), gantry rotation time of 0.35 s. Raw data were collected using a 210° scan reconstruction algorithm and a detector width of 0.625 mm. Images were reconstructed by sharp lung algorithm with a 0.625 mm slice thickness and a 50 cm field of view. Image analysis was performed using the Chest Imaging Platform (www.ChestImagingPlatform.org) in the Applied Chest Imaging Laboratory at Brigham and Women’s Hospital. Total lung volume, mass, density (median Hounsfield units of all lung voxels), airway lumen diameter, and airway % wall area were calculated from the inspiratory chest CT scans as described previously25–28. The parameters used for image acquisition and reconstruction led to substantial artifact in estimates of the percentage of low-attenuating tissue in the lungs. We present the median lung density since it is robust to such effects. By convention, the third sub-segment of the right upper lobe was selected to calculate the airway lumen diameter (an indicator of airway narrowing) and % wall area (a measure of airway wall thickening)29.
To classify CT scans by the presence or absence of visual emphysema, chest CT images were uploaded to a Picture Archiving and Communication System (PACS) workstation (Virtual Place Raijin, AZE Ltd., Tokyo, Japan) and visually evaluated for emphysema in three lung zones (upper, middle, lower) by three board-certified radiologists specializing in thoracic imaging using a modified sequential reading method30,31. After sequential reading, cases with emphysema in at least one lung zone were re-evaluated by two radiologists for consensus.
Statistical methods
We used multivariable linear regression models for continuous outcomes (lung volume, mass, median lung density, airway % wall area, and airway diameter), and multivariable logistic regression models for visual emphysema on CT. We adjusted for age at CT scan, sex, height (both as a linear and squared term), weight, median value of owner occupied housing and population density (persons/km2) in the census tract, personal educational attainment, former smoking, pack-years of smoking, any household smoking, cohort and date of CT scan. Current smokers were excluded from primary analyses out of concern that acute inflammatory effects of recent smoking might overwhelm chronic effects of pollution exposure. These covariates, and the exclusion of current smokers, were determined a priori and are consistent with our previous publications in this cohort2,6,32. We did not adjust for race/ethnicity because nearly all participants are of European ancestry, and individual-level income was not measured in this cohort.
Results from log-transformed proximity to roadway analyses were scaled as contrasting participants who lived at the 25th percentile of distance (66 m) to those who lived at the 75th percentile (429 m) from the nearest major roadway. For ease of interpretation, and consistency with prior publications6, results for all associations with annual average PM2.5 at home address were scaled to a 2 μg/m3 difference in annual average PM2.5.
We tested for evidence of differential susceptibility to pollution exposure by sex, cohort, and smoking status (including and excluding current smokers). In sensitivity analyses, we relaxed our exclusion criteria, and repeated analyses including 204 current smokers, and repeated near-roadway analyses including 321 participants living >1,000 m from a major roadway. As in prior work, we assessed the sensitivity of results to choice of PM2.5 index year, by repeating analyses using PM2.5 averaged from 2003–200824.
Scaled regression coefficients and odds ratios (ORs) were reported with 95% confidence intervals (CIs). Analyses were performed using Proc GLM and Proc LOGISTIC in SAS 9.4 (SAS Institute, Inc., Cary, NC). Figures were plotted using Stata 13 (StataCorp LP, College Station, TX). We used a threshold interaction term p value of ≤0.10 to evaluate effect modification.
Results
Study Participants
Participant characteristics and outcomes are listed in Table 1. Mean participant age was 60.1 years, with an equal representation of each sex. Nearly half were former smokers and almost a quarter of participants had lived with a smoker during adulthood. The majority attended at least some college and nearly half of participants had a college degree. By census tract, the median value of owner-occupied housing was $225,000 with a broad distribution, and population density was 1,051 and right-skewed. Overall, 11% had emphysema as determined by CT visual inspection.
Table 1.
Participant Characteristics
| Age, y, mean (SD) | 60.1 (11.9) |
|---|---|
| BMI, kg/m2, mean (SD) | 28.5 (5.4) |
| Pack-years, mean (SD) | 8.1 (14.5) |
| Pack-years among former smokers, mean (SD) | 17.0 (17.1) |
| Median census tract owner-occupied home value, $, mean (SD) | 225,000 (113,000) |
| Population density of census tract, persons/km2, mean (SD) | 1,051 (3,024) |
| Lung volume, L, mean (SD) | 4.9 (1.2) |
| Lung mass, g, mean (SD) | 849 (166) |
| Median lung density, Hounsfield units, mean (SD) | −864 (41) |
| Airway lumen diameter, mm, mean (SD) | 4.8 (0.9) |
| Airway wall area, %, mean (SD) | 53.9 (5.6) |
| Presence of visual emphysema, % | 11 |
| Male, % | 49 |
| Smoker in household in adulthood, % | 23 |
| Smoking status, % | |
| Never | 52 |
| Former | 48 |
| Education, % | |
| <High school | 1 |
| High school | 20 |
| Some college | 30 |
| College graduate | 48 |
Data calculated from 2,545 non-smoking participants enrolled in the chest computed tomography (CT) sub-study. Of these, 2,353 provided CT measures of lung volume, mass, and density, 2,013 had measures of the airway lumen diameter and wall area, and 2,430 had a determination of visual emphysema.
Exposure Distributions
Exposure distributions are summarized in Table 2. The median distance to the closest major roadway was 214 m after excluding 11.7% of participants living more than 1 km from a major road. In 2008, annual average PM2.5 concentration among study participants ranged from 1.0 – 15.6 μg/m3. The mean and median 2008 PM2.5 levels were 9.4 and 9.7 μg/m3, respectively, below the current annual PM2.5 U.S. Environmental Protection Agency (EPA) annual National Air Quality Standard (NAAQS) of 12 μg/m3. Average PM2.5 among those living >400 m from a major road was 8.8 μg/m3 compared to 9.7 μg/m3 among those < 100 m from a major road (eTable 1).
Table 2.
Distributions of Distance to Roadway and the Annual Average of PM2.5 in 2008
| Distance to major roadway, m, median (IQRw)a | 214 (363) |
|---|---|
| Distance to roadway in categoriesa, % | |
| <100 m | 31 |
| 100-<200 m | 16 |
| 200-<400 m | 24 |
| 400-<1,000 m | 27 |
| PM2.5, μg/m3, median (IQRw) | 9.7 (1.6) |
321 participants (11.7 %) who lived ≥1,000m from a major roadway were excluded from the distance to roadway primary analyses. IQRw indicates interquartile range width.
Associations with CT Lung and Airway Measures
Major findings are summarized in Tables 3 and 4. Proximity to major roadway was associated with higher inspiratory lung volume. There were no convincing associations of proximity to major road or PM2.5 with the other pulmonary CT measures. Figure 1 shows the difference in average lung volume for each roadway category compared to the 400-<1,000 m reference.
Table 3.
Associations of Major Roadway and PM2.5 Exposure with Computed Tomography Measures of the Lung and Airway
| Difference in Outcome per Unit Increase in Exposurea (95% CI) |
|
|---|---|
| Lung volume, mL | |
| Close proximity to road | 58 (11, 106) |
| PM2.5 | 24 (−31, 80) |
| Lung mass, g | |
| Close proximity to road | 1.3 (−3.3, 5.9) |
| PM2.5 | −1.9 (−7.3, 3.5) |
| Median lung density, HU | |
| Close proximity to road | −1.1 (−3.2, 0.9) |
| PM2.5 | −1.3 (−3.8, 1.2) |
| Odds of emphysema | |
| Close proximity to road | 1.11 (0.92, 1.35) |
| PM2.5 | 1.10 (0.86, 1.39) |
| Airway wall area % | |
| Close proximity to road | 0.20 (−0.12, 0.52) |
| PM2.5 | 0.03 (−0.35, 0.41) |
| Airway lumen diameter, mm | |
| Close proximity to road | −0.03 (−0.08, 0.02) |
| PM2.5 | −0.02 (−0.08, 0.04) |
Scaled from the 75th (429 m) to the 25th (66 m) percentile of the log-transformed distance to major roadway and per 2 μg/m3 for PM2.5. Adjusted for sex, age, height and height2, weight, education (no high school diploma, completed high school, some college, college degree or higher), median owner-occupied home value and population density from 2000 census tract, smoking status (former vs never), pack-years of smoking, smoking by others in household, cohort, and date of exam.
Table 4.
Associations of Distance to Major Road by Category and Lung Computed Tomography Measures
| Difference in Outcome Compared to Referencea (95% CI) |
|
|---|---|
| Lung volume, mL | |
| <100 m | 108 (8, 207) |
| 100-<200 m | 114 (−6, 235) |
| 200-<400 m | −7 (−112, 99) |
| 400-<1,000 m | Reference |
| Lung mass, g | |
| <100 m | 0.6 (−9.1, 10.3) |
| 100-<200 m | 8.7 (−3.1, 20.5) |
| 200-<400 m | −5.0 (−15.3, 5.3) |
| 400-<1,000 m | Reference |
| Median lung density, HU | |
| <100 m | −3.7 (−8.0, 0.7) |
| 100-<200 m | −3.5 (−8.8, 1.8) |
| 200-<400 m | −0.6 (−5.2, 4.0) |
| 400-<1,000 m | Reference |
| Odds of emphysema | |
| <100 m | 1.2 (0.8, 1.9) |
| 100-<200 m | 1.4 (0.8, 2.3) |
| 200-<400 m | 1.5 (1.0, 2.4) |
| 400-<1,000 m | Reference |
| Airway wall area % | |
| <100 m | 0.21 (−0.46, 0.88) |
| 100-<200 m | 0.05 (−0.77, 0.86) |
| 200-<400 m | 0.08 (−0.63, 0.79) |
| 400-<1,000 m | Reference |
| Airway lumen diameter, mm | |
| <100 m | −0.03 (−0.14, 0.07) |
| 100-<200 m | −0.01 (−0.14, 0.12) |
| 200-<400 m | 0.00 (−0.11, 0.11) |
| 400-<1,000 m | Reference |
Adjusted for sex, age, height and height2, weight, education (no high school diploma, completed high school, some college, college degree or higher), median owner-occupied home value and population density from 2000 census tract, smoking status (former vs never), pack-years of smoking, smoking by others in household, cohort, and date of exam.
Figure 1. Difference in Lung Volume (mL) by Distance to Major Roadway Category.
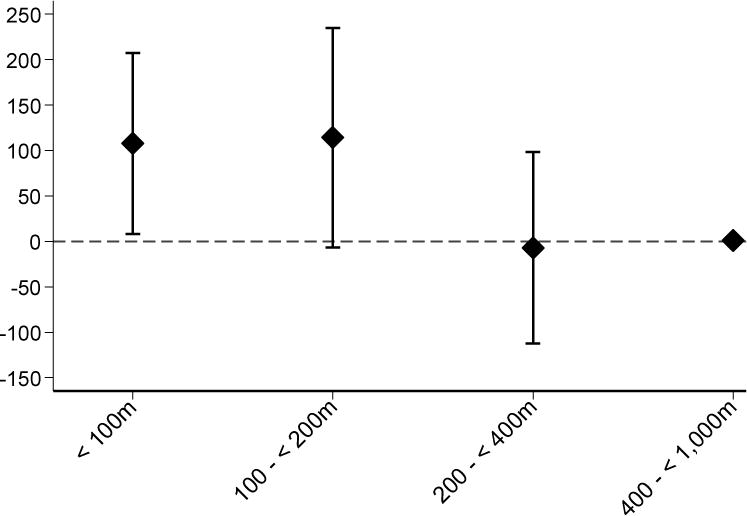
Adjusted for sex, age, height and height2, weight, education (no high school diploma, completed high school, some college, college degree or higher), median owner-occupied home value and population density from 2000 census tract, smoking status (former vs never), pack-years of smoking, smoking by others in household, cohort, and date of exam.
In testing for effect modification by sex, smoking status, and cohort, road proximity was associated with lower lung density among men (Pinteraction=0.05), and higher odds of emphysema among former smokers (Pinteraction=0.05). All other interactions had P> 0.1. Figure 2 shows the difference in lung density by distance to road category, stratified by sex. Men living <100 m from a road had a median lung density 7.7 Hounsfield units (HU) lower (95% CI −13.9, −1.5) than those living 400–1,000 m from a major road, but among women, there was no such association. Among former smokers, an interquartile range width difference in proximity to road (log-transformed) was associated with a 23% (95% CI −1, 52) higher odds of emphysema as determined by CT. This association was null among never smokers. When we included current smokers, there remained effect modification by smoking status (global Pinteraction=0.03) and there was a positive association between proximity to road and odds of emphysema among the former smokers (but not among never and current smokers). Results of these stratified analyses are shown in eTables 2–3 in the online supplement.
Figure 2. Difference in Median Lung Density (HU) by Distance to Major Roadway Category, Stratified by Sex.
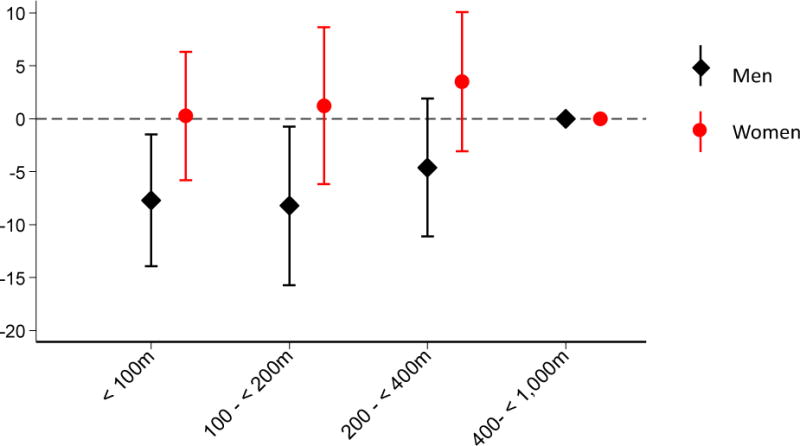
Adjusted for sex, age, height and height2, weight, education (no high school diploma, completed high school, some college, college degree or higher), median owner-occupied home value and population density from 2000 census tract, smoking status (former vs never), pack-years of smoking, smoking by others in household, cohort, and date of exam.
Sensitivity Analyses
When current smokers were included in the analyses, the association between proximity to road and lung volume was still observed and the remaining associations were unchanged. When we examined associations with distance to road without excluding those living ≥1,000 m from a major road, the association between road proximity and odds of emphysema increased slightly: in the full cohort, odds of emphysema were 17% higher (95% CI −2, 39) among those living 66 m from a major road compared to 429 m. The other associations were essentially unchanged. Associations did not change substantially when we examined the 2003-2008 average of PM2.5. Results of all sensitivity analyses are shown in eTables 4–6 in the online supplement.
Exploratory Analyses
To place the association between proximity to road and inspiratory lung volume in context, we examined associations of smoking status and airflow obstruction (defined as FEV1/FVC < 0.7) with lung volume measured by CT in the full study population, including current smokers. Each of these measures was associated with higher lung volume in parsimonious and fully adjusted models (eTable 7, online supplement). Airflow obstruction on spirometry and current smoking were each associated with more than a 400 mL higher lung volume than those with no obstruction and never smoking status, respectively. Former smoking was associated with a 237 mL (95% CI 161, 312) higher lung volume than never smoking, which is about twice the difference in inspiratory lung volume of 108 mL (95% CI 8, 207) for those living within 100 m from a major road compared to those living 400-1,000 m away (Figure 1).
Discussion
In this cohort of generally healthy adults residing in the Northeastern US, those living closer to a major road had a higher average inspiratory lung volume. Despite our previous findings in this cohort that annual PM2.5 exposure was associated with lower lung function and faster lung function decline6, PM2.5 exposure was not associated with any of the CT measures of lung or airway structure that we examined.
Taken into context with our previous findings, the association of road proximity with higher lung volume could indicate: (1) subclinical emphysema, (2) an adverse effect on the small airways, resulting in air trapping, (3) increased inspiratory effort, or a combination of these. It seems unlikely that people living closer to roads would on average inhale more deeply during the inspiratory breath-hold of a CT scan, but this is a possibility as these analyses cannot account for variability in participant effort. Airflow obstruction and current and former smoking status were strongly associated with higher inspiratory lung volume in this population. Effect sizes for smoking and airflow obstruction were 2-4 times the 100 mL higher lung volume for those living < 100 m vs 400-1,000 m from a major road (eTable 7, online supplement). This supports the notion that higher lung volume may be an indicator of obstructive lung disease. However this conclusion is not supported by the fact that we did not identify associations with emphysema and airway thickening/narrowing.
In addition to lung volume, we considered two other indicators of emphysema: lung density and visual emphysema determined by radiologist consensus opinion. Lung density has been found to be a robust indicator of subclinical emphysema among smokers and those with impaired lung function33,34, but a recent study questioned the generalizability of using lung density on CT as a measure of emphysema among those with normal lung function on average35. In our secondary analyses, road proximity was associated with higher odds of visual emphysema among former smokers, and lower median lung density among men. Taken together, these findings provide some evidence of elevated emphysema risk among those living very near major roads. Our finding that former smokers may be more susceptible to emphysema in association with living close to a major road is consistent with studies, including our prior work in Framingham, that have found greater reductions in lung function among former smokers, compared to never or current smokers, in association with traffic-related pollution6,9. It is possible that smoking-related injury may predispose the lung to injury from air pollution. Others have also found that men may have greater susceptibility to residential pollution exposure than women2,36, although the literature is inconsistent37.
A second explanation for the higher lung volume finding is an adverse effect of near-roadway pollution on the small airways. We were unable to assess for air trapping in this study because our protocol did not measure both inspiratory and expiratory films. A small number of studies have examined ambient exposures and air trapping by CT. In the COPDGene study of approximately 10,000 current and ex-smokers, occupational exposure to dust and fumes was associated with a higher percentage of lung less than −856 HU at end expiration (a measure of air trapping)12. Occupational dust/fume exposure was also associated with greater airway wall thickness among men only. In a small study of Mexican women with COPD, non-smokers exposed to biomass burning had more air trapping on CT compared to smokers, who had more emphysema38. A study examining chest X-rays of 249 healthy children identified hyperinflation in a much larger proportion of children in the polluted city of Mexico City, compared to a less polluted region of Mexico39. Among children with abnormal chest X-rays who underwent CT scanning, many had mild bronchial wall thickening and air trapping. We did not identify associations between road proximity and airway wall thickness and lumen diameter. Although the dimensions of the large airways have been found, histologically, to be a reasonable proxy of small airway dimensions, this relationship is imprecise (R2 only 0.57 for percent wall area in one study)40. The possibility that the association between traffic exposure and lung volume in our study is mediated by an effect on the very small airways remains unanswered.
There is inconsistent evidence of an association between long-term traffic-related pollution exposure at levels experienced in the US and Europe and risk of COPD. In a study of 4,757 55-year-old women in Germany in 1985-1994 (when pollution levels were substantially higher), Schikowski and colleagues found that women living within 100 m of a major road were 1.79 times (95% CI 1.06, 3.02) more likely to have COPD by GOLD criteria than those living further away41. A Swedish large-scale postal questionnaire study found that living <100 m from a busy road was associated with self-reported COPD diagnosis and chronic bronchitis symptoms42. A meta-analysis of four European cohorts found positive but imprecise associations of NOx, PM2.5, PM10 and traffic density within 100 m of the home with COPD prevalence and incidence43. There were associations between traffic density and COPD only among women and never smokers43. A separate analysis in the same cohort did not find an association between current traffic exposure and chronic bronchitis symptoms44. We found that traffic exposure was associated with lower lung density among men, and higher odds of emphysema among former smokers, but not among women and never smokers.
Associations of PM2.5 with lung volume, median density, visual emphysema and airway diameter were in the same direction as road proximity, but these associations were small in magnitude and imprecise. We have previously found associations of PM2.5 (and also proximity to road) with lower FEV1 and FVC (but not FEV1:FVC) in a repeated measures analysis in this cohort6. In comparing our CT outcomes with these measures, FEV1 and FVC were fairly strongly correlated with lung volume and lung mass (Pearson correlations ranged from 0.56 to 0.72), and modest correlations were observed with airway wall thickness and lumen diameter (0.22 and 0.19 respectively) (eTable8). After accounting for age, sex, and height, the correlations with lung volume and mass were lower, but still present (partial correlations 0.17 to 0.23, except for 0.43 between lung volume and FVC, eTable 8).
There are a number of potential explanations for the weak or null findings for PM2.5. Compared to prior analyses using more than 9,000 spirometry tests, the present analysis using up to 2,749 CT exams was less well-powered to examine associations with PM2.5. Additionally, the CT measures may have less precision compared to spirometry obtained in accordance with American Thoracic Society standards (especially lung mass, which is computed from CT measures of volume and density28). Nonetheless, the associations we observed between proximity to road and lung volume persisted after adjustment for FVC, and indicate a difference in inspiratory lung volume not entirely explained by differences in lung function: lung volume was 137 mL higher (95% CI 45, 230) if <100 m, and 135 mL higher (95% CI 24, 247) if 100 - < 200 m from a major road compared to 400 - <1,000 m, after adjusting for FVC. It is possible that the mixture of particles and gases in the immediate vicinity of a major road are responsible for the observed associations with lung volume, and not overall PM2.5 exposure. A number of studies investigating adverse health outcomes have found stronger associations with living close to a road than with modeled estimates of pollutant exposure32,45–48. Near-roadway pollution has a higher proportion of very tiny (less than 0.1 micron diameter) ultrafine particles, including metals and carbonaceous materials emitted in motor vehicle exhaust. Ultrafine particles dissipate exponentially with distance from a freeway, and the smallest of these dissipate within 90 m49. Near-roadway pollution also contains particles that are larger than PM2.5 (road dust from tires and break lining containing plastics and metals), and gases including nitrogen oxides, that may contribute to risk of lung disease. There is some evidence that larger particles (greater than 2.5 or 10 microns) may be more harmful to the lung than PM2.5, which may explain the lack of association with PM2.510,44. Compared to earlier studies, we were also limited in our power to examine PM2.5, because average PM2.5 in 2008 was not only low, but narrowly distributed (IQR width only 1.6 μg/m3). Nonetheless, other studies have found associations between annual PM2.5 at similar concentrations and adverse health measures6,21,50,51. It is possible that other near-roadway exposures, including noise or social stress, could be responsible for the associations with lung volume, even after adjustment for measures of socioeconomic status, but there is no strong biologic reason to suspect this.
Our study has some limitations. It is a cross-sectional study design. Proximity to roadway is a singular measure of distance that does not take into account how long the participant lived at a given address. The PM2.5 satellite data became available in 2003, and therefore we could not consider differences in PM2.5 exposures earlier in life in our analyses. Instead, we used the 2008 (and 2003-2008) averages as indicators of recent, longer-term exposure. Additionally, the modeled PM2.5 data is only resolved to 200 m and therefore could not capture micro-scale differences in long-term PM2.5 exposure. Future work, including adult cohort studies with longitudinal address and highly-resolved exposure data capturing a large proportion of the lifetime, may address these limitations. Our findings may not be generalizable to other ethnicities or people of very low socioeconomic position. Although we have adjusted for a robust list of potential confounders, we cannot exclude the possibility of residual confounding.
Our study also has several strengths. In this relatively large cohort of adults, we used an innovative spatial–temporal model to estimate PM2.5 at each person’s address, and accounted for demographic and lifestyle factors, and individual- and neighborhood-level indicators of socioeconomic position in our analyses. We examined novel CT measures of the lung and airways using standardized quantitative and clinical protocols to assess for radiographic evidence of emphysema and airway disease, many of which have not been examined in air pollution research before.
Conclusions
In this study, people living closer to a major road had a higher average inspiratory lung volume, but otherwise there was no evidence of an association between ambient pollution and radiographic measures of emphysema or airway disease.
Supplementary Material
Acknowledgments
The authors wish to thank the Framingham Heart Study participants for their participation in the study.
Sources of Financial Support:
This results reported herein correspond to specific aim 1 of the National Institute for Environmental Health Sciences K23 ES026204 grant to Mary B. Rice. This work was also supported by the US Environmental Protection Agency (R832416, RD834798), and the National Heart, Lung and Blood Institute (the Framingham Heart Study Contract Nos. N01-HC-25195 and HHSN268201500001I, and T32HL007575), and National Institute of General Medical Sciences (1P20GM109036-01A1). This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA; the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. Further, US EPA does not endorse the purchase of any commercial products or services.
Footnotes
Statement on Conflict of Interest: The authors have no conflicts of interest to declare.
Process to Replicate Results: Research applications can be submitted to the Framingham Heart Study Research Review Committees online at https://www.framinghamheartstudy.org.
References
- 1.Brunekreef B, Dockery DW, Krzyzanowski M. Epidemiologic studies on short-term effects of low levels of major ambient air pollution components. Environ Health Perspect. 1995;103(Suppl):3–13. doi: 10.1289/ehp.95103s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice MB, Ljungman PL, Wilker EH, et al. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med. 2013;188(11):1351–1357. doi: 10.1164/rccm.201308-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cakmak S, Dales R, Leech J, Liu L. The influence of air pollution on cardiovascular and pulmonary function and exercise capacity: Canadian Health Measures Survey (CHMS) Environ Res. 2011;111(8):1309–1312. doi: 10.1016/j.envres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Dales R, Kauri LM, Cakmak S, et al. Acute changes in lung function associated with proximity to a steel plant: a randomized study. Environ Int. 2013;55:15–19. doi: 10.1016/j.envint.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Downs SH, Schindler C, Liu L-JS, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- 6.Rice MB, Ljungman PL, Wilker EH, et al. Long-Term Exposure to Traffic Emissions and Fine Particulate Matter and Lung Function Decline in the Framingham Heart Study. Am J Respir Crit Care Med. 2015;191(6):656–664. doi: 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepeule J, Litonjua AA, Coull B, et al. Long-term Effects of Traffic Particles on Lung Function Decline in the Elderly. Am J Respir Crit Care Med. 2014;190(5):542–548. doi: 10.1164/rccm.201402-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes LJL, Kapetanakis V, Rudnicka AR, et al. Chronic exposure to outdoor air pollution and lung function in adults. Thorax. 2009;64(8):657–663. doi: 10.1136/thx.2008.109389. [DOI] [PubMed] [Google Scholar]
- 9.Franco Suglia S, Gryparis A, Schwartz J, Wright RJ. Association between traffic-related black carbon exposure and lung function among urban women. Environ Health Perspect. 2008;116(10):1333–1337. doi: 10.1289/ehp.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam M, Schikowski T, Carsin AE, et al. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J. 2015;45(1):38–50. doi: 10.1183/09031936.00130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adar SD, Kaufman JD, Diez-Roux AV, et al. Air Pollution and Percent Emphysema Identified by Computed Tomography in the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect. 2014 Oct; doi: 10.1289/ehp.1307951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchetti N, Garshick E, Kinney GL, et al. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med. 2014;190(7):756–762. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger KI, Reibman J, Oppenheimer BW, Vlahos I, Harrison D, Goldring RM. Lessons from the World Trade Center disaster: airway disease presenting as restrictive dysfunction. Chest. 2013;144(1):249–257. doi: 10.1378/chest.12-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HS, Lee CG, Kim DH, et al. Emphysema prevalence related air pollution caused by a cement plant. Ann Occup Environ Med. 2016;28:17. doi: 10.1186/s40557-016-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannson K, Balmes J, Collard H. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest. 2015;147(4):1161–1167. doi: 10.1378/chest.14-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. doi: 10.1186/1471-2458-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massoli P, Fortner EC, Canagaratna MR, et al. Pollution Gradients and Chemical Characterization of Particulate Matter from Vehicular Traffic near Major Roadways: Results from the 2009 Queens College Air Quality Study in NYC. Aerosol Sci Technol. 2012;46(11):1201–1218. [Google Scholar]
- 20.Rosenbloom JI, Wilker EH, Mukamal KJ, Schwartz J, Mittleman MA. Residential proximity to major roadway and 10-year all-cause mortality after myocardial infarction. Circulation. 2012;125(18):2197–2203. doi: 10.1161/CIRCULATIONAHA.111.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilker EH, Ljungman PL, Rice MB, et al. Relation of long-term exposure to air pollution to brachial artery flow-mediated dilation and reactive hyperemia. Am J Cardiol. 2014;113(12):2057–2063. doi: 10.1016/j.amjcard.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloog I, Chudnovsky AA, Just AC, et al. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:581–590. doi: 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 24.Dorans KS, Wilker EH, Li W, et al. Residential Proximity to Major Roads, Exposure to Fine Particulate Matter, and Coronary Artery Calcium: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36(8):1679–1685. doi: 10.1161/ATVBAHA.116.307141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross JC, Estépar RSJ, Díaz A, et al. Lung extraction, lobe segmentation and hierarchical region assessment for quantitative analysis on high resolution computed tomography images. Med Image Comput Comput Assist Interv. 2009;12(Pt 2):690–698. doi: 10.1007/978-3-642-04271-3_84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estépar RSJ, Washko GG, Silverman EK, Reilly JJ, Kikinis R, Westin C-F. Accurate airway wall estimation using phase congruency. Med Image Comput Comput Assist Interv. 2006;9(Pt 2):125–134. doi: 10.1007/11866763_16. [DOI] [PubMed] [Google Scholar]
- 27.San José Estépar R, Reilly JJ, Silverman EK, Washko GR. Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc. 2008;5(9):905–909. doi: 10.1513/pats.200809-104QC. [DOI] [PubMed] [Google Scholar]
- 28.Washko GR, Kinney GL, Ross JC, et al. Lung Mass in Smokers. Acad Radiol. 2017;24(4):386–392. doi: 10.1016/j.acra.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz AA, Bartholmai B, San José Estépar R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104(8):1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araki T, Nishino M, Zazueta OE, et al. Paraseptal emphysema: Prevalence and distribution on CT and association with interstitial lung abnormalities. Eur J Radiol. 2015;84(7):1413–1418. doi: 10.1016/j.ejrad.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki T, Nishino M, Gao W, et al. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax. 2015;70(12):1156–1162. doi: 10.1136/thoraxjnl-2015-207653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, et al. Residential Proximity to Major Roadways, Fine Particulate Matter, and Adiposity: The Framingham Heart Study. Obesity. 2016;24(12):2593–2599. doi: 10.1002/oby.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181(4):353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 34.Castaldi PJ, San José Estépar R, Mendoza CS, et al. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188(9):1083–1090. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai PS, Hang JQ, Zhang FY, et al. Imaging phenotype of occupational endotoxin-related lung function decline. Environ Health Perspect. 2016;124(9):1436–1442. doi: 10.1289/EHP195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tashkin DP, Detels R, Simmons M, et al. The UCLA population studies of chronic obstructive respiratory disease: XI. Impact of air pollution and smoking on annual change in forced expiratory volume in one second. Am J Respir Crit Care Med. 1994;149(5):1209–1217. doi: 10.1164/ajrccm.149.5.8173761. [DOI] [PubMed] [Google Scholar]
- 37.Paulin L, Hansel N. Particulate air pollution and impaired lung function. F1000Research. 2016:5. doi: 10.12688/f1000research.7108.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725–734. doi: 10.1183/09031936.00206112. [DOI] [PubMed] [Google Scholar]
- 39.Calderón-Garcidueñas L, Mora-Tiscareño A, Fordham LA, et al. Lung radiology and pulmonary function of children chronically exposed to air pollution. Environ Health Perspect. 2006;114(9):1432–1437. doi: 10.1289/ehp.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 41.Schikowski T, Sugiri D, Ranft U, et al. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6:152. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindgren A, Stroh E, Montnémery P, Nihlén U, Jakobsson K, Axmon A. Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern Sweden. Int J Health Geogr. 2009;8:2. doi: 10.1186/1476-072X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schikowski T, Adam M, Marcon A, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J. 44(3):614–26. doi: 10.1183/09031936.00132213. [DOI] [PubMed] [Google Scholar]
- 44.Cai Y, Schikowski T, Adam M, et al. Cross-sectional associations between air pollution and chronic bronchitis: an ESCAPE meta-analysis across five cohorts. Thorax. 2014;69(11):1005–1014. doi: 10.1136/thoraxjnl-2013-204352. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann B, Moebus S, Möhlenkamp S, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116(5):489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 46.Wellenius GA, Boyle LD, Coull BA, et al. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60(11):2075–2080. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckel SP, Berhane K, Salam MT, et al. Residential traffic-related pollution exposures and exhaled nitric oxide in the children’s health study. Environ Health Perspect. 2011;119(10):1472–1477. doi: 10.1289/ehp.1103516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice MB, Rifas-Shiman SL, Litonjua AA, et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am J Respir Crit Care Med. 2016;193(8):881–888. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52(9):1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 50.Mehta AJ, Zanobetti A, Bind M-AC, et al. Long-Term Exposure to Ambient Fine Particulate Matter and Renal Function in Older Men: The Veterans Administration Normative Aging Study. Environ Health Perspect. 2016;124(9):1353–1360. doi: 10.1289/ehp.1510269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nwanaji-Enwerem JC, Colicino E, Trevisi L, et al. Long-term ambient particle exposures and blood DNA methylation age: findings from the VA normative aging study. Environ epigenetics. 2016;2(2) doi: 10.1093/eep/dvw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


